Abstract
Purpose of review
Several gastrointestinal diseases including the inflammatory bowel diseases (IBD) and malignancy are associated with elevated expression of indoleamine 2,3 dioxygenase-1 (IDO1). IDO1 initiates tryptophan catabolism along a pathway that generates several bioactive kynurenine-based metabolites. Promotion of T-cell mediated tolerance and antimicrobial effects are among the variety of functions attributed to IDO1 activity. Recent advances addressing the diverse implications of gut associated IDO1 expression are herein reviewed.
Recent Findings
In active IBD IDO1 is highly expressed both in cells of the lamina propria and epithelium. Experimental models demonstrate that IDO1 promotes gut immune homeostasis by limiting inflammatory responses and protecting the epithelium. In human colon cancer, high expression of IDO1 by the neoplastic epithelium correlates with poor prognosis. The serum kynurenine:tryptophan ratio is elevated in both active Crohn’s disease and in colon cancer suggesting this measurement may prove useful as a disease biomarker. IDO1 inhibitors have moved to clinical trials providing new hope as immunotherapy for advanced malignancy.
Summary
IDO1 activity significantly shapes gastrointestinal disease pathophysiology and severity. Measures of IDO1 activity may be useful as a disease biomarker. Manipulation of IDO1 activity has great potential as treatment for both inflammatory and malignancy associated gastrointestinal disease.
Keywords: Tryptophan, colitis, cancer, biomarker, IDO, kyurenine
INTRODUCTION
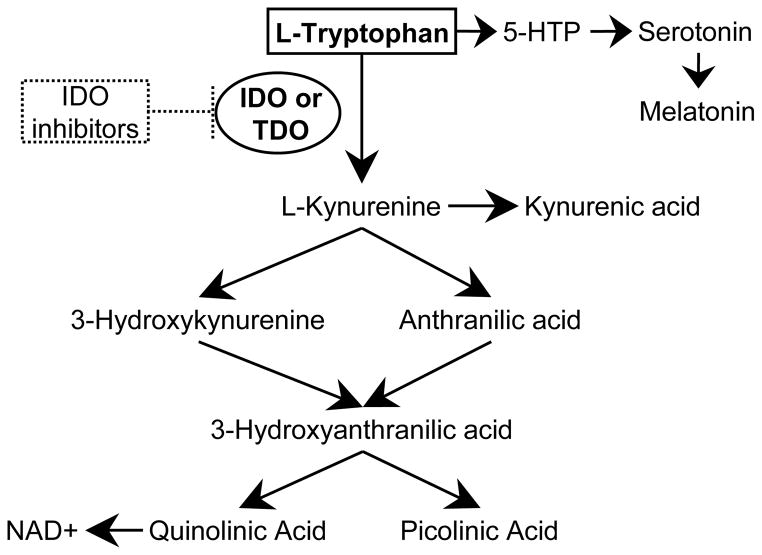
Indoleamine 2,3 dioxygenase-1 (IDO1) is the first and rate limiting step in tryptophan catabolism along the kynurenine pathway (Figure 1). Several of the downstream metabolites are biologically active and ultimately provide substrate for de novo NAD+ synthesis. Though IDO1 is not the only enzyme able to metabolize tryptophan to kynurenine, it is the most-well characterized both in the gut and in general. Of the other two enzymes, the recently described IDO2 is most highly expressed in the kidney while tryptophan 2,3 dioxygenase is most highly expressed in the liver.[1–3] All three have been found in cancer.[4, 5] The essential amino acid tryptophan, substrate for IDO1, is also the precursor for synthesis of serotonin and melatonin if metabolized along an alternate pathway.
Figure 1.
Tryptophan catabolism pathways and the IDO1 kyurenine metabolite pathway
The seminal observation that IDO1 promotes immune tolerance at the maternal-fetal interface launched myriad investigations to explore the role of this enzyme in other organ systems and disease processes.[6, 7] In this mechanistic paradigm, IDO1 activity by professional antigen presenting cells reduces local tryptophan concentrations and elevates toxic kynurenine metabolites to limit activated T-cell responses and promote regulatory T-cell activity. IDO1 is now recognized to also possess a non-enzymatic function that contributes to TGF-beta-driven tolerance in non-inflammatory contexts.[8]
In the gut, IDO1 is highly upregulated in response to inflammation and in malignancy. IDO1’s ability to potently shape gastrointestinal (GI) disease pathophysiology and severity make an important target for drug discovery. IDO1 expression in the gut mucosa also changes systemic levels of tryptophan and kynurenine. This brings to light the potential to use IDO activity as a disease biomarker and also raises the question as to whether changes in tryptophan and kynurenine metabolites may contribute to extraintestinal manifestations of human inflammatory bowel disease (IBD) including disease-activity-associated mood-disturbance. This article provides historical context and reviews recent advances for the role of IDO1 in the gastrointestinal health and disease.
IDO1 IN COLITIS AND HUMAN INFLAMMATORY BOWEL DISEASE
In the homeostatic state gut expression of IDO1 is low and mostly occurs in cells of the lamina propria. IDO1 expression is stimulated by inflammatory cytokines including IFNγ, TNFα and IL1-B. Consistent with this, IDO1 is one of the most highly upregulated genes in human IBD and animal models of colitis.[9–13]
Natural IDO1 expression modifies colitis severity
Our group was among the first to evaluate the functional significance of IDO1 expression in the gut.[14] Gurtner demonstrated that IDO1 expression and functionality was increased over baseline in acute TNBS (trinitrobenzene sulfonic acid) colitis. Disease severity was worsened in mice receiving the IDO inhibitor 1-DL-methyl tryptophan (1mT), suggesting IDO1 down-regulates Th1 inflammatory responses within the intestinal tract. Matteoli further observed that CD103+ gut dendritic cells expressing IDO1 support regulatory T-cell conversion while suppressing Th1/Th17 differentiation to promote oral tolerance and limit gut inflammation.[15] CD103+ DCs have recently been shown to accept luminal antigen from small intestinal goblet cells[16] which also appear capable of expressing IDO1.[17, 18]
IDO1 was also evaluated in two other colitis models with some relevance to IBD. Jasperson demonstrated a role for IDO1 in a model of graft versus host disease. He found IDO1 upregulation particularly apparent in the colonic epithelium of WT mice with disease and showed that IDO1−/− mice exhibited greater colitis severity, T-cell infiltration and mortality.[19] Subsequently, the authors demonstrated that induction of IDO1 primarily in professional antigen presenting cells (APC) by a TLR-7/8 agonist reduced colon injury and ameliorated lethality.[20] In an infectious colitis model using Citrobacter rodentium, IDO1−/− mice demonstrated resistance to colonization and developed an attenuated colitis compared to WT mice.[21] The authors then identified that IDO1−/− mice exhibit elevated non-specific IgA antibodies in the serum and stool at baseline. Thus, it was proposed that IDO1 mediated inhibition of B-cell responses to commensal microflora may explain these intriguing findings while still maintaining consistency with the recognized role of IDO1 as an inhibitor of lymphocyte responses in the gastrointestinal tract.[22]
Induction of IDO1 prevents colitis
We extended our initial observations by looking at IDO1 induction as a method to prevent colitis severity.[18] We demonstrated that a synthetic toll like receptor-9 (TLR9) agonist with anti-colitis effects[23] potently induced IDO1 in the colon and small intestine. In both the acute and chronic TNBS colitis models as well as the dextran sodium sulfate (DSS) model, IDO1 induction was critical to the anti-colitic effects of this agent. This study highlighted the potential of IDO1 induction as a therapeutic strategy for human IBD. Similar to our findings with the TLR9 agonist, CTLA-4 based molecules with IDO1-inducing capacity have more potent anti-colitic effects in experimental models than those which do not induce IDO1 (reference[24] and our unpublished observations). This finding may help explain why Abatacept (a CTLA4 molecule lacking IDO1 inducing capacities[25]) failed to meet endpoints in clinical trials evaluating its efficacy as an IBD therapy.[26]
Cellular source of IDO1
APCs are known to possess potent IDO1-dependent suppressive effects on T-cell proliferation[27–29] and surely mediate tolerance in the gut.[15] However, it should be appreciated that epithelial cells are represent a major source of gut IDO1 activity during inflammatory states.[9, 10, 30] IDO1 expression is particularly apparent in epithelial cells near sites of ulceration.[10] Though the function of epithelial IDO1 is not fully elucidated, antimicrobial properties may be particularly important considering the epithelial barrier dysfunction associated with IBD.[31, 32] Supporting this, IL-27 (a cytokine with Th17 cell inhibitory properties) was recently shown to block growth of intestinal bacteria and mediate epithelial barrier protection via induction of IDO1 in human and mouse intestinal epithelial cells.[33] Our work also demonstrated the epithelium to be a major source of IDO1 in response to the anti-colitis TLR9 agonist, which was associated with enhanced epithelial proliferation.[18]
Taken together the data suggest that in colitis IDO1 expressing cell types function as a negative feedback mechanism to limit the development of chronic inflammation. It is possible that IDO1 expression by APCs is critical to suppressing inflammatory T-cell responses while epithelial IDO1 activity functions predominantly to limit microbial invasion and perhaps promote epithelial repair. This supposition could be confirmed by a model enabling tissue specific deletion of IDO1 expression. The physiologic balance between IDO1-mediated tryptophan depletion and role that this essential amino acid appears to play in maintaining mucosal homeostasis[34] remains to be answered.
IDO1 EXPRESSION AS A BIOMARKER OF GI DISEASE
There is a clinical need for new biomarkers which specifically reflect gastrointestinal disease pathophysiology. Biomarkers support clinical decision making by providing supplemental information for disease diagnosis, determination of disease activity, prognosis/risk stratification and prediction of response to therapy. Many biomarkers currently in use are not disease specific, but reflect generalized inflammation. Promise is held for new, more specific biomarkers that detect differences in genomics (genotype and gene expression), proteomics and metabolomics.[35] Biomarkers should ideally be readily obtained, inexpensive to perform, consistently quantifiable across labs, and unaffected by co-morbid factors.[36] Recent studies support targeting the IDO1-mediated tryptophan catabolism pathway as a biomarker of gastrointestinal inflammatory diseases and malignancy.[35]
Biomarker in IBD
Serum changes reflective of IDO1 activation in the gut correlate with Crohn’s disease (CD) activity.[30] Kynurenine is an initial metabolite in IDO1 mediated tryptophan catabolism. The kynurenine/tryptophan (K/T) ratio is a surrogate marker for IDO1 activity in the setting of an activated immune system. Using this ratio (vs either alone) limits potential bias related to differences in dietary intake of tryptophan.[37] Examining serum from a well-characterized cohort of CD patients and controls we found that serum tryptophan was depressed while the K/T ratio was elevated in active CD. Both measurements correlated with CD activity assessments and the acute phase reactants ESR and CRP. Activity for all Montreal Classifications of CD location could be identified by the K/T ratio. Finally, serial measurements from a subgroup of CD patients revealed that as CD activity improved, tryptophan and kynurenine levels normalized as did the K/T ratio.
Other human and animal studies also point to IDO1 activity as a biomarker worth pursuing for diseases of intestinal inflammation including IBD and potentially also celiac disease.[38] A historical report identified low serum tryptophan in CD patients.[39] As kynurenine was not measured, the finding was attributed to compromised absorption. Similarly a recent detailed serum aminogram investigation identified low tryptophan in both active CD and UC patients compared to controls.[40] Serum levels of tryptophan are also reduced by nearly 80% in mice with dextran sodium sulfate induced colitis.[41] Kynurenine and kynurenic acid were found to be elevated in small cohort studies of CD and UC patients compared to controls.[42, 43] In the IL-10−/− model of experimental colitis, metabolomic profiling identified elevation of urinary xanthurenic acid, a kynurenine metabolite.[44, 45] Subsequent investigations revealed elevated plasma levels of kynurenine and 3-hydroxykynurenine in colitic mice and showed that urinary xanthurenic acid levels correlated with colitis severity.[46]
Reflecting Mucosal Inflammation
New blood or stool based biomarkers for IBD ideally will be able to assess mucosal healing in order to provide the best predictive value for treatment success and clinical prognosis.[47–49] Measurement of serum tryptophan and kynurenine pathway metabolites has great potential as an objective surrogate marker of gut mucosal immune activation and biomarker for CD activity and perhaps ulcerative colitis as well. Increased IDO1 expression in the gut reflects immune activation and inflammation. Inflammatory cytokines IFNγ and TNFα induce IDO1. IDO1 expression and the K/T ratio normalize with effective IBD therapy.[12, 30, 50] Though mucosal healing was not directly assessed in our study, we found that changes in K/T ratio with active CD were greater than in any illness outside of sepsis resulting in death.[30] These profound differences are likely attributable to the large surface area and multiple cell types expressing IDO1 within the gut mucosa during active disease. Given recent metabolomics data, confirmative studies may utilize a panel of kynurenine metabolites as well as tryptophan.
Biomarker in CRC
The IDO1-kynurenine pathway might also be exploited as a biomarker for colon cancer. Though the stage of cancer was not reported, Liu identified a near doubling of the K/T ratio in patients with CRC verses controls.[51] Reduced serum tryptophan correlated with quality of life deterioration in patients with metastatic CRC in another study.[52] Finally, a study evaluating colonic secretions found a tripling of kynurenic acid levels in patients with colon cancer and doubling in patients with tubulovillous adenomas as compared to healthy controls.[53]
IDO1 AND COLON CANCER
IDO1 is expressed by several human cancers and its presence has been linked to poor prognosis.[54] The IDO1 like enzymes TDO and IDO2 have also been identified in several cancers. As such, significant attention has been given to targeting inhibition of tryptophan-kynurenine pathways for cancer therapy. In cancers of the GI tract, IDO1 seems to be the most upregulated of these enzymes, though TDO expression has also been reported.[4, 5]
Human observational studies
IDO1 may play a role in colorectal cancer (CRC) pathophysiology. Ferdinande reported that IDO1 expression was particularly high in the neoplastic epithelium at the tumor’s invasion front. Moreover, high level IDO1 expression in the neoplastic epithelium correlated with metachronous metastases and reduced survival in this study.[55] Brandacher also found high IDO1 expression in ~40% of CRCs where it significantly associated with increased liver metastases, but not reduced mortality.[56] Another study found a correlation between high density of IDO1+ cells in tumor draining lymph nodes and reduced 5-year survival rates in colon cancer patients.[57] Even in isolation, several colon cancer cell lines constitutively express IDO1.[58]
Several questions remain with regard to the function of IDO1 expression in colon cancer and in particular IDO1 expression by the neoplastic epithelium. Available retrospective studies on human resection specimens are limited in their ability to inform us how IDO1 activity might shape colon cancer progression. It has been suggested by these studies that the function of IDO1 in colon cancer is akin to what has been reported in other cancers. In this paradigm IDO1 activity contributes to pathogenesis by reducing tumor-reactive T-cell activity and inducing regulatory T-cells to foster tumoral-immune tolerance. However, the observed relationship between IDO1 expression and T-cell infiltration has not been consistent.[55, 56]
Experimental models
In extrapolating data from other cancers, it would be assumed that breaking tolerance in CRC by inhibiting IDO1 would be a beneficial. However, a recent report evaluating potential cardiac and gastrointestinal liabilities in an IDO1 knockout mouse had findings which may contradict this supposition. Using a model of colon tumorigenesis induced by genotoxin administration followed by single episode of colonic injury with dextran sodium sulfate, IDO1 null mice developed greater colitis severity and increased tumor formation.[59] This phenotype was lost in a subsequent experiment when different doses of the genotoxin and colitic agent were used and the findings were not confirmed by use of and IDO1 inhibitor. Regardless, one would have predicted enhanced tumor immune surveillance and thus small tumors in the IDO1 null mice. These results require clarification as CRC is a major cause of death and these findings may temper enthusiasm for testing promising IDO1 inhibitors in trials for colon cancer.
IDO POTPOURRI
Other recent and interesting, yet preliminary observations relevant to IDO1, tryptophan catabolism and gastrointestinal disease deserve mention.
IDO1 and novel probiotics
Existent and novel probiotics may exert immunomodulatory effects via induction of IDO1 expression. Peptidoglycan purified from a Lactobacillus salivarius species was able to reduce experimental colitis in an IL-10-dependent manner. The protection was also correlated with an upregulation of IDO1 and favored the development of tolerogenic (CD103+) dendritic cells and regulatory T cells.[60] Early colonization of mice with a defined mixture of commensal intestinal bacteria species (Clostridium clusters IV and XIVa) was found to promote a colonic environment rich with TGF-B and regulatory T-cells while offering protection from colitis.[61] Intestinal epithelial cells from the mice colonized with the Clostridium mixture expressed high levels of IDO1.
IDO1 inhibitors
Several inhibitors of the IDO pathway have been described and some are now in phase I clinical trials in cancer including D-1mT[62, 63] and INCB023843.[51] The latter appears to potently inhibit IDO1 in a specific manner[51], efficiently reduces tryptophan metabolism in CT26 colon cancer cells in vitro[64] and was well tolerated in a phase I clinical trial which included >50% patients with CRC.[65] The proposed effect of inhibiting IDO is to break immune tolerance against tumor-associated antigens. As such these agents may be used as concomitant therapy with chemotherapy or vaccines.[66]
IDO1 and the Gut-Brain axis
Current evidence supports the role of cell mediated immune activation as an important biologic contributor to the onset of sickness and depressive behavior.[67] In animal models of chronic inflammation, associated depressive behavior is eliminated by IDO1 inhibition or genetic ablation.[68] Furthermore, brain IDO1 activity contributes to depression associated with chronic pain.[69] In human IBD anxiety and depression are common, and in both CD and UC depression and anxiety state (but not trait) are worse during periods of active disease.[70] These findings suggest a potential role for IDO1 in mediating gut-brain interactions and mood disturbance in IBD.
CONCLUSION
The IDO1 mediated trytophan catabolism pathway has important roles in the gut both in health and disease. Baseline expression of IDO1 in APCs contributes to immune tolerance. Upregulation of IDO1 occurs during inflammatory states including human IBD and colon cancer. Serum levels of tryptophan and kynurenine pathway metabolites change with IDO1 activity and may serve as a useful biomarker for mucosal immune activation. IDO1 activity may also influence a diverse set of extraintestinal manifestations including mood disturbance. Pharmacologic agents which inhibit or potentiate IDO1 expression and activity have the potential to impact a diversity of intestinal disorders.
KEY POINTS.
IDO1 expression and activity is an important mediator of intestinal homeostasis both in health and disease.
Experimental models suggest that IDO1 upregulation observed in Crohn’s disease and ulcerative colitis acts to limit the inflammatory response and may be exploitable for disease therapeutics.
IDO1 activity in the gut mucosa during active inflammatory bowel disease changes serum levels of tryptophan and kynurenine based metabolites positioning the pathway as a potential disease biomarker.
Most colon cancers express IDO1; while its presence has been linked to poor prognosis, how IDO1 contributes to colon tumorigenesis requires further exploration.
Acknowledgments
This research was funded in part by NIH grant DK089016 and a Central Society for Clinical Research Early Career Development Award.
Footnotes
No relevant conflicts of interest to declare.
References
- 1.Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Metz R, Duhadaway JB, Kamasani U, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 3.Fukunaga M, Yamamoto Y, Kawasoe M, et al. Studies on Tissue and Cellular Distribution of Indoleamine 2,3-Dioxygenase 2: The Absence of IDO1 Upregulates IDO2 Expression in the Epididymis. J Histochem Cytochem. 2012 doi: 10.1369/0022155412458926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platten M, Wick W, Van den Eynde BJ. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- 5*.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. This study demonstrates that another enzyme which catabolizes tryptophan to kynurenine (tryptophan 2,3 dioxgenase) is overexpressed in several tumor types and contributes tumor pathogenesis in brain cancer via kynurenine activation of the arylhydrocarbon receptor. While some colon cancers express TDO, the GI tract exposure to endogenous and endogenous AHR ligands is much higher at baseline than the brain, thus the impact of this pathway on GI tract cancers may be less profound. [DOI] [PubMed] [Google Scholar]
- 6.Munn DH, Shafizadeh E, Attwood JT, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 8*.Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–8. doi: 10.1038/ni.2077. Beyond its recognized ability to catabolize tryptophan, it is revealed that IDO1 also acts as a signal transducer in murine pDCs to contribute to TGF-beta-driven tolerance in noninflammatory contexts. The importance of this function to intestinal disease remains to be determined. [DOI] [PubMed] [Google Scholar]
- 9.Barcelo-Batllori S, Andre M, Servis C, et al. Proteomic analysis of cytokine induced proteins in human intestinal epithelial cells: implications for inflammatory bowel diseases. Proteomics. 2002;2:551–60. doi: 10.1002/1615-9861(200205)2:5<551::AID-PROT551>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Ferdinande L, Demetter P, Perez-Novo C, et al. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int J Immunopathol Pharmacol. 2008;21:289–95. doi: 10.1177/039463200802100205. [DOI] [PubMed] [Google Scholar]
- 11.Dieckgraefe BK, Stenson WF, Korzenik JR, et al. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics. 2000;4:1–11. doi: 10.1152/physiolgenomics.2000.4.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Wolf AM, Wolf D, Rumpold H, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hansen JJ, Holt L, Sartor RB. Gene expression patterns in experimental colitis in IL-10-deficient mice. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurtner GJ, Newberry RD, Schloemann SR, et al. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–73. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 15**.Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. In vitro and in vivo experiments demonstrate that IDO1 is a critical contributor to the tolerogenic effects attributed to a subset of gut DCs which express CD103. This extends upon the work of Gurtner (ref 14) but adds oral tolerance as a model and identifies a cell type with IDO1 activity that has particular relevance in the gut. [DOI] [PubMed] [Google Scholar]
- 16.McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–9. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell LV, Else KJ. Regulation of colonic epithelial cell turnover by IDO contributes to the innate susceptibility of SCID mice to Trichuris muris infection. Parasite Immunol. 2011;33:244–9. doi: 10.1111/j.1365-3024.2010.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010;184:3907–16. doi: 10.4049/jimmunol.0900291. IDO1 induction prior to colitic insult is demonstrated to prevent injury and facilities epithelial repair in both the DSS and TNBS models. This highlights the possibility of IDO1 induction as a therapy for IBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111:3257–65. doi: 10.1182/blood-2007-06-096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, et al. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–70. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrington L, Srikanth CV, Antony R, et al. Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infect Immun. 2008;76:3045–53. doi: 10.1128/IAI.00193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherayil BJ. Indoleamine 2,3-dioxygenase in intestinal immunity and inflammation. Inflamm Bowel Dis. 2009;15:1391–6. doi: 10.1002/ibd.20910. [DOI] [PubMed] [Google Scholar]
- 23.Katakura K, Lee J, Rachmilewitz D, et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coquerelle C, Oldenhove G, Acolty V, et al. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut. 2009;58:1363–73. doi: 10.1136/gut.2008.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis PM, Nadler SG, Stetsko DK, Suchard SJ. Abatacept modulates human dendritic cell-stimulated T-cell proliferation and effector function independent of IDO induction. Clin Immunol. 2008;126:38–47. doi: 10.1016/j.clim.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Sandborn WJ, Colombel JF, Sands BE, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143:62–69. e4. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Mellor AL, Keskin DB, Johnson T, et al. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–6. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 28.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 29.Mellor AL, Baban B, Chandler PR, et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 30**.Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn’s disease activity. Inflamm Bowel Dis. 2012;18:1214–20. doi: 10.1002/ibd.21849. This work establishes the viability of the serum kyurenine:tryptophan ratio as a biomarker of disease severity and immune activation in Crohn’s disease patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKenzie CR, Heseler K, Muller A, Daubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–44. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 32.Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Diegelmann J, Olszak T, Goke B, et al. A novel role for interleukin-27 (IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory proteins. J Biol Chem. 2012;287:286–98. doi: 10.1074/jbc.M111.294355. IL-27-induced IDO1 activity is shown to inhibit growth of intestinal bacteria through local tryptophan depletion demonstrating the role for IDO1 in mediating intestinal epithelial barrier protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–81. doi: 10.1038/nature11228. This work provides new understanding of how tryptophan is important in maintaining intestinal homeostasis. The focus of the article is on angiotensin converting enzyme 2 and its renin-angiotensin-system independent function in regulating amino acid homeostasis. The interaction between IDO dependent tryptophan depletion and the findings addressed require investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–25. doi: 10.1016/j.trsl.2012.01.001. This reviews current biomarker use in human IBD and address future biomarkers including those which exploit the IDO1 pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendoza JL, Abreu MT. Biological markers in inflammatory bowel disease: practical consideration for clinicians. Gastroenterol Clin Biol. 2009;33 (Suppl 3):S158–73. doi: 10.1016/S0399-8320(09)73151-3. [DOI] [PubMed] [Google Scholar]
- 37.Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Torres MI, Lopez-Casado MA, Lorite P, Rios A. Tryptophan metabolism and indoleamine 2,3-dioxygenase expression in coeliac disease. Clin Exp Immunol. 2007;148:419–24. doi: 10.1111/j.1365-2249.2007.03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beeken WL. Serum tryptophan in Crohn’s disease. Scand J Gastroenterol. 1976;11:735–40. [PubMed] [Google Scholar]
- 40.Hisamatsu T, Okamoto S, Hashimoto M, et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS One. 2012;7:e31131. doi: 10.1371/journal.pone.0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiomi Y, Nishiumi S, Ooi M, et al. GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm Bowel Dis. 2011;17:2261–74. doi: 10.1002/ibd.21616. [DOI] [PubMed] [Google Scholar]
- 42.Forrest CM, Gould SR, Darlington LG, Stone TW. Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Adv Exp Med Biol. 2003;527:395–400. doi: 10.1007/978-1-4615-0135-0_46. [DOI] [PubMed] [Google Scholar]
- 43.Forrest CM, Youd P, Kennedy A, et al. Purine, kynurenine, neopterin and lipid peroxidation levels in inflammatory bowel disease. J Biomed Sci. 2002;9:436–42. doi: 10.1007/BF02256538. [DOI] [PubMed] [Google Scholar]
- 44.Lin HM, Barnett MP, Roy NC, et al. Metabolomic analysis identifies inflammatory and noninflammatory metabolic effects of genetic modification in a mouse model of Crohn’s disease. J Proteome Res. 2010;9:1965–75. doi: 10.1021/pr901130s. [DOI] [PubMed] [Google Scholar]
- 45.Lin HM, Edmunds SI, Helsby NA, et al. Nontargeted urinary metabolite profiling of a mouse model of Crohn’s disease. J Proteome Res. 2009;8:2045–57. doi: 10.1021/pr800999t. [DOI] [PubMed] [Google Scholar]
- 46.Otter D, Cao M, Lin HM, et al. Identification of urinary biomarkers of colon inflammation in IL10−/− mice using Short-Column LCMS metabolomics. J Biomed Biotechnol. 2011;2011:974701. doi: 10.1155/2011/974701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pineton de Chambrun G, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 48.Stidham RW, Higgins PD. Value of mucosal assessment and biomarkers in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2010;4:285–91. doi: 10.1586/egh.10.22. [DOI] [PubMed] [Google Scholar]
- 49.Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L, Chen H, Wen Q, Zhang Y. Indoleamine 2,3-dioxygenase expression in human inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2012;24:695–701. doi: 10.1097/MEG.0b013e328351c1c2. [DOI] [PubMed] [Google Scholar]
- 51*.Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–30. doi: 10.1182/blood-2009-09-246124. The potent, IDO1 specific inhibitor INCB024360 is described. [DOI] [PubMed] [Google Scholar]
- 52.Huang A, Fuchs D, Widner B, et al. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br J Cancer. 2002;86:1691–6. doi: 10.1038/sj.bjc.6600336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walczak K, Dabrowski W, Langner E, et al. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand J Gastroenterol. 2011;46:903–12. doi: 10.3109/00365521.2011.579159. [DOI] [PubMed] [Google Scholar]
- 54*.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front Biosci (Elite Ed) 2012;4:734–45. doi: 10.2741/e414. IDO1’s role in cancer pathogenesis and the current state of targeting IDO1 inhibition for cancer therapy are reviewed in excellent detail. [DOI] [PubMed] [Google Scholar]
- 55**.Ferdinande L, Decaestecker C, Verset L, et al. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106:141–7. doi: 10.1038/bjc.2011.513. Informative study demonstrating epithelial IDO1 expression in CRC correlated with poor outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–51. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 57.Gao YF, Peng RQ, Li J, et al. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med. 2009;7:71. doi: 10.1186/1479-5876-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 59.Chang MY, Smith C, Duhadaway JB, et al. Cardiac and gastrointestinal liabilities caused by deficiency in the immune modulatory enzyme indoleamine 2,3-dioxygenase. Cancer Biol Ther. 2011:12. doi: 10.4161/cbt.12.12.18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Macho Fernandez E, Valenti V, Rockel C, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050–9. doi: 10.1136/gut.2010.232918. Demonstrates that induction of IDO1 activity may be one way in which probiotic bacteria promote tolerance and again provides connections between innate immune signaling, IL10 and IDO1. [DOI] [PubMed] [Google Scholar]
- 61*.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. The tolerance promoting Clostridium species described in this paper are demonstrated to induce IDO1 in colonic epithelial cells of colonized mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia L, Schweikart K, Tomaszewski J, et al. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: absence of toxicity due to saturating absorption. Food Chem Toxicol. 2008;46:203–11. doi: 10.1016/j.fct.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hussein Hatem, Soliman HH, Neuger A, Noyes D, et al. A phase I study of 1-methyl-D-tryptophan in patients with advanced malignancies. J Clin Oncol. 2012;30 (suppl; abstr 2501) [Google Scholar]
- 64.Koblish HK, Hansbury MJ, Bowman KJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489–98. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 65.Newton RC, Scherle PA, Bowman K, et al. Pharmacodynamic assessment of INCB024360, an inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1), in advanced cancer patients. J Clin Oncol. 2012:30. (Abstract 2500) [Google Scholar]
- 66*.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2012 doi: 10.1016/j.it.2012.10.001. Global review of IDO’s function in immunity and cancer by luminaries in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Connor JC, Lawson MA, Andre C, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Kim H, Chen L, Lim G, et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122:2940–54. doi: 10.1172/JCI61884. This study connects immune activation, IDO1 activity and depression. Though the gut is not addressed in these experiments, the effects likely have relevance to the mechanisms underlying mood disturbance associated with active inflammatory bowel disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Addolorato G, Capristo E, Stefanini GF, Gasbarrini G. Inflammatory bowel disease: a study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand J Gastroenterol. 1997;32:1013–21. doi: 10.3109/00365529709011218. [DOI] [PubMed] [Google Scholar]