Abstract
Object
Low-frequency components of the spontaneous functional MR imaging signal provide information about the intrinsic functional and anatomical organization of the brain. The ability to use such methods in individual patients may provide a powerful tool for presurgical planning. The authors explore the feasibility of presurgical motor function mapping in which a task-free paradigm is used.
Methods
Six surgical candidates with tumors or epileptic foci near the motor cortex participated in this study. The investigators directly compared task-elicited activation of the motor system to activation obtained from intrinsic activity correlations. The motor network within the unhealthy hemisphere was identified based on intrinsic activity correlations, allowing distortions of functional anatomy caused by the tumor and epilepsy to be directly visualized. The precision of the motor function mapping was further explored in 1 participant by using direct cortical stimulation.
Results
The motor regions localized based on the spontaneous activity correlations were quite similar to the regions defined by actual movement tasks and cortical stimulation. Using intrinsic activity correlations, it was possible to map the motor cortex in presurgical patients.
Conclusions
This task-free paradigm may provide a powerful approach to map functional anatomy in patients without task compliance and allow multiple brain systems to be determined in a single scanning session.
Keywords: epilepsy surgery, functional connectivity, neurosurgery, tumor
Neurosurgical removal of a brain lesion requires minimizing postoperative functional deficits while maximizing the size of the resection itself. Information about the anatomical relationship between eloquent cortex and the lesion’s borders is therefore extremely valuable in planning the operation. The traditional approach is to inspect the pathological tissue in relation to anatomical landmarks. For example, the knob-like structure in the precentral gyrus is generally a good predictor of the location of the hand representation within the primary motor area.5,49 However, normal variation and distortions due to the brain lesion make it difficult to localize functional areas precisely based on anatomical landmarks alone.4,26,35,41
In response to these challenges, functional mapping in individual patients has become an important procedure for surgical planning and risk assessment. Invasive cortical mapping is often considered the gold standard for functional mapping.12,21,27,37,48 Direct cortical stimulation is managed perioperatively in the awake patient or in the presurgical patient with implanted subdural grids. Under these conditions, stimulation-induced disruption provides information about the location of eloquent cortex. With subdural grids, it is also possible to conduct evoked potential studies.2,20,39,46 The limitation of invasive functional mapping is that it usually occurs a short time before the planned resection, leaving little time to analyze the results and discuss options. Another drawback of invasive cortical mapping is the lack of information about deep brain structures, because the subdural grids only record the electrical potential on the brain surface.
Recently, fMR imaging has been offered as a noninvasive means of presurgical functional mapping.13,23,24,33,38,40 The basic approach is to conduct an imaging session while a patient performs a task set designed to target a single domain such as language, memory, or motor function. The images thus obtained are then used prior to the surgery to identify regions of functional activity. This approach is powerful because it allows detailed assessment of functional anatomy in a timely manner, and it includes deep brain structures. However, there are significant limitations. First, some patients have difficulty performing the required tasks, especially those who have developmental brain disorders, altered levels of consciousness, or other functional impairments.22,33 If a patient is not able to perform the prescribed task, the presently available functional mapping approaches may be unreliable or prove impossible.33 Second, specific task sets must be performed to target distinct functions (for example, language vs motor function). Although optimization is possible to allow efficient cycling through multiple functional domains, it is presently not possible to map multiple brain functions simultaneously. Even within the motor system, task epochs must alternate between separate motor acts (for example, hand vs tongue movements) to map their distinct anatomical locations.
Functional mapping based on spontaneous intrinsic activity offers an alternative approach to presurgical mapping.8 The procedure relies on the observation that brain systems exhibit slow, spontaneous activity fluctuations that can be measured using fMR imaging.16 Biswal and colleagues3 were the first to demonstrate that intrinsic activity could be used to map the motor system. In their seminal study, they showed that motor areas activated by actual motor movements could also be localized using spontaneous activity correlations. To do this, they measured the signal fluctuations in a left motor region while a participant simply rested with his or her eyes closed. Activity throughout the brain that spontaneously correlated with the left motor region was then localized. Their results revealed that the right motor cortex showed strong correlation as well as multiple other regions within the motor system. This approach—often referred to as fcMR imaging—has recently been used to map brain systems linked to motor function,9 vision,34 audition,25 memory,44 and attention.15 Although the underlying physiological bases of the slow intrinsic activity fluctuations that underlie fcMR imaging remain incompletely understood,16 the measured activity patterns probably reflect a combination of direct and indirect anatomical connectivity.43
Several properties of fcMR imaging make it a particularly promising tool for presurgical planning. First, the procedure is robust in individuals.10,44 In typical situations, activation maps can be obtained in approximately 20-minute scan sessions in almost all individuals. Second, task compliance is not required. Most fcMR imaging studies have been conducted during rest and fixation states. However, even that level of task compliance appears unnecessary. These fcMR imaging maps have been obtained during sleep19 and after induction of anesthesia,43 suggesting that patient compliance will not be required and that fcMR imaging can be performed in a “task-free” manner. As direct evidence of the feasibility of task-free mapping, fcMR imaging has been used to study brain systems in human infants.17 Finally, multiple brain systems can be simultaneously mapped from the same data. Thus, while still an early-stage concept, it seems possible that a single, simple procedure could be used for many functional mapping needs.
For all of these reasons we undertook a feasibility study to explore whether fcMR imaging is appropriate for functional mapping in presurgical patients. Most of the findings discussed above are based on the normal population. In clinical patients with tumors and epilepsy, not only the cortical anatomy but also the location of functional networks is distorted due to various pathological changes.24,29,30,47 It is thus of great interest to investigate whether fcMR imaging is applicable in clinical functional mapping, and furthermore to determine specifically if fcMR mapping is comparable to traditional task-based fMR imaging in patients being studied. If successful, fcMR imaging could provide a novel method for presurgical evaluation of eloquent cortex and free the patients from task compliance.
Motivated by this goal, we used fcMR imaging to study 6 surgical candidates with tumors or epileptic foci near the motor cortex. The fcMR imaging, in which the patients simply fixated their gaze on a small crosshair with no other task instruction, was conducted first. Approximately 3–5 minutes later, a traditional task-based fMR imaging study was obtained, in which the patients performed hand and tongue movements. This allowed us to compare task-elicited activation of the motor system directly to activation obtained from intrinsic activity correlations. In 1 patient, cortical stimulation was also conducted to define the hand motor area and tongue motor area for comparison with the fcMR imaging results.
Methods
Study Participants
Six epileptic patients with tumors or epileptic foci near the motor cortex participated in this study before surgery. Informed consent was obtained in accordance with the guidelines, and approval of the institutional review board at Massachusetts General Hospital and Children’s Hospital Boston was received. The individuals studied were typical of the patients for whom presurgical planning is conducted. The sex, age, and cause of epilepsy for each patient are listed in Table 1.
TABLE 1.
Characteristics of 6 patients with epilepsy who underwent task-free presurgical mapping*
| Case No. | Age (yrs), Sex | Handedness | Tumor Type | Location of Epileptic Foci |
|---|---|---|---|---|
| 1 | 12, F | rt | ependymoma | lt central area |
| 2 | 17, F | rt | oligodendroglioma | rt central area |
| 3 | 20, M | rt | NA | rt central area |
| 4 | 16, M | rt | not determined | bilat central areas |
| 5 | 16, F | rt | ependymoma | rt occipital area |
| 6 | 19, F | lt | venous angioma | lt sylvian fissure |
NA = not applicable.
Data Acquisition With MR Imaging
Images were acquired on a 3-T Tim Trio MR imaging unit with the vendor’s 12-channel head coil (Siemens). Pillows and padded clamps were used for head stabilization. Stimuli were displayed on a Macintosh laptop computer (Apple Computer, Inc.) using the Psychophysics Toolbox extensions6,36 in MATLAB (The MathWorks, Inc.). A liquid crystal display projected stimuli onto a screen at the head of the magnet bore, which was viewable via a mirror attached to the head coil. Structural images were acquired using a sagittal magnetization-prepared rapid gradient echo T1-weighted sequence (TR 2 seconds, TE 2.37 msec, flip angle 90°, slice number 160, 1-mm isotropic voxels).
The functional study consisted of blocked trial runs of actual motor movements (either hand or tongue) and rest runs during which no task was performed. All 6 participants performed 3 runs of the hand movement task. Four participants (the patients in Cases 2, 4, 5, and 6) also performed 3 runs of the tongue movement task. Images were acquired using an echo planar imaging gradient echo sequence sensitive to blood oxygen level–dependent contrast (TR 2 seconds, TE 30 msec, flip angle 90°, slice number 33, 3-mm isotropic voxels). Each task run consisted of three 36-second blocks of the movement task and four 28-second blocks of fixation. Eight seconds of dummy scans were acquired in the beginning of the run to allow for longitudinal magnetization stabilization. An addition 8 seconds of fixation was acquired at the end of the run to capture the full evolution of the hemodynamic response.
During the movement task blocks, a picture of a hand or tongue was displayed centrally on the screen for 2 seconds, and the participants were instructed to make a hand or tongue movement as soon as they saw the picture. Blocks contained movements of only 1 type. The inter-stimulus interval was 1 second. For the hand movement task, the participants were instructed to move only the hand contralateral to the tumor or epileptic foci. For the tongue movement task, the participants were instructed to move the tongue to touch the teeth on one side and then move it to the other side, while minimizing jaw and mouth movements.
All participants performed 4 passive fixation runs. Each run was 380 seconds long, and a black crosshair was centered on a white screen during the entire run. The participants simply fixated on the crosshair for the duration of each run. They were instructed to stay awake and to minimize head movement.
We acquired the images using 2 sets of parameters. Exploiting the high sensitivity of fcMR imaging, the patients in Cases 1–4 underwent scanning using high-resolution and long-TR-gradient echo planar imaging sequences (TR 5 seconds, TE 30 msec, flip angle 90°, slice number 55, 2-mm isotropic voxels). The patients in Cases 5 and 6 underwent scanning with lower-resolution and short TR sequences to match exactly the parameters used for the task scan (TR 2 seconds, TE 30 msec, flip angle 90°, slice number 33, 3-mm isotropic voxels).
Analysis of MR Imaging Data
Both the task and the resting-state data were preprocessed using the following steps: 1) slice timing correction (Statistical Parametric Mapping version 2, Wellcome Department of Cognitive Neurology); 2) rigid body correction for head motion;28 3) normalization for global mean signal intensity across runs; and 4) transformation of the data into a standard atlas space. The second step provided a record of head position that was later used as a nuisance regressor for functional connectivity analysis (see below). Atlas registration was achieved by computing affine transforms connecting the first image volume of the first functional run with the T1-weighted structural images (RIB Software). Our atlas representative template included data from 12 normal young adults made to conform to the Montreal Neurological Institute template by using previously described methods.7 Motion correction and atlas transformation were combined in 1 step to yield a motion-corrected volumetric time series resampled to 2-mm isotropic voxels.
Task data were analyzed using the general linear model18 as implemented in Statistical Parametric Mapping program, version 2. Regressors of no interest included motion correction parameters and low frequency drift. The task blocks use gamma function convolved with a boxcar function to model the hemodynamic response function.32
The resting-state data were analyzed using region-based fcMR imaging analysis as applied by Fox et al.15 and described in detail by Vincent et al.44 The steps are briefly described here. First, a 6-mm full-width half-maximum Gaussian spatial smoothing program was applied to the data. For each voxel, the blood oxygen level–dependent signal was then filtered by a 0.01- to 0.08-Hz Butterworth bandpass filter. The data were further processed to regress out several sources of spurious or regionally nonspecific variance, including the 6 motion parameters obtained from motion correction, the signal averaged over the whole brain, the signal averaged over the lateral ventricles, and the signal averaged over a region centered in the deep cerebral white matter.
To map the motor areas of the unhealthy hemisphere affected by tumor or epileptic foci, we defined seed regions in the healthy hemisphere based exclusively on the anatomical data and analyzed the functional connectivity between both hemispheres. In this manner, the motor areas as determined by fcMR imaging were identified completely independent of the task data and could therefore be compared in an unbiased manner to assess their spatial similarity. The mean time course in the seed region was extracted, and then its correlation with every other voxel was calculated. The correlation map was converted to a z-map by using the Fisher z transform. Finally, the z-map was registered back to the individual structural MR image for visualization.
Seed Region Selection
Seed regions localized to the approximate hand and tongue motor areas were selected on the healthy hemisphere based on anatomical characteristics. The hand motor area was identified as the knob-like structure in the precentral gyrus,49 just posterior to the junction of the superior frontal sulcus with the precentral sulcus. The high-resolution structural MR images were visually inspected. Once the typical knob-like structure was identified, a spherical seed region with a radius of 8 mm was positioned to cover the largest portion of the knob-like structure and transformed into the standard brain atlas for fcMR imaging analysis. The tongue motor areas are located anterior to the central sulcus and just superior to the sylvian fissure. Using subdural electrodes, Urasaki et al.42 found that the mean location of tongue motor area was 0.77 ± 0.93 cm anterior to the central sulcus and 1.6 ± 0.85 cm superior to the sylvian fissure. Based on the findings of Urasaki et al., a spherical seed region of 8 mm was positioned 1 cm anterior to the central sulcus and 2 cm superior to the sylvian fissure to estimate the presumed tongue motor cortex in the healthy hemisphere.
Cortical Stimulation
Motor function mapping in which direct cortical stimulation was used was performed in the patient in Case 2, in whom subdural electrode grids were subsequently implanted to localize the epileptic foci. The grids consisted of electrodes 3 mm in diameter with center-to-center distances of 10 mm. The placement of the grids is illustrated in the intraoperative photograph (see Fig. 4 upper, discussed in more detail later). Postimplantation CT images were coregistered to the preoperative structural MR images by using FreeSurfer38 to determine the location of the electrodes. Bipolar stimulation was applied on the electrodes on the motor and somatosensory cortex by using stimulus trains of 3-second pulses at 60 Hz. The current was increased from 1 to 15 mA, with a step of 2 mA. The stimulation on a pair of electrodes was stopped if the patient had a sensation or movement response.
Fig. 4.
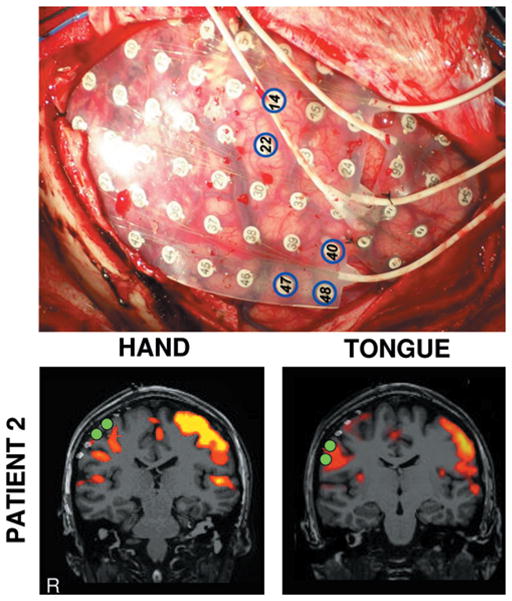
Comparison of results of direct cortical stimulation and fcMR imaging. The intraoperative photograph in the upper panel shows the grid placement for the patient in Case 2 and the locations of the electrodes that disrupted hand (14, 22) and tongue (40, 47, 48) movements. The fcMR analysis results are displayed in the lower panels; the hand motor region (left column) and the tongue motor region (right column) are displayed. Activation maps show the results of fcMR imaging analysis from Fig. 2. Filled green circles show the locations of cortical stimulation electrodes that selectively disrupted hand and tongue movements. The hand and tongue regions defined by fcMR imaging correlate with the estimates of the regions based on stimulation.
Results
Functional Mapping Based on fcMR Imaging is Highly Similar to Task-Based Localization
The hand motor regions localized based on the spontaneous activity correlations (Fig. 1, center column) were quite similar to the regions defined by actual movement tasks (Fig. 1, left column). Note that the visual cortex was also activated in the task runs because the patients performed the movements following a visual cue. Overlapping areas derived from these two functional mapping techniques are also shown (Fig. 1, right column). Note the overlap of the estimate of the motor area’s location in relation to the lesion location. These results indicated that the fcMR imaging is able to localize the motor area precisely.
Fig. 1.
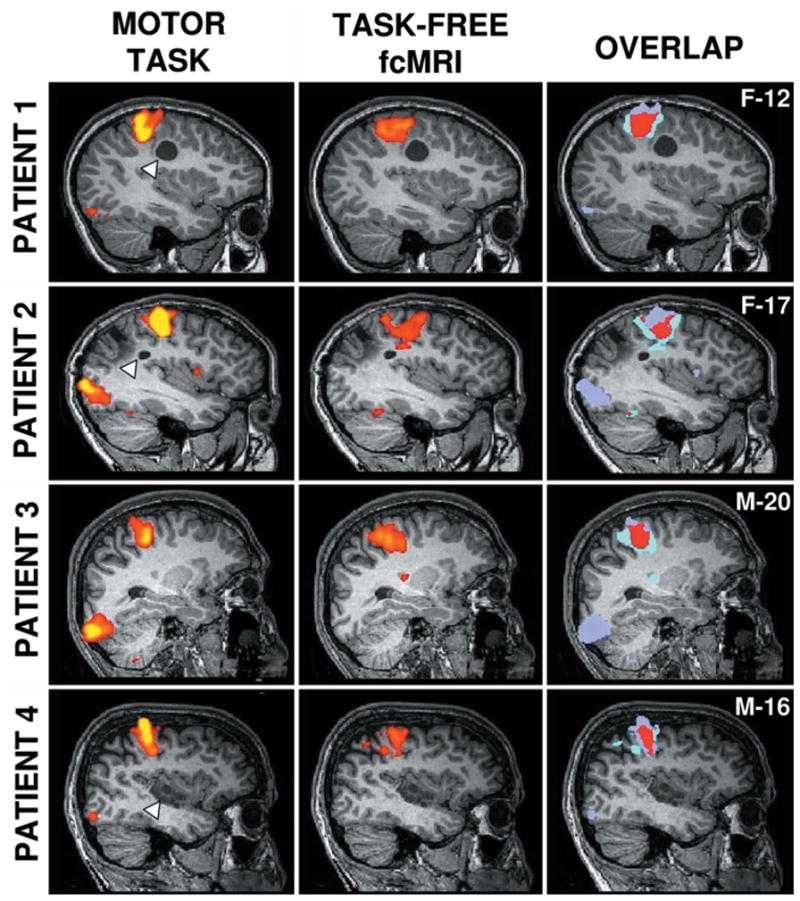
Task and fcMR imaging–based mapping localizing similar regions. Hand motor regions defined by actual motor task movements (left column) and task-free fcMR imaging (center column) are plotted on sagittal sections for each patient (overlaid on their structural image). Colors represent Z values, with the threshold set to Z = 0.4–0.5. The overlap of the two techniques is shown in red (right column). Each row displays a different patient, with the sex (M or F) and age (in years) indicated in the rightmost panel. Brain lesions are indicated by the white triangles for the patients in Cases 1, 2, and 4; the patient in Case 3 has no visible lesion.
Functional Mapping Based on fcMR Imaging Shows Selectivity for Hand and Tongue Regions
Although hand and tongue regions are separated by only a few centimeters on the motor “homunculus,”1 they can be clearly distinguished using this task-free mapping technique. The tongue regions localized by fcMR imaging (Fig. 2, center column) are highly similar to the regions defined by actual movements (Fig. 2, left column).
Fig. 2.
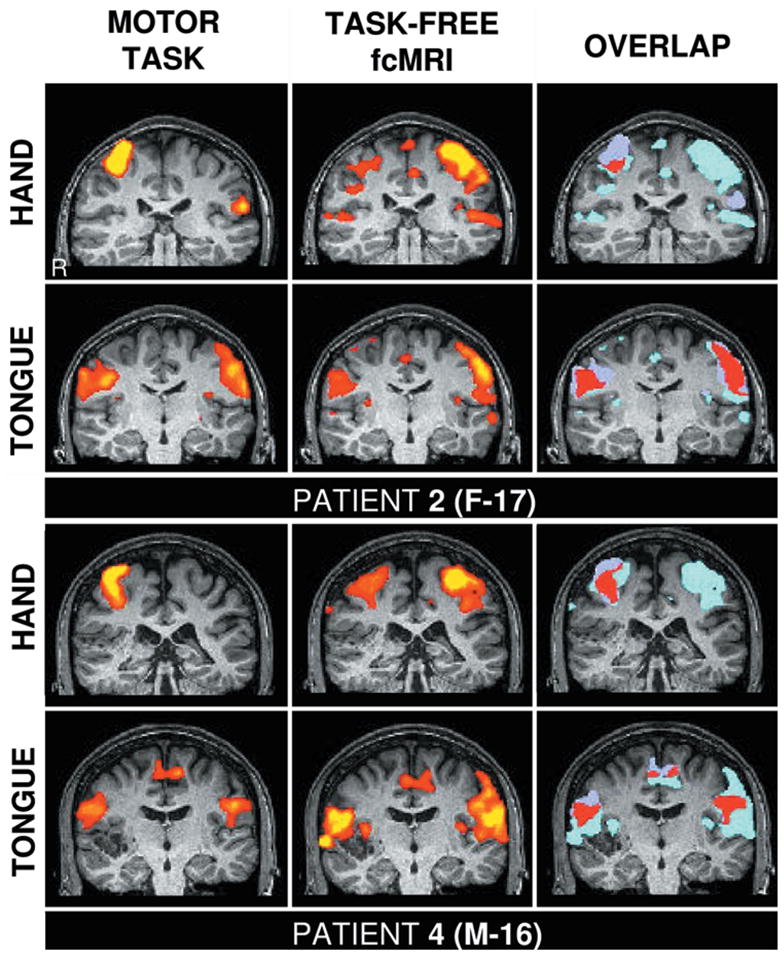
Functional mapping based on fcMR imaging is anatomically specific. These studies are comparisons of hand and tongue motor regions defined by actual motor task movements (left columns) and task-free fcMR imaging (center columns). The overlap of the two techniques is shown in red (right columns). The upper panels show data from the patient in Case 2, and the lower panels from the patient in Case 4. The right hemisphere is displayed on the right side of the panel. Note the systematic shift of the location of the hand and tongue regions in each patient, which is present for the fcMR analysis. The fcMR analysis of the hand region in the patient in Case 2 is less stable than other measures, possibly due to the location of the seizure activity (see text).
We also found that the correlation between left and right hand regions was weaker in the patient in Case 2 than in the other patients. The hand motor region defined by fcMR imaging showed some discontinuity, although the location was correct. This variation could be due to methodological noise, although it is interesting to note that, based on subdural electrode recordings, the epileptic focus in this patient was localized in or near the hand motor region in the right hemisphere. The finding of weakened correlation between left and right hand regions indicates that functional connectivity between the hemispheres may be disrupted due to the epileptic discharges. We consider this a preliminary observation, but nonetheless draw attention to it because of its implications both for fcMR imaging mapping and for the potential use of this technique to detect diseased tissue.
Functional Mapping Based on fcMR Imaging is Robust Across Different Image Resolutions
One technical question of fcMR imaging is how robust the mapping is when acquisition resolutions change. In the first 4 patients, we used 2-mm isotropic voxels as we tried to push the limits of achievable resolution with fcMR images. In the next 2 patients, we acquired the images by using a larger voxel size and sequence parameters matched to the task scan. The hand and tongue regions were defined for the patients in Cases 5 and 6 (Fig. 3). The maps demonstrate that fcMR imaging and task mapping produce similar results when the image resolution is held constant. Moreover, our observations suggest that fcMR imaging is robust across acquisition resolution, and thus is likely to provide a powerful method across a range of parameter selections.
Fig. 3.
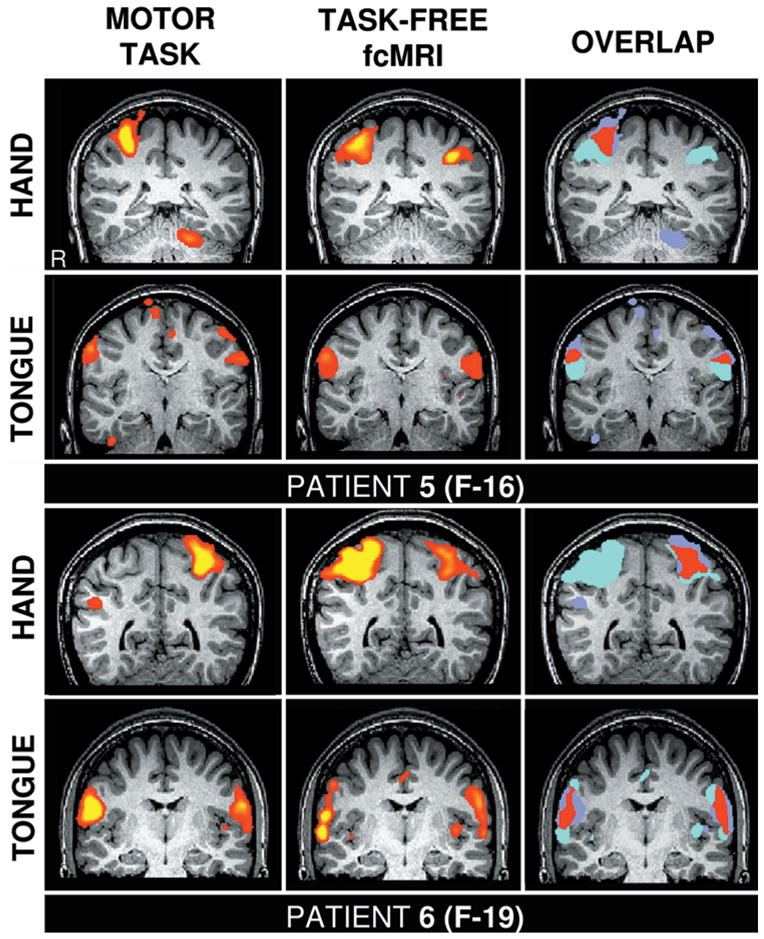
Functional mapping based on fcMR imaging is robust across different image resolutions. These studies are comparisons of hand and tongue motor regions defined by actual motor task movements (left column) and task-free fcMR imaging (center column), similar to those in Fig. 2. The resting-state images were acquired using 3-mm isotropic voxels and 2-second TR, as in the task scans.
Functional Mapping Based on fcMR Imaging is Correlated With Direct Cortical Stimulation
The patient in Case 2 was further studied using direct cortical stimulation during her surgery. Stimulation of electrodes 14 and 22 (marked by blue circles in Fig. 4 upper) caused left hand movement, indicating that the hand motor area lay beneath these electrodes. Note that based on analysis of clinical seizures, the epileptic foci were also localized to the area covered by these 2 electrodes. Electrodes 40, 47, and 48 caused tongue movement, indicating that the tongue motor area lay beneath these electrodes. To determine correspondence, the CT and fcMR images were both overlaid onto the structural MR images (Fig. 4 lower left). The electrodes responsible for hand and tongue movements are marked in green. Our results indicated that the fcMR imaging functional mapping is consistent with the findings from cortical stimulation (Fig. 4 lower right).
Discussion
In the present study, we investigated the feasibility of using fcMR imaging for presurgical mapping. Many studies have explored the topography of brain systems by measuring intrinsic brain activity in healthy participants3,9,11,15,25,31,34,44 (for a recent review, see Fox and Raichle16). Here we demonstrated that, in patients with tumors and focal epilepsy, fcMR imaging was capable of mapping motor cortex by using seed regions selected based on the anatomical features in the healthy hemisphere. Of the 6 patients, 5 had clear anatomical distortion near the motor cortex. The fcMR imaging was nonetheless able to provide functional mapping of the motor cortex and yield results consistent with those derived from traditional task-based localization. Four of the 6 patients received surgical treatments after the presurgical mapping. None of these patients experienced a new neurological deficit after the surgery. These findings provide a proof of the concept that task-free methods of presurgical mapping can use intrinsic activity and complement task-based approaches. Task-free methods may be particularly useful for patients who are not able to perform prescribed tasks in the scanner due to young age, altered level of consciousness, or other functional disorders.
The fMR imaging method has been an important tool for presurgical mapping, but it typically requires the participation of the patient in a task and is limited by the number of paradigms that can be run in a single scanning session. This limitation can be overcome by the task-free fcMR imaging paradigm used here. In principle, multiple functional systems can be determined based on a single scanning session. To date, a variety of brain systems have been explored using fcMR imaging, including motor,3,9 visual,34 auditory,25 memory,44 and attention15 systems. Of direct relevance, all of these explorations used procedures that are similar to those applied here to study presurgical patients. Therefore, fcMR imaging provides a unique opportunity to evaluate multiple brain functions simultaneously.
A critical problem in presurgical mapping is to determine the dominant hemisphere for language and memory. Lateralization is often accomplished through anesthetization of one brain hemisphere by using the invasive Wada45 test, which has risks and discomfort. The need to replace the Wada test with less invasive and more reliable techniques has long been recognized.1 Cordes et al.9 used fcMR imaging to study the language system and produced a map similar to that obtained using a word-generation task. Vincent et al.44 recently used fcMR imaging to identify a hippocampal-cortical memory system that was similar to that activated by tasks involving remembering. These studies suggest that intrinsic activity may be amenable to functional mapping well beyond the motor system. In particular, functional mapping based on fcMR studies may eventually lead to new language and memory lateralization techniques.
An unexpected implication of the present results is that fcMR imaging may reflect the deficit caused by pathological changes that are not discernible using traditional fMR studies. In the patient in Case 2, epileptic foci were localized to the hand motor area in the right hemisphere. The symptoms of the seizure included left hand “floppiness” and loss of control of the left hand. In traditional task-based fMR imaging, there is no indication of the deficit in the hand motor area. However, in the fcMR map, the functional connectivity between the left and right hand motor areas was weakened and disrupted, while the connectivity between tongue areas remained intact (Fig. 2).
Conclusions
Although only a preliminary observation, this finding suggests that fcMR imaging may be sensitive to the functional changes associated with the diseased tissue. Future studies will be required to determine both the implications of this effect on the robustness of fcMR procedures for task-free mapping and also to explore directly whether such effects can be used intentionally to identify diseased tissue.
Acknowledgments
The authors thank Abraham Snyder and Itamar Kahn for assistance with fcMR imaging procedures, and the Athinoula A. Martinos Center for Biomedical Imaging for support. They also thank M. Takeoka, A. Pinto, and S. Manganaro for assistance with cortical stimulation.
Abbreviations used in this paper
- fcMR
functional connectivity MR
- fMR
functional MR
Footnotes
Disclosure
This work was supported by National Center for Research Resources (P41RR14074), National Institutes of Health Grant No. K08MH067966, the Mental Illness and Neuroscience Discovery Institute, and the Howard Hughes Medical Institute. Dr. Buckner has applied for a patent for using functional connectivity analysis. The authors report no other conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Abou–Khalil B, Schlaggar BL. Is it time to replace the Wada test? Neurology. 2002;59:160–161. doi: 10.1212/wnl.59.2.160. [DOI] [PubMed] [Google Scholar]
- 2.Berger MS, Cohen WA, Ojemann GA. Correlation of motorcortex brain mapping data with magnetic resonance imaging. J Neurosurg. 1990;72:383–387. doi: 10.3171/jns.1990.72.3.0383. [DOI] [PubMed] [Google Scholar]
- 3.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–554. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 4.Bittar RG, Olivier A, Sadikot AF, Andermann F, Pike GB, Reutens DC. Presurgical motor and somatosensory cortex mapping with functional magnetic resonance imaging and positron emission tomography. J Neurosurg. 1999;91:915–921. doi: 10.3171/jns.1999.91.6.0915. [DOI] [PubMed] [Google Scholar]
- 5.Boroojerdi B. Localization of the motor hand area using transcranial magnetic stimulation and functional magnetic resonance imaging. Clin Neurophysiol. 1999;110:699–704. doi: 10.1016/s1388-2457(98)00027-3. [DOI] [PubMed] [Google Scholar]
- 6.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 7.Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 10.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg. 1999;91:238–250. doi: 10.3171/jns.1999.91.2.0238. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez G, de Greiff A, von Oertzen J, Reuber M, Lun S, Klaver P, et al. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. Neuroimage. 2001;14:585–594. doi: 10.1006/nimg.2001.0854. [DOI] [PubMed] [Google Scholar]
- 14.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 15.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 17.Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 19.Fukunaga M, Horovitz S, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Gregorie EM, Goldring S. Localization of function in the excision of lesions from the sensorimotor region. J Neurosurg. 1984;61:1047–1054. doi: 10.3171/jns.1984.61.6.1047. [DOI] [PubMed] [Google Scholar]
- 21.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Haughton VM, Turski PA, Meyerand B, Wendt G, Moritz CH, Ulmer J. The clinical applications of functional MR imaging. Neuroimaging Clin N Am. 1999;9:285–293. [PubMed] [Google Scholar]
- 23.Hirsch J, Ruge MI, Kim KH, Correa DD, Victor JD, Relkin NR, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery. 2000;47:711–721. doi: 10.1097/00006123-200009000-00037. [DOI] [PubMed] [Google Scholar]
- 24.Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol. 2000;21:1415–1422. [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter MD, Eickhoff SB, Miller TW, Farrow TF, Wilkinson ID, Woodruff PW. Neural activity in speech-sensitive auditory cortex during silence. Proc Natl Acad Sci U S A. 2006;103:189–193. doi: 10.1073/pnas.0506268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki S, Nakagawa H, Fukusumi A, Kichikawa K, Kitamura K, Otsuji H, et al. Identification of pre- and postcentral gyri on CT and MR images on the basis of the medullary pattern of cerebral white matter. Radiology. 1991;179:207–212. doi: 10.1148/radiology.179.1.2006278. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Thompson RM, Butts RK, Sharbrough FW, Kelly PJ, Hanson DP, et al. Sensory motor cortex: correlation of pre-surgical mapping with functional MR imaging and invasive cortical mapping. Radiology. 1994;190:85–92. doi: 10.1148/radiology.190.1.8259434. [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 29.Krings T, Töpper R, Willmes K, Reinges MH, Gilsbach JM, Thron A. Activation in primary and secondary motor areas in patients with CNS neoplasms and weakness. Neurology. 2002;58:381–390. doi: 10.1212/wnl.58.3.381. [DOI] [PubMed] [Google Scholar]
- 30.Lee M, Reddy H, Johansen-Berg H, Pendlebury S, Jenkinson M, Smith S, et al. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol. 2000;47:606–613. [PubMed] [Google Scholar]
- 31.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 33.Mueller WM, Yetkin F, Zerrin H, Thomas A, Morris GL, Swanson SJ, et al. Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors. Neurosurgery. 1996;39:515–521. doi: 10.1097/00006123-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Ojemann GA. Individual variability in cortical organization of language. J Neurosurg. 1979;50:164–169. doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- 36.Pelli DG. The VideoToolbox software for visual psychophysics. Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- 37.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 38.Roux FE, Boulanouar K, Ranjeva JP, Tremoulet M, Henry P, Manelfe C, et al. Usefulness of motor functional MRI correlated to cortical mapping in rolandic low-grade astrocytomas. Acta Neurochir (Wien) 1999;141:71–79. doi: 10.1007/s007010050268. [DOI] [PubMed] [Google Scholar]
- 39.Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678–685. [PubMed] [Google Scholar]
- 40.Stapleton SR, Kiriakopoulos E, Mikulis D, Drake JM, Hoffman HJ, Humphreys R, et al. Combined utility of functional MRI, cortical mapping, and frameless stereotaxy in the resection of lesions in eloquent areas of brain in children. Pediatr Neurosurg. 1997;26:68–82. doi: 10.1159/000121167. [DOI] [PubMed] [Google Scholar]
- 41.Steinmetz H, Furst G, Freund HJ. Variation of perisylvian andcalcarine anatomic landmarks within stereotaxic proportional coordinates. AJNR Am J Neuroradiol. 1990;11:1123–1130. [PMC free article] [PubMed] [Google Scholar]
- 42.Urasaki E, Uematsu S, Gordon B, Lesser RP. Cortical tongue area studied by chronically implanted subdural electrodes–with special reference to parietal motor and frontal sensory responses. Brain. 1994;117:117–132. doi: 10.1093/brain/117.1.117. [DOI] [PubMed] [Google Scholar]
- 43.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 44.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 45.Wada J. A new method for determination of the side of cerebral speech dominance: a preliminary report on the intracarotid injection of sodium amytal in man. Igaku Seibutsugaku. 1949;14:221–222. [Google Scholar]
- 46.Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg. 1979;51:476–506. doi: 10.3171/jns.1979.51.4.0476. [DOI] [PubMed] [Google Scholar]
- 47.Wunderlich G, Knorr U, Herzog H, Kiwit JC, Freun HJ, Seitz RJ. Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery. 1998;42:18–26. doi: 10.1097/00006123-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Yetkin FZ, Mueller WM, Morris GL, McAuliffe TL, Ulmer JL, Cox RW, et al. Functional MR activation correlated with intraoperative cortical mapping. AJNR Am J Neuroradiol. 1997;18:1311–1315. [PMC free article] [PubMed] [Google Scholar]
- 49.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]


