Introduction
The mechanisms underlying cell polarity and lumen formation are well described within epithelial structures of mammalian systems, and invertebrate model organisms. Only recently has the molecular control of endothelial polarity and vessel lumen formation undergone similar investigation. The endothelial layer requires similar organization including a requisite apical-basolateral polarity corresponding to luminal and abluminal membranes. In addition, the endothelium also exhibits features of planar cell polarity traditionally described in drosophila eye and wing discs (1), where in response to flow endothelial cells orient in a planar fashion. Coincident with providing a barrier to flow, the endothelial layer also functions to regulate permeability. Key molecules known to regulate endothelial permeability have been shown to control polarity, suggesting the processes may be linked. The integrity of the polarized endothelial layer becomes paramount when initiating new vascular growth. New vascular sprouts require breaking the existing symmetry within a vascular tube, and the adoption of a migratory phenotype typified by the front-rear polarity previously described in leukocytes (2). We will review the various types of polarity as they apply to endothelial cells. In addition, we will touch upon vascular lumen formation and how polarity plays an integral role in the process.
Balancing migratory polarity with permeability
Endothelial cells require directed migration in response to a variety of growth factors and matrix signals (review in (3)). One physiologic example of a front-rear polarized endothelial cell is the tip cell that leads an angiogenic front. While tip cells are discussed in detail elsewhere in this issue [ref], there exist a number of coordinated events in the initiating vascular sprout that require the integration of opposing polarity cues. When presented with a local migratory cue, such as VEGF, the responsive endothelial cell (or selected tip cell) will orient filopodia and lamellipodia to the leading front while the cell undergoes significant cytoskeletal changes (4). To account for the generation and assembly of actin based structures that make up the filopodial and lamellipodial extensions, the cell must counterbalance forces by integration of stress fibers that associate with focal adhesion proteins at membrane protrusion initiation points, and ECM and cell-cell attachment (5–7). RhoA, Rac1, and Cdc42 all belong to a family of Rho GTPases that have demonstrated integrin mediated activation at extracellular matrix (ECM) cell contacts (review in (8)), and localize to focal adhesions which serve as initiation points for membrane protrusion (6). In endothelium, caveolin-1 (the main component of caveolae) is polarized to the trailing edge of a migrating cell on 2D surfaces and serves to participate in β1 integrin endocytosis (9–11). However caveolin-1 polarization has been shown to localize to the leading edge of endothelial cells in 3D environments suggesting dependence on local contextual cues (10,12). Regardless of localization, one function of caveolae during migration is the recycling of integrins, and mediating integrin interactions with Rho GTPases (Figure 1)(13–15). Biosensor activity of Rho GTPases in mouse fibroblasts implicates RhoA in the initial activation at the site of protrusion, where Rac1 and Cdc42 function to propagate the initiating event via actin assembly and stabilization (fig. 1) (14). Specific loss of endothelial Rac1 results in absence of focal adhesion formation and reduced lamellipodia and filopodia extensions culminating in loss of polarized cell migration and attachment (16). In leukocytes, localized Rac1 activity leads to cellular tension mediated by actin assembly and polarized membrane protrusion which allows for the maintenance of cell front-rear polarity (17). It is possible that leading tip cells and/or other migrating endothelial cells adopt a similar tension mechanism to maintain their polarized membrane extensions. Unlike leukocytes however, endothelial cells at the angiogenic front migrate in a coordinated group dynamic where the polarized rear of the migrating tip cell is associated with another endothelial stalk cell via cell-cell adhesion. As stretch has been demonstrated to induce endothelial proliferation via Rac1 activation, and requires VE-cadherin cell-cell adhesion (18), it is conceivable that endothelial tip cell membrane protrusion opposes the force of tip-stalk cell-cell attachment to generate activation “stretch”. In addition to the migration and proliferation of endothelial cells that make up the angiogenic front (19), the growing network must also modulate vascular permeability through tight junction regulation. Loss of endothelial Rac1, which disrupts migration, also creates a resistance to VEGF induced vascular permeability (16), suggesting that the growing endothelial sprout may compromise tight junction stability for migratory ability.
Figure 1.
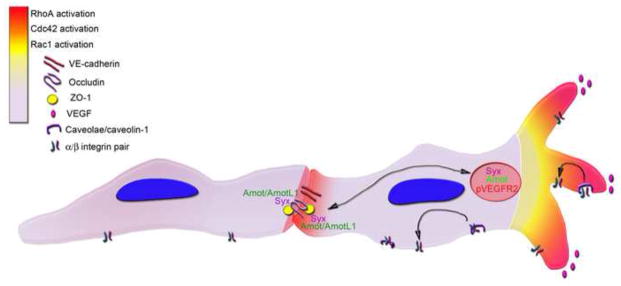
Balancing migration and cell-cell adhesion. In response to growth factor cues (in this example VEGF, pink), the migratory endothelial cell will orient filopodia and lamellipodia to the leading front while the cell undergoes cytoskeletal rearrangement. Caveolin1 interacts with 31 integrin as the cell migrates, and participates in the endocytosis and recycling of integrins. In response to polarity and matrix cues, RhoA is initiated at the site of protrusion and actin assembly. Cdc42 and Rac1 function to propagate the initiating event. Amot and AmotL1 interact with Syx (and can interact with each other) to regulate RhoA both at the cell-cell tight junctions (TJ) and in the polarized leading edge. Phosphorylated VEGFR2 (pVEGFR) is co-trafficked with Amot and Syx and function in TJ disassembly. TJ is represented by Occludin, ZO-1 and VE-cadherin.
The tip-stalk designation within a growing vascular sprout is not static. Recent data demonstrates that the leading angiogenic front is made up of a migratory network of cells that change position frequently, instead of immutably specified tip and stalk cells (20,21). Thus, membrane protrusions, cell-ECM, and cell-cell adhesion are all dynamically interchangeable while vascular permeability is regulated within the trailing lumenized vascular network. Angiomotin (Amot) and angiomotin-like1 (AmotL1), proteins interact with apical Crbs:Pals:Patj polarity complex members through their PDZ binding domain to regulate downstream RhoA (22–24). In the leading angiogenic front, it appears AmotL1 functions to stabilize tight junctions in stalk cells, while Amot functions primarily in polarization and migration of the tip cell (22). Angiopoietin-1 and VEGF have opposing effects on junctional stability and vascular permeability through localization of Syx, a RhoGEF (Rho guanine nucleotide exchange factors) specific for RhoA (25). Syx acts downstream of Crumbs polarity complexes to localize to tight junctions (TJs) and maintain barrier integrity (25). Angiopoietin stabilizes Syx localization to TJs, while VEGF results in loss of Syx from TJs (25). Amot may cooperate with VEGF in the disruption of junctional complexes for cell migratory behavior, as Syx is co-trafficked with Amot and pVEGFR2 (fig. 1) in Rab13 vesicles (26,27). Thus a migratory tip cell that encounters VEGF, will via its downstream signaling displace Syx from VE-cadherin and ZO (zona occludens) with subsequent tight junction disassembly (26). Similar to Amot proteins, Scrib protein stabilizes adherens junctions (as part of the Scribbled basolateral polarity complex which includes lethal giant larvae (Lgl) and discs large (Dlg)), and via its PDZ domain recruits the GEF βPIX, which regulates Rac1 and Cdc42 activation for directed cell migration (28,29). While Scrib localizes to endothelial cell-cell contacts, it also associates with α5 integrin in the basolateral surface of migrating endothelial cells, and serves to protect α5 integrin from Rab7a dependent lysosomal degradation (30). In epithelial structures, the Crumbs apical complex and Scrib-Lgl-Dlg basolateral complex play antagonistic roles in apical/basal polarity (31). In endothelial migration the two complexes seem to play similar roles in directed chemotactic migration. Whether they do so within distinct localized regions of the cell (i.e. front-rear polarized domains) remains to be investigated. To maintain vascular plexus growth and tissue perfusion, multiple tip cells in the angiogenic front need to coalesce into a functioning vascular network. Recent data has suggested that as proximal endothelial cells form cell-cell junctions they appear to do so via a lamellipodia bridge transition (32). Upon lamellipodia retraction, thin cytoplasmic processes remain and remodel into VE-cadherin rich filopodia-like structures between cells, with subsequent expansion of the cell-cell boundary, with presumed loss of front-rear polarity (32). Loss of front-rear polarity and incorporation into an endothelial layer requires the subsequent acquisition of apical-basal polarity, regulation of permeability, and lumen formation.
Apical/basolateral polarity and barriers to permeability
Apical and basolateral polarity has been well delineated in mammalian epithelium in vivo, and in vitro with the use of self organizing 3D structures comprised of mammary or kidney epithelium (33). In epithelial structures, tall cuboidal shaped cells display distinct complexes that identify apical and basal regions by segregation of phosphoinositides and protein complexes to different cell surfaces. Critical to this process in a variety of cell types are the polarity complex proteins Par3, Par6 and atypical protein kinase C (aPKC) (34). Par3 is known to interact at epithelial cellular junctions, while Par6 and aPKC delineate the apical domain (33). Crumbs (apical) and Scribble (basolateral) complexes interact with the par complex machinery to orchestrate cell polarity in epithelium (31,35). Thus far, endothelial angiomotins (36) are known to interact with members of the Crumbs complex which include Crbs:Pals:Patj to orchestrate endothelial polarity and vascular morphogenesis (22). In mammals, the complex consists of the Crbs transmembrane proteins, and scaffolding proteins Pals1 and PatJ (35). Crbs has a role in the stability of apical cell junctions, and can associate with Pals1 PDZ domains, that in turn bind PatJ (a multi-PDZ domain protein that interacts with zonula occludens (ZO) junctional proteins) (35). The crumbs signaling pathway intersects with the par polarity complex through Par6 binding to Pals1 N-terminus, possibly recruiting Par6 to Crb in apical junctions (37). Amot and angiomotin like proteins AmotL1 and AmotL2 localize to endothelial tight junctions, as evidenced by colocalization with ZO-1, and bind via their PDZ domain to PatJ (22,23,38). As previously described, Syx localization to TJs via Amot plays an important role in tight junction integrity, and interestingly loss of Amot, AmotL1 and Syx all demonstrate increased vascular permeability with reduced sprouting (36,39–41). Intersection of this particular pathway (AmotL1/PatJ) with the par complex in the endothelium, and possible downstream effects on polarized endothelial proteins remains to be seen. The Scribble complex includes two membrane-associated scaffold proteins Scrib and Dlg, each with PDZ domains that allow their mutual association at the basolateral cortex (42). Lgl (lethal giant larvae) is genetically linked to the Scrib/Dlg complex, and its localization is regulated in part by its phosphorylation by aPKC (42). While there is not extensive data implicating a role of the Scrib-Lgl-Dlg complex in endothelial apical-basal polarity, loss of Scrib does exhibit vascular hemorrhaging suggestive of defective permeability barriers and a likely role in endothelial polarity (30). The Scrib complex has recently been shown to regulate hippo pathway members (43). Amot family proteins also intersect with the Hippo signaling pathway, where Amot and AmotL1 can bind YAP and TAZ transcriptional activators (44). As the Hippo pathway has recently been implicated in apical polarity (45,46), independent of its well known role in organ and tissue size, the intersection of Amot, Crbs, Scrib, Par and now Hippo may account for multiple layers of cell polarity regulation that remain to be fully investigated in the endothelium.
Endothelial Par3 has been demonstrated to regulate endothelial polarity in the context of 31 integrin loss (47), VE-cadherin loss (48–50), and Ras interacting protein 1, Rasip1, loss (which results in decreased Cdc42 and Rac1 activity) (51). The requisite flattened cell shape of an endothelial cell makes detection of truly segregated apical and basal complexes challenging. Yet, a growing body of work suggests that many of the epithelial apical-basolateral polarity mechanisms also play a critical role in endothelial polarity. Endothelial specific loss of β1 integrin results in a transcriptional decrease in Par3 resulting in cuboidal endothelial cell shape and circumferential expression of adhesion proteins (including VE-cadherin) with mislocalization of apical markers (47). This is partially rescued by Par3 restoration (47). In contrast, total loss of function of Par3 in a wildtype background is not reported to exhibit abnormal endothelial cell shape or polarity in vivo (52), suggesting that other cues may be sufficient to maintain endothelial cell polarization. Yet Par3 knockdown in vitro demonstrates luminal defects in endothelial tube formation assays (49). Par3 and separately Par6, in absence of aPKC, are able to directly complex with VE-cadherin in the endothelium (48,50). Loss of VE-cadherin results in abnormal endothelial cell polarity with mis-expression of apical and basal markers, as well as mislocalization of Par3 (49). However, Par3 knockdown (or Tiam, a Rac exchange factor known to interact with the par complex for migration (53)), does not result in mislocalization of VE-cadherin (49). Knockdown of CCM1, a gene deleted in cerebral cavernous malformation that also localizes to VE-cadherin at endothelial cell adherens junctions, results in mislocalizaton of Par3 as well as VE-cadherin (49). Thus, endothelial Par3 and VE-cadherin may rely on a variety of partners to maintain endothelial polarity and function.
Lumen formation
Not surprisingly, formation of a lumen requires polarization of participating cells and hence many of the pathways already described above play a critical role in endothelial lumen formation. Detailed review of endothelial lumen formation has been described elsewhere (review in (54)), but here we will briefly outline the various mechanisms of lumen formation and the key pathways implicated.
As tip and stalk cells progress in building a functional vascular network, lumen formation must occur shortly after the initial branches of the network are formed. The leading tip cell is by definition not lumenized, however the stalk cells that make up the trailing network do rapidly form lumens. This process has been described as cord (or cell) hollowing, and has been noted to occur via two proposed mechanisms. The first is through the coalescence of intracellular vacuoles within single stalk cells. The vacuoles fuse intracellularly to form a contiguous lumen in a new vessel sprout, and has been depicted in both 3D human endothelial cell matrices and in live imaging of zebrafish (55). A second mechanism involves either unicellular membrane invaginations or multicellular cord hollowing. Both are mediated through cell rearrangements after coalescence of fused junctional rings comprised of adhesion proteins (ZO-1 and VE-cadherin), as visualized in the zebrafish (56). Whether the two mechanisms of cord hollowing (vacuolar coalescence versus adhesive boundary fusion) are mutually exclusive within vascular beds remains to be seen. Vascular lumen stability must be counterbalanced with sprouting angiogenesis. This is seen in Scrib mutants where loss of Scrib-regulated endothelial migration results in enhanced tubulogenesis (30). This is in stark contrast to the role of Scrib in the mammalian lung where Scrib mutants exhibit absence of epithelial lumens within the developing lung airways (57). The luminal defect in the lung epithelia appears mediated through the planar cell polarity pathway (PCP) (57). As Scrib has been demonstrated to complex with TAZ of the hippo pathway in breast cancer epithelia (58), it may be that Scrib regulation of endothelial tubulogenesis occurs via regulation of hippo signaling in absence of PCP pathways. Angiomotin like 2 (amotL2), a protein important in migration with ties to Crumb polarity complexes as well as the Hippo signaling pathways, demonstrates decreased endothelial tubulogenesis after knockdown in matrigel assays (59). In epithelial cysts, AmotL2 knockdown resulted in a similar phenotype with abnormal cell-filled lumens and was noted to be YAP and TAZ dependent (60). Thus, the opposing functions of Crumbs/Amot and Scrib complexes in epithelial cell polarity may also regulate Hippo pathway members in an opposing fashion to regulate endothelial migration, polarity and lumen formation.
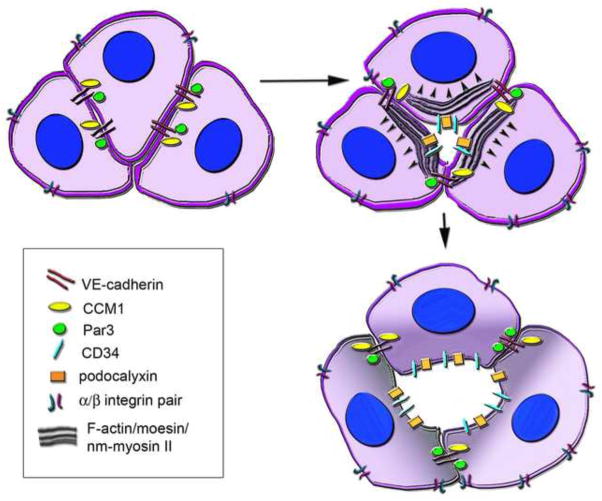
Formation of the paired aortae requires migration of angioblasts with self-assembly into single lumenized structures. Rearrangement of cellular adhesion molecules and polarity mediators appears to be a common theme in lumen formation of the developing aortae. A study by Strilic et al. 2009, demonstrated that the aortic lumens develop initially through small extracellular gaps between endothelial cells that progress to larger diameters (61). The initial separations between cells occur after the apical distribution of CD34 and podycalyxin sialomucins (Figure 2), suggesting that cells polarize prior to forming a lumen, and that the acquisition of luminal/abluminal polarity is VE-cadherin dependent (61). VE-cadherin and CCM1, mentioned previously as apical basal polarity mediators, also demonstrate luminal defects after loss of function (49). VE-cadherin is noted to play a role in Moesin and F-actin recruitment to cell-cell contacts (and subsequently non muscle (nm) myosin II, which induces cell shape changes) (61). Protein kinase C (PKC) is responsible for linking CD34 to the actin cytoskeleton via Moesin phosphorylation, while ROCK (Rho associated protein kinase) activity is critical for recruitment of nm Myosin II, and subsequent cell shape changes. Together, the data suggest that apical polarity is acquired first, with sialomucins creating membrane repulsion (due to their negatively charged residues), and subsequent events lead to force generation of cytoskeletal and cell shape changes to support an apically located lumen (fig. 2) (61). The Rho GTPase family, integral to lumen formation in vitro where it interacts with the par complex (62), has been recently confirmed as the pathway responsible for failure of aortic lumen formation after loss of endothelial-restricted Rasip1 (51). Loss of function of Rasip1 (a regulator of GTPase signaling) or Arghap29 (a RhoA-specific GTPase activating protein (GAP)) results in a decrease in Cdc42/Rac1 signaling leading to abnormal aortic lumen formation (51). In the context of Rasip1 or Arghap29 loss, cells exhibit mislocalization of Par3, decreased activation of 31 integrin, but retain normal localization of CD34, podoxalyxin, moesin, and nm myosin II (51). However, mutant Rasip1 endothelium also demonstrated an overabundance of phosphorylated myosin light chain protein, which is required for nm myosin II activity, resulting a hypercontractile phenotype (51). Thus, both models suggest downstream cytoskeletal contractile forces are critical in lumen formation; with too little (as evidenced by PKC and ROCK inhibition (61)) or too much (in Rasip1 mutants with increased ROCK activity (51)) resulting in loss of a patent vascular lumen. The requisite balance of contractile forces for endothelial lumen formation is further evidenced in a recent study that demonstrates critical role for a controlled level of acetylated tubulins in endothelial lumen formation (63). Thus, the apical luminal membrane in endothelium is formed through a balance of oppositional extracellular and intracellular forces.
Figure 2.
Lumen formation within the aorta. The contact sites of neighboring endothelial cells are comprised of junctional proteins, one of which is VE-cadherin. VE-cadherin can associate with both CCM-1 and Par3 proteins, which regulate apical/basal polarity and lumen formation. As the luminal membrane becomes polarized, apical localization of sialomucins (CD34 and podocalyxin) is noted. Negatively charged sialomucins cause membrane repulsion, as moesin associates with CD34 and recruits F-actin, and eventually nm myosin II, to the apical membrane. This actin/myosin recruitment and assembly generates contractile forces that regulate cell shape changes resulting in a patent lumen. β1 integrin is located in the basolateral membrane has been demonstrated to also play a role in cell shape and lumen formation, possibly by acting to counterbalance apical forces in the process.
Once endothelium becomes polarized and flattened with delineated apical/luminal and basolateral/abluminal surfaces, it is typified as a single cell layer. When 31 integrin ECM cues are lost after this vascular morphology is established, the aortic endothelium adopts a multilayered morphology while mid-sized arterial vessels exhibit luminal defects (47). 31 integrin blockade during early aortic cord formation results in an absence of a patent lumen (64), as does its blockade in endothelial tube formation assays (65) and in the developing retina (47). Loss of cell polarity due to 31 integrin loss is exemplified by decreased Par3 expression and mislocalization of apical and basal markers, but preferentially affects arterial vascular beds at later developmental timepoints (47). A recent intriguing study ties ephrinB2 and Par3 to VEGF receptor endocytosis in the growing vascular front (66). As ephrinB2 is preferentially expressed in arterial vessels, this interaction of ephrinB2 and Par3 may lend insight into the differential luminal defects across endothelial subtype noted later in development (47). Thus, endothelial cells comprising different vascular beds or undergoing different vascular processes (angiogenesis, vasculogenesis, remodeling) likely employ a myriad of available mechanisms to achieve lumen formation. Further investigation into lumen formation and cell polarity in both developmental and pathological contexts with respect to regional and endothelial subtype may uncover how the various mechanisms and signaling pathways are employed.
Planar cell polarity and flow-responsive patterning
Sheer stress has been demonstrated to induce cell shape and cytoskeletal changes in a directional manner when exposed to fluid forces (67). Rho and Cdc42 have been implicated in this response as they both demonstrate activation of downstream signaling upon shear stress (68,69). While the alignment of endothelium in response to flow has been questioned as being truly dependent on planar cell polarity (PCP), recent data suggests that the endothelium can display hallmarks of PCP, as endothelial microtubule systems (MTOC) are polarized uniformly to one side of the cell nuclei and are reversibly oriented under blockade of glycogen synthase kinase 33 (GSK-33, a protein kinase in the Wnt signaling pathway) (70). In addition, disruption of Wnt PCP (i.e. non-canonical signaling) pathway mediators, dishevelled (Dvl) and Wnt5a known to be involved in epithelial PCP, result in endothelial proliferation and patterning defects (71,72). Most recently a frizzled receptor (Fzd4), also implicated in non-canonical Wnt PCP signaling has been shown to play an important role in angiogenesis, with mutants exhibiting impaired endothelial proliferation, migration and lumen formation (73,74). Thus, it is likely that the same pathways implicated in PCP and microtubule/cytoskeletal polarity in epithelial structures have similar functions in endothelial morphogenesis and patterning. This would place Wnt, dishevelled, and frizzled of the non-canonical Wnt PCP signaling pathway upstream of microtubule and cytoskeletal rearrangements mediated by GSK333and Rho GTPases.
In addition to cytoskeletal cell orientation along the vascular axis, endothelial cells have also been shown to mitotically divide perpendicularly along the vessel long axis (70,75). The divisional cues are noted to be downstream of VEGF, as divisional axis is disrupted in VEGF receptor mutants (75). However, the ordered divisional axes are noted in newly formed vessels that lack blood flow suggesting that PCP cell orientation and polarized division is patterned independent of flow (75). Thus in vivo, endothelial cells may use PCP pathways for initial patterning and then rely on flow mechanosensor complexes such as PECAM-1, VE-Cadherin, and VEGFR2 (76) to further activate integrins and signaling in response to shear forces. There still remains the difficult task of understanding the separate contributions of PCP mechanisms and flow mechanotransduction to vascular morphogenesis.
Concluding remarks
The creation and maintenance of both newly formed and mature vascular beds requires a complex balance of physical forces, polarity determinants, adhesive and permeable properties, as well as the ability to remodel in response to growth and flow cues. Hence the endothelium must retain quiescent states but be poised for action when new vascular growth is needed, or changes in fluid dynamics demand for plasticity. All this must occur in addition to its function as a barrier. Cellular polarity plays an integral role in all these processes. The mediators of polarity that are implicated in endothelial biology comprise a dizzying list of pathways that in many cases have incomplete connections to one another. However, as the longstanding epithelial models begin to provide a more precise picture of pathway determinants and intersections between them, and endothelial models gain more traction, we are starting to see some overarching concepts. In particular, the machinery used for cell-cell adhesion is also co-opted for endothelial migration. Sprouting angiogenesis and endothelial barrier integrity play opposing roles with similar mediators. Lastly, the complexes required for requisite apical basal polarity remain critical for lumen formation as well. The master regulators that emerge in these processes have ties to longstanding polarity pathways described in non-mammalian systems. The Scrib and Crumbs polarity complexes, with ties to par polarity complexes, Rho GTPases and Hippo family members are emerging as important endothelial signaling pathways. As the investigation into how these pathways interact with one another to regulate endothelial biology proceeds, we may see similar principles take shape that are not only specific to the endothelium but may be applicable across various cell types and organisms.
Highlights.
Key molecules known to regulate endothelial permeability also control polarity.
Complexes required for apical basal polarity remain critical for lumen formation.
Angiogenesis and barrier integrity play opposing roles with similar mediators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wehrle-Haller B, Imhof BA. Actin, microtubules and focal adhesion dynamics during cell migration. Int J Biochem Cell Biol. 2003 Jan;35(1):39–50. doi: 10.1016/s1357-2725(02)00071-7. [DOI] [PubMed] [Google Scholar]
- 3.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circulation Research. 2007 Mar 30;100(6):782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. The Journal of Cell Biology. 2003 Jun 23;161(6):1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002 Aug;16(10):1195–204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 6.Xia N, Thodeti CK, Hunt TP, Xu Q, Ho M, Whitesides GM, et al. Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation. FASEB J. 2008 Jun;22(6):1649–59. doi: 10.1096/fj.07-090571. [DOI] [PubMed] [Google Scholar]
- 7.Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000 Feb;12(1):63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 8.Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003 Oct;15(5):590–7. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 9.Shi F, Sottile J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. Journal of Cell Science. 2008 Jul;121(Pt 14):2360–71. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parat M-O, Anand-Apte B, Fox PL. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol Biol Cell. 2003 Aug;14(8):3156–68. doi: 10.1091/mbc.E02-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beardsley A, Fang K, Mertz H, Castranova V, Friend S, Liu J. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J Biol Chem. 2005 Feb 4;280(5):3541–7. doi: 10.1074/jbc.M409040200. [DOI] [PubMed] [Google Scholar]
- 12.Santilman V, Baran J, Anand-Apte B, Fox PL, Parat M-O. Caveolin-1 polarization in migrating endothelial cells is directed by substrate topology not chemoattractant gradient. Cell Motil Cytoskeleton. 2006 Nov;63(11):673–80. doi: 10.1002/cm.20153. [DOI] [PubMed] [Google Scholar]
- 13.del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-García A, Anderson RGW, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005 Aug 21;7(9):901–8. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009 Sep 3;461(7260):99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B, Radel C, Hughes D, Kelemen S, Rizzo V. p190 RhoGTPase-activating protein links the 31 integrin/caveolin-1 mechanosignaling complex to RhoA and actin remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011 Feb;31(2):376–83. doi: 10.1161/ATVBAHA.110.217794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008 Jun;22(6):1829–38. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 17.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012 Jan 20;148(1–2):175–88. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 Are Differentially Required for Stretch-Mediated Proliferation in Endothelial Versus Smooth Muscle Cells. Circulation Research. 2007 Jul 12;101(5):e44–e52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 19.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010 Oct;22(5):617–25. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, et al. Nat Cell Biol. 10. Vol. 12. Nature Publishing Group; 2010. Sep 26, Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting; pp. 943–53. [DOI] [PubMed] [Google Scholar]
- 21.Blum Y, Belting H-G, Ellertsdóttir E, Herwig L, Lüders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Developmental Biology. 2008 Apr;316(2):312–22. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Vertuani S, Nyström S, Audebert S, Meijer I, Tegnebratt T, et al. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circulation Research. 2009 Jul 31;105(3):260–70. doi: 10.1161/CIRCRESAHA.109.195156. [DOI] [PubMed] [Google Scholar]
- 23.Ernkvist M, Luna Persson N, Audebert S, Lecine P, Sinha I, Liu M, et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009 Jan 1;113(1):244–53. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bratt A, Birot O, Sinha I, Veitonmäki N, Aase K, Ernkvist M, et al. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005 Oct 14;280(41):34859–69. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- 25.Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. The Journal of Cell Biology. 2012 Dec 24;199(7):1103–15. doi: 10.1083/jcb.201207009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Horowitz A. Membrane traffic as a coordinator of cell migration and junction remodeling. Commun Integr Biol. 2011 Nov 11;4(6):703–5. doi: 10.4161/cib.17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C, Agrawal S, Vasanji A, Drazba J, Sarkaria S, Xie J, et al. Rab13-dependent Trafficking of RhoA Is Required for Directional Migration and Angiogenesis. Journal of Biological Chemistry [Internet] 2011 Jun 24;286(26):23511–20. doi: 10.1074/jbc.M111.245209. Available from: http://www.jbc.org/cgi/doi/10.1074/jbc.M111.245209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank SR, Bell JH, Frödin M, Hansen SH. A 3PIX-PAK2 complex confers protection against Scrib-dependent and cadherin-mediated apoptosis. Curr Biol. 2012 Oct 9;22(19):1747–54. doi: 10.1016/j.cub.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007 Apr 5;26(16):2272–82. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 30.Michaelis UR, Chavakis E, Kruse C, Jungblut B, Kaluza D, Wandzioch K, et al. The Polarity Protein Scrib is Essential for Directed Endothelial Cell Migration. Circulation Research. 2013 Jan 29; doi: 10.1161/CIRCRESAHA.112.300592. [DOI] [PubMed] [Google Scholar]
- 31.Martín-Belmonte F, Perez-Moreno M. Nature Reviews Cancer. 1. Vol. 12. Nature Publishing Group; 2011. Dec 15, Epithelial cell polarity, stem cells and cancer; pp. 23–38. [DOI] [PubMed] [Google Scholar]
- 32.Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol Biol Cell. 2012 Jan;23(2):310–23. doi: 10.1091/mbc.E11-08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008 Apr;20(2):227–34. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein B, Macara IG. The PAR Proteins: Fundamental Players in Animal Cell Polarization. Dev Cell [Internet] 2007 Nov 6;13(5):609–22. doi: 10.1016/j.devcel.2007.10.007. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1534580707003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochimica et Biophysica Acta (BBA) - Biomembranes [Internet] 2008 Mar;1778(3):614–30. doi: 10.1016/j.bbamem.2007.08.029. Available from: http://linkinghub.elsevier.com/retrieve/pii/S000527360700332X. [DOI] [PubMed] [Google Scholar]
- 36.Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, et al. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes & Development. 2007 Aug 15;21(16):2055–68. doi: 10.1101/gad.432007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003 Feb;5(2):137–42. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- 38.Bratt A, Birot O, Sinha I, Veitonmäki N, Aase K, Ernkvist M, et al. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005 Oct 14;280(41):34859–69. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- 39.Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. The Journal of Cell Biology. 2012 Dec 24;199(7):1103–15. doi: 10.1083/jcb.201207009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Vertuani S, Nystrom S, Audebert S, Meijer I, Tegnebratt T, et al. Angiomotin-Like Protein 1 Controls Endothelial Polarity and Junction Stability During Sprouting Angiogenesis. Circulation Research. 2009 Jul 30;105(3):260–70. doi: 10.1161/CIRCRESAHA.109.195156. [DOI] [PubMed] [Google Scholar]
- 41.Garnaas MK, Moodie KL, Liu ML, Samant GV, Li K, Marx R, et al. Syx, a RhoA Guanine Exchange Factor, Is Essential for Angiogenesis In Vivo. Circulation Research. 2008 Aug 14;103(7):710–6. doi: 10.1161/CIRCRESAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enomoto M, Igaki T. Journal of Genetics and Genomics. 10. Vol. 38. Elsevier Limited and Science Press; 2011. Oct 20, Deciphering tumor-suppressor signaling in flies: Genetic link between Scribble/Dlg/Lgl and the Hippo pathways; pp. 461–70. [DOI] [PubMed] [Google Scholar]
- 43.Verghese S, Waghmare I, Kwon H, Hanes K, Kango-Singh M. Scribble acts in the Drosophila fat-hippo pathway to regulate warts activity. PLoS ONE. 2012;7(11):e47173. doi: 10.1371/journal.pone.0047173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. Journal of Biological Chemistry. 2011 Mar 4;286(9):7018–26. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011 Jun 1;436(2):213–24. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- 46.Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. Journal of Cell Science. 2009 Jul 15;122(Pt 14):2351–9. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010 Jan 19;18(1):39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iden S, Rehder D, August B, Suzuki A, Wolburg-Buchholz K, Wolburg H, et al. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep. 2006 Dec;7(12):1239–46. doi: 10.1038/sj.embor.7400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, et al. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. Journal of Cell Science. 2010 Apr 1;123(Pt 7):1073–80. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 50.Tyler RC, Peterson FC, Volkman BF. Distal interactions within the par3-VE-cadherin complex. Biochemistry. 2010 Feb 9;49(5):951–7. doi: 10.1021/bi9017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, et al. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell. 2011 Apr 19;20(4):526–39. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirose T. PAR3 is essential for cyst-mediated epicardial development by establishing apical cortical domains. Development. 2006 Apr 1;133(7):1389–98. doi: 10.1242/dev.02294. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Watanabe T, Matsuzawa K, Katsumi A, Kakeno M, Matsui T, et al. Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. The Journal of Cell Biology. 2012 Oct 15;199(2):331–45. doi: 10.1083/jcb.201202041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009 Feb;16(2):222–31. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006 Jul 27;442(7101):453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 56.Herwig L, Blum Y, Krudewig A, Ellertsdóttir E, Lenard A, Belting H-G, et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011 Nov 22;21(22):1942–8. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, Hilton H, et al. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Developmental Biology. 2013 Jan 15;373(2):267–80. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. Cell. 4. Vol. 147. Elsevier Inc; 2011. Nov 11, The Hippo Transducer TAZ Confers Cancer Stem Cell-Related Traits on Breast Cancer Cells; pp. 759–72. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Li Z, Xu P, Huang L, Tong J, Huang H, et al. Angiomotin-like2 Gene (amotl2) Is Required for Migration and Proliferation of Endothelial Cells during Angiogenesis. Journal of Biological Chemistry. 2011 Nov 18;286(47):41095–104. doi: 10.1074/jbc.M111.296806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes & Development. 2011 Jan 4;25(1):51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.StriliC B, KuCera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009 Oct;17(4):505–15. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. Journal of Cell Science. 2008 Apr 1;121(Pt 7):989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 63.Kim DJ, Martinez-Lemus L, Davis GE. EB1, p150Glued and Clasp1 control endothelial tubulogenesis through microtubule assembly, acetylation and apical polarization. Blood. 2013 Feb 26; doi: 10.1182/blood-2012-11-470179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drake CJ, Davis LA, Little CD. Antibodies to beta 1-integrins cause alterations of aortic vasculogenesis, in vivo. Dev Dyn. 1992 Jan;193(1):83–91. doi: 10.1002/aja.1001930111. [DOI] [PubMed] [Google Scholar]
- 65.Sacharidou A, Stratman AN, Davis GE. Molecular Mechanisms Controlling Vascular Lumen Formation in Three-Dimensional Extracellular Matrices. Cells Tissues Organs. 2012;195(1–2):122–43. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HCA, et al. Nat Cell Biol. 3. Vol. 15. Nature Publishing Group; 2013. Jan 27, Spatial regulation of VEGF receptor endocytosis in angiogenesis; pp. 249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. Journal of Cell Science. 1996 Apr;109( Pt 4):713–26. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 68.Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, et al. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest. 1999 Apr;103(8):1141–50. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simmers MB, Pryor AW, Blackman BR. Arterial shear stress regulates endothelial cell-directed migration, polarity, and morphology in confluent monolayers. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1937–46. doi: 10.1152/ajpheart.00534.2007. [DOI] [PubMed] [Google Scholar]
- 70.McCue S, Dajnowiec D, Xu F, Zhang M, Jackson MR, Langille BL. Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circulation Research. 2006 Apr 14;98(7):939–46. doi: 10.1161/01.RES.0000216595.15868.55. [DOI] [PubMed] [Google Scholar]
- 71.Cirone P, Lin S, Griesbach HL, Zhang Y, Slusarski DC, Crews CM. A role for planar cell polarity signaling in angiogenesis. Angiogenesis. 2008;11(4):347–60. doi: 10.1007/s10456-008-9116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sundberg TB, Darricarrere N, Cirone P, Li X, McDonald L, Mei X, et al. Disruption of Wnt planar cell polarity signaling by aberrant accumulation of the MetAP-2 substrate Rab37. Chem Biol. 2011 Oct 28;18(10):1300–11. doi: 10.1016/j.chembiol.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Descamps B, Sewduth R, Ferreira Tojais N, Jaspard B, Reynaud A, Sohet F, et al. Frizzled 4 regulates arterial network organization through noncanonical Wnt/planar cell polarity signaling. Circulation Research. 2012 Jan 6;110(1):47–58. doi: 10.1161/CIRCRESAHA.111.250936. [DOI] [PubMed] [Google Scholar]
- 74.Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009 Oct 16;139(2):285–98. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng G, Taylor SM, McColm JR, Kappas NC, Kearney JB, Williams LH, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007 Feb 15;109(4):1345–52. doi: 10.1182/blood-2006-07-037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005 Sep 15;437(7057):426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]