Abstract
Multisynaptic neural and endocrine pathways from the suprachiasmatic nucleus of the hypothalamus have been hypothesized to communicate circadian and photic information to the adrenal glands. In humans, light exposure has been reported to have no effect, increase, or decrease cortisol levels. These inconsistent findings in humans may be related to differences among studies including the intensity (∼500 to 5500 lux), duration (15 min to 4 h), and circadian phase of light exposure. The authors assessed the influence of exposure to bright light on cortisol levels in humans during the rising and descending phases of the circadian rhythm of cortisol, that is, when cortisol levels are high. Twenty healthy men and women were studied using a within-subject research design. Subjects were studied in an environment free of time cues for 9 to 10 days. Subjects received a 6.7-h exposure of bright light (∼10,000 lux; equivalent to ambient light intensity just after sunrise or just before sunset) or dim light (∼3 lux; equivalent to candlelight) during the biological night and morning. Bright light exposure significantly reduced plasma cortisol levels at both circadian phases studied, whereas dim light exposure had little effect on cortisol levels. The finding of an acute suppressive effect of bright light exposure on cortisol levels supports the existence of a mechanism by which photic information can acutely influence the human adrenal glands.
Keywords: circadian rhythm, biological clock, diurnal, circadian phase, adrenal gland
The steroid hormone cortisol serves a variety of important functions in the human body. Cortisol has been reported to influence metabolic (Porterfield, 1997; Brillon et al., 1995), immune (Norbiato et al., 1997), muscle (Holmäng and Björntorp, 1992), and brain functions (Porterfield, 1997; Deleon et al., 1997). Cortisol production and secretion is regulated primarily by the hypothalamic-pituitary-adrenal (HPA) axis through release of corticotrophin releasing hormone (CRH) from the hypothalamus and adrenocorticotrophin hormone (ACTH) from the anterior pituitary gland (Angeli et al., 1992). Cortisol feeds back to the hippocampus (Spencer et al., 2001), hypothalamus, and the anterior pituitary, inhibiting CRH and ACTH, thus influencing its own production via negative feedback (Porterfield, 1997). The suprachiasmatic nucleus (SCN) of the hypothalamus regulates the circadian rhythm of corticosteroids (Kalsbeek et al., 1996; Skene et al., 1999; Czeisler and Klerman, 1999). In humans, cortisol levels decrease across the habitual waking day and are lowest near habitual bedtime after which time they increase across the habitual night and peak near habitual wake time, regardless of continuous wakefulness or sleep (Weibel et al., 1995; Leproult et al., 1997a; Czeisler and Klerman, 1999). Superimposed on this circadian rhythm are fluctuations in cortisol associated with the pulsatile release of the hormone. Factors such as anxiety (Czeisler et al., 1976), stress (Porterfield, 1997), immune challenge (Späth-Schwalbe et al., 1998), physical activity (Leproult et al., 1997b; DeRijk et al., 1997), posture changes (Hennig et al., 2000), blood glucose levels (Porterfield, 1997), sleep onset (Weitzman et al., 1983), sleep loss (Leproult et al., 1997a; Spiegel et al., 2004), awakening from sleep (Weibel et al., 1995), and exposure to light (Scheer and Buijs, 1999; Leproult et al., 2001; Kostoglou-Athanassiou et al., 1998) have been reported to alter cortisol levels acutely.
Light exposure is known to reset the phase of the internal biological clock and to affect human physiology and behavior acutely (Czeisler and Wright, 1999; Duffy and Wright, 2005; Foret et al., 1993; Wright et al., 1997b; Lavoie et al., 2003; Cajochen et al., 2002; Cajochen et al., 2005; Scheer et al., 1999; Wright et al., 1997a). The findings on the acute effect of light on cortisol have been inconsistent however. Light exposure has been reported to acutely decrease (Kostoglou-Athanassiou et al., 1998), increase (Scheer and Buijs, 1999; Leproult et al., 2001), or have little effect (Scheer and Buijs, 1999; Rüger et al., 2006; Leproult et al., 1997b; Thalén et al., 1997; Leproult et al., 2001; McIntyre et al., 1992; Beck-Friis et al., 1985; Lavoie et al., 2003) on cortisol levels. It is possible that these findings are dependent in part upon study differences in the intensity, duration, and circadian phase of light exposure as these factors are known to strongly influence the effects of light on human physiology. We therefore examined the acute effect of bright light exposure on plasma cortisol levels in humans when cortisol levels were high, during the rising and descending circadian phases of the cortisol rhythm.
Materials and Methods
Subjects
Data for this analysis were obtained from healthy subjects who participated in studies that assessed the phase-shifting effects of bright light exposure, some of which have contributed to a previous publication (Khalsa et al., 2003). Twenty subjects (5 females) aged 25.3 ± 6.4 y (mean ± SD) who received bright or dim light during the rising or descending phases of the cortisol rhythm were included in the current analysis. Thirteen subjects were exposed to bright light during the rising or descending phases of the cortisol rhythm and 7 subjects to dim light during the rising phase of the cortisol rhythm.
Subjects provided written informed consent and study procedures were conducted in accordance with the Declaration of Helsinki. The Institutional Review Board approved the research protocol.
General Protocol
Subjects lived in a suite on the General Clinical Research Center at the Brigham and Women's Hospital for 9 to 10 days in an environment free of time cues. Data from the first 6 days were used for the current analyses. During 3 baseline days of the protocol (Fig. 1), subjects were scheduled to sleep for 8 h at their self-selected habitual bedtime, as determined by the prior week of ambulatory actigraphy (Khalsa et al., 2003). The baseline days ensured that prior light history was similar for subjects prior to dim or bright light exposure. On the 4th and 5th days, a constant routine (CR) was used to assess the circadian phase of the melatonin rhythm and baseline cortisol levels (Khalsa et al., 2003; Duffy and Wright, 2005). Blood was sampled every 30 min with an indwelling intravenous catheter located in a forearm vein. Subjects were randomized to a predetermined CR duration so that following an 8-h recovery sleep episode, each subject would be exposed to light at a different circadian phase. The current analysis examined those subjects who were exposed to light when cortisol levels were high on the rising and descending phases of the circadian rhythm of cortisol. We used the circadian rhythm of melatonin to describe the circadian phase at which subjects were exposed to light because melatonin sampled on a constant routine in dim light is an accurate marker of circadian phase in humans (Klerman et al., 2002).
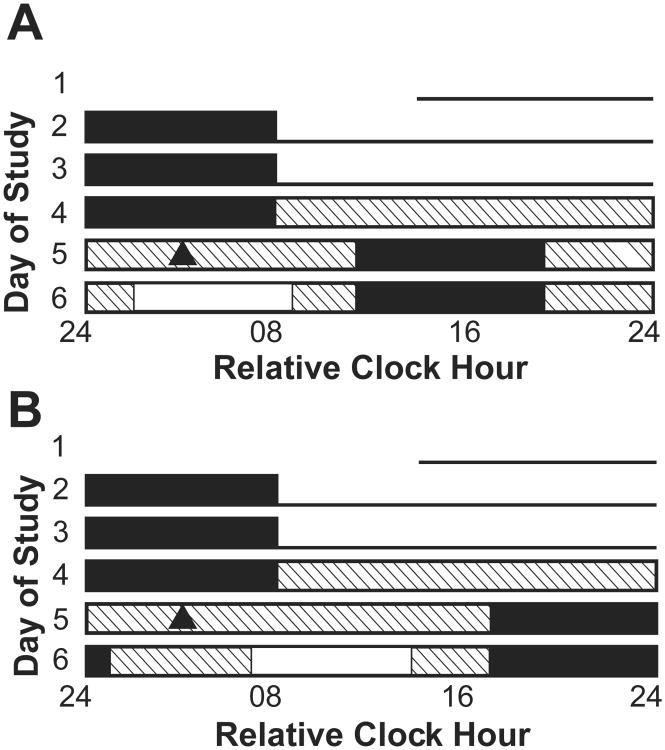
Figure 1.
Experimental protocol. Relative clock time with habitual wake time assigned to 0800 h is represented on the horizontal axis and the day of the study is on the vertical axis. Three 24 h baseline days consisted of a habitual sleep-wake cycle including 16 h of scheduled wakefulness with < 150 lux of light intensity (lines) followed by 8 h of scheduled sleep in darkness (filled boxes). Four of the subjects in the dim light condition had their light exposure reduced to ∼1.5 lux on the second half of day 3. On day 4, a constant routine (CR) with a light intensity of ∼3 lux (boxes filled with diagonal lines) was continued until the 8-h sleep episode on day 5. Note that the CR duration for 4 subjects exposed to dim light during the rising phase of the circadian rhythm in cortisol lasted 2 instead of 1 circadian cycle and thus they had another day on the CR compared to other subjects. Day 6 consisted of ∼3 lux during scheduled wake-fulness, except during exposure to the bright (∼10,000 lux) or dim (∼3 lux—no change in intensity) light stimulus (open box) timed either during the rising (A) or descending (B) phase of the cortisol rhythm. Cortisol data used in the current analyses are from the CR on day 5 (baseline) and the light exposure session on day 6. Circadian timing of the average light exposure session for each condition is represented by the time of light exposure relative to the average timing of the melatonin midpoint (closed triangle; also see Materials and Methods).
Cortisol and Melatonin Assessment
Blood samples from the CR and the day of light exposure 24 h later were used for the current analysis. Dim light melatonin onset (DLMO25%) and offset (DLMOff25%) were defined as the linear interpolated point in time that melatonin levels were consistently above and below 25% of the 3-harmonic peak-to-trough amplitude of the melatonin rhythm, respectively (Wright and Czeisler, 2002; Gronfier et al., 2004). Melatonin midpoint was defined as the time point midway between DLMO25% and DLMOff25% (Gronfier et al., 2004). Plasma melatonin levels were determined by 125I radioimmunoassay (DiagnosTech, Osceola, WI); sensitivity, 2.5 pg/mL; intra- and interassay coefficients of variation, 5.9% and 9.9%, respectively. Plasma cortisol levels were determined by chemiluminescent assay (Beckman Coulter, Chaska, MN); sensitivity, 0.4 mg/dL; intra- and interassay coefficients of variation, 6.4% and 7.9%, respectively.
Light Exposure
Lighting was generated using ordinary commercially available ceiling-mounted broad-spectrum fluorescent lamps (4100 K; Phillips Lighting, Eindhoven, the Netherlands) and transmitted through a UV-stable filter (Lextran 9030 with prismatic lens; GE Plastics, Pittsfield, MA). Illuminance and irradiance measures were conducted using an IL1400 radiometer/powermeter with SEL-033/Y/W and SEL-033/F/W detectors, respectively (International Light., Newburyport, MA). Light intensity during scheduled wakefulness of baseline days was <150 lux (0.48 W/m2) maximum in the room at ∼187 cm with the sensor in the vertical angle. During the remainder of the protocol, subjects were exposed to dim light (∼3 lux [∼0.0087 W/m2] in the horizontal angle of gaze with the light sensor at 137 cm from the ground; <15 lux maximum at ∼187 cm with the light sensor pointed in the direction of the ceiling fixtures) during scheduled wakefulness and darkness during scheduled sleep, except for light exposure sessions.
The light exposure on day 6 consisted of bright light (∼10,000 lux, in the angle of gaze ∼30 W/m2; equivalent to sunlight at dawn or dusk; n = 13) for 6.7 h in the middle of that 16-h wake episode or continuous ambient dim light (∼3 lux; equivalent to candle light; n = 7). Prior light-dark and scheduled wakefulness-sleep conditions were similar for those exposed to dim or bright light exposure. Table 1 shows the start of the light exposure relative to the melatonin midpoint for each of the light exposure conditions and phases of the cortisol rhythm. Posture was controlled by having subjects seated 12 min prior to and throughout the light exposure session. Technicians monitored subjects to ensure that they kept their eyes open and stayed seated during the light exposure session. During the light exposure sessions (both dim and bright), subjects alternated between free gaze around the room and fixed gaze at a target on the wall every 6 min throughout the 6.7-h session (however, fixed gaze and posture control occurred for only for 1 h in 4 subjects exposed to dim light). Subjects in the bright light condition received ∼10,000 lux during the fixed gaze sessions and ∼5000 to 9000 lux during the free gaze sessions. Subjects in the dim light condition received ∼3 lux during both fixed and free gaze sessions.
Table 1.
Condition characteristics.
| Age | Start of Light Exposure Relative to Melatonin Midpointa | |||
|---|---|---|---|---|
|
|
|
|||
| Condition (Light; Phase of Cortisol Profile) | Participants (n) | Mean ± SD (y) | Mean ± SD (h) | Range (h) |
| Bright light, rising phase | 8 | 23.5 ± 4.3 | −1.9 ± 1.1 | −3.0 to 0.3 |
| Bright light, descending phase | 5 | 29.0 ± 8.9 | 3.2 ± 1.7 | 1.5 to 5.7 |
| Dim light, rising phase | 7 | 24.7 ± 6.1 | −1.9 ± 1.4 | −3.4 to 0.0 |
A negative number represents timing of light exposure onset prior to the melatonin midpoint in hours, determined 24 h earlier. A positive number represents timing of light exposure onset after the melatonin midpoint in hours.
Statistical Analysis
Repeated measures ANOVA tested for differences between baseline and treatment day (baseline night vs. light exposure night), light exposure condition (bright light vs. dim light group during the rising phase of the cortisol rhythm only as there were insufficient subjects for a dim light descending phase analysis), and time into light exposure (hours from onset of light exposure). Data for the rising and descending phases of the cortisol rhythm were analyzed separately. The influence of bright light on cortisol levels beginning 30 min prior to, during, and up to ∼4.5 h following light exposure was examined. These times were selected because of blood sampling problems for some subjects prior to the light exposure session. To control for differences in circadian phase among subjects, cortisol data from the same subject collected 24 h earlier on the CR was used as baseline. Huynh-Feldt correction factors were used to correct for violations of homogeneity of variance. Multiple planned comparisons were examined with Fisher's least significant tests combined with a modified Bonferroni correction to reduce type I error (Keppel, 1991). We also computed the trapezoidal area under the curve (AUC; Wright and Czeisler, 2002) for cortisol levels during light exposure and the equivalent clock hours during the dim light baseline 24 h earlier to determine the percent change in cortisol in response to the bright light exposure. Independent t tests were used to compare the percent change in cortisol AUC between light exposure conditions for the rising phase of the cortisol rhythm and circadian phase of the light exposure between the light conditions during the rising phase of the cortisol rhythm. Two-tailed single-sample t tests were used to compare the percent change in cortisol AUC from the baseline to the light exposure nights.
Results
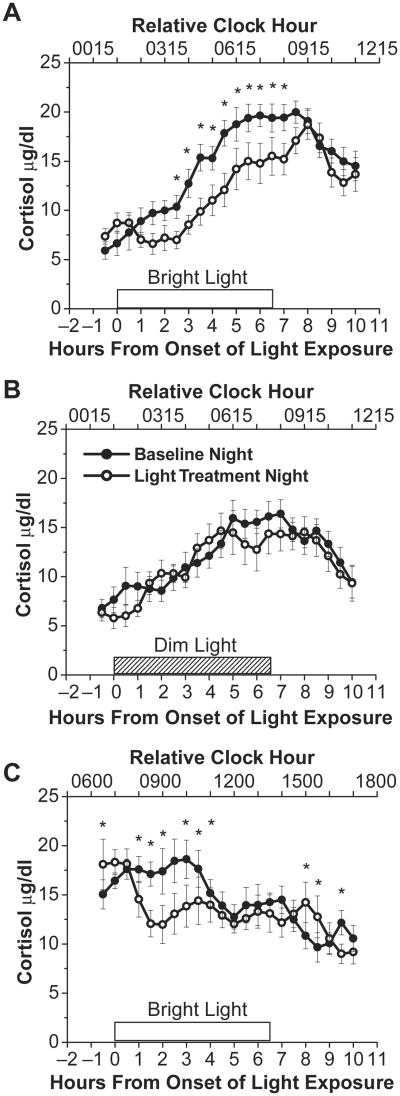
The circadian phase of light exposure did not differ statistically between bright and dim light conditions for the rising phases of the cortisol rhythm (independent t13 = −0.07, p = 0.95). Figure 2A shows that exposure to bright light on the rising phase of the cortisol rhythm significantly decreased cortisol levels when compared to exposure to dim light the day prior, whereas Figure 2B shows that exposure to dim light on the light treatment night had relatively little effect on cortisol levels (light exposure condition × day × time into light exposure interaction, F21,273 = 2.01, p < 0.01). As shown in Figure 2A, significant decreases in cortisol levels were observed ∼2.5 h after the onset of bright light exposure and continued until ∼0.8 h after the offset of light exposure.
Figure 2.
Bright light and dim light exposure group curves. Panels A and B show cortisol levels on the rising phase of the cortisol rhythm during the baseline night (∼3 lux; closed circles) and immediately before, during, and after bright (∼10,000 lux; n = 8; open circles) or dim (∼3 lux; n = 7; open circles) light exposure, respectively. Panel C shows cortisol levels on the descending (n = 5) phase of the cortisol rhythm during the baseline night (∼3 lux; closed circles) and during exposure to bright light (∼10,000 lux; open circles). Open box indicates timing of bright light exposure on the light exposure night. Box filled with diagonal lines indicates timing of dim light on the light exposure night; however, in reality dim light subjects were continuously exposed to dim light for the entire scheduled wakefulness episode. Note that the last time point shown for the light exposure session is an average of the last 12 min of light exposure and the first 18 min of dim light exposure. * denotes significant differences in cortisol levels between baseline and light exposure nights (p < 0.024; modified Bonferroni correction). Error bars represent SEM.
Figure 2C shows that exposure to bright light on the descending phase of the cortisol rhythm also significantly decreased cortisol levels when compared to exposure to dim light the day prior (day × time of day interaction, F21,84 = 4.46, p < 0.01). As seen in Figure 2C, significant decreases in cortisol were observed beginning ∼1.0 h after the onset of light exposure and continued for several hours. In addition, after bright light exposure ended there were 2 time points during which cortisol levels were significantly higher and 1 time point during which cortisol levels were significantly lower on the light exposure night compared to the baseline night.
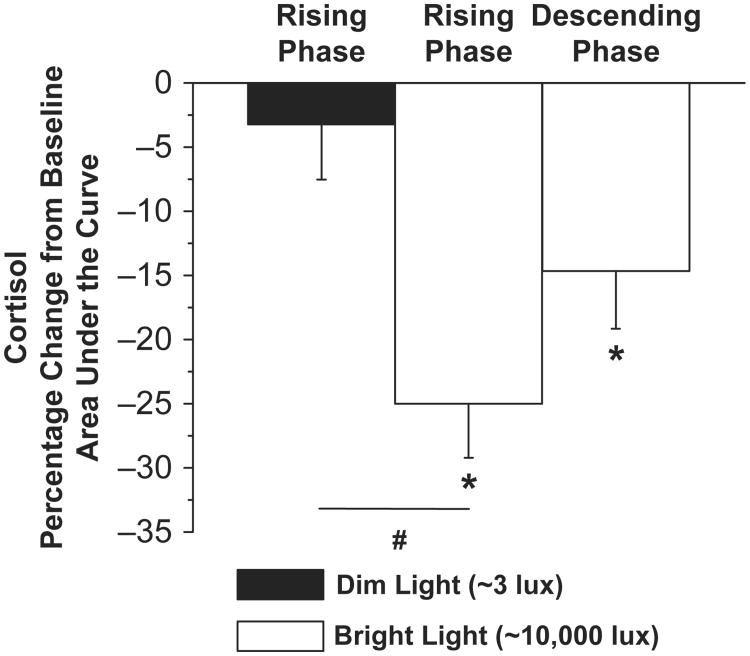
Area under the curve analysis indicated that the percent change in cortisol from baseline was significantly reduced during the bright light exposure session (single-sample t7 = 5.96, p < 0.01) but not during the dim light exposure session (single-sample t6 = 0.75, p = 0.48) on the rising phase of the cortisol rhythm (Fig. 3). In addition, the percent reduction in cortisol AUC from baseline was significantly greater in the bright light versus the dim light group (independent t13 = –3.61, p < 0.01). Furthermore, the change in cortisol from baseline was significantly reduced during the bright light exposure session (single-sample t4 = 3.26, p < 0.01) on the descending phase of the cortisol rhythm (Fig. 3).
Figure 3.
An area under the curve analysis. Percentage change from baseline for area under the curve cortisol levels during the light exposure session for the rising and descending phases of the cortisol rhythm. *denotes significant decreases in cortisol levels between baseline (∼3 lux) and bright light exposure (∼10,000 lux) nights (within groups, p < 0.017; modified Bonferroni correction). # denotes significant decreases in cortisol levels between dim and bright light exposure conditions on the rising phase of the cortisol rhythm (between group, p < 0.017; modified Bonferroni correction). Error bars represent SEM.
Discussion
In the current study, we found that exposure to ∼10,000 lux of bright light on the rising and descending phases of the cortisol rhythm (i.e., when cortisol levels are high) decreased cortisol levels in humans. Cortisol levels during bright light exposure were lower than cortisol levels during dim light exposure the day prior, thus controlling for circadian phase within individuals. Exposure to bright light on the rising phase of the cortisol rhythm significantly decreased cortisol levels beginning ∼2.5 h after light exposure onset when compared to baseline. Cortisol levels remained decreased throughout the light exposure session and were then similar to the baseline night levels shortly after bright light exposure ended, suggesting no immediate large phase delay shift of the cortisol rhythm, although we cannot exclude a larger phase delay of an evening oscillator as compared to a morning oscillator (Liu and Borjigin, 2005).
Subjects exposed to dim light during the rising phase of the cortisol rhythm showed similar levels of cortisol compared to the baseline night. Exposure to bright light on the descending phase of the cortisol rhythm also significantly decreased cortisol levels beginning ∼1 h after light exposure onset when compared to baseline. Cortisol levels were decreased for the first half of the light exposure session but were similar to baseline night levels during the second half of the light exposure session suggesting no immediate phase advance shift of the cortisol rhythm. The absence of a sustained effect of light on cortisol levels at the end of the descending phase of the cortisol rhythm may be due to exposure at a circadian phase at which cortisol is less sensitive to light exposure (Scheer and Buijs, 1999).
While we found a suppressing effect of light on cortisol levels in this study, there is also evidence that light can increase or have little effect on cortisol levels in humans. Cortisol levels in humans might be affected by the duration, intensity, and biological timing of the light exposure. During the afternoon, between ∼1200 and 1600 h when cortisol levels are relatively low, exposure to light intensities of 4500 to 5000 lux has consistently been reported to have no significant influence on cortisol levels (Leproult et al., 2001; Rüger et al., 2006). Exposure to bright light during the afternoon was not examined in the current study. During the night and early morning between 2000 h and 0600 h, exposure to 600 to 3000 lux broad spectrum light (Beck-Friis et al., 1985; McIntyre et al., 1992; Lavoie et al., 2003; Scheer and Buijs, 1999; Petterborg et al., 1991; Thalén et al., 1997) and exposure to ∼10 to 12 μW/cm2 460 and 555 nm light (Lockley et al., 2006) have been reported to cause no change in cortisol levels with the exception of one study (Leproult et al., 2001). In three studies that exposed subjects to a light intensity of ≥5000 lux, conflicting findings were reported, however. Specifically, exposure to 5000 lux between ∼2200 h and 1000 h (Leproult et al., 1997b) or between 2400 h and 0400 h (Rüger et al., 2006) was reported to induce no change in cortisol levels, whereas exposure to 5500 lux between 2000 h and 0200 h (Kostoglou-Athanassiou et al., 1998) was reported to decrease mean cortisol levels when compared to a within-subjects baseline but not to a between-subjects control. Our finding of reductions in cortisol levels during exposure to ∼10,000 lux on the rising and descending phases of the circadian rhythm of cortisol, when levels are high, suggests that the intensity of bright light may be important in determining the effects of light on cortisol levels. Such a finding would be consistent with the findings of intensity response curves for melatonin suppression (Zeitzer et al., 2000). Examination of multiple light intensities would be necessary to determine if there is an intensity response curve for the effects of light on cortisol.
During the morning, light exposure has been reported to increase (Scheer and Buijs, 1999; Leproult et al., 2001) or cause no change in cortisol levels (Beck-Friis et al., 1985; Leproult et al., 1997b). Specifically, Scheer and Buijs, (1999) exposed subjects to 1 h of 800 lux at habitual wake time and reported significantly higher salivary cortisol levels in the first 40 min of light exposure and that after 1 h of light exposure cortisol levels were similar to baseline. Leproult et al. (2001) reported increased plasma cortisol levels within the first 15 min of a stepwise light intensity pattern of 2000 up to 4500 lux between 0500 h and 0800 h. The transient increases in cortisol in response to light exposure were observed when cortisol was sampled frequently (every 15-20 min). In the current study, we found no significant change in plasma cortisol levels near the beginning of the light exposure; although the first time we sampled cortisol was 30 min after light exposure onset and thus more immediate changes could not be examined.
Inconsistencies are also found in nonhumans with respect to the influence of light on corticosteroids. It also appears that the short- vs. long-duration light exposures on glucocorticoids are different between diurnal and nocturnal mammals. These inconsistencies might be related to light intensity, duration of light exposure, and the circadian phase in which the animal was exposed to light (Perlow et al. 1981; Buijs et al., 1999; Ishida et al., 2005; Mohawk et al., 2007; Hatanaka et al., 2008).
Changes in corticosterone levels were reported to be dependent upon an intact SCN and were not related to a change in ACTH levels (Buijs et al., 1999; Ishida et al., 2005). Retrograde virus labeling showed a polysynaptic neural pathway from the SCN to the paraventricular nucleus, the spinal cord, and the adrenal gland, suggesting that the SCN has direct neural influence on adrenal function (Buijs et al., 1999). Furthermore, when sympathetic nerve input to the adrenal gland was severed, the effect of light exposure on corticosterone levels subsequent to the end of light exposure was not observed (Ishida et al., 2005). Also, when pituitary adenylate cyclase-activating polypeptide (PACAP)-deficient mice are exposed to light, the effects of light exposure on corticosterone levels subsequent to the end of light exposure were not observed (Hatanaka et al., 2008). The latter may indicate that PACAP is involved in this neural pathway. It is unknown if the reduced cortisol levels we observed in the current study were achieved by stimulation of the same neuroanatomical pathway. Future studies in humans could assess ACTH and cortisol levels to determine whether reduced cortisol levels in response to bright light exposure are associated with changes in ACTH levels.
A factor that may have influenced the current results, as well as the results of some prior studies, is the influence of total sleep deprivation vs. sleep on cortisol levels. Sleep deprivation has been reported to increase cortisol levels during the quiescent interval of the circadian rhythm of cortisol (Leproult et al., 1997a; Gronfier and Brandenberger 1998). Although subjects were sleep deprived in the current study on the baseline night prior to light exposure, sleep deprivation appeared to have a minimal effect on the current results because subjects exposed to dim light during the rising phase of the cortisol rhythm on both nights showed similar cortisol levels on both days. Furthermore, cortisol levels were higher prior to the light exposure session on the light exposure night compared to baseline night—opposite to what would be expected if sleep deprivation were to acutely increase cortisol levels during the sampling window on the baseline night. Another factor that might influence the acute effects of bright light on cortisol levels is prior light exposure history. Specifically, following exposure to dim light on the constant routine, the subsequent effect of bright light on cortisol levels may be enhanced as has been reported for the effect of light on melatonin suppression (Hebert et al., 2002; Smith et al., 2004).
Like many other studies, our primary comparison was conducted using a within-subjects baseline condition in dim light. Cortisol levels were reduced for subjects exposed to bright light; whereas subjects exposed to dim light during the rising phase of the cortisol rhythm showed similar cortisol levels on both nights. Cortisol levels were reduced until ∼0.8 h after the bright light exposure session ended during the rising phase of the circadian rhythm of cortisol. Therefore, it is unlikely that our current results are representative of an immediate large phase shift of the cortisol rhythm. If a rapid phase delay had occurred in response to evening light exposure (Khalsa et al., 2003), cortisol levels should have been consistently higher after the bright light exposure. However, it is possible that bright light exposure at this phase induced considerable reduction in endogenous circadian amplitude, which could account for the observed reduction in peak cortisol levels, as reported by Jewett et al. (1991). It is unlikely that a rapid phase advance could account for the observed response to morning light exposure on the descending phase of the cortisol rhythm (Khalsa et al., 2003), as cortisol levels would then have been consistently lower after bright light exposure ended. Last, the finding that dim light exposure caused little change in cortisol levels suggests that the protocol of sleep deprivation per se had a small influence on our findings.
In conclusion, exposure to ∼10,000 lux of bright light for 6.7 h during the rising and descending phases of the cortisol rhythm reduced cortisol levels. A multisynaptic neural pathway from the retina, to the SCN, to adrenal glands, that bypasses the HPA axis has been hypothesized to be responsible for the acute influence of light on corticosteroid levels (Buijs et al., 1999). Whether this neural pathway may also mediate the effects of light on cortisol in humans remains to be tested. The current study is the first to expose subjects to a long duration light intensity of ∼10,000 lux and examine the acute effect on cortisol levels in humans. A review of findings from other studies suggests that the biological timing of light exposure may also be an important determinant of the effect of light on cortisol (proposed in Scheer and Buijs, 1999), and our data are consistent with such a window of sensitivity because suppression was greatest at the end of the rising phase and start of the descending phase, when cortisol levels are highest. The implications of our current findings that bright light decreased cortisol levels on the rising phase of the cortisol rhythm are perhaps most applicable to night and early morning shift workers and jet travelers who are exposed to bright light during the biological night. The finding that bright light exposure reduced cortisol levels on the descending phase of the cortisol rhythm suggests that exposure to morning sunlight may have a greater effect on adrenal cortex physiology than previously recognized. Future studies should examine the physiological consequences of reduced cortisol levels during exposure to bright light.
Acknowledgments
Research supported by NIH R01-MH45130, NIH R01-HL081761, by General Clinical Research Center Grant GCRC-M01-RR-02635 from the National Center for Research Resources, and by the Undergraduate Research Opportunities Program in collaboration with the Biological Sciences Initiative at the University of Colorado–Boulder.
Footnotes
The online version of this article can be found at: http://jbr.sagepub.com/cgi/content/abstract/25/3/208
References
- Angeli A, Gatti G, Masera R. Chronobiology of the hypothalamic-pituitary-adrenal and rennin-angiotensin-aldosteron systems. In: Touitou Y, Haus E, editors. Biological Rhythms in Clinical and Laboratory Medicine. Berlin: Springer-Verlag; 1992. pp. 292–314. [Google Scholar]
- Beck-Friis J, Borg G, Wetterberg L. Rebound increase of nocturnal serum melatonin levels following evening suppression by bright light exposure in healthy men: Relation to cortisol levels and morning exposure. Ann N Y Acad Sci. 1985;453:371–375. [Google Scholar]
- Brillon DJ, Zheng B, Campbell RG, Matthews DE. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 1995;268:E501–E513. doi: 10.1152/ajpendo.1995.268.3.E501. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MGP, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Moore-Ede MC, Regestein QR, Kisch ES, Fang VS, Ehrlich EN. Episodic 24-hour cortisol secretory patterns in patients awaiting elective cardiac surgery. J Clin Endocrinol Metab. 1976;42:273–283. doi: 10.1210/jcem-42-2-273. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–132. [PubMed] [Google Scholar]
- Czeisler CA, Wright KP. Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Regulation of Sleep and Circadian Rhythms. New York: Marcel Dekker; 1999. pp. 149–180. [Google Scholar]
- de Leon MJ, McRae T, Rusinek H, Convit A, De Santi S, Tarshish C, Golomb J, Volkow N, Daisley K, Orentreich N, et al. Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer's disease. J Clin Endocrinol Metab. 1997;82:3251–3259. doi: 10.1210/jcem.82.10.4305. [DOI] [PubMed] [Google Scholar]
- DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, Paciotti G, Gold PW, Sternberg EM. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: High sensitivity of TNF alpha and resistance of IL-6. J Clin Endocrinol Metab. 1997;82:2182–2191. doi: 10.1210/jcem.82.7.4041. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Wright KP. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;28:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- Foret J, Aguirre A, Touitou Y, Clodoré M, Benoit O. Effect of morning bright light on body temperature, plasma cortisol and wrist motility measured during 24 hour of constant conditions. Neurosci Lett. 1993;155:155–158. doi: 10.1016/0304-3940(93)90696-i. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Brandenberger G. Ultradian rhythms in pituitary and adrenal hormones: Their relations to sleep. Sleep Med Rev. 1998;2:17–29. doi: 10.1016/s1087-0792(98)90051-x. [DOI] [PubMed] [Google Scholar]
- Hatanaka M, Tanida M, Shintani N, Isojima Y, Kawaguchi C, Hashimoto H, Kakuda M, Haba R, Nagai K, Baba A. Lack of light-induced elevation of renal sympathetic nerve activity and plasma corticosterone levels in PACAP-deficient mice. Neurosci Lett. 2008;444:153–156. doi: 10.1016/j.neulet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Friebe J, Ryl I, Krämer B, Böttcher J, Netter P. Upright posture influences salivary cortisol. Psychoneuroendocrinology. 2000;25:69–83. doi: 10.1016/s0306-4530(99)00037-2. [DOI] [PubMed] [Google Scholar]
- Holmäng A, Björntorp P. The effects of cortisol on insulin sensitivity in muscle. Acta Physiol Scand. 1992;144:425–431. doi: 10.1111/j.1748-1716.1992.tb09316.x. [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991;350:59–62. doi: 10.1038/350059a0. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Vliet J, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: A reverse microdialysis study. J Neuroendocrinol. 1996;8:299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher's Handbook. 3rd. Upper Saddle River, NJ: Prentice Hall; 1991. pp. 169–170. [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Kostoglou-Athanassiou I, Treacher DF, Wheeler MJ, Forsling ML. Bright light exposure and pituitary hormone secretion. Clin Endocrinol (Oxf) 1998;48:73–79. doi: 10.1046/j.1365-2265.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Paquet J, Selmaoui B, Rufiange M, Dumont M. Vigilance levels during and after bright light exposure in the first half of the night. Chronobiol Int. 2003;20:1019–1038. doi: 10.1081/cbi-120025534. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, L'Hermite-Balériaux M, Van Cauter E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab. 2001;86:151–157. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997a;20:865–870. [PubMed] [Google Scholar]
- Leproult R, Van Reeth O, Byrne MM, Sturis J, Van Cauter E. Sleepiness, performance, and neuroendocrine function during sleep deprivation: Effects of exposure to bright light or exercise. J Biol Rhythms. 1997b;12:245–258. doi: 10.1177/074873049701200306. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Reentrainment of the circadian pacemaker through three distinct stages. J Biol Rhythms. 2005;20:441–450. doi: 10.1177/0748730405279388. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Melatonin, cortisol and prolactin response to acute nocturnal light exposure in healthy volunteers. Psychoneuroendocrinology. 1992;17:243–248. doi: 10.1016/0306-4530(92)90063-d. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Pargament JM, Lee TM. Circadian dependence of corticosterone release to light exposure in the rat. Physiol Behav. 2007;92:800–806. doi: 10.1016/j.physbeh.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbiato G, Bevilacqua M, Vago T, Taddei A, Clerici M. Glucocorticoids and the immune function in the human immunodeficiency virus infection: A study in hypercortisolemic and cortisol-resistant patients. J Clin Endocrinol and Metab. 1997;82:3260–3263. doi: 10.1210/jcem.82.10.4304. [DOI] [PubMed] [Google Scholar]
- Perlow MJ, Reppert SM, Boyar RM, Klein DC. Daily rhythms in cortisol and melatonin in primate cerebrospinal-fluid—Effects of constant light and dark. Neuroendocrinology. 1981;32:193–196. doi: 10.1159/000123157. [DOI] [PubMed] [Google Scholar]
- Petterborg LJ, Kjellman BF, Thalén BE, Wetterberg L. Effect of a 15 minute light-pulse on nocturnal serum melatonin levels in human volunteers. J Pineal Res. 1991;10:9–13. doi: 10.1111/j.1600-079x.1991.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Porterfield SP. Endocrine Physiology. St. Louis: Mosby-Year Book Inc; 1997. pp. 139–140. [Google Scholar]
- Rüger M, Gordijn MCM, Beersma DGM, De Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: Comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1313–R1320. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- Scheer FAJL, Van Doornen LJ, Buijs RM. Light and diurnal cycle affect human heart rate: Possible role for the circadian pacemaker. J Biol Rhythms. 1999;14:202–212. doi: 10.1177/074873099129000614. [DOI] [PubMed] [Google Scholar]
- Scheer FAJL, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab. 1999;84:3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- Skene DJ, Lockley SW, James K, Arendt J. Correlation between urinary cortisol and 6-sulphatoxymelatonin rhythms in field studies of blind subjects. Clin Endocrinol (Oxf) 1999;50:715–719. doi: 10.1046/j.1365-2265.1999.00714.x. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Späth-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in health men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Kalman BA, Dhabhar FS. Role of endogenous glucocorticods in immune system function: regulation and counterrregulation. In: Mcewen B, editor. Handbook of Physiology Coping with the Environment: Neural and Endocrine Mechanisms. IV. New York: Oxford University Press; 2001. pp. 381–424. [Google Scholar]
- Spiegel K, Leproult R, L'Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clinical Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Thalén BE, Mørkrid L, Kjellman BF, Wetterberg L. Cortisol in light treatment of seasonal and non-seasonal depression: Relationship between melatonin and cortisol. Acta Psychiatr Scand. 1997;96:385–394. doi: 10.1111/j.1600-0447.1997.tb09934.x. [DOI] [PubMed] [Google Scholar]
- Weibel L, Follenius M, Spiegel K, Ehrhart J, Brandenberger G. Comparative effect of night and daytime sleep on the 24-hour cortisol secretory profile. Sleep. 1995;18:549–556. [PubMed] [Google Scholar]
- Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56:352–358. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- Wright KP, Badia P, Myers BL, Plenzler SC. Combination of bright light and caffeine as a counter-measure for impaired alertness and performance during extended sleep deprivation. J Sleep Res. 1997a;6:26–35. doi: 10.1046/j.1365-2869.1997.00022.x. [DOI] [PubMed] [Google Scholar]
- Wright KP, Badia P, Myers BL, Plenzler SC, Hakel M. Caffeine and light effects on nighttime melatonin and temperature levels in sleep-deprived humans. Brain Res. 1997b;747:78–84. doi: 10.1016/s0006-8993(96)01268-1. [DOI] [PubMed] [Google Scholar]
- Wright KP, Czeisler CA. Absence of circadian phase resetting in response to bright light behind the knees. Science. 2002;297:571. doi: 10.1126/science.1071697. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]