Abstract
Background
Chronic kidney disease (CKD) impairs intestinal barrier function which leads to endotoxemia and systemic inflammation. We have found depletion of intestinal epithelial tight junction (TJ) proteins in animals with CKD. We further showed that addition of ESRD patients' plasma to the culture medium provokes marked drop in transepithelial electrical resistance (TER) and depletion of TJ proteins in cultured human enterocytes. These effects were less severe with post- than pre-hemodialysis plasma suggesting the role of dialyzable agent(s). This study tested the hypothesis that intestinal barrier dysfunction in uremia may be due to diffusion of urea into the gut and its conversion to ammonia by microbial urease.
Methods
Human enterocytes (T84 cells) were seeded on Transwell plates and utilized when TER exceeded 1,000 Ω.cm2 to ensure full polarization and TJ formation. Confluent cells were then incubated for 24 hr in media containing zero, 42, or 74 mg/dl urea or urea plus urease to simulate presence of microbial flora.
Results
At clinically-relevant concentrations, urea caused a concentration-dependent fall in TER and the key TJ protein; cluadin-1, occludin, and ZO1. The effects of urea were dramatically amplified by urease causing cells detachment, dissipation of TER, and massive loss of TJ proteins.
Conclusions
uremia-induced disruption of intestinal TJ and barrier function is, in part, mediated by urea which is generally considered to be a nontoxic retained metabolite. These findings reveal a novel mechanism for salutary effect of urea-lowering strategies e.g. low protein diet and longer and more frequent dialysis regimens in advanced CKD.
Keywords: Endotoxin, Inflammation, Gastrointestinal pathology, End-stage renal disease, uremia, cardiovascular disease
Introduction
There is emerging evidence pointing to intestinal barrier dysfunction and its role in the pathogenesis of systemic inflammation in humans and animals with advanced CKD [1,2]. In an earlier study we found marked depletion of the key trans-cellular (claudin-1 and occludin) and intra-cellular (ZO1) constituents of colonic epithelial tight junction (TJ) in the rats with CKD induced by either 5/6 nephrectomy or adenine-induced tubulointerstitial nephropathy. The reduction in the abundance of the tight junction proteins was accompanied by their normal even elevated mRNA abundance [3] suggesting post-transcriptional or post-translational nature of the down regulation of these proteins. In a recent study [4] we asked whether uremia-induced intestinal epithelial barrier dysfunction and the TJ protein depletion is mediated by the retained uremic toxins/metabolites and if so whether they can be removed by dialysis. To this end we tested the effects of addition to the culture media of pre- and post-hemodialysis plasma samples from ESRD patients and plasma from healthy controls on the polarized T84 human colonic epithelial cells. When seeded on Transwell plates, T84 cells form a polarized, impermeable monolayer that exhibits many of the functional characteristics of the intestinal epithelial cells in vivo, including vectorial solute transport and barrier function [5,6]. The study showed that compared with the control plasma, incubation in media containing pre-dialysis plasma from ESRD patients caused a marked drop in transepithelial electrical resistance (TER) denoting increased epithelial permeability. This was accompanied by significant reductions in trans-cellular i.e. cluadin-1 and occludin and intra-cellular i.e. zonula occludens (ZO1) protein constituents of epithelial tight junction apparatus. The severity of TJ damage and dysfunction was significantly less in cells exposed to the post-dialysis than per-dialysis plasma. These findings pointed to the presence of as-yet unidentified, but partially dialyzable, product(s) in the uremic plasma capable of degrading epithelial TJ and impairing intestinal epithelial barrier function. The reduction in urinary excretion of nitrogenous waste products in advanced CKD results in their accumulation in the body fluids. The most abundant among these metabolites is urea which has been considered to be a non-toxic and inert retained uremic metabolite. The rise in urea concentration in the intra-cellular and extra-cellular fluid compartments results in its heavy influx into the gastro-intestinal tract (7). Within the intestinal lumen urea is hydrolyzed by microbial urease forming large quantities of ammonia [CO(NH2)2 +H2O → CO2+2NH3] which is readily converted to ammonium hydroxide [NH3 + H2O→NH4OH] (8,9). Ammonium hydroxide, in turn, leads to a modest rise in the luminal fluid's pH, causes mucosal irritation and promotes enterocolitis [10,11]. Based on these observations we hypothesize that ammonium hydroxide generated from hydrolysis of urea by microbial urease in the intestinal lumen of uremic patients may contribute to the epithelial barrier dysfunction and erosion of the TJ protein constituents. The present study was designed to test this hypothesis by exploring the effect of clinically-relevant concentrations of urea alone and urea plus urease on intestinal epithelial barrier function and structure in vitro.
Methods
Cell culture and incubation studies
T84 cells were obtained from American Type Culture Collection (Manassas, VA) and were grown in DMEM/F12 medium (Invitrogen Inc. Carlsbad, California) containing sodium bicarbonate 1.2 g/L, l-glutamine 2.5 mM, HEPES 15 mM, and sodium pyruvate 0.5 mM (Invitrogen) supplemented with 10% fetal calf serum. To form polarized monolayers cells were seeded (60,000 cells/cm2) in 12- well or 6-well plates with Transwell inserts (Fisher Scientific Pittsburgh PA) for measurement of TER and Western blot analysis. The T84 monolayers were maintained for 21 days at 37° C in complete medium in an incubator containing a mixture of air with 5% carbon dioxide. The medium was changed every other day and the trans-epithelial electrical resistance (TER) was measured regularly using Millicell ERS-2 meter (EMD Millipore Inc. Billerica, MA). When a TER exceeding 1,000 mΩ/cm2 was reached the monolayers were incubated for 24 hr at 37° C in DMEM/F12 medium alone or that containing 42mg/dl or 72mg/dl (70, or 120 uM) urea (Sigma-Aldrich, St. Louis, MO) with or without urease (Sigma Inc.). The given urea concentrations were chosen to approximate the pre- and post-hemodialysis values generally found in ESRD patients and as such were considered to be clinically relevant. Urease was added to the medium at 1 unit / uM urea. At the conclusion of the 24-hr incubation period, the TER was measured and cells were harvested and processed for Western blot analysis.
Western Blot analyses
The cells were lysed with M-PER Mammalian Protein Extraction Reagent (Fisher Scientific, Pittsburgh PA). Protein concentration in the cell lysate was determined by BSA assay kit (Pierce Rockford, IL) and 20 µg of total protein from each sample were fractionated on 4–12% Bis-Tris gradient gel (Invitrogen) at 120 V for two hours and transferred to a Nitrocellulose Membrane. The membrane was then incubated with rabbit anti-claudin-1 or rabbit anti-occludin or mouse anti-ZO 1 (Invitrogen, Carlsbad, CA) antibodies at 1:250 dilutions, and anti-actin antibody (Sigma6 Aldrich, St. Louis, MO) at 1:10,000 dilution overnight. The appropriate horseradish peroxidase–conjugated secondary antibodies (Sigma-Aldrich) were used at a 1:5000 dilution. The membrane was visualized with SuperSignal West Pico (Pierce) and developed by autoluminography.
Data analysis
Analysis of variance and Student's t-test were used in statistical evaluation of the data which are presented as mean ± SD. P values less than 0.05 were considered significant.
Results
Effect of urea and urea plus urease on TER and PH
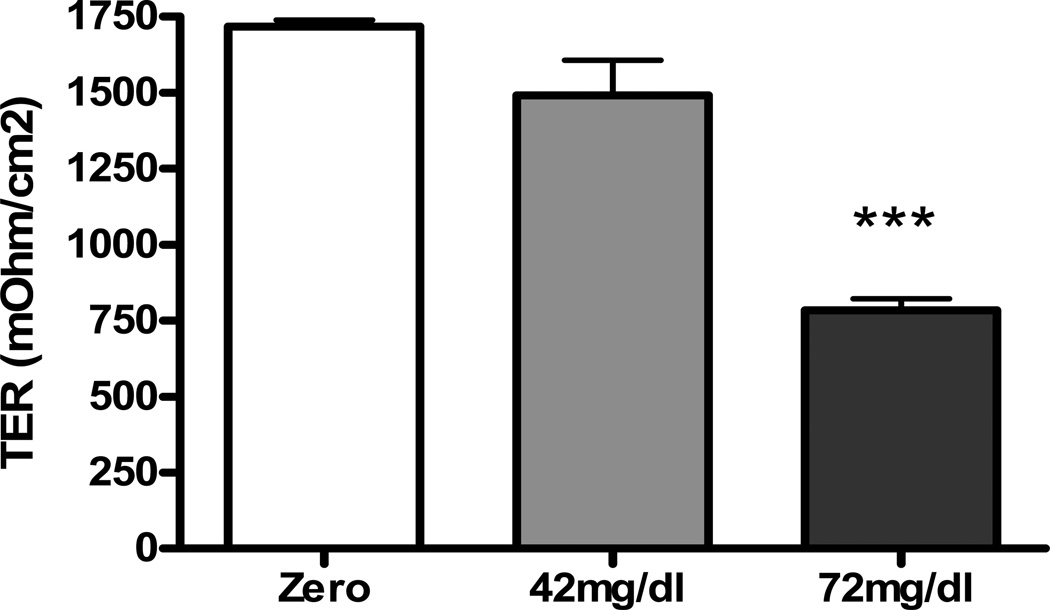
Incubation in media containing urea at 42 or 72 mg/dl resulted in a significant concentration-dependent fall in basalapical electrical resistance pointing to the ability of urea to impair epithelial barrier function (Figure 1). Epithelial monolayers incubated in media containing both urea and urease exhibited detachment which precluded the ability to measure TER. Addition of urease alone to the culture medium had no significant effect on TER. The average PH of the culture medium rose from 7.1 to 7.3, 24 hr after addition of urea plus urease.
Figure 1.
Bar graphs depicting the trans-epithelial electrical resistance (TER) in intestinal epithelial T84 cell monolayers incubated for 24 hr in regular media and those incubated in media containing 42 or 72 mg/dl urea.
Effect of urea and urea plus urease on epithelial TJ proteins
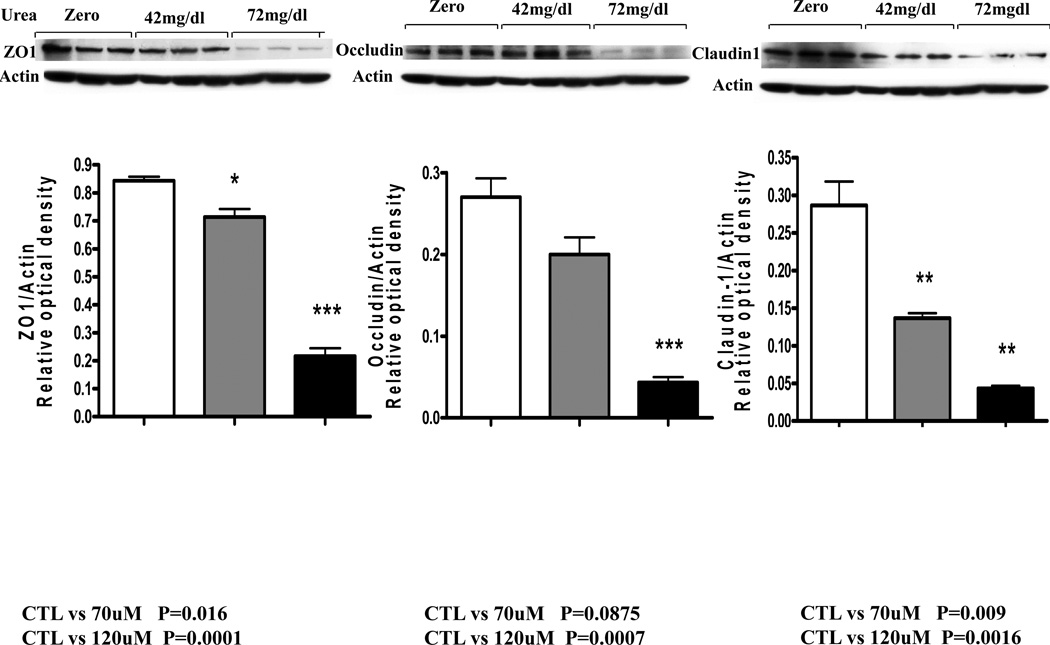
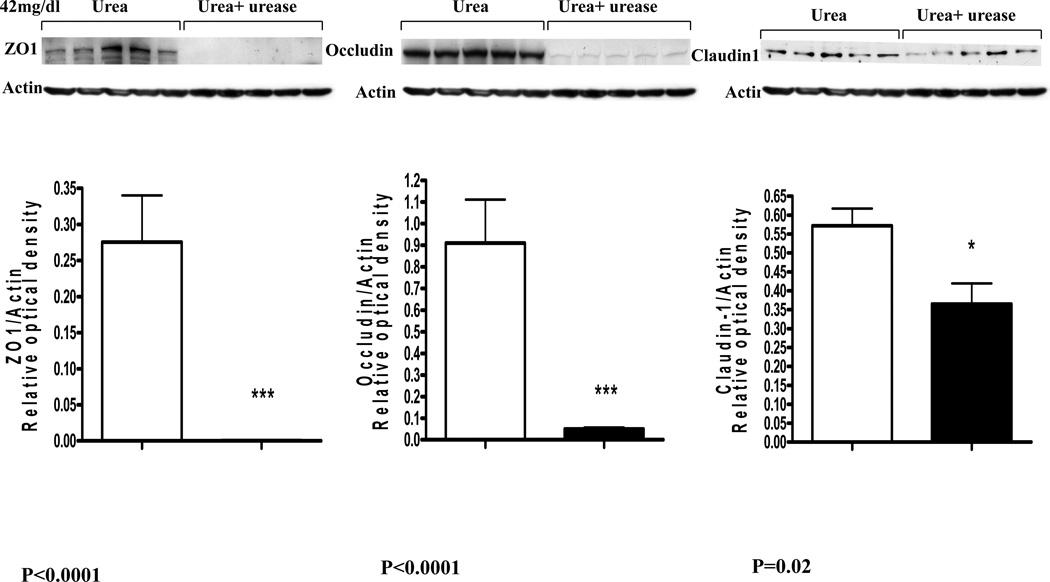
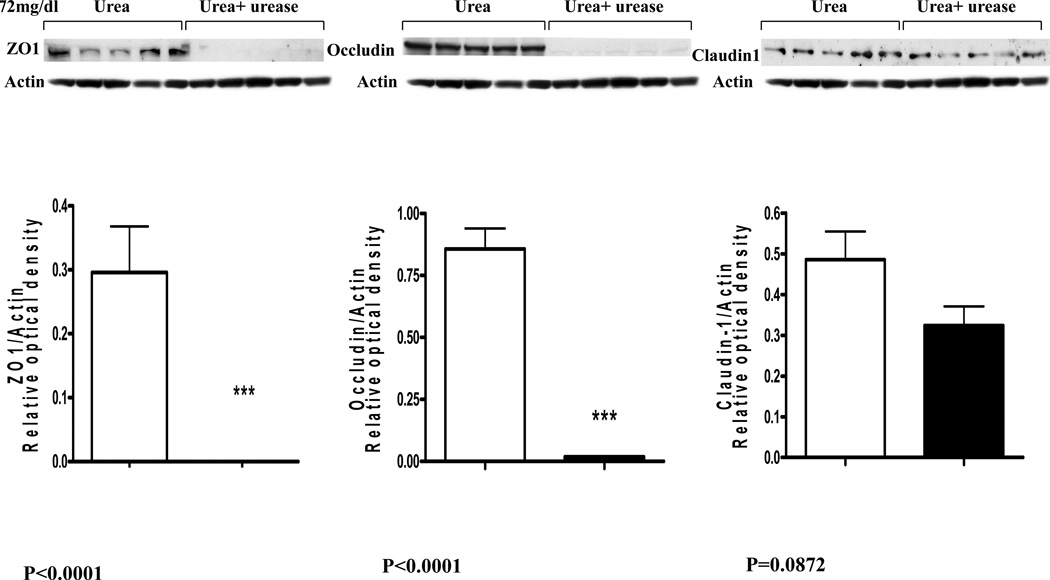
The fall in the monolayer TER in cells incubated in urea-containing media was associated with significant concentration dependent reductions in cluadin-1, occludin, and ZO1 abundance (Data are depicted in figures 2 and 3). Addition of urease to the incubation media designed to simulate presence urease-possessing colonic bacteria lead to a further and drastic fall in claudin-1, occludin, and ZO1 abundance (Figure 4).
Figure 2.
Representative Western blots and group data depicting protein abundance of occludin, claudin1 and ZO1 in intestinal epithelial T84 cell monolayers incubated for 24 hr in regular media and media containing 42 or 72 mg/dl urea.
Figure 3.
Representative Western blots and group data depicting protein abundance of occludin, claudin1 and ZO1 in intestinal epithelial T84 cell monolayers incubated for 24 hr in media containing 42 mg/dl urea alone and those incubated in media containing 42 mg urea plus urease.
Figure 4.
Representative Western blots and group data depicting protein abundance of occludin, claudin1 and ZO1 in intestinal epithelial T84 cell monolayers incubated for 24 hr in media containing 72 mg/dl urea alone and those incubated in media containing 72 mg urea plus urease.
Discussion
Systemic inflammation is a constant feature and a major mediator of cardiovascular disease, cachexia, anemia and numerous other morbidities in patients with advanced CKD [12–16]. Inflammation in these patients is commonly associated with endotoxemia in the absence of clinical infection [17–19]. The most likely source of endotoxemia in the infection-free patients is the gut microbial flora. However entry of endotoxin from the intestinal lumen to the circulation can occur only when the mucosal barrier is impaired. As summarized in recent reviews [1,2] there is compelling evidence supporting the impairment of the intestinal barrier function and its potential contribution to the prevailing inflammation in the uremic humans. This is based on the reported increase in intestinal permeability to high molecular weight polyethylene glycols [20 17], presence of endotoxemia [17,19], and histological evidence of chronic inflammation throughout the gastrointestinal tract in this population [10,11]. The tight junction apparatus is essential for the epithelial polarity and restriction of paracellular permeability of intestinal epithelium. Consequently disruption of the intestinal epithelial TJ can facilitate the influx of noxious microbial and other luminal contents in the intestinal wall and their subsequent release throughout the body via the vascular and lymphatic channels. This can, in turn, lead to local and systemic inflammation and accumulation of uremic toxins generated by the gut microbiota.
The present study revealed that at clinically-relevant concentrations, urea significantly lowered the TER in the tight junction-forming polarized human colonic epithelial cell monolayer in vitro. The effect of urea was dramatically amplified by urease which was used to simulate the effect of urease-possessing microbial species in the colon. These observations illustrate the potential contribution of influx of urea into the intestinal tract in the pathogenesis of the uremia-induced intestinal barrier dysfunction in the uremic patients. The observed fall in the TER was accompanied by and was largely due to the urea-concentration-dependent depletion of occludin, claudin 1, and ZO1 which are the key protein constituents of the epithelial TJ. The erosive effect of urea on the measured TJ proteins was greatly intensified in the presence of urease leading to near complete depletion of ZO1 and occludin and severe reduction in claudin 1 abundance. As noted above, accumulation of urea in the intra- and extra-cellular fluid compartments in patients and animals with advanced CKD results in its heavy influx into the gastro-intestinal tract via passive diffusion and incorporation in the glandular secretions (7–9). As noted earlier within the intestinal lumen urea is hydrolyzed spontaneously and by microbial urease forming large quantities of ammonia [8,9]. Ammonia is, in turn, converted to ammonium hydroxide which is a caustic base capable of causing cytotoxicity and tissue damage. Recent studies conducted by our group have shown marked changes in the composition of microbial flora in humans and animals with advanced CKD [21]. Five of the 10 microbial families which were most abundant in the CKD patients possessed functional urease [2,21]. Thus massive influx of urea into the gastrointestinal tract together with the dominance of urease-possessing bacteria in the uremic individuals, work in concert to promote formation of ammonia and ammonium hydroxide in the intestinal tract [8,9]. This can account for the dramatic impairment of the barrier function and destruction of TJ apparatus observed in the present in vitro experiments designed to simulate the effect of uremia in vivo.
In conclusion the CKD-induced disruption of intestinal TJ and impairment of the gut barrier function is, in part, mediated by urea which heretofore was considered to be a nontoxic uremic retained metabolite. These findings illustrate the adverse effect of the high urea levels and reveal a novel mechanism for the salutary effect of urea-lowering strategies such as low protein diet and longer and more frequent dialysis regimens in patients with advanced CKD.
ACKNOWLEDGEMENTS
This study was in part funded by the NIH grants RR026138 and MD000182.
Footnotes
CONFLICT OF INTEREST: The authors have no conflict of interest.
References
- 1.Ritz E. Intestinal-renal syndrome: mirage or reality? Blood Purif. 2011;31:70–76. doi: 10.1159/000321848. [DOI] [PubMed] [Google Scholar]
- 2.Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine - A major link to inflammation and uremic toxicity. Current Opinion in Nephrology & Hypertension. 2012 Sep 24; doi: 10.1097/MNH.0b013e328358c8d5. [Epub ahead of print] PMID: 23010760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol Dial Transplant. 2012;27:2686–2693. doi: 10.1093/ndt/gfr624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri ND, Goshtasby N, Yuan J, Jellbauer S, Moradi H, Raffatellu M, r Kalantar-Zadeh K. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrology. 2012;36(5):438–443. doi: 10.1159/000343886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madara JL, Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J. Cell Biol. 1985;101:2124–2133. doi: 10.1083/jcb.101.6.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectoral electrolyte transport. Am. J. Physiol. 246 (Gastrointest. Liver Physiol. 9) 1984:G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 7.Lee YT. Urea concentration in intestinal fluids in normal and uremic dogs. J Surg Oncol. 1971;3(2):163–168. doi: 10.1002/jso.2930030210. [DOI] [PubMed] [Google Scholar]
- 8.Bourke E, Milne MD, Stokes GS. Caecal pH and ammonia in experimental uraemia. Gut. 1966 Oct;7(5):558–561. doi: 10.1136/gut.7.5.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swales JD, Tange JD, Evans DJ. Intestinal ammonia in uraemia: the effect of a urease inhibitor, acetohydroxamic acid. Clin Sci. 1972 Jan;42(1):105–112. doi: 10.1042/cs0420105. [DOI] [PubMed] [Google Scholar]
- 10.Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci. 1993;38:257–268. doi: 10.1007/BF01307542. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri ND, Dure-Smith B, Miller R, et al. Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am J Gastroenterol. 1985;80:608–611. [PubMed] [Google Scholar]
- 12.Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012 Jan;22(1):149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease-what have we learned in 10 years? Semin Dial. 2010;23:498–509. doi: 10.1111/j.1525-139X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 14.Himmelfarb J, Stenvinkel P, Ikizler TA, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end stage renal disease. Kidney Int. 2007;71:167–172. doi: 10.1038/sj.ki.5002019. [DOI] [PubMed] [Google Scholar]
- 16.Gollapudi P, Yoon J-W, Gollapudi S, et al. Effect of End Stage Renal Disease and Hemodialysis on Expression and Activities of Leukocyte Toll-like Receptors (TLR) . Am J Nephrol. 2010;31:247–254. doi: 10.1159/000276764. [DOI] [PubMed] [Google Scholar]
- 17.Feroze U, Kalantar-Zadeh K, Sterling KA, Molnar MZ, Noori N, Benner D, Shah V, Dwivedi R, Becker K, Kovesdy CP, Raj DS. Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J Ren Nutr. 2012;22(3):317–326. doi: 10.1053/j.jrn.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves S, Pecoits-Filho R, Perreto S, et al. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21:2788–2794. doi: 10.1093/ndt/gfl273. [DOI] [PubMed] [Google Scholar]
- 19.Szeto CC, Kwan BC, Chow KM, et al. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnusson M, Magnusson KE, Sundqvist T, et al. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut. 1991;32:754–759. doi: 10.1136/gut.32.7.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaziri ND, Wong J, Pahl MV, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters the composition of intestinal microbial flora. Kidney Int. 2012 Sep 19; doi: 10.1038/ki.2012.345. doi: 10.1038/ki.2012.345. [Epub ahead of print] PMID: 22992469. [DOI] [PubMed] [Google Scholar]