Abstract
Purpose
Herpes simplex virus-type 2 (HSV-2) can cause Acute Retinal Necrosis (ARN), which can lead to exudative and rhegmatogenous retinal detachment; yet, little is know about the cellular and molecular mechanisms of HSV-2 entry into retinal pigment epithelial (RPE) cells. The goal of this study was to establish the identity of the critical receptors utilized by the virus for infection.
Methods
A reporter HSV-2 virus, which expresses beta-galactosidase, was used to quantify entry into RPE cells and viral replication was ascertained using a plaque assay. Flow cytometry and immunocytochemistry were used to determine cellular expression of entry receptors. Localization of these receptors to either the apical or basal surface of RPE cells was determined using immunocytochemistry. The necessity of these receptors, both individually and in combination, for viral entry was established using both receptor-specific antibodies and siRNAs.
Results
RPE are highly susceptible to HSV-2 entry and replication. Several assays demonstrated the expression of the entry receptors nectin-1, HVEM, and PILR-alpha and their localization primarily to the apical surface of RPE cells. Receptor-specific antibodies and siRNA knockdown of receptors significantly reduced viral entry and implicated nectin-1 as an important receptor with HVEM and PILR-alpha potentially also contributing to entry.
Conclusions
HSV-2 is capable of developing a productive infection in RPE cells by utilizing nectin-1 as an important entry receptor. To lesser degrees, HVEM and PILR-alpha may also contribute to HSV-2 entry into RPE cells.
INTRODUCTION
Herpes simplex virus (HSV) is the leading cause of infectious blindness in developed nations.1 It is also known to infect a variety of cell types and cause numerous ocular diseases including blepharitis, stromal keratitis, chorioretinitis, and retinitis, among others.2,3 Each year there are 500,000 primary cases of HSV-2 infection in the US and at least 22% of the population has a latent infection.4,5 It has been accepted that virtually all patients infected with HSV-2 will have recurrent disease.3 The urgency of developing a better understanding of HSV-2 pathogenesis is increasing given the discovery of new drug-resistant strains.6 The prevalence of perinatal infections ranges from 1 in 2000 to 1 in 5000 births per year in the US.7 HSV-2 infection of the retina is also the leading cause of acute retinal necrosis in persons younger than 25.8,9
HSV-2 induced retinitis and acute retinal necrosis (ARN) are devastating infections seen in both immunocompromised and immunocompetent patients.10-14 ARN is a blinding disease marked by rapidly progressive peripheral retinal necrosis and was first described in humans by Urayama.15 It has been found that ARN caused by HSV may be the result of direct viral invasion or a recurrence of a previous episode of retinitis or keratitis caused by the virus.3,16,17 Latent infections have been known to be triggered by such events as trauma, neurosurgery, or high-dose corticosteroids.18 The disease is typically characterized by inflammatory orbitotopathy, proptosis and optic nerve involvement and can lead to exudative and rhegmatogenous retinal detachment.19-21 Due to the escalating prevalence of HSV-2, it has been predicted that the incidence of retinitis and ARN will grow even higher.22
In general, HSV entry into host cells is a multi-step process that begins with the specific binding of viral envelope glycoproteins to the host-cell surface receptors. Glycoproteins B and C (gB and gC) mediate the initial attachment of the virions to certain cell-surface glycosaminoglycans, most notably heparan sulfate.23,24 Following the interaction with heparan sulfate, a conformational change allows glycoprotein D (gD) to bind to its receptor.25-27 Then, a concerted action involving gD, its receptor, three additional HSV glycoproteins (gB, gH, and gL), a gB co-receptor, and possibly other gH co-receptors trigger fusion of the viral envelope with the host cell membrane.28-31 Recently, paired immunoglobulin-like type 2 receptor-alpha (PILR-α), has been implicated as a gB-coreceptor for HSV-1 entry.31 The significance of PILR-α, however, is not known for HSV-2 entry into its host cells.
HSV-1 and HSV-2 may differ in which cellular receptor gD binds to. There are several known gD receptors categorized into three structurally unrelated families. These include nectin-1 and nectin-2, members of the immunoglobulin superfamily;32-34 HVEM, a member of the tumor necrosis factor (TNF) receptor family;35 and a modified form of heparan sulfate: 3-O-sulfated heparan sulfate (3-OS HS).24,32,36 Nectin-1 and nectin-2 mediate entry of HSV-1 and HSV-2, although nectin-2's HSV-1 entry mediating activity is limited to some mutant strains.25,33,34 Nectin-1 is extensively expressed in human cells of epithelial and neuronal origin, while nectin-2 is widely expressed in many human tissues, but has limited expression in neuronal cells.33,37 HVEM mediates entry of HSV-1 into T-lymphocytes and trabecular meshwork cells and HSV-2 entry into corneal fibroblasts.35,38,39 HSV-1, but not HSV-2, entry is known to be mediated by 3-OS HS, which is expressed in many human cell lines.24,36
Retinal pigment epithelial cells have a vital role in the maintenance of the human retina, and HSV-2 infection in these cells has disastrous consequences for vision. Despite this, the identity of important HSV-2 entry receptors in RPE cells remains unknown. In this study we sought to characterize the cellular and molecular mechanisms of HSV-2 entry into RPE cells. This study demonstrates that nectin-1, HVEM, and PILR-α are primarily expressed on the apical surface of RPE cells. We also demonstrate that HSV-2 utilizes nectin-1 to enter into RPE cells, yet is also capable of utilizing HVEM and possibly, PILR-α for entry. This is the first study to investigate a role for PILR-α in HSV-2 infection and the first report of a natural target cell type in which nectin-1 was found to significantly contribute to HSV-2 entry.
MATERIALS AND METHODS
Cells, Viruses, and Antibodies
RPE cells were provided by B.Y.J.T. Yue (University of Illinois at Chicago). They were originally obtained from ATCC (Manassas, VA) and are spontaneously arising ARPE-19 cells. The cells were grown in L-glutamine containing Dulbecco's Modified Eagles Medium (DMEM) from Invitrogen Corp. (Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). Cells were trypsinized and passaged after reaching confluence.
Patricia Spear (Northwestern University, Chicago, IL) provided African green monkey kidney (Vero) cells, and murine melanoma (B78H1) cells. These cells were cultured as previously described.35,40 HSV-2(333) and HSV-2(333)gJ-viruses were also provided by Patricia Spear.41 The virus stocks were propagated in complementing cell lines and titered on Vero cells and stored at -80°C.
Anti-nectin-1 antibodies used were poliovirus receptor related 1 (PRR1) antibody, specific for nectin-1 (Beckman Coulter, Fullerton, CA) and monoclonal mouse anti-nectin-1 (Zymed Laboratories, San Francisco, CA, catalog no. 37-5900). Monoclonal mouse anti-HVEM antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, catalog no. sc-74089). Monoclonal rat anti-PILR-α antibody was purchased from Dendritics (Lyon, France, catalog no. ddx0230). FITC-conjugated anti-mouse IgG and FITC-conjugated anti-rat IgG antibodies were obtained from Sigma-Aldrich (St. Louis, MO).
Viral Entry Assays
Viral entry assays were based on the quantification of β-galactosidase expressed from the viral genome. RPE cells and naturally resistant B78H1 cells40 were washed with 1x phosphate buffered saline (PBS) and exposed to 50 μL of serially diluted recombinant HSV-2(333)gJ-, which expresses β-galactosidase after entry into cells. Virus was serially diluted in PBS with 3% bovine serum albumin (BSA). After 6 hours, cells were washed with 1x PBS and incubated with the β-galactosidase substrate, o-nitrophenyl-β-D-galactopyranoside (ImmunoPure ONPG; Pierce, Rockford, IL), as previous described.38 The enzymatic activity was monitored at 410 nm by spectrophotometry (Molecular Devices spectra MAX 190, Sunnyvale, CA). HSV entry into RPE and B78H1 cells was also confirmed qualitatively by 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) assays, as previously described.39 In essence, cells were infected for 6 hours with the reporter virus, then washed, fixed, permeabilized, and incubated with the X-gal substrate, which produces a blue color in cells when β-galactosidase acts on it. Microscopy was performed using the 20X objective of the inverted microscope (Zeiss, Axiovert 100M). The slide book version 3.0 was used for images. All experiments were repeated a minimum of three times unless otherwise noted.
Time Point Plaque Assay
Confluent monolayers of RPE, B78H1, and Vero cells (approximately 106) in 24 well culture dishes were infected with HSV-2(333) at 0.01 PFU/cell or mock infected for 2 hours at 37°C. After removal of the inoculum, 1 mL of the cell type-appropriate media was added and the dishes were incubated at 37°C. At different time points: 0, 24, 48, and 72 hours post-infection, the cells were fixed using fixative buffer (2% formaldehyde and 0.2% glutaradehyde) at room temperature for 20 min, followed by Giemsa staining for 25 min. The cells were then washed five times with nanopure water, and the numbers of plaques were counted as a measure of viral replication. The images were taken using the 10x objective of a Zeiss Axiovert 100 microscope.
Flow Cytometry
Flow cytometry analysis was performed to determine cell surface expression of nectin-1, HVEM and PILR-α. Monolayers of approximately 2×106 RPE cells were transferred to 1.5 mL Eppendorf tubes, washed with 1x PBS, and incubated at 4°C for 90 min with either anti-nectin-1 (1:50 dilution), anti-HVEM (1:20 dilution), or anti-PILR-α (1:50) antibody. Cells were then washed with 1x PBS and incubated at 4°C for 45 min with the appropriate secondary antibody (1:1000 dilution). FITC-conjugated anti-mouse IgG (Sigma) was used for nectin-1 and HVEM and FITC-conjugated anti-rat IgG (Sigma) was used for PILR-α. RPE cells stained with only with FITC-conjugated secondary anti-mouse IgG or FITC-conjugated anti-rat IgG were used as background controls. Cells were then examined by fluorescence-activated cell sorter (FACS) analysis.
Immunocytochemistry
Chamber slides (Lab-Tek; Nunc, Rochester, NY) were plated with monolayers of RPE cells in order to visualize the receptors on the cell-surface. Cells were incubated at 4°C for 45 minutes with either anti-nectin-1 (1:25 dilution), anti-HVEM (1:10 dilution), or anti-PILR-α (1:25 dilution) primary antibody. They were then washed 6 times with cold PBS and fixed with acetone for 10 minutes at -20°C. Cells were then blocked with PBS containing 10% calf serum for 15 minutes and incubated at 37°C for 30 min with the appropriate secondary antibodies (1:250 dilution), as described for flow cytometry. After incubation with secondary antibody, cells were washed 6 times with PBS and imaged using laser-scanning spectrum confocal microscopy (TCS SP2; Leica). Cells treated only with the secondary antibody were used as controls.
Immunocytochemistry was also used to image receptor expression on the apical and basal surfaces of RPE cells. Cell-culture inserts (Millicell; Millipore, Billerica, MA) with a 1 μm pore size were plated with monolayers of RPE cells and exposed to primary antibodies on either their apical or basal surface. The specific antibodies, their dilutions, and the remainder of the procedure were the same as those used in the previous immunocytochemistry assay. The side not exposed to the antibodies was incubated with an equivalent volume of PBS containing 3% BSA. Cells only exposed to the appropriate secondary antibody on either their apical or basal surfaces were used as controls.
Antibody Blocking of Receptors
RPE cells were plated onto 96-well plates, and the appropriate antibody was added in serial dilution. Anti-PRR1 (specific for nectin-1), anti-HVEM, anti-PILR-α antibodies were used to block the corresponding receptor. The plate was incubated with primary antibody at room temperature for 2 hours. Viral entry assays were then performed utilizing identical doses of HSV-2(333)gJ- as described previously.39 Cells incubated with a control primary antibody (α-TGFβR II) were used as a control. The experiment was repeated three times with similar results.
siRNA Interference of Major Receptors
siRNAs that downregulated nectin-1 (NM_002855_1, Sigma), HVEM (NM_003820_1, Sigma), and PILR-α (Santa Cruz Biotech., Santa Cruz, CA, catalog no. sc-89726) were used to interfere with the expression of these receptors. RPE cells were plated onto 6-well culture dishes and were transfected with the corresponding RNA duplexes or control scrambled RNA duplexes. After 48 hours, cells were loosened with Cell Dissociation Buffer (Invitrogen Corp.) and replated onto 96-well plates. An aliquot of the cells was set aside to be used for Western Blot analysis. Cells were also transfected with both nectin-1 and HVEM siRNA or all three siRNAs to determine any cumulative effect. Viral entry assays were then performed, as previously described, with serial dilutions of HSV-2(333)gJ-. β-galactosidase activity was quantified using a spectrophotometer (Molecular Devices) at an optical density of 410 nm.
Western Blot Analysis of Receptor Expression
The siRNA downregulated receptors were tested for expression via Western Blot. Whole cell lysates of the siRNA transfected cells previously set aside were immediately boiled for 10 minutes, and equal amounts of protein were subjected to 10% SDS-PAGE and electroblotted onto a nitrocellulose membrane. Non-specific binding was blocked using 5% nonfat milk in PBS for 2 hours at 37°C. The membrane was incubated with primary antibodies to HVEM (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), nectin-1 (1:1000; Zymed Laboratories, San Francisco, CA), and PILR-α (1:500; Dendritics, Lyon, France) or β-actin (1:5000, Sigma) overnight at 4°C. The membrane was then washed 5 times for 5 minutes each time with 0.1% Tween 20/PBS, followed by incubation for 1 hour with HRP-conjugated anti-mouse secondary antibody for nectin-1 and HVEM (1:20000; Jackson ImmunoResearch, West Grove, PA; catalog no. 115-035-062) and HRP-conjugated-streptavidin for PILR-α (1:10000; ThermoScientific, Rockford, IL). The membrane was again washed 5 times for 5 minutes each time with 0.1% Tween 20/PBS, and bands were visualized by exposure to X-ray film after the addition of chemiluminescent substrate (ECL; Thermo Scientific).
Statistical Analysis
All experiments were conducted a minimum of three times with similar results and quantitative data are expressed as mean ±SD. A paired t-test was performed for quantitative data to establish statistical differences between experimental and control groups (P < 0.05).
RESULTS
Viral Entry and Replication in RPE Cells
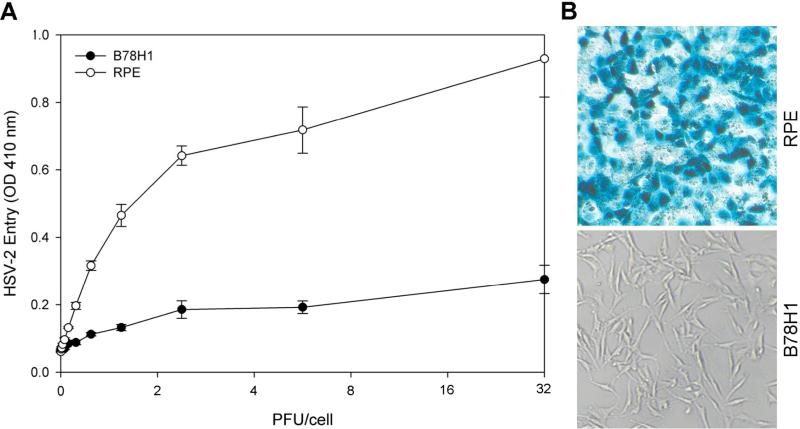
To establish HSV-2 entry in RPE cells, confluent monolayers of cells were plated in 96-well culture dishes and infected with serial dilutions of recombinant HSV-2(333)gJ-, which expresses β-galactosidase upon entry into cells. Naturally resistant B78H1 cells were used as a negative control. Viral entry was measured after 6 hours of infection. As shown in Figure 1A, there was significantly more entry in RPE cells than in B78H1 cells in a dose-dependant manner. Similar results were also obtained when the cells were analyzed for HSV-2 entry using the insoluble β-galactosidase substrate X-gal (Figure 1B). As expected, no β-galactosidase activity was observed in B78H1 cells, but activity was observed in RPE cells (blue color).
Figure 1.
HSV-2 can enter into cultured RPE cells. (A) Dose response curve of HSV-2 entry into RPE cells. Cultured RPE cells along with naturally HSV-2 resistant B78H1 cells were plated in 96-well culture dishes and inoculated with two-fold serial dilutions of β-galactosidase-expressing recombinant HSV-2(333)gJ- virus at the plaque forming units (PFU) indicated. After 6 hours, the cells were washed, permeablized, and incubated with ONPG substrate for quantification of β-galactosidase activity. The enzymatic activity was measured at an optical density of 410 nm (OD 410 nm) using a spectrophotometer. In this and other figures each value shown is the mean of three or more determinations (± SD). (B) Confirmation of HSV-1 entry into RPE cells by X-gal staining. RPE cells grown (4 × 106 cells) in six well dishes were challenged with β-galactosidase-expressing recombinant HSV-2(333)gJ- at 20 PFU/cell. B78H1 cells were also infected in parallel as a negative control. After 6 h of infection at 37° C, cells were washed, fixed and permeabilized, and incubated with X-gal, which yields an insoluble blue product upon hydrolysis by β-galactosidase. Blue cells (representing viral entry) were seen as shown. Microscopy was performed using a 20 × objective of a Zeiss Axiovert 100 microscope. The slide book version 3.0 was used for images.
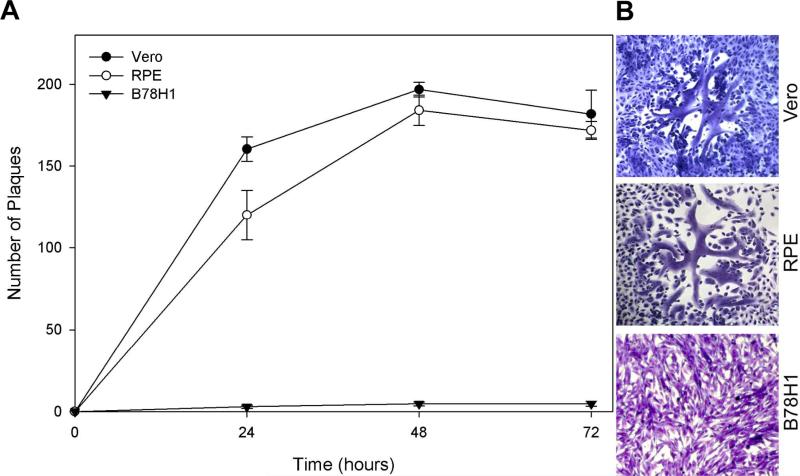
Accepting the ability of HSV-2 to enter RPE cells, we sought to determine whether HSV-2 entry into RPE cells leads to productive viral replication. As shown in Figure 2A, RPE and Vero cells exposed to wild-type HSV-2(333) at 0.01 MOI both produced a large number of plaques over time as compared to the naturally-resistant B78H1 cells. Vero cells were used as a positive control. The number and size of plaques were similar between RPE and Vero cells. Typical plaques formed in each of the cells after 48 hours of infection are shown in Figure 2B. These results demonstrate the ability of HSV-2 to both enter and replicate in RPE cells.
Figure 2.
HSV-2 is capable of productively replicating in RPE cells. (A) Confluent monolayers of Vero, RPE, and B78H1 cells were infected with wild-type HSV-2(333) at 0.01 PFU/cell for 2 hours at 37°C. Cells were fixed at 24, 48, and 72 hours post-infection and stained with Gimesa. The number of plaques were counted and the data represents the mean ± SD of results performed in triplicate in a representative experiment. (B) Typical Gimesa-stained plaques in Vero, RPE, and B78H1 cells 48 hours post-infection with HSV-2(333) are shown. Microscopy was performed with the 10x objective of a Zeiss Axiovert 100 microscope and the slide book version 3.0 was used for images.
Identification and Expression of Entry Receptors
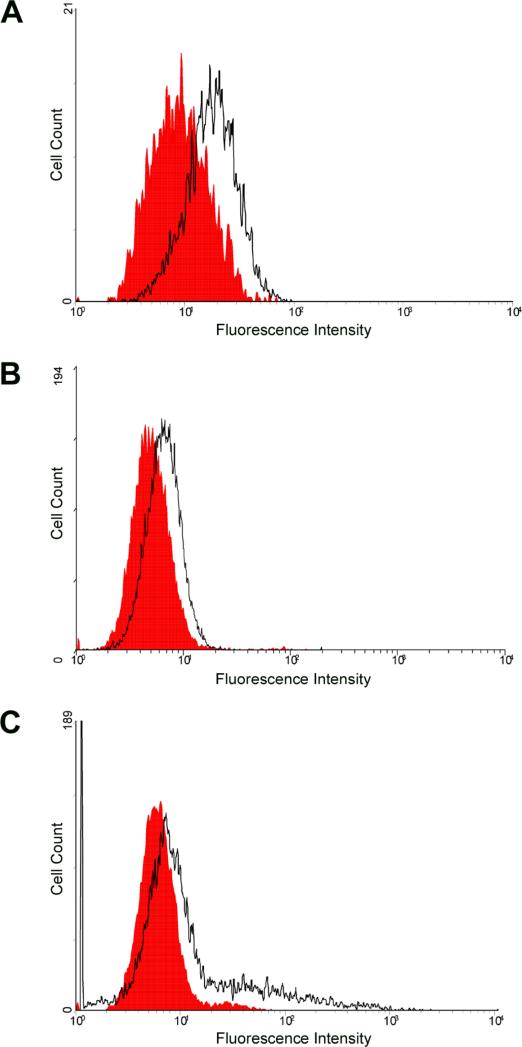
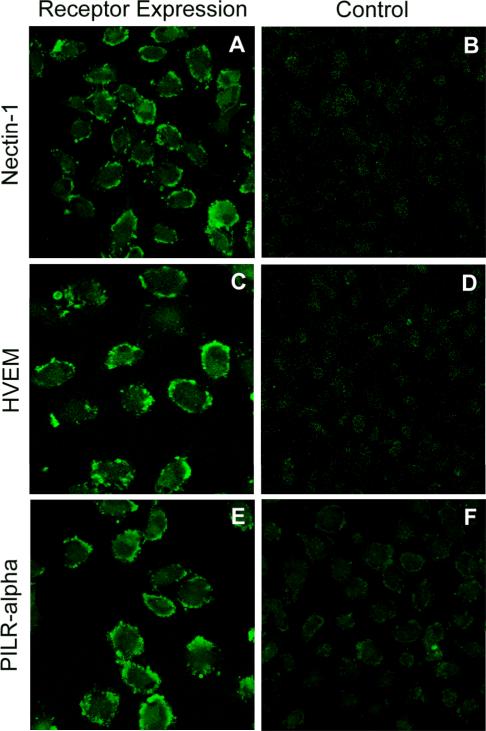
Next, using flow cytometry and immunofluorescence, we determined which of the known HSV-2 entry receptors are expressed on RPE cells. Flow cytometry demonstrated the expression of nectin-1 and HVEM. Interestingly, PILR-α, a reported co-receptor for HSV-1 entry, was also expressed. While the expression of nectin-1 was most discernable (Fig. 3A), HVEM and PILR-α (Figs. 3B, C) were also clearly detected on the surface of RPE cells. To further verify and visualize the cell-surface expression of the receptors, laser-scanning confocal microscopy was used for an immunoflurorescence assay. Receptor-specific primary antibodies and FITC-conjugated secondary antibodies were used. The results shown in Figure 4, confirmed the expression of nectin-1, HVEM, and PILR-α on RPE cells. Control cells that were only incubated with FITC-conjugated secondary antibody did not demonstrate any significant cell surface fluorescence (Fig. 4B, D, F).
Figure 3.
Flow cytometry analysis of cell-surface receptor expression. Expression was detected using FACS analysis on cells treated with primary antibodies to nectin-1 (A), HVEM (B), or PILR-α (C). RPE cells stained only with FITC-conjugated secondary antibody were used as background controls and are shown in red in the figure.
Figure 4.
Visualization of receptors on RPE cell membrane using immunocytochemistry. Cells were mock-treated with PBS containing 3% BSA (B, D, F) or treated with primary antibody for nectin-1 (A), HVEM (C), or PILR-α (E). All cells were then treated with FITC-conjugated secondary antibody and imaged using confocal microscopy. The presence of green indicates receptor expression.
Localization of Receptor expression
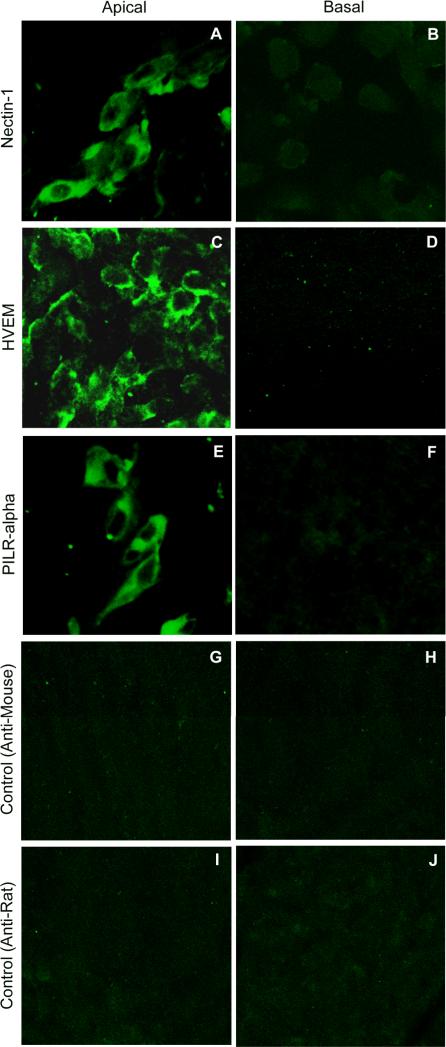
With the knowledge that nectin-1, HVEM, and PILR-α are all expressed by RPE cells, a determination of whether the receptors are preferentially expressed on the apical or basal cell surface was made. A transwell culture system was utilized to allow selective access to either the apical or basal surface of the RPE cells. Receptor-specific primary antibodies and the appropriate FITC-conjugated secondary antibodies were used in an immunofluorescence asssy to analyze the respective surface. Nectin-1, HVEM, and PILR-α were all highly expressed on the apical, but not basal cell surfaces (Fig. 5A-F). Cells only treated with the appropriate FITC-conjugated secondary antibody were used as control, and did not exhibit significant fluorescence (Fig. 5G-J). Controls were made for each receptor, but for clarity only one set of controls for each type of secondary antibody used is shown.
Figure 5.
Receptor expression on the apical and basal surfaces of RPE. Cells were plated in transwell hanging cell culture inserts to allow access to both the apical and basal cell surfaces. Cells were treated on either their apical or basal surfaces with primary antibody to nectin-1 (A,B), HVEM (C,D), or PILR-α (E,F). Cells mock treated with PBS containing 3% BSA were used as controls (G-J). All cells were then incubated with FITC-conjugated secondary antibody on the appropriate surface and imaged by confocal microscopy. FITC-conjugated anti-mouse secondary antibody was used for nectin-1 and HVEM and FITC-conjugated anti-rat secondary antibody was used for PILR-α. The presence of green indicates receptor expression. Controls were made for each of the three receptors, but for clarity one set of controls is shown for each type of secondary antibody.
Antibody-Blocking and Downregulation of Receptors
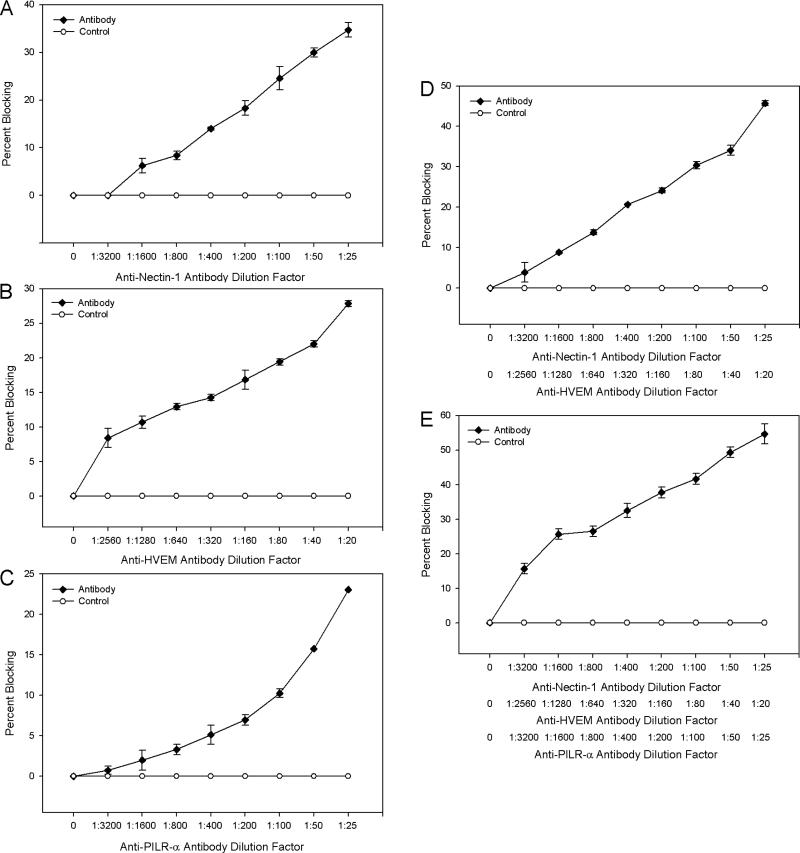
Selective expression of nectin-1, HVEM, and PILR-α at the apical surface of RPE cells raised the possibility that any of them or all of them could be important for HSV-2 entry into RPE cells. To test this, receptor-specific antibodies were used to block viral access to the receptors. Data was analyzed in terms of percent blocking, the relative amount that entry was reduced compared to control cells. An approximately 40 %, 30%, and 25% blocking of HSV-2 entry was observed for cells treated with anti-nectin-1, anti-HVEM, and anti-PILR-α antibody, respectively (Fig. 6A-C). Blocking multiple receptors resulted in an increased effect with an approximately 50% and 60% reduction in entry when anti-nectin-1 and anti-HVEM antibodies or antibodies to all three receptors were used, respectively (Fig. 6D, E).
Figure 6.
Antibodies to the receptors block HSV-2 entry. Monolayers of cells plated in 96-well culture dishes were incubated with serial dilutions of primary antibodies to nectin-1 (A), HVEM (B), PILR-α (C), nectin-1 and HVEM (D), or all three receptors (E) for 2 hours. Cells were then exposed to identical doses of HSV-2(333)gJ- (50 PFU/cell) and viral entry was measured 6 hours post-infection using a spectrophotometer. The percent difference in entry between cells treated with control antibody (α-TGFβR II) and those treated with receptor-specific antibody is reported as the percent blocking. Data shown are the means of triplicate determinations and are representative of three independent experiments.
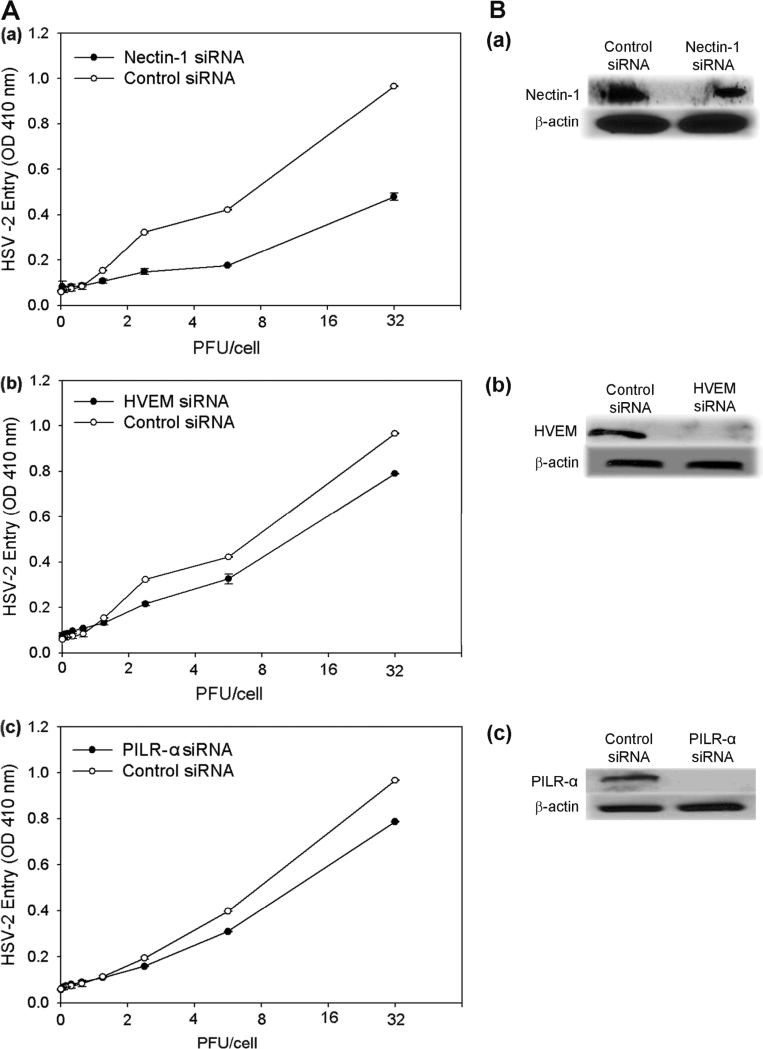
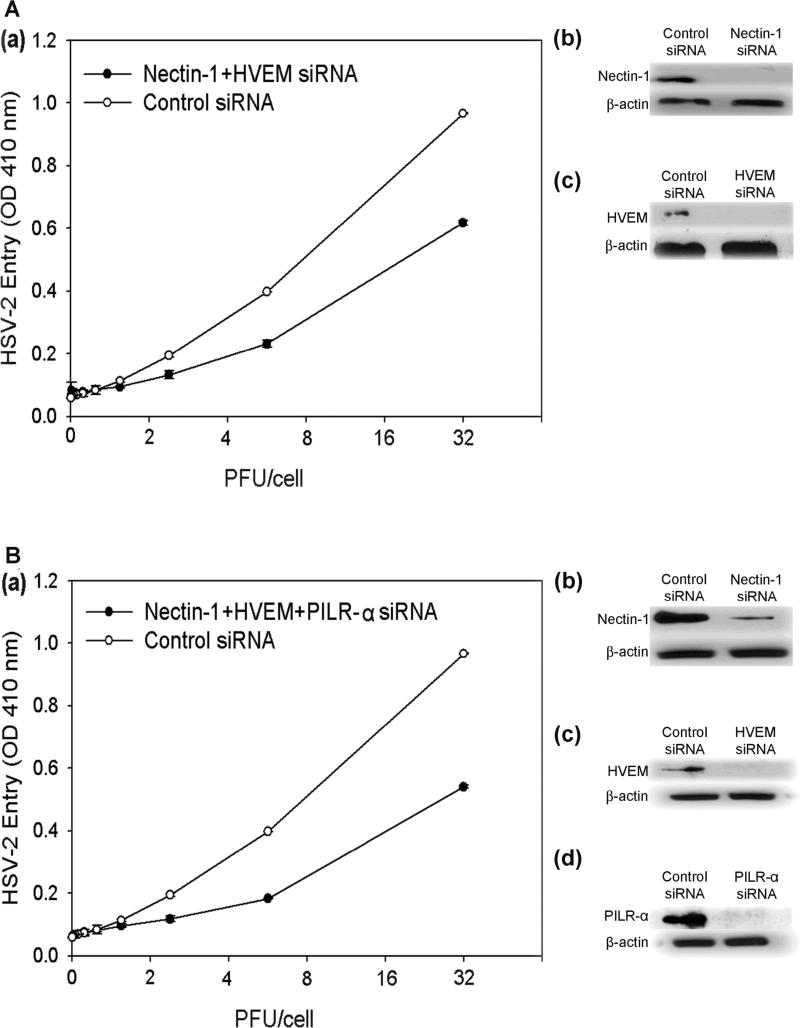
These results were confirmed by receptor-specific siRNA downregulation of nectin-1, HVEM, and PILR-α expression (Fig. 7Aa-c). The trend of downregulation of nectin-1 demonstrating the largest reduction in entry followed by HVEM and PILR-α was also verified. Downregulation of nectin-1, HVEM, and PILR-α together also resulted in less entry than downregulation of only nectin-1 and HVEM together, confirming the importance of all three receptors (Fig. 8Aa, Ba). Receptor downregulation from the cell surface was verified in parallel by Western Blot (Fig. 7Ba-c, 8Ab-c, 8Bb-d).
Figure 7.
siRNA knockdown of receptor expression. (A) HSV-2 entry was determined in cells transfected with siRNA against the entry receptors nectin-1 (a), HVEM (b), and PILR-α (c). Cells that were treated with an equivalent amount of scrambled siRNA were used as a control. (B) Western blot analysis of receptor expression. Western Blot was performed with cells transfected with siRNA against the receptors nectin-1 (a), HVEM (b), and PILR-α (c). Cells that were treated with an equivalent amount of scrambled siRNA were used as a control. Primary antibodies specific for each receptor and secondary antibodies conjugated to HRP were used for the Western Blot.
Figure 8.
Combination siRNA knockdown of receptor expression. HSV-2 entry was determined in cells transfected with siRNA against both nectin-1 and HVEM (Aa) or nectin-1, HVEM, and PILR-α (Ba). Western Blot analysis was done to verify downregulation of individual receptor expression in each siRNA combination (Ab-c, Bb-d), as indicated. Cells that were treated with an equivalent amount of scrambled siRNA were used as a control. Primary antibodies specific for each receptor and secondary antibodies conjugated to HRP were used for the Western Blot.
DISCUSSION
Retinitis is known to occur in persons in all stages of life. In particular, acute retinal necrosis is known to be caused by a few viruses, and among them, HSV-2 is known to be the major cause of disease in persons less than 25 years of age, especially children.8,42 Children with congenital and neonatal HSV-2 infections often develop chorioretinitis and have a higher morbidity than from any other viral infection.43 Additionally, because of the neural route the virus takes after an anterior chamber infection, all patients with an anterior chamber viral infection or keratitis can develop retinitis.17 Because of the virus’ ability to establish a latent infection, anyone with a primary infection is at risk for developing retinal disease throughout their life.11,17,22,44,45 Reports of HSV-2 induced acute retinal necrosis continue to increase.9,18,44,46-48
Acute retinal necrosis and other retinal infections are devastating and often blinding conditions, yet little is know about the cellular and molecular mechanisms of viral entry into retinal cells. We have demonstrated that RPE cells are highly susceptible to HSV-2 infection and that nectin-1, HVEM, and PILR-α are primarily localized to the apical cell surface. The study also reveals the novel result that the virus utilizes nectin-1, and is also capable of utilizing HVEM and PILR-α to enter into RPE cells. Ours is the first study to demonstrate the use of nectin-1 as an important receptor for HSV-2 entry into a natural target cell type. Until now, nectin-1 was mostly implicated in HSV-1 entry into its target cells. It is also the first to investigate a role for PILR-α in HSV-2 infections and the first report to raise the possibility that HSV can use all three receptors to infect one target cell type.25,31,39 Although, the fact that neither the siRNA interference nor the antibodies could block all viral entry suggests the presence of an, as of yet, unknown receptor. The use of multiple receptors by the virus became evident due to the substantial reduction in viral entry both in cells incubated with individual or a combination of receptor-specific antibodies and cells transfected with individual or a combination of receptor-specific siRNAs (Figs 6-8). Based on the relative entry blocking effect of the three receptors, it appears that nectin-1 is clearly important, followed by HVEM and possibly, PILR-α. Interestingly, maximal reduction of entry was found when all three receptors were blocked, suggesting the ability of the virus to utilize multiple receptors, potentially including a currently unknown receptor, for entry into the same cell type. The reduction in entry observed when all three receptors were blocked can be explained by the virus being significantly hindered in its ability to bind to an entry receptor. When only one receptor was blocked, the virus was able to utilize the other receptors and still enter RPE cells. Residual entry even after all three receptors were blocked, hints to the interesting possibility that other receptor(s) exist that can allow HSV-2 entry. It must also be considered that the receptor-specific antibodies and siRNAs may not have blocked all the receptors present or may have provided incomplete blocking. Given the current understanding of the role of PILR-α in HSV infection, PILR-α can not be considered to act as a substitute receptor for nectin-1 or HVEM. Rather it may potentially contribute as a coreceptor for HSV-2, as it has been described for HSV-1.31 Its role in HSV-2 entry, however, may be of less significance than HSV-1. Viral use of multiple cell surface receptors can be recognized as a means to maximize entry.
The observation that nectin-1 is highly expressed on the apical surface of RPE cells is consistent with previous findings.49 We have confirmed and extended these findings as well as discovered that HVEM and PILR-α are also highly expressed on the apical surface of RPE cells. This has important implications in providing a molecular rationale for the accepted description of how anterior chamber infection with HSV leads to contralateral retinal necrosis and sparing of the ipsilateral retina. This is known as the Von-Szily reaction.50 Briefly, it is believed that the virus travels from the ipsilateral ciliary ganglion, through the ipsilateral Edinger-Westphal and suprachiasmatic nuclei, and then crosses over and spreads down the contralateral optic nerve to the ganglion cells of the contralateral retina to the inner nuclear layer and then to the RPE. The ipsilateral retina is spared due to effective immune protection.17,51-60 The accepted direction of viral travel within the retina would allow the virus to enter RPE cells from the apical surface utilizing nectin-1, but also HVEM and PILR-α. Glycosaminoglycans in the interphotoreceptor matrix61 may also assist the virus in attaching to the apical surface of RPE cells and in causing infection. Interestingly, it has been reported that HSV-2 induced retinitis in mice impacts the both the ipsilateral and contralateral retina, which may suggest variations in the exact neural travel mechanisms used by HSV-1 and HSV-2.62-64
The apical surface of RPE cells is known to be specialized to phagocytose photoreceptor outer segments as a part of the RPE cells’ normal maintenance of the retina itself. Portions of PILR-α are also known to have some homology with tyrosine-based sorting signals that are involved in the internalization of cell-surface molecules.65 Thus, it is possible that HSV's use of PILR-α in RPE cells takes advantage of the cells inherent phagocytic nature and assists in viral uptake. It is also known that PILR-α has an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its intracellular portion. Activation of this receptor leads to downregulation of immune response via Src homology-2 (SH2) domain-containing protein tyrosine phosphatase 2 (SHP-2), which inhibits phophorylations induced by activation signals.66 Thus, binding of HSV glycoprotein B to PILR-α on RPE cells may inhibit immune response. It has also been found that RPE cells play a role in the immune privilege of the subretinal space, so HSV infection of the RPE can also further suppress immunity and cause greater infection.67 The inflammatory cytokines that have been described to be produced in acute retinal necrosis, especially TNF-α, IFN-γ, and IL-4,68 may allow for granulomatous inflammation and the fundoscopic findings characteristic of ARN.
Future studies should analyze potential receptors that are localized to the basolateral surface of RPE cells as infection from that side has been noted in vitro,69 although Bruch's membrane may serve as a physical barrier to this in vivo. Additionally, the specific role of nectin-1, HVEM, and PILR-α in vivo can now be determined given that their role in viral entry has been demonstrated. Much work still needs to be done to fully understand viral pathogenesis in the retina, yet our study elucidates unique insights that may provide for therapies to control retinal infection and preserve sight.
REFERENCES
- 1.Liesegang TJ, Melton LJd, Daly PJ, et al. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–9. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 2.Stanberry LR, Jorgenson DM, Nahmias AJ. Herpes simplex viruses 1 and 2. In: Evans AS, Kaslow RA, editors. Viral Infections of Humans. 4th ed Plenum; New York: 1997. pp. 419–454. [Google Scholar]
- 3.Liesgang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20(1):1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Leone P, Fleming DT, Gilsenan AW, Li L, Justus S. Seroprevalence of herpes simplex virus-2 in suburban primary care offices in the United States. Sex Transm Dis. 2004;31(5):311–6. doi: 10.1097/01.olq.0000123651.84697.d6. [DOI] [PubMed] [Google Scholar]
- 5.Gorbach SL, Bartlett JG, Blacklow NR. Infectious Diseases. 2nd ed. Saunders; Philadelphia: 1998. [Google Scholar]
- 6.Reyes M, Shaik NS, Graber JM, Nisenbaum R, Wetherall NT, Fukuda K, Reeves WC. Task Force on Herpes Simplex Virus Resistance. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Arch Intern Med. 2003;163(1):76–80. doi: 10.1001/archinte.163.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Hartnett ME, Capone A, Trese M. Pediatric Retina. Lippincott, Williams, and Wilkins; Philadelphia: 2004. [Google Scholar]
- 8.Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TG. Viral causes of the acute retinal necrosis syndrome. Am. J. Ophthalmol. 2000;129:166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 9.Van Gelder RN, Willig JL, Holland GN, Kaplan HJ. Herpes simplex virus type 2 as a cause of acute retinal necrosis syndrome in young patients. Ophthalmology. 2001;108(5):869–76. doi: 10.1016/s0161-6420(01)00556-5. [DOI] [PubMed] [Google Scholar]
- 10.Atherton SS. Acute retinal necrosis: insights into pathogenesis from the mouse model. Herpes. 2001;8:69–73. [PubMed] [Google Scholar]
- 11.Kim C, Yoon YH. Unilateral acute retinal necrosis occurring 2 years after herpes simplex type 1 encephalitis. Ophthalmic Surg. Lasers. 2002;33:250–252. [PubMed] [Google Scholar]
- 12.Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114:756–762. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Usui Y, Takeuchi M, Goto H, Mori H, Kezuka T, Sakai J, Usui M. Acute retinal necrosis in Japan. Ophthalmology. 2008;115(9):1632–3. doi: 10.1016/j.ophtha.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Hamasaki DI, Atherton SS, Dix RD. HSV-2 alters retinal physiology and morphology bilaterally in mice. Invest Ophthalmol Vis Sci. 1990;31(6):1056–69. [PubMed] [Google Scholar]
- 15.Urayama A, Yamada N, Susaki T, et al. Unilateral acute uveitis with retinal periarteritis and detachment. Jpn J Clin Ophthalmol. 1971;25:607–619. [Google Scholar]
- 16.Duker JS, Nielsen JC, Eagle RC, Jr, Bosley TM, Granadier R, Benson WE. Rapidly progressive acute retinal necrosis secondary to herpes simplex virus, type 1. Ophthalmology. 1990;97(12):1638–43. doi: 10.1016/s0161-6420(90)32356-4. [DOI] [PubMed] [Google Scholar]
- 17.Smith LK, Kurz PA, Wilson DJ, Flaxel CJ, Rosenbaum JT. Two patients with the von Szily reaction: herpetic keratitis and contralateral retinal necrosis. Am J Ophthalmol. 2007;143(3):536–8. doi: 10.1016/j.ajo.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 18.Tran TH, Stanescu D, Caspers-Velu L, Rozenberg F, Liesnard C, Gaudric A, Lehoang P, Bodaghi B. Clinical characteristics of acute HSV-2 retinal necrosis. Am J Ophthalmol. 2004;137(5):872–9. doi: 10.1016/j.ajo.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Nussenblatt RB, Palestine AG. Acute retinal necrosis. Uveitis: fundamentals and clinical practice. Year Book Medical; Chicago: 1989. pp. 407–414. [Google Scholar]
- 20.Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35(5):327–43. doi: 10.1016/0039-6257(91)90183-g. [DOI] [PubMed] [Google Scholar]
- 21.Usui Y, Goto H. Overview and diagnosis of acute retinal necrosis syndrome. Semin Ophthalmol. 2008;23(4):275–83. doi: 10.1080/08820530802111325. [DOI] [PubMed] [Google Scholar]
- 22.Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol. 2006;141(3):547–557. doi: 10.1016/j.ajo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Herold BC, WuDunn D, Soltys N, Spear PG. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65(3):1090–8. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108(4):503–10. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spear PG. Herpes simple virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 26.Krummenacher C, Rux AH, Whitbeck JC, Ponce-de-Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty RJ, Spear PG, Eisenberg RJ, Cohen GH. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol. 1999;73(10):8127–37. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J Virol. 2000;74(23):10863–72. doi: 10.1128/jvi.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry C, Bell S, Minson T, Browne H. Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3 integrins. J Gen Virol. 2005;86(Pt 1):7–10. doi: 10.1099/vir.0.80567-0. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Romero P, Perez A, Capul A, Montgomery R, Fuller AO. Herpes simplex virus entry mediator associates in infected cells in a complex with viral proteins gD and at least gH. J Virol. 2005;79(7):4540–4. doi: 10.1128/JVI.79.7.4540-4544.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scanlan PM, Tiwari V, Bommireddy S, Shukla D. Cellular expression of gH confers resistance to herpes simplex virus type-1 entry. Virology. 2003;312(1):14–24. doi: 10.1016/s0042-6822(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 31.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, Arase N, Shiratori I, Tanaka S, Kawaguchi Y, Spear PG, Lanier LL, Arase H. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132(6):935–44. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla D, Dal Canto MC, Rowe CL, Spear PG. Striking similarity of murine nectin-1alpha to human nectin-1alpha (HveC) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J Virol. 2000;74(24):11773–81. doi: 10.1128/jvi.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77(19):10179–85. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246(1):179–89. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87(3):427–36. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 36.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99(1):13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 37.Shukla D, Scanlan PM, Tiwari V, Sheth V, Clement C, Guzman-Hartman G, Dermody TS, Valyi-Nagy T. Expression of nectin-1 in normal and herpes simplex virus type 1-infected murine brain. Appl Immunohistochem Mol Morphol. 2006;14(3):341–7. doi: 10.1097/00129039-200609000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Tiwari V, Clement C, Scanlan PM, Kowlessur D, Yue BY, Shukla D. A role for herpesvirus entry mediator as the receptor for herpes simplex virus 1 entry into primary human trabecular meshwork cells. J Virol. 2005;79(20):13173–9. doi: 10.1128/JVI.79.20.13173-13179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari V, Shukla SY, Yue BY, Shukla D. Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J Gen Virol. 2007;88(Pt 8):2106–10. doi: 10.1099/vir.0.82830-0. [DOI] [PubMed] [Google Scholar]
- 40.Shukla D, Rowe CL, Dong Y, Racaniello VR, Spear PG. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol. 1999;73(5):4493–7. doi: 10.1128/jvi.73.5.4493-4497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez WM, Spear PG. Amino acid substitutions in the V domain of nectin-1 (HveC) that impair entry activity for herpes simplex virus types 1 and 2 but not for Pseudorabies virus or bovine herpesvirus 1. J Virol. 2002;76(14):7255–62. doi: 10.1128/JVI.76.14.7255-7262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan JCH, Byles D, Stanford MR, Frith PA, Graham EM. Acute retinal necrosis in children caused by herpes simplex virus. Retina. 2001;21(4):344–7. doi: 10.1097/00006982-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Baskin HJ, Hedlund G. Neuroimaging of herpesvirus infections in children. Pediatr Radiol. 2007;37(10):949–63. doi: 10.1007/s00247-007-0506-1. [DOI] [PubMed] [Google Scholar]
- 44.Schlingemann RO, Bruinenberg M, Wertheim-van Dillen P, Feron E. Twenty years’ delay of fellow eye involvement in herpes simplex virus type 2-associated bilateral acute retinal necrosis syndrome. Am J Ophthalmol. 1996;122(6):891–2. doi: 10.1016/s0002-9394(14)70390-3. [DOI] [PubMed] [Google Scholar]
- 45.Thompson WS, Culbertson WW, Smiddy WE, Robertson JE, Rosenbaum JT. Acute retinal necrosis caused by reactivation of herpes simplex virus type 2. Am J Ophthalmol. 1994;118:205–211. doi: 10.1016/s0002-9394(14)72900-9. [DOI] [PubMed] [Google Scholar]
- 46.Rahhal FM, Siegel LM, Russak V, Wiley CA, Tedder DG, Weinberg A, Rickman L, Freeman WR. Clinicopathologic correlations in acute retinal necrosis caused by herpes simplex virus type 2. Arch Ophthalmol. 1996;114(11):1416–9. doi: 10.1001/archopht.1996.01100140616019. [DOI] [PubMed] [Google Scholar]
- 47.Markomichelakis NN, Zafirakis P, Karambogia-Karefillidi P, Drakoulis N, Vagiakou-Boudri E, Paterakis G, Apostolopoulos M. Herpes simplex virus type 2: a cause of acute retinal necrosis syndrome. Ocul Immunol Inflamm. 2001;9(2):103–9. doi: 10.1076/ocii.9.2.103.3974. [DOI] [PubMed] [Google Scholar]
- 48.Miserocchi E, Modorati G, Azzolini C, Foster CS, Brancato R. Herpes simplex virus type 2 acute retinal necrosis in an immunocompetent patient. Eur J Ophthalmol. 2003;13(1):99–102. doi: 10.1177/112067210301300118. [DOI] [PubMed] [Google Scholar]
- 49.Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J Virol. 2006;80(24):12209–18. doi: 10.1128/JVI.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von Szily A. Experimental endogenous transmission of infection from bulbus to bulbus. Klin Monatsbl Augenheilkd. 1924;72:593–602. [Google Scholar]
- 51.Zheng M, Fields MA, Liu Y, Cathcart H, Richter E, Atherton SS. Neutrophils protect the retina of the injected eye from infection after anterior chamber inoculation of HSV-1 in BALB/c mice. Invest Ophthalmol Vis Sci. 2008;49(9):4018–25. doi: 10.1167/iovs.08-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fields MA, Zheng M, Wall P, Oberg S, Atherton SS. Uniocular anterior chamber inoculation of a tumor necrosis factor alpha-expressing recombinant of herpes simplex virus type 1 results in more rapid destruction and increased viral replication in the retina of the uninoculated eye. J Virol. 2008;82(10):5068–78. doi: 10.1128/JVI.00082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kezuka T, Atherton SS. Acute retinal necrosis. Chem Immunol Allergy. 2007;92:244–53. doi: 10.1159/000099275. [DOI] [PubMed] [Google Scholar]
- 54.Tanigawa M, Bigger JE, Kanter MY, Atherton SS. Natural killer cells prevent direct anterior-to-posterior spread of herpes simplex virus type 1 in the eye. Invest Ophthalmol Vis Sci. 2000;41(1):132–7. [PubMed] [Google Scholar]
- 55.Matsubara S, Atherton SS. Spread of HSV-1 to the suprachiasmatic nuclei and retina in T cell depleted BALB/c mice. J Neuroimmunol. 1997;80(1-2):165–71. doi: 10.1016/s0165-5728(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 56.Atherton SS, Streilein JW. Two waves of virus following anterior chamber inoculation of HSV-1. Invest Ophthalmol Vis Sci. 1987;28:571–579. [PubMed] [Google Scholar]
- 57.Vann VR, Atherton SS. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32(9):2462–72. [PubMed] [Google Scholar]
- 58.Lewis ML, Culbertson WW, Post JD, Miller D, Kokame GT, Dix RD. Herpes simplex virus type 1. A cause of the acute retinal necrosis syndrome. Ophthalmology. 1989;96(6):875–8. doi: 10.1016/s0161-6420(89)32823-5. [DOI] [PubMed] [Google Scholar]
- 59.Mahjoub SB, Ganley JP, Misra RP, Langford MP. Isolation of a herpes simplex virus type 2 that is retinovirulent in mice. Curr Eye Res. 1989;8(7):687–95. doi: 10.3109/02713688909025803. [DOI] [PubMed] [Google Scholar]
- 60.Whittum JA, McCulley JP, Niederkorn JY, Streilein JW. Ocular disease induced in mice by anterior chamber inoculation of herpes simplex virus. Invest Ophthalmol Vis Sci. 1984;25(9):1065–73. [PubMed] [Google Scholar]
- 61.Landers RA, Hollyfield JG. Proteoglycans in the mouse interphotoreceptor matrix. VI. Evidence for photoreceptor synthesis of chondroitin sulfate proteoglycan using genetically fractionated retinas. Exp Eye Res. 1992;55(2):345–56. doi: 10.1016/0014-4835(92)90199-3. [DOI] [PubMed] [Google Scholar]
- 62.Zierhut M, Hemady R, Zhao TZ, Merchant A, Foster CS. Herpes simplex virus type 2 induced retinal necrosis in BALB/c mice. Acta Ophthalmol (Copenh) 1994;72(2):211–7. doi: 10.1111/j.1755-3768.1994.tb05018.x. [DOI] [PubMed] [Google Scholar]
- 63.Atherton SS, Streilein JW. Virus-specific DTH prevents contralateral retinitis following intracameral inoculation of HSV-2. Curr Eye Res. 1987;6(1):133–9. doi: 10.3109/02713688709020080. [DOI] [PubMed] [Google Scholar]
- 64.Dix RD, Streilein JW, Cousins S, Atherton SS. Histopathologic characteristics of two forms of experimental herpes simplex virus retinitis. Curr Eye Res. 1987;6(1):47–52. doi: 10.3109/02713688709020067. [DOI] [PubMed] [Google Scholar]
- 65.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269(5232):1872–5. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 66.Fournier N, Chalus L, Durand I, Garcia E, Pin JJ, Churakova T, Patel S, Zlot C, Gorman D, Zurawski S, Abrams J, Bates EE, Garrone P. FDF03, a novel inhibitory receptor of the immunoglobulin superfamily, is expressed by human dendritic and myeloid cells. J Immunol. 2000;165(3):1197–209. doi: 10.4049/jimmunol.165.3.1197. [DOI] [PubMed] [Google Scholar]
- 67.Farrokh-Siar L, Rezai KA, Semnani RT, Patel SC, Ernest JT, Peterson EJ, Koretzky GA, van Seventer GA. Human fetal retinal pigment epithelial cells induce apoptosis in the T-cell line Jurkat. Invest Ophthalmol Vis Sci. 1999;40(7):1503–11. [PubMed] [Google Scholar]
- 68.Zheng M, Atherton SS. Cytokine profiles and inflammatory cells during HSV-1-induced acute retinal necrosis. Invest Ophthalmol Vis Sci. 2005;46(4):1356–63. doi: 10.1167/iovs.04-1284. [DOI] [PubMed] [Google Scholar]
- 69.Topp KS, Rothman AL, Lavail JH. Herpes virus infection of RPE and MDCK cells: polarity of infection. Exp Eye Res. 1997;64(3):343–54. doi: 10.1006/exer.1996.0209. [DOI] [PubMed] [Google Scholar]