Abstract
The current study investigates the interactive behavior of titanium alloy particle-challenged osteoblastic bone marrow stromal cells (BMSCs) and macrophage lineage cells in a murine knee-prosthesis failure model. BMSCs were isolated from male BALB/c mice femurs and induced in osteogenic medium. At 24 hours after isolation, BMSCs in complete induction medium were challenged with 1, 3, or 5mg/ml titanium particles for 7 days. Culture media were collected at 2, 4 and 6 days and cells were harvested at 7 days for alkaline phosphatase (ALP) assay/stains. Cell proliferation in the presence of Ti particles was periodically evaluated by MTT assay. Mice implanted with titanium-pin tibial implants were given an intra-articular injection of 50μl medium containing 5×105 Ti particles-challenged bone marrow derived osteoblastic cells, followed by a repeat injection at 2 weeks post-op. Control mice with titanium-pin implants received a naïve osteoblastic cell transfusion. After sacrifice at 4 week, the implanted knee joint of each group was collected for biomechanical pin-pullout testing, histological evaluation and RT-PCR analysis of mRNA extracted from the joint tissues. Ti-particles significantly stimulated the proliferation of BMSC-derived osteoblastic cells at both high and low particle concentrations (p<0.05), with no marked differences between the particle doses. ALP expression was diminished following Ti-particle interactions, especially in the high dose particle group (p<0.05). In addition, the culture media collected from short-term challenged (48 hours) osteoblasts significantly increased the numbers of TRAP+ cells when added to mouse peripheral blood monocytes cultures, in comparison with the monocytes cells receiving naïve osteoblasts media (p<0.05). Intra-articular introduction of the osteoblastic cells to the mouse pin-implant failure model resulted in reduced implant interfacial shear strength and thicker peri-implant soft-tissue formation, suggesting that titanium particles-challenged osteoblasts contributed to periprosthetic osteolysis. Comparison of the gene expression profiles among the peri-implant tissue samples following osteoblast injection did not find significant difference in RunX2 or Osterix/Sp7 between the groups. However, MMP-2, IL-1, TNF-α, RANKL, and TRAP gene expressions were elevated in the challenged-osteoblast group (p<0.05). In conclusion, titanium alloy particles were shown to interfere with the growth, maturation, and functions of the bone marrow osteoblast progenitor cells. Particle-challenged osteoblasts appear to express mediators that regulate osteoclastogenesis and peri-prosthetic osteolysis.
Keywords: titanium debris particles, osteolysis, bone marrow, osteoblast, osteoclast
1. Introduction
Aseptic loosening has become the most common cause of long term failure following total joint replacement, occurring in up to 25% of implant recipients [1, 2]. As no effective drug therapy is currently available to inhibit osteolysis, surgical revision is usually required. Investigations have suggested that particulate wear debris generated at the interface between implant component and surrounding bone play critical roles in pathogenesis of aseptic loosening [3–5]. Evidence suggests that various types of cells (such as macrophages, osteoclast precursor cells, osteoblasts, lymphocytes, fibroblasts) exposed to wear debris at interface between implant component and the surrounding bone may be involved in periprosthetic osteolysis and the pathogenesis of aseptic loosening. [6]. Inflammatory cytokines, including interleukin-1β, tumor necrosis factor-α, interleukin-6, and other mediators are elevated locally due to wear debris challenges [7, 8]., These mediators concomitantly affect the functions of cells in either an autocrine or paracrine manner of distinct signaling mechanisms. As a result, this process eventually leads to the imbalance of bone metabolism and the aseptic loosening of artificial joints.
Osteoclasts derived from the monocyte/macrophage cell lineage, and osteoblasts rooted in bone marrow are accepted as the principal and immediate cell types participating in bone metabolism. It has been recognized that particulate wear debris are potent stimuli for osteoclast differentiation and maturation [9]. Circulating peripheral blood monocytes (PBMCs) are among the first cells to colonize an inflammation site, and cells of the monocyte/macrophage lineage differentiate and maturate into phagocytic macrophages [10]. Macrophages appear to be the key cells in responding to the stimulus of wear debris. We previously reported that various debris particles activated systemic human monocytes to traffic to and participate in the inflammation that characterizes the peri-prosthetic tissue associated aseptic loosening [11, 12]. Recently, wear debris has been shown to have an adverse effect on bone formation by bone marrow stromal cells [1, 13–15], and in vitro studies suggested that osteoblasts play a pivotal role in osteoclastogenesis process through the production of RANKL and CSF-1 [16, 17].
Bone marrow stromal cells (BMSCs) including osteoblast progenitor cells are naturally present within the prosthesis site and in close contact with the prosthetic component and may be critical contributors to the maintenance of bone homeostasis at the bone/prosthesis interface. Perturbation of BMSCs by implants and wear debris may affect bone ingrowth and interface stability, leading to increased osteoclastogenesis and bone resorption [14, 18]. While extensive studies have focused on wear particles promoting osteoclastogenesis and osteolysis by monocyte/macrophage lineage cells, we hypothesize that debris particles challenged osteoblasts may play an equally important role in regulating the balance of the bone turn-over and the differentiation/activation of osteoclasts. The current study intends to test the hypothesis to investigate the interactive behaviors of the titanium particle-challenged osteoblastic bone marrow stromal cells (BMSCs) and macrophage lineage cells in a murine knee-prosthesis failure model.
2. Materials and Methods
2.1 Biomaterial particles
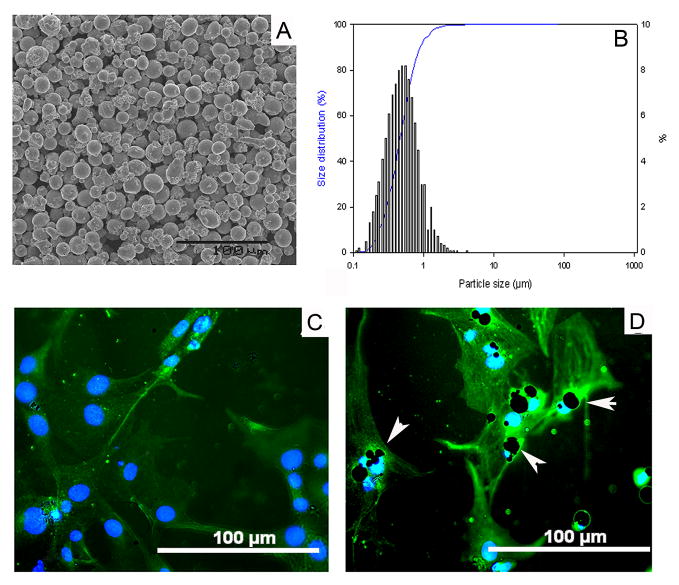
Titanium alloy particles (Ti–6Al–4V, the medium particle size 0.67μm, range 0.1 – 7.19μm) used in this project were generated by the Zimmer Corporation (Warsaw, Indiana). The size distribution of the particles was evaluated using a Coulter particle counter equipped with interchangeable (100, 30, and 15μm pore) attachments, and by scanning electron microscopy (SEM). Particles for SEM analysis were dispersed on a 0.1μm Isopore membrane filter, and dried for 24 hours. Samples were imaged at 800x magnification to visualize particle size characteristics and particle concentration distribution (Fig. 1A and 1B), which was analyzed using the ImagePro+ software package (Media Cybernetics, Maryland). Prior to use, particles were washed in 70% ethanol solution to remove endotoxin, which was confirmed using the Limulus assay (Endosafe; Charles Rivers, Charlestown, SC).
Fig. 1.
(A) Scanning electron microscopy (SEM) appearance of the particles used in experiments (800x), and (B) the histogram of the particle distribution pattern. Panel (C) shows typical osteoblastic cells when PKH2-labeled bone marrow cells were cultured in osteogenic-induction medium for 7 days (200x magnification). (D) Titanium particles were engulfed into osteoblastic cells (white arrows, 200x).
2.2 Primary osteoblast induction and characterization
Bone marrow cells were obtained from femurs of male BALB/c mice (6–8 weeks of age). Using density gradient centrifugation over Histopaque®-1083 (Sigma-Aldrich, St. Louis, MO), mononuclear cells were isolated, and then further purified by differential speed adherence. For the differentiation of osteoblasts, BMSCs were cultured in DMEM culture medium supplemented with 10% FBS, 10mM β-glycerol phosphate, 100mM L-ascorbic acid, and 10nM dexamethasone, 2mM glutamine, 100U/ml penicillin and 100mg/ml streptomycin [19]. After labeled with 2μM of PKH2 green fluorescent dye (Sigma-Aldrich, St. Louis, MO), cell morphology was observed under a fluorescent microscope throughout the culture period (Fig. 1C). A FluoroShield™ with DAPI mount medium (Sigma-Aldrich, St. Louis, MO) was used to visualize the nuclei of cells.
At day 7 after induction, alkaline phosphatase (ALP) staining was performed using a commercial Kit (Sigma-Aldrich, St. Louis, MO) to assess osteoblastogenesis. Briefly, alkaline-dye mixture was freshly prepared by reconstituting a Fast Violet B capsule and 2ml Naphthol AS_MX Phosphate Alkaline solution to a final volume of 50ml with deionized water at 37°C. Following fixation by immersing in citrate buffered acetone for 30 seconds, cells were incubated in alkaline-dye mixture for 30 minutes at 26°C and counterstaining was performed using Mayer’s Hematoxylin for 10 minutes.
2.3 Osteoblast formation challenged by Ti-particles in vitro
At 24 hours after isolation the bone marrow mononuclear cells were cultured in complete induction medium, without or with 1, 3, or 5mg/ml titanium particles for 7 days. Culture media were collected at 2, 4 and 6 days and cells were harvested at 7 days for ALP stain and ALP assay in cell lysate, respectively. After lysis by CelLytic™ M (Sigma-Aldrich, St. Louis, MO) and centrifuged for 15 minutes at 12000 rpm, the supernatant was retained for protein assay. Cell lysates were assayed using the ALP Kit (Sigma-Aldrich, St. Louis, MO). Based on the colorimetric reaction with hydrolysis of p-nitrophenyl phosphate, ALP activity was determined by OD values read at 405 nm wavelength on a microplate photospetrometer (Molecular Devices, Menlo Park, CA), and normalized against total protein concentration. Cell proliferation and viability were also periodically (1, 3, 5, 7 day) evaluated by MTT assay in the presence of Ti particles at 0, 0.63, 1.25, 2.5 or 5 mg/ml, with the methodology reported previously [20]. Cell responses were expressed as a proliferation index, which was calculated by normalization with the baseline proliferation (non-particle controls).
2.4 In vitro study of osteoclastogenesis initiated by conditioned osteoblast
Peripheral blood monocytes (PBMCs) were isolated from Balb/c mice by centrifugation of pooled whole blood sample layered on Histopaque-1077 and PBS gradients. At 24 hours after incubation in RPMI 1640 medium +10%FBS, 1ml of media from osteoblast cultures (with or without Ti-particle stimulation) were transferred to the wells of mouse PBMC cultures and maintained at 37°C and 5% CO2 for 48 hours. As negative control, PBMC culturing in 1ml of RPMI 1640 medium +10%FBS was included. To identify osteoclastogenesis from PBMCs, tartrate-resistant acid phosphatase (TRAP) staining was performed using a commercial staining kit (Sigma Chemicals) in accordance with the manufacture’s protocol as published elsewhere [21, 22].
2.5 Establishment of the mouse pin-implantation model
All animal procedures used in this study have been approved and monitored by Wichita State University Animal Care and Use Committee (IACUC). Balb/c mice (Jackson Labs, Bar Harbor, ME) aged 10–12 weeks were quarantined in our animal facility for 2 weeks prior to experimentation. Titanium-alloy pins of 0.8mm diameter and 5mm long with a flat large top of 1.4mm diameter were provided by Stryker Orthopaedic, Inc. (Mahwah, NJ). The mouse model was established as described previously [23]. Briefly, mice were anesthetized with Xylazine and Ketamine, and a 5mm medial parapatellar surgical incision made to expose the patellar ligament. The patella was dislocated laterally to expose the tibial plateau, and the cartilage of the tibial plateau was partially removed using a #11 scalpel. The center of the tibial plateau was reamed with a 0.8mm dental drill to expose the proximal 5mm of the tibial intramedullary canal. A titanium-alloy pin was then press-fit into the canal in a manner that the surface of pin head became flush with the cartilaginous surface of the tibial plateau and did not interfere with the motion of the knee. To mimic prosthetic wear, 10μl of a titanium-alloy particle suspension (4×104 particles of Ti–6Al–4V) was pipetted into the tibia canal before insertion of pin implant during surgery, and 40μl of Ti particles were intra-articularly injected into the prosthetic joint every 2 weeks following surgery.
2.6 Intra-articular injection of Ti particles-challenged osteoblast
Osteoblastic cells successful induced from BMSCs were cultured with 2mg/ml titanium alloy particles for 48 hours. The mice with the pin implantation and Ti-particle challenges were divided into 2 groups post-op: (1) mice in the OBs group were given an intra-articular injection of 50μl medium containing 5×105 Ti particles-challenged OBs, and the cell infusion was repeated at 2 weeks post-op (n=12); (2) loosening control mice received an intra-articular injection of 50μl cells-free medium (n=12). The third group of mice only received the pin implantation without ti-particle stimulation and osteoblastic cell infusion, as stable control (n=12). All mice were sacrificed at 4 weeks, and the implanted knee joints were collected for pin-pullout test, histological evaluation and RT-PCR analysis.
2.7 Micro-computerized tomography (microCT) scans
Micro-CT scanning was performed immediately following surgery and at the animal sacrifice using a SCANCO microCT System (vivaCT 40, SCANCO Medical, Switzerland). The parameters for the scans were 30μm isotropic voxel size, 400 projections, 200ms exposure time, 70 KW voltages, and 114μA current. Following acquisition and reconstruction, the image data were analyzed with MicroCT Evaluation program V6.5-1 software to generate isosurfaces of the volume of interest (VOI) and to calculate the bone mineral densities (BMD) of the tibia bones surrounding the titanium pins.[23].
2.8 Interfacial shear strength test
Following sacrifice of the animal, the mouse limb with the implant intact was removed by disarticulating the knee joint. All soft tissue around the prosthetic joint was carefully removed to expose the implanted pin surface and proximal tibia. A custom aluminum fixture was used to securely hold and align the pin for extraction. The test fixture utilized two razor blades which were tightened onto the head of pin to securely grip the pin for removal. The pin and tibia were mounted to this fixture and the pin alignment checked to insure that its axis was parallel with direction of extraction and orthogonal to the razor blade grips. With the tibia held by the fixture mounted on the load frame, approximately 1cm of the distal portion of the tibia was then cemented into a potting box using high strength dental cement. The cement was cured for at least 30 minutes before testing. A Bose 3200 ElectroForce™ load frame (Bose Corporation, Eden Prairie, MN) was used to conduct the pin extraction test. The test was run under displacement control at a rate of 1mm/min and actuator position and applied load were recorded as a function of time using Bose WinTest® software.
2.9 Histological analysis and TRAP staining
The peri-implant proximal tibiae were fixed in formalin and decalcified before paraffin embedding, processing and cutting to generate 6μm sections. Sections were stained with hematoxylin and eosin to examine new bone formation or bone erosion around the prosthetic pin, and the evidence of debris-associated inflammation (peri-prosthetic tissue formation and cellular infiltration). The width of the soft-tissue membrane at the implant and surrounding bone interface was measured using the Image-Pro+ software. Histological TRAP staining (Sigma-Aldrich, St Louis, MO, USA) on the paraffin-sectioned prosthetic joints was performed after pin removal as described previously [12, 21, 22]. The level of the positive stains was evaluated in randomly selected six different fields of the each peri-implant tissue section.
2.10 Gene expression profile assessment
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed to assess the effect of Ti particles-challenged osteoblasts on osteoclastogenesis and osteolysis. Proximal tibiae surrounding pin-implant were minced on dry ice followed by a Polytron (PT-MR2100; Kinematica, Lucerne, Switzerland) homogenization. Total RNA from cells homogenates was extracted using a commercial kit following the manufacturer’s instructions (Tel-Test, Friendswood, TX). The quantity and purity of the RNA and DNA were examined by spectrophotometric analysis at 260nm and 280nm wavelengths. The cDNA was reverse transcribed from 0.5μg of total RNA in a 20μl reaction mixture containing 1×PCR buffer, 500μM each of deoxynucleotide triphosphates, 0.5 units/μl of RNase inhibitor, 2.5μM random hexamers, 5.5mM MgCl2, and 1.25 units/μl of RT (Perkin-Elmer Cetus, Norwalk, CT). The reaction mixture was incubated in a Veriti 96-well Thermal Cycler (Applied Biosystems) at 25 °C for 10 minutes, 48°C for 25 minutes, followed by 95°C for 5 minutes. The expression of Osx, NFATc1, RunX2, MCP-1, IL-1, RANKL, and TRAP was determined by real-time PCR using StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) as described previously [24]. With the Ct value of GAPDH as an internal control and mean Ct value of target gene expression from stable control group (no Ti-particle challenge) as the calibrator sample (named 0% expression), the comparative quantification of gene expression of the samples from Ti-particle challenged group (osteolysis control) and OBs-treated group were calculated according to the formula given in the manufacture’s manual. Selected RT-PCR products were electrophoresis on 1.8% agarose to confirm the correct amplification of the target gene.
2.11 Statistical analysis
Data from 3 independent experiments were analyzed for this study. A total of 36 animals were investigated in the experiments, with 12 animals for each group. Statistical analysis between groups was performed by single factor analysis of variance (ANOVA) test with the Schafer formula for post hoc multiple comparisons, using the SPSS 17.0.1 software package (SPSS, Chicago, IL, USA). A p-value of less than 0.05 was considered as significant difference. The values were expressed as arithmetic mean and standard error.
3. Results
3.1 Osteoblast induction and characterization
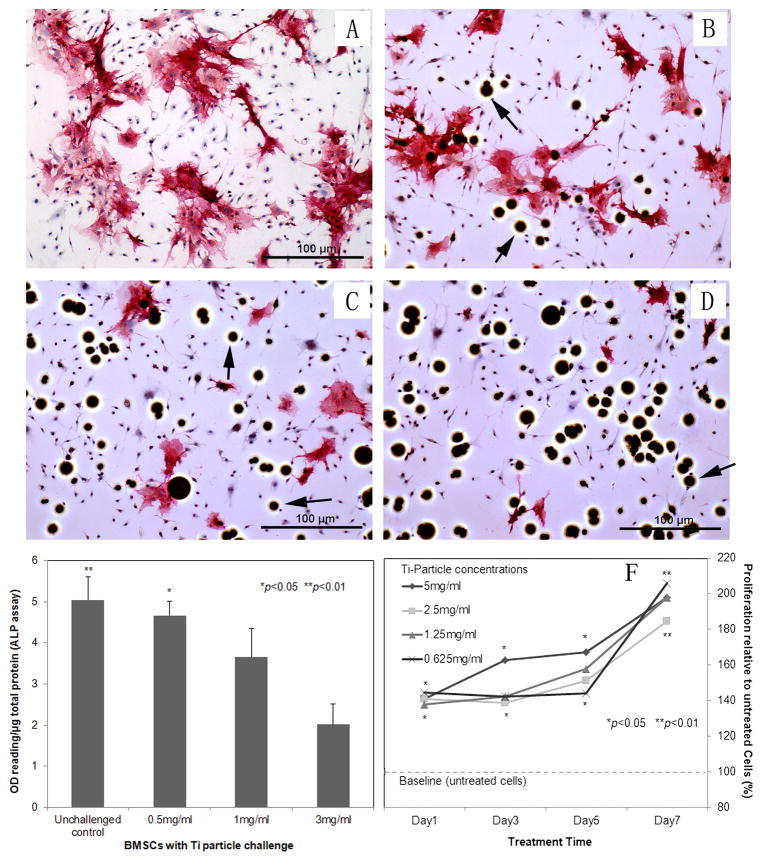
As differentiation and proliferation proceeded, the BMSCs exhibited osteoblast-like spindle or polygonal morphology in the osteoblast-induction medium (Fig. 1C), and the phenotype of osteoblastic cells was confirmed by positive ALP stains. ALP expression was observed in most conditioned cells (72.38 ± 3.98%; Fig. 2A).
Fig. 2.
(A–D) Immunostain reveals alkaline phosphatase (ALP) expression on bone marrow cells: challenged the cells by different concentrations of titanium particles (0, 0.5, 1, 3 mg/ml) in osteogenic-induction medium (100× magnification); (E) ALP activity assay of cell lysate: The OD values of ALP activity were normalized against the total protein concentration in the sample and expressed as arbitrary unit. The control sample was from cells lysate without particle treatment; (F) MTT assay to periodically estimate the viability and proliferation of BMSCs treated by various concentrated Ti particles. Stimulation indexes were calculated by comparison with baseline proliferation (particle-free controls, *p<0.05, **p<0.01)
3.2 Effects of Ti-particles on Cell Proliferation and Osteogenesis
At 24 hours after exposure to titanium particles, Ti–6Al–4V particles were readily identified within osteoblastic cells (Fig. 1D). Ti-particles significantly stimulated the proliferation of BMSC-derived osteoblastic cells at both high and low particle concentrations, and there was no marked difference between the particle doses (Fig. 2F). However, the ALP positive stained cells challenged by different concentrations of titanium particles (0.5, 1, 3 mg/ml) in osteogenic-induction medium revealed that Ti-particles resulted in a dose-dependant inhibitive effect of osteoblastogenesis (Fig. 2A–D). The ALP assay of cell lysate (Fig. 2E) indicated that ALP expression was diminished following Ti-particle interaction, especially in the high dose group (p<0.01), which is consistent with the results of ALP staining.
3.3 Osteoclastogenesis initiated by conditioned osteoblast in vitro
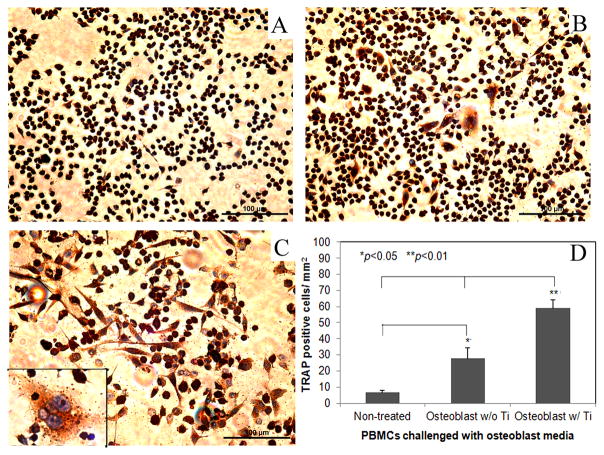
Figure 3 shows the TRAP+ cells in the mouse peripheral blood monocytes cultured in conditioned media from osteoblast cultures with or without titanium particle stimulation. Both conditioned media significantly promoted TRAP expression in PBMCs compared to the cell cultures in regular medium (p<0.05). However, culture media collected from short-term Ti-challenged (48 hours) osteoblasts resulted in significantly more TRAP+ cells in the cultures in comparison with the PBMC cultures in conditioned media from naïve osteoblastic cells (p<0.01, Fig. 3D).
Fig. 3.
tartrate-resistant acid phosphatase (TRAP) staining of peripheral blood monocytes (PBMCs) following different treatments (200×): (A) cells receiving naïve osteoblasts media; (B) conditioned media from osteoblast culture without titanium particle stimulation was added; (C) cells culturing in osteoblast media with Ti particles stimulation, the insert shows a blowup of multinucleated TRAP+ osteoclasts (400×); (D) summarization of the number of TRAP+ cells in treated PBMCs among groups (*p<0.05, **p<0.01).
3.4 Implant stability assessment
The mechanical stability of the implant after wear debris challenge and OBs injection was evaluated by the pin pull-out test. The dental cement was sufficiently strong to fasten distal limbs during the pin pullout. The average pulling force to dissociate the pin-implant from surrounding bone was 18.45N ± 2.24 in non-challenged stable controls and 9.47N ± 1.39 in the Ti-particle challenged loosening group. Introduction of exogenous OBs further decreased the implant stability with only 6.29N ± 1.99 of extraction force required to pull out the implant from surrounding bone (p<0.05, Fig. 4).
Fig. 4.
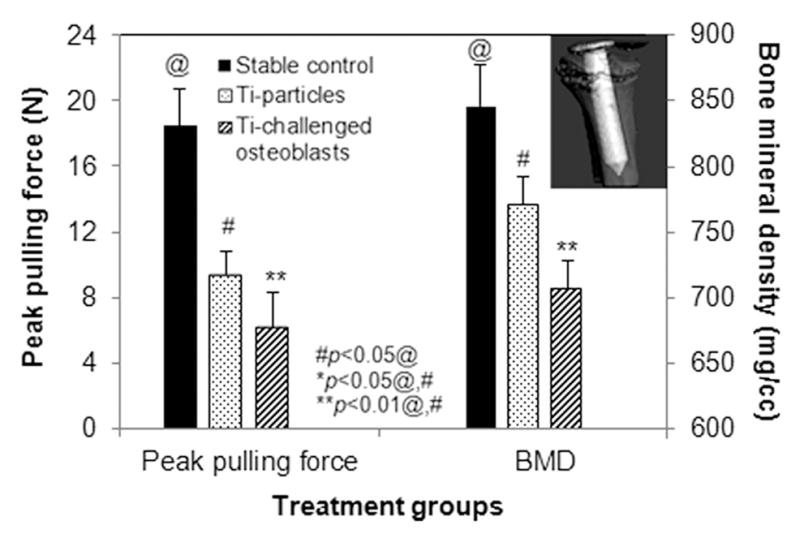
The average of peak pulling force required to pull out the implants at 4 weeks after different treatments (left plot). Plot on the right side summarizes the periprosthetic bone mineral density (BMD) determined using the SCANCO Evaluation program V6.5-1 software to generate isosurfaces of the region of interest (ROI). The insert illustrated a microCT reconstructed 3-D isosurfaces image. (#p<0.05 than@, *p<0.05 than @ and #, **p<0.01 than @ and #)
3.5 MicroCT evaluation
MicroCT scans indicated that the implants were well fixed with no obvious migration in the animals without particle challenge, while titanium particle injections resulted in a marked periprosthetic bone resorption. Figure 4 summarizes the changes in periprosthetic bone mineral density (BMD) between the experiment groups, with a significant reduction in BMD observed in the Ti particle treated group. The introduction of Ti-particles challenged OBs further reduced the BMD of peri-implant bone at the termination of the experiment compared to the BMD immediately following the pin implantation surgery (p<0.05, Fig. 4).
3.6 Histological analyses
Histological evaluation of the pin prosthesis model clearly indicated new bone growth in the particle-free stable pin-implantation group (Fig. 5A). Consistent with our previous results, peri-prosthetic pseudo-membranes developed in mice with Ti-particle challenges at the bone–implant interface (Fig. 5B). Further, local transfusion of Ti-particle activated osteoblastic cells resulted in more severe inflammation with thicker peri-implant membrane formation and more inflammatory cells infiltration (p<0.05; Fig. 5C). Quantification of the periprosthetic membrane thickness was accomplished using a computerized image analysis system (Fig. 5D).
Fig. 5.
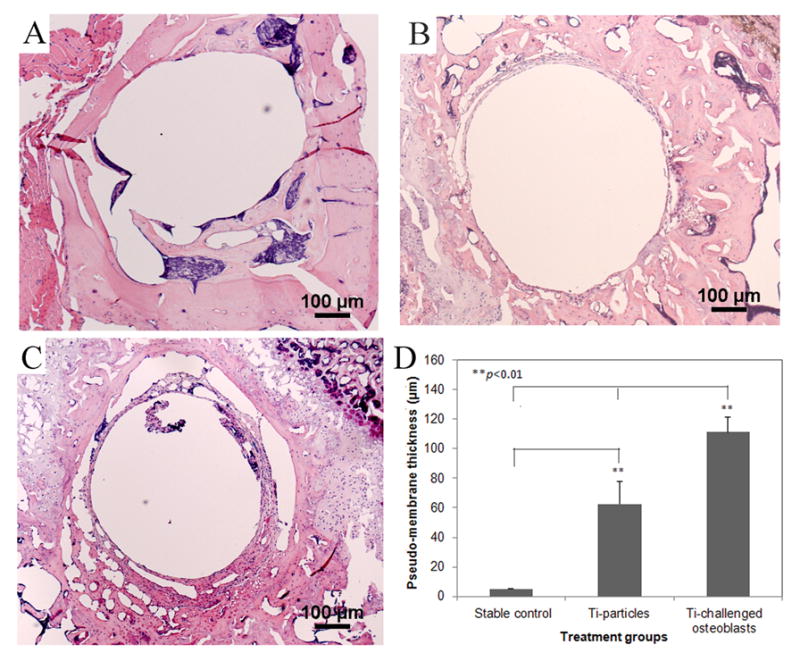
(A–C) represent the histological appearances of pin-implanted tibiae at 4 weeks following different treatments (40×): (A) stable pin implantation without particles stimulation; (B) Ti particles treated prosthesis failure control; (C) the injection of Ti particles-challenged OBs significantly result in more severe inflammation with thicker membrane generation. (D) The comparison of periprosthetic membrane thickness in response to different treatments (**p<0.01).
TRAP staining was performed to reveal the osteoclastogenesis at the bone-implant interface. Significantly increased TRAP+ cells were observed in sections from Ti-challenged-OBs transfused group compared to the other two groups (P<0.01, Fig. 6).
Fig. 6.
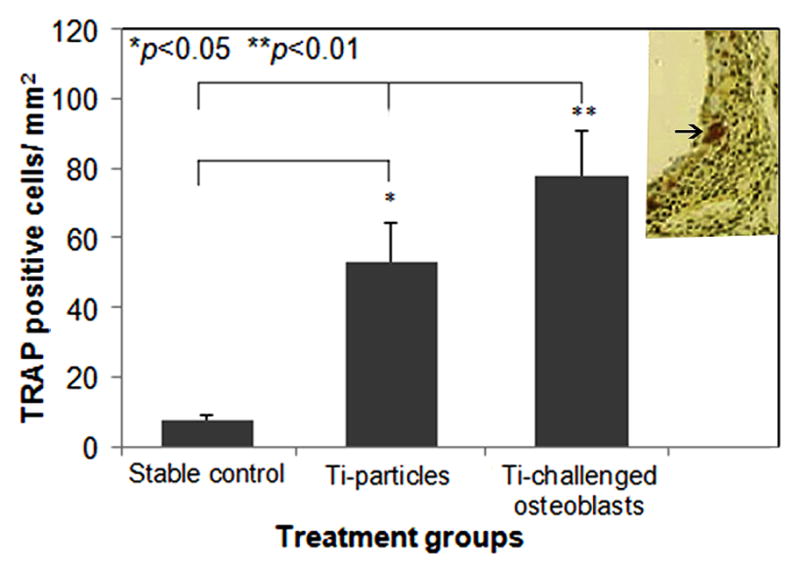
TRAP positive cell counts in the sections among groups were determined using a computerized image analysis system with ImagePro+ software (*p<0.05, **p<0.01). The inserted image illustrates a TRAP stained peri-implant tissue section with purple stained osteoclastic cells.
3.7 Molecular and immunologic analyses
Gene expression profiles of osteogenesis, pro-inflammatory cytokines, and osteoclastogenesis in the peri-implant bone were examined by real-time PCR. Figure 7 shows the effects of Ti-particles challenged osteoblasts on the expression of the genes related to osteolysis. There was no meaningful difference in the gene expression of RunX2 or Osterix/Sp7 between the loosening control group and Ti-particle challenged OBs-treated group. However, the mice following osteoblast injection exhibited significantly elevated gene expressions of MMP-2, IL-1, TNF-α, RANKL, and TRAP in comparison with non-treated loosening group (p<0.05), indicating that the particle-activated osteoblastic cells play a pivotal role in wear debris associated peri-prosthesis osteolysis and osteoclastogenesis.
Fig. 7.
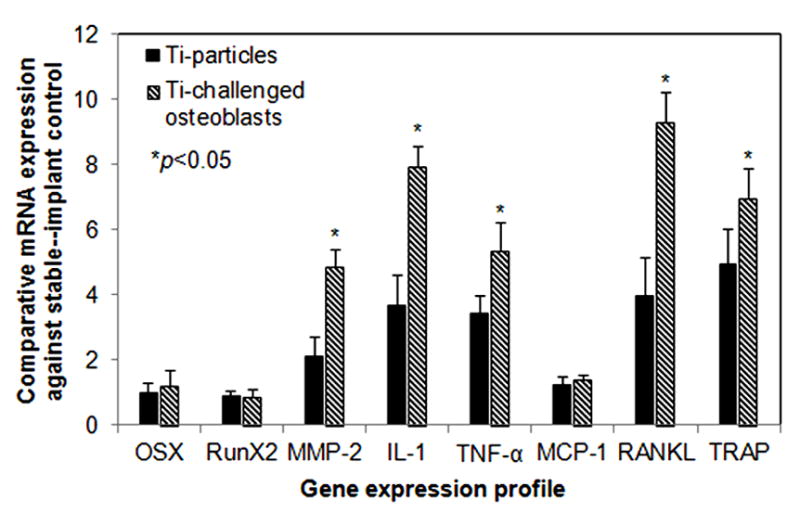
Gene expression profiles of the peri-implant tissue following Ti-particle treatment or the injection of Ti-particles challenged osteoblasts. The data are expressed as comparative gene copies against readings from the stable pin-implantation control (see Material and methods, *p<0.05).
4. Discussion
Implant wear is an inevitable consequence in patients receiving total joint replacement (TJA) due to the normal functions of the prosthetic joint; and the wear debris associated chronic inflammation and bone resorption appear to play critical roles in pathogenesis of aseptic loosening [4, 5, 25]. Particulate wear debris activate a variety of types of cells at the interface between implant components and the surrounding bone through either up- or down-regulation of proinflammatory cytokines and chemokines [6]. It is generally accepted that the maturation and activation of osteoclasts (OCs) represent the principal pathology of aseptic loosening. As OCs are derived from osteoclast progenitor cells (OCPs) from the monocyte/macrophage lineage, recruitment of OCPs from blood and the generation of functional OCs at the interface between an implant component and the surrounding bone plays a critical role in periprosthetic osteolysis and bone resorption [9, 11, 12, 26]. As first noted in 1990, the in vitro maturation of osteoclasts requires the presence of marrow stromal cells or their osteoblast progenitor cells [27]. The current study tested our hypothesis that wear debris particles also alter the maturation and functions of osteoblastic progenitors (bone marrow stromal cells) which participate in and play a critical role in the pathology of osteoclastogenesis and periprosthetic bone resorption in this murine model of a weight-bearing knee pin implant.
Our laboratory has recently developed a mouse model of knee implantation with a metal pin on proximal tibia. With a large flat-top partially forming the articulating surface, it models a weight-bearing prosthetic joint, which may be sustained for up to 6 months without signs of loosening [23]. This model can be manipulated to mimic debris-associated implant failure condition and is suitable for biomechanical, histological, and molecular evaluations. In situ microCT may be used to confirm the initial position of the implant, and periodic microCT monitoring provides kinetic observation of bone-implant interaction and bone mineral density changes. Combined with histology evaluation, microCT can illustrate the stable prosthesis with new bone formation and ingrowth, if no particulate biomaterial debris interactions at the bone-implant interface. After periprosthetic titanium particle challenges, an inflammatory pseudo-membrane at the interface between the pin-implant and surrounding bone was observed as early as 2 weeks, and periprosthetic bone resorption was also noticed on microCT images at 3 weeks [23].
BMSCs, generally considered the major osteoprogenitor cell type and located in bone marrow stroma in high numbers [28] are proposed to be critically affected during particle-mediated osteolysis. During the course of the implant life, particularly in the case of poor initial implant-fixation with excessive micromotion and constant accumulation of wear particles over time, bone marrow cells are continuously exposed to high concentrations of debris throughout the stage of bone repair or reconstruction. As a result, the prolonged exposure to particles may disrupt the normal osteogenic differentiation processes and subsequently lead to a diminished population of functional osteoblasts and compromised osseointegration at the bone-implant interface.
This study demonstrates that titanium alloy particles interfere with the differentiation, maturation, and functions of osteoblasts. In agreement with previous studies that metallic or polymeric particles impaired the ability of mature osteoblastic cells to synthesize collagen α1(I) and mineralize matrix [13, 29, 30], this study provides evidence that Ti particles provoked the proliferation of BMSCs and sustained Ti-particle stimulation resulted in a dose-dependent inhibition of osteoblastogenesis with diminished ALP activity. Simultaneously, the gene expression data suggested that particle-stimulated osteoblastic cells regulated many genes including pro-inflammatory cytokines and MMPs. It has been shown that IL-1 and TNF-α are potent mediators of bone resorption, activating osteoclast or precursor cells by direct stimulation of macrophages, and providing activation signals to lymphocytes [31]. As a result, the lymphocyte derived IL-2, IL-6, IL-17 or γ-IFN can influence osteoclastic activity and bone remodeling [32]. In other studies, enzymatic bone resorption through MMPs has been shown to be involved in debris-associated periprosthetic osteolysis [33].
Another significantly important function of osteoblastic cells impacted by our study is the production of RANKL. It is accepted that altered RANKL/OPG ratio plays a crucial role in the differentiation of OCs from macrophage or osteoclast precursors. The balance between the expression of the stimulator of osteoclastogenesis (RANKL) and the inhibitor (OPG) dictates the quantity of bone resorption and the kinetics of remodeling [12, 22, 34]. Thus wear debris particles challenged osteoblasts may contribute to the periprosthetic osteolysis through RANKL mediated osteoclast maturation and activation. Our study demonstrated that particle-challenged osteoblasts appeared to express mediators that regulate the differentiation of osteoclasts in vitro and peri-prosthetic osteolysis in vivo, with particular augmention of RANKL expression typically associated with elevated osteoclastogenesis.
5. Conclusion
This study demonstrates pivotal effects of OBs in the process of wear particle-associated osteoclastogenesis and osteolysis at the interface between the implant and surrounding bone. Besides direct effect of Ti particles stimulating osteoblasts to promote osteoclastogenesis through RANKL, several other possibilities may be considered in the peri-implant osteolysis problem, including TNF-α induced osteoclastogenesis by direct stimulation of macrophages and enzymatic bone resorption through MMPs. Indeed, it might be possible that these different scenarios (RANKL, MCP-1, TNF-α, MMPs) are simultaneously involved in the peri-implant osteolysis; and titanium particle-stimulated osteoblastic cells play a central role in the pathogenesis of osteolytic aseptic loosening.
Acknowledgments
This work was supported by a research grant from National Institutes of Health (5R03AR054929, S.-Y.Y.) and research funding from Orthopaedic Research Institute, Via Christi Health. The authors wish to thank Ms. Zheng Song, Mr. Joel White, and Ms. Nora Zacharias for their excellent technical assistance.
Footnotes
Disclosures
All authors report no conflicts of interest and are responsible for the content of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, et al. The epidemiology of revision total knee arthroplasty in the United States. Clinical orthopaedics and related research. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J of Bone Joint Surg [AM] 2009;91:128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Kadoya Y, Revell PA, Kobayashi A, al-Saffar N, Scott G, Freeman MA. Wear particulate species and bone loss in failed total joint arthroplasties. Clinical orthopaedics and related research. 1997:118–29. doi: 10.1097/00003086-199707000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Chiba J, Rubash HE. In vivo and in vitro analysis of membranes from hip prostheses inserted without cement. J Bone Joint Surg Am. 1994;76:172–80. doi: 10.2106/00004623-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Maloney WJ, Smith RL, Schmalzried TP, Chiba J, Huene D, Rubash H. Isolation and characterization of wear particles generated in patients who have had failure of a hip arthroplasty without cement. J Bone Joint Surg Am. 1995;77:1301–10. doi: 10.2106/00004623-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. The Journal of bone and joint surgery British volume. 1998;80:531–9. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 7.Hundric-Haspl Z, Pecina M, Haspl M, Tomicic M, Jukic I. Plasma cytokines as markers of aseptic prosthesis loosening. Clinical orthopaedics and related research. 2006;453:299–304. doi: 10.1097/01.blo.0000229365.57985.96. [DOI] [PubMed] [Google Scholar]
- 8.Stea S, Visentin M, Granchi D, Ciapetti G, Donati ME, Sudanese A, et al. Cytokines and osteolysis around total hip prostheses. Cytokine. 2000;12:1575–9. doi: 10.1006/cyto.2000.0753. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Ferguson DJ, Quinn JM, Simpson AH, Athanasou NA. Biomaterial particle phagocytosis by bone-resorbing osteoclasts. The Journal of bone and joint surgery British volume. 1997;79:849–56. doi: 10.1302/0301-620x.79b5.7780. [DOI] [PubMed] [Google Scholar]
- 10.Rao AJ, Gibon E, Ma T, Yao Z, Smith RL, Goodman SB. Revision joint replacement, wear particles, and macrophage polarization. Acta biomaterialia. 2012;8:2815–23. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SY, Zhang K, Bai L, Song Z, Yu H, McQueen DA, et al. Polymethylmethacrylate and titanium alloy particles activate peripheral monocytes during periprosthetic inflammation and osteolysis. J Orthop Res. 2011;29:781–6. doi: 10.1002/jor.21287. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K, Jia TH, McQueen D, Gong WM, Markel DC, Wooley PH, et al. Circulating blood monocytes traffic to and participate in the periprosthetic tissue inflammation. Inflammation research : official journal of the European Histamine Research Society [et al] 2009;58:837–44. doi: 10.1007/s00011-009-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermes C, Chandrasekaran R, Jacobs JJ, Galante JO, Roebuck KA, Glant TT. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. J Bone Joint Surg Am. 2001;83-A:201–11. doi: 10.2106/00004623-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Okafor CC, Haleem-Smith H, Laqueriere P, Manner PA, Tuan RS. Particulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debris. J Orthop Res. 2006;24:461–73. doi: 10.1002/jor.20075. [DOI] [PubMed] [Google Scholar]
- 15.Haleem-Smith H, Argintar E, Bush C, Hampton D, Postma WF, Chen FH, et al. Biological responses of human mesenchymal stem cells to titanium wear debris particles. J Orthop Res. 2012;30:853–63. doi: 10.1002/jor.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granchi D, Amato I, Battistelli L, Ciapetti G, Pagani S, Avnet S, et al. Molecular basis of osteoclastogenesis induced by osteoblasts exposed to wear particles. Biomaterials. 2005;26:2371–9. doi: 10.1016/j.biomaterials.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Pioletti DP, Kottelat A. The influence of wear particles in the expression of osteoclastogenesis factors by osteoblasts. Biomaterials. 2004;25:5803–8. doi: 10.1016/j.biomaterials.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Chiu R, Smith KE, Ma GK, Ma T, Smith RL, Goodman SB. Polymethylmethacrylate particles impair osteoprogenitor viability and expression of osteogenic transcription factors Runx2, osterix, and Dlx5. J Orthop Res. 2010;28:571–7. doi: 10.1002/jor.21035. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang SY. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30:508–17. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 20.Wooley PH, Petersen S, Song Z, Nasser S. Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. J Orthop Res. 1997;15:874–80. doi: 10.1002/jor.1100150613. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Jia TH, Zacharias N, Gong W, Du HX, Wooley PH, et al. Combination gene therapy targeting on interleukin-1beta and RANKL for wear debris-induced aseptic loosening. Gene therapy. 2012 doi: 10.1038/gt.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Jia TH, Chong AC, Bai L, Yu H, Gong W, et al. Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene therapy. 2010;17:1262–9. doi: 10.1038/gt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SY, Yu H, Gong W, Wu B, Mayton L, Costello R, et al. Murine model of prosthesis failure for the long-term study of aseptic loosening. J Orthop Res. 2007;25:603–11. doi: 10.1002/jor.20342. [DOI] [PubMed] [Google Scholar]
- 24.Yang SY, Nasser S, Markel DC, Robbins PD, Wooley PH. Human periprosthetic tissues implanted in severe combined immunodeficient mice respond to gene transfer of a cytokine inhibitor. J Bone Joint Surg [AM] 2005;87:1088–97. doi: 10.2106/JBJS.D.02052. [DOI] [PubMed] [Google Scholar]
- 25.Ollivere B, Wimhurst JA, Clark IM, Donell ST. Current concepts in osteolysis. The Journal of bone and joint surgery British volume. 2012;94:10–5. doi: 10.1302/0301-620X.94B1.28047. [DOI] [PubMed] [Google Scholar]
- 26.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 27.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:7260–4. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis research & therapy. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ML, Nesti LJ, Tuli R, Lazatin J, Danielson KG, Sharkey PF, et al. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. J Orthop Res. 2002;20:1175–84. doi: 10.1016/S0736-0266(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 30.Vermes C, Roebuck KA, Chandrasekaran R, Dobai JG, Jacobs JJ, Glant TT. Particulate wear debris activates protein tyrosine kinases and nuclear factor kappaB, which down-regulates type I collagen synthesis in human osteoblasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:1756–65. doi: 10.1359/jbmr.2000.15.9.1756. [DOI] [PubMed] [Google Scholar]
- 31.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. The Journal of clinical investigation. 2000;106:1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manolagas SC, Jilka RL, Girasole G, Passeri G, Bellido T. Estrogen, cytokines, and the control of osteoclast formation and bone resorption in vitro and in vivo. Osteoporosis Int. 1993;3 (Suppl 1):114–6. doi: 10.1007/BF01621882. [DOI] [PubMed] [Google Scholar]
- 33.Takagi M. Neutral proteinases and their inhibitors in the loosening of total hip prostheses. Acta orthopaedica Scandinavica Supplementum. 1996;271:3–29. [PubMed] [Google Scholar]
- 34.Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheumat. 2002;46:2514–23. doi: 10.1002/art.10527. [DOI] [PubMed] [Google Scholar]