Abstract
A general, mild, and efficient 1,2-migration/cycloisomerization methodology toward multisubstituted 3-thio-, seleno-, halo-, aryl-, and alkyl-furans and pyrroles, as well as fused heterocycles, valuable building blocks for synthetic chemistry, has been developed. Moreover, regiodivergent conditions have been identified for C-4 bromo- and thio-substituted allenones and alkynones for the assembly of regioisomeric 2-hetero substituted furans selectively. It was demonstrated that, depending on reaction conditions, ambident substrates can be selectively transformed into furan products, as well as undergo selective 6-exo-dig or Nazarov cyclizations. Our mechanistic investigations have revealed that the transformation proceeds via allenylcarbonyl or allenylimine intermediates followed by 1,2-group migration to the allenyl sp carbon during cycloisomerization. It was found that 1,2-migration of chalcogens and halogens predominantly proceeds via formation of irenium intermediates. Analogous intermediate can also be proposed for 1,2-aryl shift. Furthermore, it was shown that the cycloisomerization cascade can be catalyzed by Brønsted acids, albeit less efficiently, and commonly observed reactivity of Lewis acid catalysts cannot be attributed to the eventual formation of proton. Undoubtedly, thermally induced or Lewis acid-catalyzed transformations proceed via intramolecular Michael addition or activation of the enone moiety pathways, whereas certain carbophilic metals trigger carbenoid/oxonium type pathway. However, a facile cycloisomerization in the presence of cationic complexes, as well as observed migratory aptitude in the cycloisomerization of unsymmetrically disubstituted aryl- and alkylallenes, strongly supports electrophilic nature for this transformation. Full mechanistic details, as well as the scope of this transformation, are discussed.
Introduction
Furans and pyrroles are ubiquitous heterocycles, broadly found in naturally occurring and biologically active compounds,1,2 as well as in material science.3 Among these, heterosubstituted furans and pyrroles represent an important subclass, both as synthons4 and themselves as functionalized heterocycles. Approaches toward functionalized five-membered heterocycles can be divided into two groups: functionalization of a preexisting heterocyclic core, and assembly of the ring from acyclic precursors.5 Among the two, the latter route has greater potential for rapidly obtaining diversity in functionalized heterocycles. Within this group,6 the variations of Paal-Knorr synthesis5 has proven to be the most powerful method for the synthesis of furans and pyrroles. However, this approach is unsuitable for acid-sensitive substrates, as well as C-2 unsubstituted heterocycles, owing to the instability of the precursors. As an alternative, focus has shifted lately to catalytic approaches toward furans7 and pyrroles8 from acyclic substrates, which often employ milder conditions and provide easy access to multisubstituted heterocyclic cores. Atom-economical cycloisomerization methods are particularly attractive. Among them, transition metal-catalyzed cycloisomerizations of allenyl ketones 1 introduced by Marshall7m–q (Cat = Ag) and then elaborated by Hashmi7i (Cat = Au) have become one of the most powerful methods for assembly of furan ring 2. Along this line, we have recently developed a mild and efficient Cu-catalyzed cycloisomerization of alkynyl imines9 and ketones 310 into the respective heterocycles 2 (Scheme 1). The reaction conditions, which were developed, are compatible with both acid- and basesensitive substrates.9,10 Moreover, this method proved to be especially efficient for the synthesis of C-2 unsubstituted pyrroles 2, which are not readily available through traditional condensation methods (Scheme 1).5 Mechanistic investigations revealed that the reaction proceeds through an allenylimine or-ketone intermediate 1, and that the propargylic protons ultimately reside at the C-3 and C-4 positions of the ring (Scheme 1).9,11
Scheme 1.
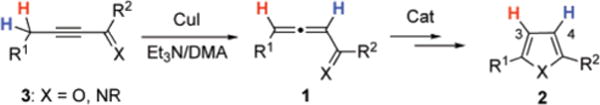
General Cu-Catalyzed Cycloisomerization of Alkynyl Ketones and Imines toward Furans and Pyrroles
Despite a number of advantages of these protocols, their scope is limited to the preparation of C-3 and C-4 unsubstituted heterocycles only. We reasoned that this problem could be alleviated if one of the hydrogens at C-4 in 4 is replaced with a suitable migrating group Y. Thus, aiming at expanding the scope of the migrating group, we have recently developed a set of cascade methods for the synthesis of C-3 substituted pyrroles and furans 7 proceeding via 1,2-shift of thio-,12 halogen-,13 and aryl/alkyl-14 groups in allene 5 (Scheme 2).
Scheme 2.
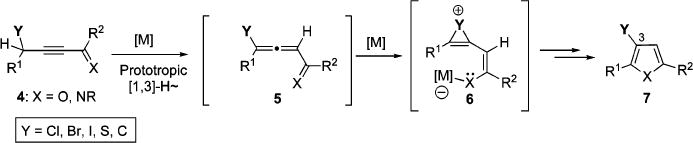
Introduction of Different Migrating Groups toward Trisubstituted Heterocycles
Herein, we describe a more detailed study of these transformations, the synthesis of seleno-heterocycles including unprecedented 1,2-selenium migration, as well as a more thorough mechanistic investigation of these unique cascade cycloisomerizations.
Results and Discussion
1,2-Sulfur Migration in the Synthesis of Heterocycles
1,2-Migration of chalcogenides15 is an important chemical transformation, which is extensively used in carbohydrate chemistry for substitution at the anomeric center,16,17 as well as in the synthesis of stereodefined, nonaromatic heterocycles18,19 and allylsulfides.18 Furthermore, 1,2-thio-shift is known to occur in aromatic rings20 and to carbenoid centers.21 Known 1,2-migrations of chalcogens can be classified as two types: 1,2-migration from sp3 center to adjacent sp3 center via a thiiranium or seleniranium intermediate16 and 1,2-migration from either sp3 or sp2 center to another sp2-carbon.7an,20,22 However, prior to our work,12 there were no reports on 1,2-migration of the thio-group from an olefinic sp2 carbon to an sp center.
During investigation of the scope of the recently found Cu-catalyzed transformation of alkynyl ketones and imines 3 into 2,5-disubstituted furans10 and pyrroles 2,9 it was discovered that heating of thioalkynyl ketone 8 in DMA in the presence of CuI (10 mol %) not only produced the targeted 2,5-disubstituted furan 10 but also a small amount of the 2,4-disubstituted furan 11 (Scheme 3).
Scheme 3.
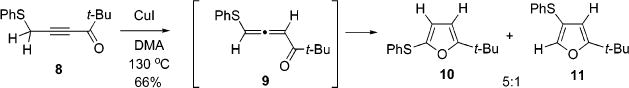
1,2-H Vs 1,2-S Migration during Cycloisomerization
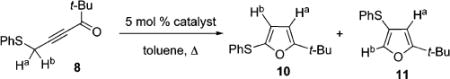
Brief optimization of this reaction revealed that AuCl3, PtCl2, and PdCl2(MeCN)2 led largely to decomposition. However, employment of (Et3P)AuCl afforded a 20:1 mixture of regioisomeric furans favoring “normal” product 10, likely resulting from an even more preferential 1,2-hydrogen vs 1,2-thio shift in 9 (Table 1).
Table 1.
Optimization of Cycloisomerization of Thioalkynones
| entry | catalyst | GC ratio 10:11 | NMR yield (%)a |
|---|---|---|---|
| 1 | CuI | 5:1 | 66b |
| 2 | AuCl3 | 1:1.4 | 5 |
| 3 | PtCl2 | 1:2.5 | traces |
| 4 | Pd(MeCN)2Cl2 | 1:3.5 | 2 |
| 5 | (Et3P)AuCl | 20:1 | 63 |
NMR yields calculated using dibromomethane as an internal standard.
DMA used as solvent.
Intrigued by the unexpected observation of the 3-thiofuran 11, we endeavored to investigate the formation of this product more thoroughly. Initially, we hypothesized that the two products arise from a common allenyl ketone intermediate 9.9,23 The “normal” product 10 forms from the copper-assisted ring closure, followed by the base-assisted intramolecular proton transfer.9,10 The regioisomeric furan 11 was thought to result from the intramolecular Michael addition of sulfur at the allenic carbon, forming an intermediate aromatic thiirenium zwitterion 6 (Scheme 2),24,25 which underwent further cycloisomerization to give 11. It occurred to us that if the above concept is correct, then replacement of one of the propargylic hydrogens in 8 with any other nonmigrating group should enforce selective 1,2-migration of the thio group to produce the 3-thio substituted furan. To examine this proposal, thioalkynone 12a was subjected to the cycloisomerization conditions described above. Remarkably, cycloisomerization of 12a proceeded smoothly to give 3-thio substituted furan 14a as a single regioisomer in excellent yield (Scheme 4).
Scheme 4.
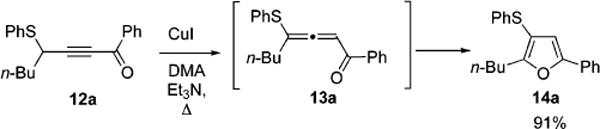
Selective 1,2-Sufur Migration during Cycloisomerization of 12a
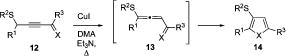
Naturally, next we investigated the scope of a selective migrative cycloisomerization of substituted propargylsulfides en route to 3-thiosubstituted heterocycles. Accordingly, a series of alkyl-substituted propargyl sulfides 12 were synthesized and subjected to the cycloisomerization reaction (Table 2). Cycloisomerization of thiopropargylketones 12a,b,c proceeded uneventfully, affording the trisubstituted furans 14a,b,c in good to excellent yields (entries 1–3). Gratifyingly, thiopropargyla-ldehyde 12d underwent smooth and selective cycloisomerization, producing 2-butyl-3-(phenylthio)furan (14d) in 71% yield as a single reaction product (Table 2, entry 4). Cycloisomerization of (phenylthio)propargylketones possessing alkenyl-(12e), ester-(12f), and tetrahydro-2H-pyran-2-yloxy-(12g) functionalities in the side chain proceeded readily, affording the corresponding trisubstituted furans 14e–g in good to very high yields (entries 5–7). The alkylsulfanyl group migrated with efficiency comparable to its phenylsulfanyl-analog to give the corresponding furan 14h in 72% yield (entry 8). Moreover, it was found that thiopropargyl imines 12i–n underwent a similar transformation in the presence of CuI to give the corresponding 3-thio-substituted pyrroles 14i–n in good yields (entries 9–14). Again, the dodecylsulfanyl-group (entry 10) migrated comparably to the phenylsulfanyl-analog (entry 9) and the THP-protected alcohol functionality was tolerated (entry 14). It is worth mentioning that all synthesized pyrroles have deprotectable groups at the nitrogen atom, such as tert-butyl- (14i,j entries 9,10),26 trityl- (14k, entry 11),27 and 3-(ethylbutyryl)- (EB)9,28 (14l–n, entries 12–14), and thus can be easily further functionalized at the nitrogen site. In addition, fused pyrroloheterocycle 14o was smoothly synthesized in a preparative scale from 12o.
Table 2.
Cycloisomerization of Thioalkynones and Thioalkynimines 12 into 3-Thiofurans 14
| Entry | Substrate | Product | Yield (%)a | |
|---|---|---|---|---|
| 1 |
|
|
14a | 91 |
| 2 |
|
|
14b | 76 |
| 3 |
|
|
14c | 89 |
| 4 |
|
|
14d | 71 |
| 5 |
|
|
14e | 95 |
| 6 |
|
|
14f | 71 |
| 7 |
|
|
14g | 93 |
| 8 |
|
|
14h | 72 |
| 9 |
|
|
14i | 78 |
| 10 |
|
|
14j | 86 |
| 11 |
|
|
14k | 85 |
| 12 |
|
|
141 | 74 |
| 13 |
|
|
14m | 67 |
| 14 |
|
|
14n | 78 |
| 15 |
|
|
14o | 53b |
Isolated yields, reactions were performed on 1 mmol scale.
Reaction was performed on 3.89 mmol scale under the following conditions: 0.5 equiv CuBr, 1:7 Et3N:DMA, 0.08M, 150 °C, 12 h.
Generally, transformation of thiopropargyl-ketones or imines 12 into furans and pyrroles 14 required presence of 0.2–5 equiv of triethylamine as a base.11 However, when phenylthiopropargylketone 12g possessing tetrahydro-2H-pyran-2-yloxy moiety was subjected to the cycloisomerization conditions in the absence of the base, dihydro-2H-pyran-6-yl derivative 15 was formed in 93% yield along with the trace amounts of the expected furan 14g (Scheme 5). Normally, in the presence of base, 12g undergoes a facile prototropic rearrangement to allenylsulfide 13g, which via a putative thiirenium intermediate i, transforms into furan 14g. We hypothesized that in the absence of base this route is unlikely. Instead, 12g undergoes a competitive 6-exo-dig cyclization to form 2-yledene-tetrahydro-2H-pyranium intermediate ii which, upon fragmentation with the loss of 3,4-dihydro-2H-pyran, gives enone iii. Subsequent Cu-assisted isomerization of the latter produces the thermodynamically more stable enone 15. This was confirmed by DFT calculations (B3LYP; 6-31G*: + 3.6 kcal/mol and + 6.6 kcal/mol ground state energy differences for (E)-iii and (Z)-iii over 15, accordingly).
Scheme 5.
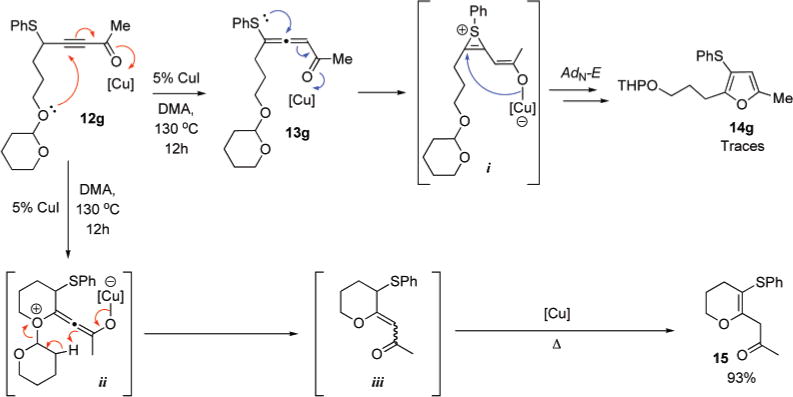
Competitive 6-exo-dig Cyclization of 12g in the Absence of the Base
1,2-Selenium Migration in the Synthesis of Heterocycles
Motivated by the successful 1,2-thio migration during the cycloisomerization reaction of alkynyl ketones and imines, we next attempted to incorporate 1,2-selenium migration into the cycloisomerization cascade. Selenoheterocycles are important units which have found broad applications in biological studies as cytotoxic antitumor agents,29 as NMR active tracers, and in protein–enzyme interaction studies.30 Synthesis of nonaromatic selenoheterocycles, including selenolactones, is well known. The majority of syntheses involve electrophilic activation of an unsaturated bond followed by intramolecular ring closure.31 In contrast, only a few scattered methods have been reported for the synthesis of 3-seleno-furans and pyrroles, including a Paal-Knorr approach,32 unselective electrophilic selenation,33 and halogen–selenium exchange.34 These methods suffer from significant drawbacks, including limited scope of products, imperfect regioselectivity, and low yields. We reasoned that our 1,2-migration/cycloisomerization methodology might be a perfect and convenient solution for the synthesis of 3-seleno-furans and pyrroles.
To this end, cycloisomerization of propargylselenoalkynone 16a toward furan 17a was examined in the presence of different transition metal catalysts (Table 3). Surprisingly, cycloisomerization of 16a in the presence of gold, platinum, and palladium catalysts (entries 1–3) provided formation of furan 17a along with notable amounts of regioisomeric furan 18, product of the competitive 1,2-alkyl migration/cyclization cascade. It was found that heating of 16a in toluene without catalyst afforded the target furan 17a almost exclusively, albeit in 38% yield (entry 4). The selectivity and yield were improved by employing CuCl as the catalyst, affording 17a as the sole regioisomer in 57% yield (entry 5). Employment of CuCl catalyst in DMA:Et3N solvent mixture at room temperature produced furan 17a in 96% yield (entry 6).
Table 3.
Cycloisomerization of Selenoalkynone 16a into Furans 17a and 18
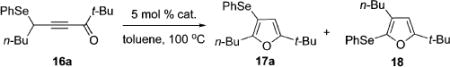
| entry | catalyst | 17a (%)a | 18 (%)a |
|---|---|---|---|
| 1 | AuCl3 | 24 | 34 |
| 2 | PtCl2 | 70 | 30 |
| 3 | Pd(MeCN)2Cl2 | 13 | 33 |
| 4 | none | 38 | – |
| 5 | CuCl | 57b | – |
| 6 | CuCl | 96c | – |
NMR yields calculated using dibromomethane as an internal standard for reactions performed on 0.1 mmol scale.
Reaction was performed on 0.5 mmol scale under the following conditions: 5 mol % CuCl, 10:1 DMA:Et3N, rt.
Next, the scope of this transformation was examined employing the optimized reaction conditions. It was found that, in addition to the t-butyl ketone 16a, alkynal 16b and methyl- and phenyl ketones 16c and 16d underwent cycloisomerization cleanly to afford the corresponding furans 17b–d in good yields (Table 4, entries 1–3). A benzylic group was also tolerated, affording trisubstituted furan 17e in 71% yield and even disubstituted furan 17f in 53% yield (entries 4, 5).35 Next, we tested the feasibility of applying this methodology for the synthesis of selenopyrroles. Indeed, it was found that propargylseleno alkynylimines 16g,h smoothly underwent cycloisomerization at room temperature to afford the corresponding N-protected9,26–28 pyrroles 17g,h in 74 and 57% yields, respectively (Table 4, entries 6, 7). However, cycloisomerization of sterically hindered N-trityl alkynyl imine 16i required heating at 110 °C to give pyrrole 17i in 54% yield (Table 4, entry 8).
Table 4.
Synthesis of 3-Seleno-Furans and Pyrroles 17
| Entry | Substrate | Product | Yield (%)a,b | |
|---|---|---|---|---|
| 1 |
|
|
17b | 74 |
| 2 |
|
|
17c | 71c |
| 3 |
|
|
17d | 84 |
| 4 |
|
|
17e | 71 |
| 5 |
|
|
17f | 53 |
| 6 |
|
|
17g | 74d |
| 7 |
|
|
17h | 57d |
| 8 |
|
|
17i | 54e |
Isolated yields for reactions performed on 0.5 mmol scale.
15 mol % CuCl, 20% Et3N, 0.5 M in DMA, rt.
5 mol % CuCl, 10:1 DMA:Et3N, rt.
30 mol % CuCl, 5 equiv Et3N, 0.5 M in DMA, rt.
30 mol % CuCl, 5 equiv Et3N, 0.5 M in DMA, 110 °C.
1,2-Halogen Migration in the Synthesis of Halofurans
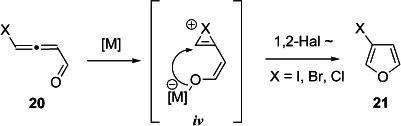
Transformations involving selective 1,2-halogen migration have not been reported until recently,36 when Iwasawa and then Fürstner disclosed 1,2-iodine,37 and 1,2-iodine and –bromine migration,38 respectively, in alkynyl halides to produce fused haloarenes. Furthermore, Liu showed 1,2-iodine shift in a Ru alkylidene complex.39 Some of these transformations involved metal carbenoid intermediates and were used in the synthesis of carbocycles.38,39 To the best of our knowledge, the synthesis of halogenated heterocycles proceeding through a halirenium intermediate has not been previously reported.
Halofurans, important building blocks, are traditionally obtained by electrophilic halogenation of furans,40 via halogen-induced cyclizations,41 or cyclocondensations of halogenated precursors.42 Most of these approaches require employment of strongly electrophilic reagents, thus limiting their application to substrates lacking acid-sensitive functionalities. With the successful development of 1,2-thio- and seleno migration/cycloisomerization approach for the synthesis of trisubstituted furans and pyrroles, we sought to further expand the scope of this methodology. Thus, we turned our attention to the synthesis of 3-halofurans.
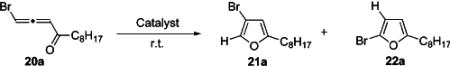
We hypothesized that replacement of chalcogens (X = RS and RSe) with halogen (X = Cl, Br, I) in the proposed intermediate iv12 might provide convenient access to 3-halofurans (eq 1). To test this idea, the Cu-catalyzed cycloisomerization of bromoallenyl ketone 20a43 was examined, which, indeed, led to the
 |
(1) |
formation of 3-bromofuran 21a, albeit in poor yield (Table 5, entries 1–2). In contrast, AgBF4, which proved efficient in cycloisomerization of different allenyl ketones,44 did not catalyze this reaction at all (entry 3). Employment of PtCl2, however, produced 3-bromofuran 21a in 50% yield along with small amounts of 2-bromofuran 22a (entry 4). To our delight, employment of AuCl3 afforded 3-bromofuran 21a in 86% yield with high selectivity (Table 5, entry 5).43,45 Surprisingly, switching solvent to THF caused a dramatic change in selectivity, affording 2-bromofuran 22a as a major product (entry 6). The latter was also exclusively obtained in the presence of Au-(PEt3)Cl (entry 8). It was found that selective cycloisomerization of 20a can be also achieved in the presence of AlCl3 and even silica gel, affording 3-bromofuran 21a, though in low yield (entries 9–10).
Table 5.
Catalyst Optimization for Cycloisomerization of Bromoallenyl Ketone 20a
| entry | cat. (mol %) | solvent | time | GC yield, % (21a:22a) |
|---|---|---|---|---|
| 1 | CuCl (10) | toluene | 1 day | 29 (21a only) |
| 2 | CuI (10) | toluene | 1 day | 21 (21a only) |
| 3 | AgBF4 (5) | DCM | 1 day | traces |
| 4 | PtCl2 (5) | toluene | 3h | 50 (96:4) |
| 5 | AuCl3 (1) | toluene | 5 min | 86 (95:5) |
| 6 | AuCl3 (1) | THF | 5 min | 78 (5:95) |
| 7 | Au(PPh3)Cl (1) | toluene | 9h | N/D (16:84) |
| 8 | Au(PEt3)Cl (1) | toluene | 9h | N/D (< 1:99) |
| 9 | AlCl3 (50) | toluene | 16 h | 27 (21a only) |
| 10 | SiO2 | toluene | 16 h | 21 (21a only) |
Next, we investigated the scope of this cascade transformation. Thus, differently substituted haloallenyl ketones were subjected to Au(III)-catalyzed cycloisomerization (Table 6). It was found that a variety of alkyl and aryl-substituted bromoallenyl ketones and aldehydes 20 underwent smooth cycloisomerization, affording 3-bromofurans 21 in good to excellent yields (entries 1–5). Remarkably, this method allowed for efficient synthesis of halofurans possessing hydroxymethyl (21e) and alkene (21f) functionalities, which are incompatible with known methods employing electrophilic reagents. It was found that fully substituted iodoallenyl ketone 20g reacted more slowly than its bromo-analogs, producing corresponding furans 21g in good yield (entry 6). Gratifyingly, ambident disubstituted allenyl iodides 20h,i underwent exclusive 1,2-iodine migration to afford 2-alkyl and -aryl substituted iodofurans 21h,i in 97 and 71% yields, respectively (entries 7,8). Chloroallene 20j also underwent this transformation to produce 3-chlorofuran 21j. However, the observed much more sluggish reaction of 20j was attributed to the decreased ability of chlorine to form halirenium species iv (eq 1). Cycloisomerization of ambident trisubstituted allenyl iodide 20k possessing more bulky n-propyl group at C-2 than that in iodoallenes 20h,i produced of 2:1 mixture of 3- and 2-iodofurans 21k and 21l, respectively.
Table 6.
1,2-Halogen Migration/Cycloisomerization toward Halofurans 21
| Entry | Substrate | Time | Product | Yield (%)a | |
|---|---|---|---|---|---|
| 1 |
|
1 day |
|
21b | 75 |
| 2 |
|
1 day |
|
21c | 73 |
| 3 |
|
1 hr |
|
21d | 73 |
| 4 |
|
1 day |
|
21e | 61 |
| 5 |
|
0.5 hr |
|
21f | 88 |
| 6 |
|
3 days |
|
21g | 73 |
| 7 |
|
5 min |
|
21h | 97 |
| 8b |
|
1 hr |
|
21i | 71 |
| 9 |
|
3 days |
|
21j | 48 |
| 10b |
|
1 hr |
|
21k | 67c |
|
|
21l | ||||
Isolated yields, reactions were performed on 0.29–1 mmol scale with 1 M concentration of 20.
Mixture of allene and corresponding propargyl isomer was employed (see Supporting Information).
Mixture (2:1) of 21k and 21l by 1H NMR.
1,2-Alkyl/Aryl Migration in the Synthesis of Furans
As discussed above, cycloisomerization of C-4 monosubstituted allenyl ketones 23 in the presence of transition metal catalysts can be used as an efficient approach for the assembly of the furan ring via formal 1,2-hydrogen shift (eq 2).7i,m–q
 |
(2) |
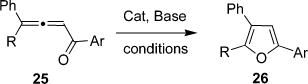
Inspired by the observation of competitive 1,2-alkyl migration during cycloisomerization of selenoalkynone 16a into 3-alkyl-furan 18 (Table 3, entries 1–3), we envisioned that development of a cascade transformation involving a 1,2-migration of an alkyl/aryl group8k,46,47,48,49 in allenylketones is also feasible. If successful, this approach may allow for the rapid assembly of fully carbon-substituted furans. To this end, the possible cycloisomerization of allene 25 into furan 26 in the presence of different catalysts was tested (Table 7). It was found that employment of Au(I) and Au(III) halides gave low yields of furan 26. Gratifyingly, switching to cationic Au(I) complexes lead to formation of expected furan in nearly quantitative yield (entries 3–4). Analogously to gold halides, Pt(II), Pt(IV), and Pd(II) salts were inefficient in this reaction (entries 5–7). Use of Cu(I) halides resulted in no reaction, whereas employment of cationic Ag(I), Cu(I), and Cu(II) salts produced 26 in moderate to high yields. Encouraged by these results, we have also examined main group metals in this reaction. Surprisingly, Al-, Si-, Sn-, and In triflates provided moderate to excellent yields of desired furan 26. Although Au(PPh3)OTf, AgOTf, In-(OTf)3, Sn(OTf)2, and TIPSOTf were nearly equally efficient in the cascade cycloisomerization of 25 to 26, In(OTf)3 appeared to be a more general catalyst with respect to the substrate scope.11
Table 7.
Optimization of Reaction Conditions for Cycloisomerization of 25
| entry | cat | mol % | solvent | T, °C | yield (%)a |
|---|---|---|---|---|---|
| 1 | AuBr3 | 5 | tolueneb | 100 | 23 |
| 2 | AuI | 5 | tolueneb | 100 | traces |
| 3 | Au(PPh3)OTf | 1 | tolueneb | 100 | 100 (89) |
| 4 | Au(PPh3)OTf | 5 | DCMc | rt | 99 |
| 5 | PtCl2 | 5 | toluened | 100 | 21 |
| 6 | PtCl4 | 5 | toluened | 100 | 21 |
| 7 | Pd(PhCN)2Cl2 | 5 | toluened | 100 | 35 |
| 8 | CuX (X = Cl, Br, I) | 5 | toluened | 100 | 0 |
| 9 | [CuOTf]2•PhH | 5 | toluened | 100 | 42 |
| 10 | Cu(OTf)2 | 5 | toluenee | 100 | 95 |
| 11 | AgPF6 | 5 | toluenee | 100 | 47 |
| 12 | AgOTf | 5 | toluenee | 100 | (80) |
| 13 | AgOTf | 20 | DCMc | rt | 70 (62) |
| 14 | Al(OTf)3 | 5 | toluenee | 100 | 64 |
| 15 | Zn(OTf)2 | 5 | toluenee | 100 | 39 |
| 16 | TMSOTf | 20 | DCMd | rt | 82 (62) |
| 17 | In(OTf)3 | 5 | toluenee | 100 | 91 (81) |
| 18 | Sn(OTf)2 | 5 | toluenee | 100 | 97 (81) |
| 19 | TIPSOTf | 5 | toluenee | 100 | 100 (81) |
| 20 | TMSNTf2 | 5 | toluenee | 100 | 72 |
aNMR yield, isolated yield in parentheses (entries 1–4: Ar = p-Br–C6H4; entries 5–20: Ar = Ph).
Solution (0.05 M) of 25.
Solution (0.02 M) of 25.
Solution (1 M) of 25.
Solution (0.1 M) of 25.
In light of the recent observations that eventual Brønsted acids are the true catalysts in some transition metal-catalyzed transformations,50 we investigated what role, if any, Brønsted acids may play in the herein described cycloisomerization reaction. To this end, the cycloisomerization of 25 by several catalysts in the presence of proton scavenger, TTBP, was examined (Table 8).51 It was found that cycloisomerization of allenyl ketone 25 at 100 °C in toluene in the presence of TfOH or Sn-(OTf)2 provided furan 26 in almost quantitative NMR yield with comparable rates (entries 1 and 10). The same result was observed for reactions performed in 1,2-dichloroethane for AgOTf, TMSOTf, and TfOH catalysts (entries 4, 6, and 8). Addition of the TTBP negligibly affected cycloisomerization reaction for both catalysts in toluene solvent series (entries 2 and 3), owing to the most probable dissociation of Lewis acid–Lewis base complex at the elevated temperature. In contrast, addition of TTBP for the 1,2-dichloroethane experiments completely suppressed the cycloisomerization reaction at room temperature for TfOH and TMSOTf, and even at 80 °C for AgOTf (entries 5, 8, and 11). However, elevation of the reaction temperature allowed for the formation of furan 26 albeit in lower yields and increased reaction times (entries 6, 9 and 12). Accordingly, TMSOTf-TTBP pair provided 61% of furan product, whereas only 36% yield was achieved for the TfOH-TTBP pair after more prolonged reaction time. Thus, taking into consideration the more efficient cycloisomerization in the presence of TTBP for TMSOTf vs TfOH, observed reactivity for the Lewis acid catalysts cannot be attributed to the formation of eventual Brønsted acid catalyst. It should be emphasized, however, that TfOH, indeed, is able to catalyze cycloisomerization of 25 into 26 even with slightly better efficiency for some 4,4-diaryl substituted allenyl ketones (entry 14 vs 15). However, cycloisomerization of 4-methyl-1,4-diphenyl allenylketone in the presence of TfOH catalyst appeared to be notably less efficient (entry 16) compared to that in the presence of In(OTf)3 (entry 17).
Table 8.
Comparison of Lewis and Brønsted Acid Catalysts for Cycloisomerization of Allenyl Ketones
| entry | R | Ar | cat (mol %) | additive (mol %) | solvent | T, °C | time, h | NMR yield (%)a,b |
|---|---|---|---|---|---|---|---|---|
| 1 | Ph | p-C6H4-CN | TfOH (10) | – | toluene | 100 | 1.5 | 96 |
| 2 | Ph | p-C6H4-CN | TfOH (10) | TTBP (40) | toluene | 100 | 2.0 | > 99 |
| 3 | Ph | p-C6H4-CN | Sn(OTf)2 (5) | TTBP (20) | toluene | 100 | 2.0 | 91 |
| 4 | Ph | p-C6H4-CN | TfOH (20) | – | DCE | rt | 1.0 | 96 |
| 5 | Ph | p-C6H4-CN | TfOH (20) | TTBPc (40) | DCE | rt | 24 | 0 |
| 6 | Ph | p-C6H4-CN | TfOH (20) | TTBP (40) | DCE | 95 | 48 | 36 |
| 7 | Ph | p-C6H4-CN | TMSOTf (20) | – | DCE | rt | 1.0 | > 99 |
| 8 | Ph | p-C6H4-CN | TMSOTf (20) | TTBP (40) | DCE | rt | 4.0 | 0 |
| 9 | Ph | p-C6H4-CN | TMSOTf (20) | TTBP (40) | DCE | 95 | 24 | 61 |
| 10 | Ph | p-C6H4-CN | AgOTf (20) | – | DCE | 80 | 2.0 | > 99 |
| 11 | Ph | p-C6H4-CN | AgOTf (20) | TTBP (40) | DCE | 80 | 2.0 | 0 |
| 12 | Ph | p-C6H4-CN | AgOTf (20) | TTBP (40) | DCE | 95 | 48 | 19 |
| 13 | Ph | p-C6H4-CN | Sn(OTf)2 (5) | – | toluene | 100 | 1.5 | > 99 |
| 14 | Ph | p-C6H4-OMe | TfOH (10) | – | toluene | 100 | 1.0 | 88 |
| 15 | Ph | p-C6H4-OMe | In(OTf)3 (5) | – | toluene | 100 | 2.0 | 79 |
| 16 | Me | Ph | TfOH (10) | – | toluene | 100 | 1.0 | 52 |
| 17 | Ph | Ph | In(OTf)3 (5) | – | toluene | 100 | 12 | 77 |
Reactions were performed on 0.1 mmol scale.
Dibromomethane was used as the standard.
TTBP = 2,4,6-tris-tert-butylpyrimidine.
Having in hand a set of optimized conditions, cycloisomerization of differently substituted allenyl ketones 25a–n was examined (Table 9). Cycloisomerization of 4,4-diphenyl substituted allenyl ketones 25a–d proceeded smoothly to provide good to high yields of furans 26a–d. Selective 1,2-migration of phenyl over methyl group occurred in allenyl ketone 25e to give 26e in 72% yield (entry 5). In contrast to the methyl-, 1,2-migration of the ethyl group competed with the phenyl group in 25f, which resulted in formation of a 2.3:1 mixture of regioisomeric furans 26f and 26g, respectively (entry 6).52 Cyclopentylideneallenyl ketone 25h underwent smooth cyclization with ring expansion to give fused furan 26h in 75% yield (entry 7). Not surprisingly, cycloisomerization of allenyl ketone 25i, possessing more thermodynamically stable 6-membered ring or 25j, having two methyl groups, provided corresponding furans 26i and 26j in low yields only (entries 8 and 9). It was also demonstrated that a variety of functional groups such as methoxy- (entry 10), bromo- (entry 11), nitro- (entry 12), and cyano- (entry 13) were perfectly tolerated under these reaction conditions.
Table 9.
Metal-Catalyzed Synthesis of Furans 26
![]()
| Entry | Substrate | Cat (mol%) | T,°C | Product | Yield (%)a,b | |
|---|---|---|---|---|---|---|
| 1 |
|
Sn(OTf)2 (5) | 100 |
|
26a | 81 |
| 2 |
|
In(OTf)3 (10) | 115 |
|
26b | 64 |
| 3 |
|
In(OTf)3 (5) | 100 |
|
26c | 90 |
| 4 |
|
AgOTf(20) | 140 |
|
26d | 79c |
| 5 |
|
In(OTf)3 (5) Au(PPh3)OTf(2) | 100 |
|
26e | 72 52d |
| 6 |
|
In(OTf)3 (5) Au(PPh3)OTf (1) | ″ |
|
26f | 88e |
|
|
26g | 76df | ||||
| 7 |
|
In(OTf)3 (5) | ″ |
|
26h | 75 |
| 8 |
|
″ | ″ |
|
26i | 18d |
| 9 |
|
″ | ″ |
|
26j | 10d |
| 10 |
|
″ | ″ |
|
26k | 62 |
| 11 |
|
In(OTf)3 (5) Au(PPh3)OTf(l) | ″ |
|
26l | 93 89 |
| 12 |
|
Sn(OTf)2 (5) | ″ |
|
26m | 85 |
| 13 |
|
″ | ″ |
|
26n | 94 |
Isolated yield.
Reactions were performed on 0.25–0.8 mmol scale.
p-Xylene was used as a solvent.
NMR yield.
Mixture (2.3:1) of 26f: 26g by 1H NMR.
Mixture (2.2:1) of 26f:26g by 1H NMR.
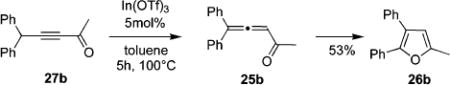
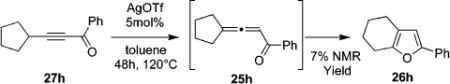
It was also shown that trisubstituted furan 26b can be directly obtained from alkynyl ketone 27b (eq 3), albeit the yield for this one-pot transformation was somewhat lower compared to that for cycloisomerization of allene 25b (Table 9, entry 2). The intermediacy of 25b has been confirmed by GC/MS monitoring of the reaction course. However, this approach is moderately efficient only for the propargylic systems which can undergo facile alkynyl-allenyl isomerization, such as in the 27b, as attempts on direct cycloisomerization of cyclopentyl-substituted alkynone 27h failed (eq 4).
 |
(3) |
 |
(4) |
It should be noted that the cycloisomerization course of C-1 phenyl substituted allenyl ketones in the presence of Lewis acid catalysts is greatly affected by the bulkiness of C-2 substituent. Thus, cycloisomerization of C-2 methyl substituted allenylketone 25d in the presence of main group triflates produced furan 26d along with notable amounts of methylene-indan-1-one 28d.11 Employment of TMSOTf allowed for the formation of 28d in 95% yield as a sole product (Scheme 6). We hypothesized that activation of the carbonyl function in 25d by a Lewis acid produces rotamers v and vi. The latter, in the case of 25d, is favored over v, which suffers the repulsion between methyl and phenyl groups. A facile aromatic Nazarov cyclization of vi produces indanone 28d53 in nearly quantitative yield (Scheme 6).
Scheme 6.
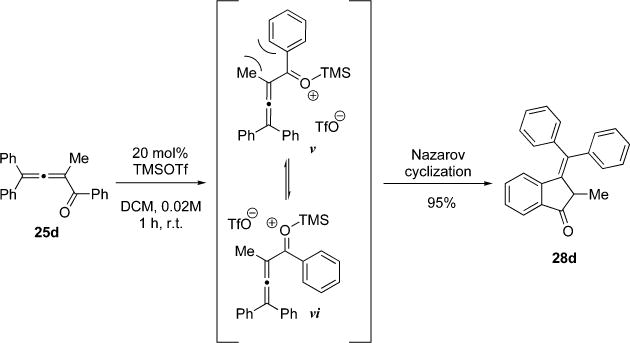
Nazarov Cyclization of Tetrasubstituted Phenyl-allenylketone 25d
Mechanistic Discussion
Naturally, we were interested in the investigation of the mechanisms of herein described 1,2-migration/cycloisomerization cascade transformations of alkynyl- or allenylketones and -imines into corresponding furans and pyrroles. Our thorough studies revealed many similarities observed during cascade cycloisomerizations of C-4 diversely substituted alkynyl and allenyl systems involving 1,2-migration of various groups as the key step in the assembly of heterocyclic cores.
Initially, we hypothesized that migrative cycloisomerization of thioalkynones 12 involves a Cu-assisted prototropic rearrangement, thus proceeding via involvement of a reactive allenyl intermediate 9/13.9,10,12,54 Analogous allenyl intermediate 19 was proposed for 1,2-selenium migrative cycloisomerization of selenoalkynones 16, whereas 1,2-halogen or 1,2-alkyl/aryl migrations were achieved utilizing the corresponding allenyl compounds 20 and 25 as starting materials. Indeed, failure to perform efficient cycloisomerization directly from propargylic ketones (eq 3 vs eq 4), where essential propargyl-allenyl isomerization is largely suppressed, confirmed the allenyl system to be a viable and necessary intermediate in the 1,2-alkyl/aryl migrative cycloisomerization.
Moreover, to gain the support for the involvement of allenyl intermediate 13, thioallenones 13a,p were prepared by independent methods and subjected to the cycloisomerization conditions described above (see Table 2). Remarkably, it was found that thioallenyl arylketone 13a, even in the absence of CuI catalyst, underwent quantitative thermal transformation to14a. In contrast, attempts to perform analogous thermal cycloisomerization of thioallenylalkylketone 13p resulted in a total decomposition of the starting material, whereas 82% of 14p was isolated when reaction was performed at room temperature in the presence of 5 mol % of CuI (Figure 1).
Figure 1.
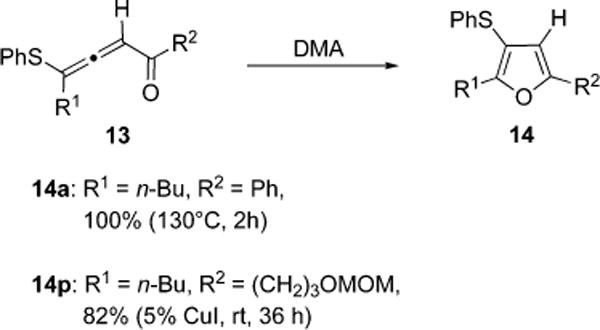
Direct Cycloisomerization of Thioallenones 13 into Furans 14.
Furthermore, we hypothesized that, considering the enhanced acidity of the propargylic proton of selenoalkynones 16,55 cycloisomerization of the latter should involve very facile propargyl-allenyl isomerization, leading to allenone intermediate 19. Indeed, when a subcatalytic loading of copper chloride was used (Scheme 7 vs Table 4, entry 1), allenal 19b accumulated in the reaction mixture (Scheme 7). Subsequent treatment of the isolated allenal 19b with copper chloride in DMA at room temperature afforded furan 17b in good yield (Scheme 7).56
Scheme 7.
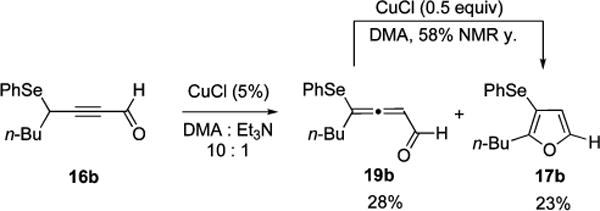
Direct Observation of Selenoallenic Intermediate 19b
Thus, based on the experimental data disclosed above, it is believed that all of the herein reported 1,2-migration/cycloisomerization cascade transformations most likely proceed via allenyl intermediates.
As discussed above, both thioallenone 13a (Figure 1) and seleno alkynone 16a (Table 3, entry 4) in the absence of the copper catalyst underwent thermal 1,2-migration/cycloisomer-ization transformation to give corresponding 3-chalcogeno-furans. Such reactivity can only be rationalized by involvement of intramolecular Michael addition of chalcogen at the enone moiety of the allenone to give intermediate thiirenium24,25 or selenirenium57 zwitterions vii and viii respectively (Scheme 8). Subsequent nucleophilic attack by oxygen or nitrogen at the irenium moiety, followed by either AdN-E or SN2-vin25 processes, furnishes the formation of furan 14. Employment of transition metal catalysts, such as Cu, Au, Pd, and Pt, in similar cycloisomerizations facilitated the propargyl-allenyl isomerization, and potentially also stabilized the formed enolate or enaminate in irenium species ix and x, which undergent analogous to vii and viii cyclization into 3-chalcogeno-furans 17 (Scheme 8).58
Scheme 8.
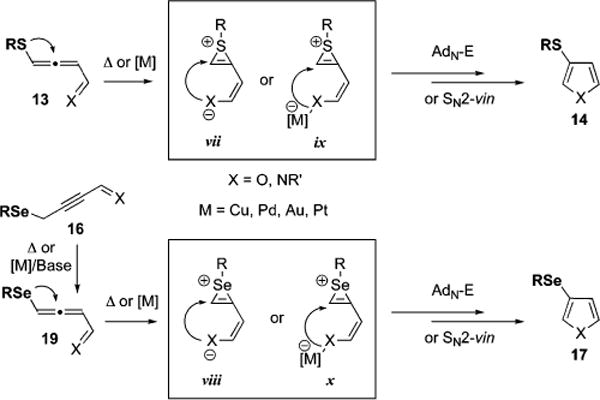
Proposed Irenium Intermediates in the 1,2-Chalcogen Migration/Cycloisomerization Cascade
An analogous scenario involving 1,2-migration of nucleophilic entities to the electrophilic sp center of allenone is responsible for the migrative cycloisomerization catalyzed by Lewis or Brønsted acids. In these cases, activation of enone moiety by these catalysts dramatically increases electrophilicity at C-3 of allenyl intermediate and, thus, provokes a more facile 1,2-migration of an adjacent group. Indeed, observed selective cycloisomerization of bromoallenylketone 20a into 3-bromofuran 21a in the presence of AlCl3 or silica gel (Table 5, entries 9–10) could only be explained by involvement of a similar to vii–x halirenium intermediate xi.59 This, taken together with the reasonably high oxophilicity of AuCl3 in noncoordinating media,60 suggests that 1,2-halogen migration/cycloisomerization cascade proceeds via analogous to 1,2-chalcogen migration pathway involving intermediate xii to give 3-halofuran 21 (Scheme 9). The reversal of regioselectivity observed in the AuCl3-catalyzed reaction in THF (Table 5, entry 6), can be attributed to a decreased oxophilicity of Au(III) complex in ethereal solvent. The same reactivity was observed for more π-philic Au(I) species (Table 5, entries 7 and 8). To verify whether selective formation of 2-bromofuran 22 proceeds through any type of carbenoid intermediates, we subjected deuterated allenyl ketone 4-d-20m to the cycloisomerization conditions (Scheme 10). This reaction produced a mixture of 2- and 3- bromo-furans d-22m and d-21m in a ratio of 2.4:1 respectively without a detectable loss of deuterium.61,62 It appears that rapid AuCl3-catalyzed propargyl-allenyl isomerization is responsible for partial incorporation of deuterium in position 3 of d-21m (4 for d-22m). Nonetheless, observation of the clean 1,2-hydride shift63 was rationalized by involvement of resonance intermediates xiv and xv (Scheme 10). Accordingly, more π-philic Au species (AuCl3 in ethereal solvents, as well as R3PAu(I)Cl catalysts) coordinate to the distal double bond of allene (xiii), activating it toward intramolecular nucleophilic attack of oxygen followed by tautomerization to form gold carbenoid species xv. The latter furnishes 2-bromofuran d-22m after subsequent 1,2-hydride shift.63
Scheme 9.
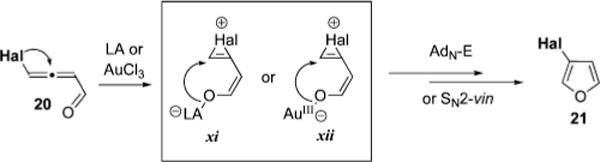
Proposed Halirenium Intermediates in the 1,2-Halogen Migration/Cycloisomerization Cascade
Scheme 10.
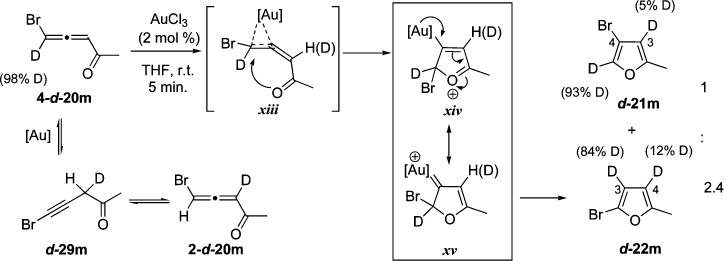
Deuterium Labeling Study of Bromoallenone 4-d-20m
As it was proposed for 1,2-halogen migration in haloallenones 20 in the presence of oxophilic catalysts (Scheme 9), 1,2-migration of alkyl/aryl group in allenylketones 25, which required employment of highly cationic metal triflates or strong Brønsted acids, could be, in turn, rationalized only via involvement of the similar intermediates xvi–xviii (Scheme 11). Thus,1,2-alkyl or -aryl migration in the intermediate Lewis acid-activated enone moiety of allenone 25, xvi,64 produces either vinyl cation xvii65 or phenonium intermediate xviii. Direct cyclization of xvii or, alternatively, sequence of either AdN-E or SN2-vin processes from xviii furnishes furan 26 (Scheme 11).
Scheme 11.
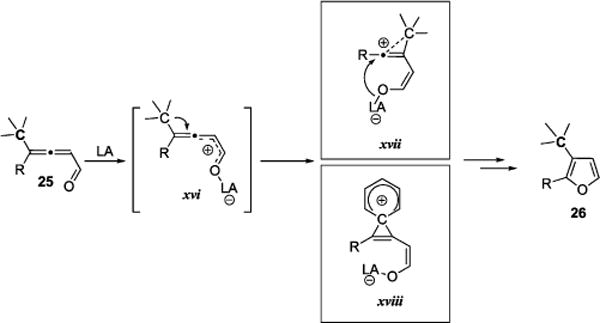
Proposed Cationic Intermediates in the 1,2-Alkyl/Aryl Migration/Cycloisomerization Cascade Triggered by Lewis Acid Catalysts
Taking into account the successful transformation of 25 into 26 employing cationic Au(I), Ag(I), and Cu(I) catalysts, we hypothesized that in the case of π-acids, migrative cascade cycloisomerization of allenone 25 follows the pathway analogous to that proposed for 1,2-halogen migration66 (Scheme 10) and involves similar to xiv and xv resonance metal-oxonium xix and carbenoid xx intermediates. Thus, sequence of 1,5-alkyl/aryl shift67 and metal elimination or direct 1,2-alkyl/aryl shift63 in xix or xx, respectively, produces furan 26 (Scheme 12).
Scheme 12.
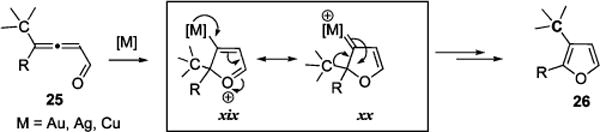
Proposed Intermediates in the Transition Metal-catalyzed 1,2-Alkyl/Aryl Migration/Cycloisomerization Cascade
Considering all the experimental data disclosed above, a generalized mechanism for the synthesis of furans involving 1,2-migration of different migrating groups is outlined in Scheme 13. It is proposed that a thermally induced and Cu-catalyzed 1,2-migration of chalcogenides (Y = SR and SeR) proceeds via paths A and B, respectively.68 Alternatively, Lewis or Brønsted acid-catalyzed cycloisomerization of allenones (X = O) involving 1,2-shifts of halogen (Y = Hal), alkyl, and aryl (Y = C) groups is postulated to follow path B, whereas carbophilic catalysts trigger reaction which proceeds through path C. Nevertheless, employment of transition metal catalysts in the 1,2-chalcogen migration/cycloisomerization cascade, such as Au(I), Au(III), Pd(II), and Pt(II),69 may involve a competitive π-system activation pathway C proceeding via 1,2-21 or 1,5-chalcogen migration in the carbenoid/oxonium intermediates 36/37.
Scheme 13.
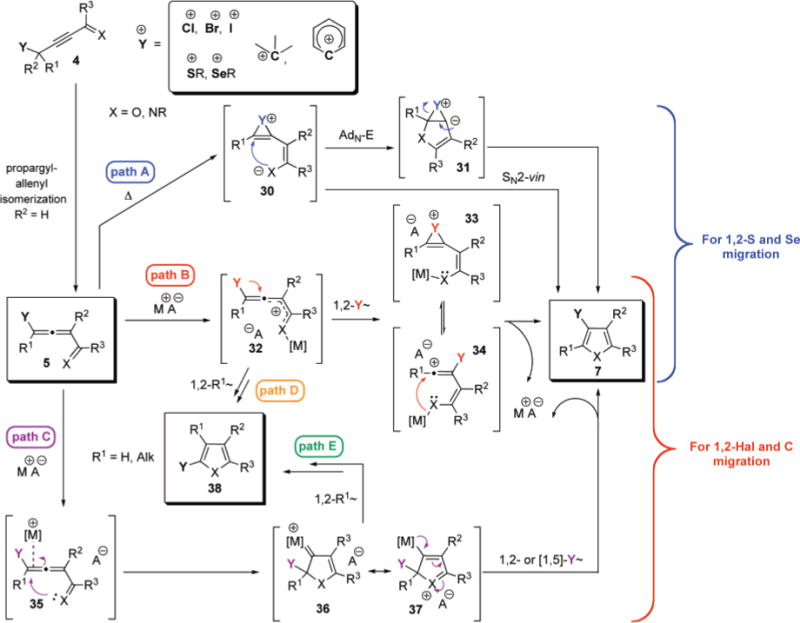
Generalized Mechanism for the Metal-Catalyzed Synthesis of Furans via Allene Intermediate Involving 1,2-Migration of Different Migrating Groups
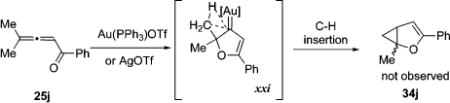
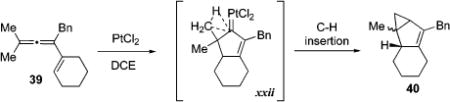
The observed competitive 1,2-hydrogen migration for thioalkynone 4 (R1, R2 = H, 8, Table 1, entries 2–5), and competing 1,2-migration of butyl group in selenoalkynone 4 (R1 = Bu, R2 = H, 16a, Table 3, entries 1–3), in case of π-philic catalysts, can be attributed to the 1,2-hydride or -alkyl shifts to the electrophilic center in 36/37 (Path E). Alternatively, 1,2-shifts65 of these groups can also occur through the activated enone intermediate 32 via equally feasible path D. In contrast to that discussed above, selective/competitive 1,2-hydrogen vs -halogen migration in haloallenones 5 (R1 = H, 20, Table 5), catalyzed by carbophilic gold catalysts, can only be rationalized via the path E. The observed migratory aptitude trends during 1,2-alkyl/aryl migration/cycloisomerization cascade strongly support predominant involvement of cationic intermediate represented by the resonance structure 37 over metal-carbenoid resonance structure 36 for Au and Ag triflate catalysts. Thus, the migratory aptitude of phenyl- vs methyl group (> 100:1) is in a good agreement with that reported in literature for the cationic rearrangements.70,71 In addition, no cyclopropanation product 34j was observed in the cycloisomerization of dimethylallenyl ketone 25j in the presence of Au(I) and Ag(I) catalysts, although this transformation proceeding via carbenoid intermediate xxii was reported48a to give fused cyclopropane 40 as a major product in the cycloisomerization of a carbocyclic analog of 25j, 39 (eq 5 and 6). Thus, although carbenoid intermediate, such as xxi or 37, and/or its attributed reactivity cannot be completely ruled out at this point for 1,2-alkyl/aryl migrative cyclization, it is considered to be substantially less likely.
 |
(5) |
 |
(6) |
Conclusion
In conclusion, a mild, efficient, and functional group-tolerant migration/cycloisomerization approach toward multisubstituted heterocycles has been developed. This cascade reaction has proven to be a powerful methodology toward diversely substituted heterocycles. The cycloisomerization approach is general: a variety of propargyl sulfides and selenides, as well as haloallenes or aryl- and alkylallenones, have been successfully employed to produce hetero-substituted furans, pyrroles, and even an indolizine in good to excellent yields. Moreover, regiodivergent conditions have been identified for cycloisomerization of bromo- and thioallenones to obtain regioisomeric 2-hetero substituted furans selectively. Mechanistic studies strongly support the involvement of an irenium type intermediate in all cases where migration occurs. Additionally, mechanistic studies indicate that propargyl chalcogenides undergo necessary isomerization into the corresponding allene during the cascade cycloisomerization. Even though the involvement of π-system activation pathway for certain transition metal-catalyzed cycloisomerizations of chalcogenoalkynones or -allenones could not be completely ruled out, it is considered as less likely. Facile cycloisomerization in the presence of cationic complexes, as well as observed migratory aptitude in the cycloisomerization of unsymmetrically substituted aryl- and alkylallenes, strongly supports electrophilic mechanism for this transformation.
Supplementary Material
Acknowledgments
The support of the National Institute of Health (GM-64444) and the National Science Foundation (CHE 0710749) is gratefully acknowledged.
Footnotes
Supporting Information Available: Experimental procedures and analytical and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.See for example:; (a) Bellina F, Rossi R. Tetrahedron. 2006;62:7213. [Google Scholar]; (b) Hou XL, Yang Z, Wong HNC. In: Progress in Heterocyclic Chemistry. Gribble GW, Gilcrist TL, editors. Vol. 15. Pergamon; Oxford: 2003. p. 167. [Google Scholar]; (c) Keay BA, Dibble PW. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 2. Elsevier; Oxford: 1997. p. 395. [Google Scholar]; (d) Donnelly DMX, Meegan MJ. In: Comprehensive Heterocyclic Chemistry. Katritzky AR, Rees CW, editors. Vol. 4. Pergamon; Oxford: 1984. p. 657. [Google Scholar]; (e) Dean FM. Naturally Occurring Oxygen Ring Compounds. Butterworths; London: 1963. p. 1. Chapter 1. [Google Scholar]
- 2.For most important examples, see:; (a) Fürstner A, Weintritt H. J Am Chem Soc. 1998;120:2817. [Google Scholar]; (b) Fürstner A. Angew Chem., Int Ed. 2003;42:3582. doi: 10.1002/anie.200300582. [DOI] [PubMed] [Google Scholar]; (c) Fürstner A, Reinecke K, Prinz H, Waldmann H. ChemBioChem. 2004;5:1575. doi: 10.1002/cbic.200400135. [DOI] [PubMed] [Google Scholar]; (d) Fürstner A, Grabowski EJ. Chem Bio Chem. 2001;2:706. doi: 10.1002/1439-7633(20010903)2:9<706::AID-CBIC706>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]; (e) Pettit GR, McNulty J, Herald DL, Doubek DL, Chapuis JC, Schmidt JM, Tackett LP, Boyd MR. J Nat Prod. 1997;60:180. doi: 10.1021/np9606106. [DOI] [PubMed] [Google Scholar]
- 3.(a) Miyata Y, Nishinaga T, Komatsu K. J Org Chem. 2005;70:1147. doi: 10.1021/jo048282z. [DOI] [PubMed] [Google Scholar]; (b) Heylen M, Van den Broeck K, Boutton C, van Beylen M, Persoons A, Samyn C. Eur Polym J. 1998;34:1453. [Google Scholar]; (c) Ferrero F, Napoli L, Tonin C, Varesano A. J Appl Polym Sci. 2006;102:4121. [Google Scholar]; (d) Venkatatraman S, Kumar R, Sankar J, Chandrashekar TK, Senhil K, Vijayan C, Kelling A, Senge MO. Chem.–Eur J. 2004;10:1423. doi: 10.1002/chem.200305558. [DOI] [PubMed] [Google Scholar]; (e) Facchetti A, Abbotto A, Beverina L, van der Boom ME, Dutta P, Evmenenko G, Pagani GA, Marks TJ. Chem Mater. 2003;15:1064. [Google Scholar]; (f) Zhang LZ, Chen CW, Lee CF, Wu CC, Luh TY. Chem Commun. 2002:2336. doi: 10.1039/b207489c. [DOI] [PubMed] [Google Scholar]; (g) Novak P, Müller K, Santhanum KSV, Haas O. Chem Rev. 1997;97:207. doi: 10.1021/cr941181o. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lee HK, Chan KF, Hui CW, Yim HK, Wu XW, Wong HNC. Pure Appl Chem. 2005;77:139. [Google Scholar]; (b) Wong HNC, Yu P, Yick CY. Pure Appl Chem. 1999;71:1041. [Google Scholar]; (c) Lipshutz BH. Chem Rev. 1986;86:795. [Google Scholar]
- 5.For recent reviews, see:; (a) König B. In: Product Class 9: Furans, in Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Maas G, editor. Georg Thieme; Stuttgart: 2001. pp. 183–285. [Google Scholar]; (b) Patil NT, Yamamoto Y. ARKIVOC. 2007;10:121. [Google Scholar]; (c) Kirsch SF. Org Biomol Chem. 2006;4:2076. doi: 10.1039/b602596j. [DOI] [PubMed] [Google Scholar]; (d) Brown RCD. Angew Chem., Int Ed. 2005;44:850. doi: 10.1002/anie.200461668. [DOI] [PubMed] [Google Scholar]; (e) Jeevanandam A, Ghule A, Ling YC. Curr Org Chem. 2002;6:841. [Google Scholar]; (f) Keay BA. Chem Soc Rev. 1999;28:209. [Google Scholar]; (g) Hou XL, Cheung HY, Hon TY, Kwan PL, Lo TH, Tong SY, Wong HNC. Tetrahedron. 1998;54:1955. [Google Scholar]; (h) Schröter S, Stock C, Bach T. Tetrahedron. 2005;61:2245. [Google Scholar]; (i) Schmuck C, Rupprecht D. Synthesis. 2007:3095. [Google Scholar]
- 6.For recent examples, see:; (a) Galliford CV, Scheidt KA. J Org Chem. 2007;72:1811. doi: 10.1021/jo0624086. [DOI] [PubMed] [Google Scholar]; (b) St Cyr DJ, Martin N, Arndtsen BA. Org Lett. 2007;9:449. doi: 10.1021/ol062773j. [DOI] [PubMed] [Google Scholar]; (c) Zhang J, Schmalz HG. Angew Chem., Int Ed. 2006;45:6704. doi: 10.1002/anie.200601252. [DOI] [PubMed] [Google Scholar]; (d) Kamijo S, Kanazawa C, Yamamoto Y. J Am Chem Soc. 2005;127:9260. doi: 10.1021/ja051875m. [DOI] [PubMed] [Google Scholar]; (e) Yao T, Zhang X, Larock RC. J Org Chem. 2005;70:7679. doi: 10.1021/jo0510585. [DOI] [PubMed] [Google Scholar]; (f) Larionov OV, de Meijere A. Angew Chem., Int Ed. 2005;44:5664. doi: 10.1002/anie.200502140. [DOI] [PubMed] [Google Scholar]; (g) Duan X-h, Liu X-y, Guo L-n, Liao M-c, Liu W-M, Liang Y-m. J Org Chem. 2005;70:6980. doi: 10.1021/jo050908d. [DOI] [PubMed] [Google Scholar]; (h) Lee KY, Lee MJ, Kim JN. Tetrahedron. 2005;61:8705. [Google Scholar]; (i) Jung CK, Wang JC, Krische MJ. J Am Chem Soc. 2004;126:4118. doi: 10.1021/ja049377l. [DOI] [PubMed] [Google Scholar]; (j) Dhawan R, Arndtsen BA. J Am Chem Soc. 2004;126:468. doi: 10.1021/ja039152v. [DOI] [PubMed] [Google Scholar]; (k) Nishibayashi Y, Yoshikawa M, Inada Y, Milton MD, Hidai M, Uemura S. Angew Chem., Int Ed. 2003;42:2681. doi: 10.1002/anie.200351170. [DOI] [PubMed] [Google Scholar]; (l) Braun RU, Zeitler K, Müller TJJ. Org Lett. 2001;3:3297. doi: 10.1021/ol0165185. [DOI] [PubMed] [Google Scholar]; (m) Brown CD, Chong JM, Shen L. Tetrahedron. 1999;55:14233. [Google Scholar]; (n) Shiraishi H, Nishitani T, Sakaguchi S, Ishii Y. J Org Chem. 1998;63:6234. doi: 10.1021/jo980435t. [DOI] [PubMed] [Google Scholar]; (o) Shindo M, Yoshimura Y, Hayashi M, Soejima H, Yoshikawa T, Matsumoto K, Shishido K. Org Lett. 2007;9:1963. doi: 10.1021/ol0705200. [DOI] [PubMed] [Google Scholar]; (p) Tejedor D, Gonzalez-Cruz D, Garcia-Tellado F, Marrero-Tellado JJ, Rodriguez ML. J Am Chem Soc. 2004;126:8390. doi: 10.1021/ja047396p. [DOI] [PubMed] [Google Scholar]
- 7.For catalytic approaches toward furans, see:; (a) Davies HML, Romines KR. Tetrahedron. 1988;44:3343. [Google Scholar]; (b) Heilbron IM, Jones ERH, Smith P, Weedon BCL. J Chem Soc. 1946:54. [Google Scholar]; (c) Marshall JA, DuBay WJ. J Org Chem. 1993;58:3435. [Google Scholar]; (d) Marshall JA, Bennett CE. J Org Chem. 1994;59:6110. [Google Scholar]; (e) Seiller B, Bruneau C, Dixneuf PH. Tetrahedron. 1995;51:13089. [Google Scholar]; (f) Gabriele B, Salerno G, dePascali F, Tomasi Sciano G, Costa M, Chiusoli GP. Tetrahedron Lett. 1997;38:6877. [Google Scholar]; (g) Gabriele B, Salerno G, Lauria E. J Org Chem. 1999;64:7687. doi: 10.1021/jo994013a. [DOI] [PubMed] [Google Scholar]; (h) Gabriele B, Salerno G, dePascali F, Costa M, Chiusoli GP. J Org Chem. 1999;64:7693. [Google Scholar]; (i) Hashmi ASK, Schwarz L, Choi JH, Frost TM. Angew Chem., Int Ed. 2000;39:2285. doi: 10.1002/1521-3773(20000703)39:13<2285::aid-anie2285>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]; (j) McDonald FE, Shultz CC. J Am Chem Soc. 1994;116:9363. [Google Scholar]; (k) Lo CY, Guo H, Lian JJ, Shen FW, Liu RS. J Org Chem. 2002;67:3930. doi: 10.1021/jo020004h. [DOI] [PubMed] [Google Scholar]; (l) Hashmi ASK, Sinha P. Adv Synth Catal. 2004;346:432. [Google Scholar]; (m) Marshall JA, DuBay WJ. J Am Chem Soc. 1992;114:1450. [Google Scholar]; (n) Marshall JA, Robinson ED. J Org Chem. 1990;55:3450. [Google Scholar]; (o) Marshall JA, Wang X. J Org Chem. 1991;56:960. [Google Scholar]; (p) Marshall JA, Wang X. J Org Chem. 1992;57:3387. [Google Scholar]; (q) Marshall JA, Bartley GS. J Org Chem. 1994;59:7169. [Google Scholar]; (r) Hashmi ASK, Schwarz L. Chem Ber./Recueil. 1997;130:1449. [Google Scholar]; (s) Hashmi ASK, Rupert TL, Knofel T, Bats JW. J Org Chem. 1997;62:7295. doi: 10.1021/jo970837l. [DOI] [PubMed] [Google Scholar]; (t) Ma S, Zhang J. Chem Commun. 2000:117. [Google Scholar]; (u) Ma S, Li L. Org Lett. 2000;2:941. doi: 10.1021/ol0055871. [DOI] [PubMed] [Google Scholar]; (v) Ma S, Gu Z, Yu Z. J Org Chem. 2005;70:6291. doi: 10.1021/jo0507441. [DOI] [PubMed] [Google Scholar]; (w) Sheng H, Lin S, Huang Y. Tetrahedron Lett. 1986;27:4893. [Google Scholar]; (x) Sheng H, Lin S, Huang Y. Synthesis. 1987:1022. [Google Scholar]; (y) Fukuda Y, Shiragami H, Utimoto K, Nozaki H. J Org Chem. 1991;56:5816. [Google Scholar]; (z) Wakabayashi Y, Fukuda Y, Shiragami H, Utimoto K, Nozaki H. Tetrahedron. 1985;41:3655. [Google Scholar]; (aa) Ma S, Zhang J. J Am Chem Soc. 2003;125:12386. doi: 10.1021/ja036616g. [DOI] [PubMed] [Google Scholar]; (ab) Ma S, Zhang J. Angew Chem., Int Ed. 2003;42:183. [Google Scholar]; (ac) Suhre MH, Reif M, Kirsch SF. Org Lett. 2005;7:3925. doi: 10.1021/ol0514101. [DOI] [PubMed] [Google Scholar]; (ad) Yao T, Zhang X, Larock RC. J Am Chem Soc. 2004;126:11164. doi: 10.1021/ja0466964. [DOI] [PubMed] [Google Scholar]; (ae) Patil NT, Wu H, Yamamoto Y. J Org Chem. 2005;70:4531. doi: 10.1021/jo050191u. [DOI] [PubMed] [Google Scholar]; (af) Aurrecoechea JM, Pérez E. Tetrahedron. 2004;60:4139. [Google Scholar]; (ag) Aurrecoechea JM, Pérez E. Tetrahedron Lett. 2003;44:3263. [Google Scholar]; (ah) Aurrecoechea JM, Pérez E. Tetrahedron Lett. 2001;42:3839. [Google Scholar]; (ai) Aurrecoechea JM, Pérez E, Solay M. J Org Chem. 2001;66:564. doi: 10.1021/jo0014257. [DOI] [PubMed] [Google Scholar]; (aj) Sniady A, Durham A, Morreale MS, Wheeler KA, Dembinski R. Org Lett. 2007;9:1175. doi: 10.1021/ol062539t. [DOI] [PubMed] [Google Scholar]; (ak) Diaz-Alvarez AE, Crochet P, Zablocka M, Duhayon C, Cadierno V, Gimeno J, Majoral JP. Adv Synth Catal. 2006;348:1671. [Google Scholar]; (al) Zhou CY, Chan PWH, Che CM. Org Lett. 2006;8:325. doi: 10.1021/ol052696c. [DOI] [PubMed] [Google Scholar]; (am) Kawai H, Oi S, Inoue Y. Heterocycles. 2006;67:101. [Google Scholar]; (an) Peng L, Zhang X, Ma M, Wang J. Angew Chem., Int Ed. 2007;46:1905. doi: 10.1002/anie.200604299. [DOI] [PubMed] [Google Scholar]
- 8.For catalytic approaches toward pyrroles, see:; (a) Yu M, Pagenkopf B. Org Lett. 2003;5:5099. doi: 10.1021/ol036180+. [DOI] [PubMed] [Google Scholar]; (b) Tseng HR, Luh TY. J Org Chem. 1997;62:4568. [Google Scholar]; (c) Tseng HR, Lee CF, Yang LM, Luh TY. J Org Chem. 1999;64:8582. [Google Scholar]; (d) Lee CF, Yang LM, Hwu TY, Feng AS, Tseng JC, Luh TY. J Am Chem Soc. 2000;122:4992. [Google Scholar]; (e) Schulte KE, Reisch J, Walker H. Chem Ber. 1965;98:98–103. [Google Scholar]; (f) Chalk AJ. Tetrahedron Lett. 1972;13:3487. [Google Scholar]; (g) Chalk AJ. Tetrahedron. 1974;30:1387. [Google Scholar]; (h) Ramathan B, Keith AJ, Armstrong D, Odom AL. Org Lett. 2004;6:2957. doi: 10.1021/ol0489088. [DOI] [PubMed] [Google Scholar]; (i) Utimoto K, Miura H, Nozaki H. Tetrahedron Lett. 1981;22:4277. [Google Scholar]; (j) Dieter RK, Yu H. Org Lett. 2001;3:3855. doi: 10.1021/ol016654+. [DOI] [PubMed] [Google Scholar]; (k) Gorin DJ, Davis NR, Toste FD. J Am Chem Soc. 2005;127:11260. doi: 10.1021/ja053804t. [DOI] [PubMed] [Google Scholar]; (l) Gabriele B, Salerno G, Fazio A, Bossio MR. Tetrahedron Lett. 2001;42:1339. [Google Scholar]; (m) Gabriele B, Salerno G, Fazio A. J Org Chem. 2003;68:7853. doi: 10.1021/jo034850j. [DOI] [PubMed] [Google Scholar]; (n) Nedolya NA, Brandsma L, Tarasova OA, Verkruijsse HD, Trofimov BA. Tetrahedron Lett. 1998;39:2409. [Google Scholar]; (o) Tarasova OA, Nedolya NA, Vvedensky V Yu, Brandsma L, Trofimov BA. Tetrahedron Lett. 1997;38:7241. [Google Scholar]; (p) Dahwan R, Arndsten BA. J Am Chem Soc. 2004;126:468. [Google Scholar]; (q) Smith CR, Bunnelle EM, Rhodes AJ, Sarpong R. Org Lett. 2007;9:1169. doi: 10.1021/ol0701971. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Binder JT, Kirsch SF. Org Lett. 2006;8:2151. doi: 10.1021/ol060664z. [DOI] [PubMed] [Google Scholar]; (s) Istrate FM, Gagosz F. Org Lett. 2007;9:3181. doi: 10.1021/ol0713032. [DOI] [PubMed] [Google Scholar]
- 9.Kel’in AV, Sromek AW, Gevorgyan V. J Am Chem Soc. 2001;123:2074. doi: 10.1021/ja0058684. [DOI] [PubMed] [Google Scholar]
- 10.Kel’in AV, Gevorgyan V. J Org Chem. 2002;67:95. doi: 10.1021/jo010832v. [DOI] [PubMed] [Google Scholar]
- 11.See Supporting Information for details.
- 12.Kim JT, Kel’in AV, Gevorgyan V. Angew Chem, Int Ed. 2003;42:98. doi: 10.1002/anie.200390064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sromek AW, Rubina M, Gevorgyan V. J Am Chem Soc. 2005;127:10500. doi: 10.1021/ja053290y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudnik AS, Gevorgyan V. Angew Chem, Int Ed. 2007;46:5195. doi: 10.1002/anie.200701128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) For, recent review on 1,2-sulfur migrations, see:; Sromek AW, Gevorgyan V. Topics Curr Chem. 2007;274:77. [Google Scholar]; (b) For, 1,2-sulfur migration in the synthesis of pyrroles, see:; Peng L, Zhang X, Ma J, Zhong Z, Wang J. Org Lett. 2007;9:1445. doi: 10.1021/ol070205d. [DOI] [PubMed] [Google Scholar]; (c) For a formal 1,4-sulfur migration in the synthesis of furans, see ref 7an.
- 16.For most recent work, see:; (a) Fox DJ, House D, Warren S. Angew Chem., Int Ed. 2002;41:2462. doi: 10.1002/1521-3773(20020715)41:14<2462::AID-ANIE2462>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; (b) Baldwin IC, Briner P, Eastgate MD, Fox DG, Warren S. Org Lett. 2002;4:4381. doi: 10.1021/ol0268384. [DOI] [PubMed] [Google Scholar]; (c) Caggiano L, Davies J, Fox DJ, Moody DC, Warren S. Chem Commun. 2003:1648. [Google Scholar]; (d) Caggiano L, Fox DJ, Warren S. Chem Commun. 2002:2528. [Google Scholar]; (e) Caggiano L, Davies J, Fox DJ, Moody DC, Warren S. Chem Commun. 2003:1650. [Google Scholar]; (f) Fox DJ, Morley TJ, Taylor S, Warren S. Org Biomol Chem. 2005;3:1369. doi: 10.1039/b503068b. [DOI] [PubMed] [Google Scholar]; (g) House D, Kerr F, Warren S. J Chem Soc Perkin Trans. 2002;1:2652. [Google Scholar]; (h) Carlisle J, Fox DJ, Warren S. Chem Commun. 2003:269. doi: 10.1039/b308609g. [DOI] [PubMed] [Google Scholar]; (i) Eastgate MD, Fox DJ, Morley TJ, Warren S. Synthesis. 2002:2124. [Google Scholar]
- 17.(a) Gruttadauria M, Aprile C, D’Anna F, Lo Meo P, Riela S, Noto R. Tetrahedron. 2001;57:6815. [Google Scholar]; (b) Gruttadauria M, Noto R. Terahedron Lett. 1999;40:8477. [Google Scholar]; (c) Gruttadauria M, Lo Meo P, Noto R. Targets Heterocycl Syst. 2001;5:31. [Google Scholar]; (d) Rouessac F, Zamarlik H. Tetrahedron Lett. 1981:2643. [Google Scholar]
- 18.(a) Carmona O, Greenhouse R, Landeros R, Muchowski JM. J Org Chem. 1980;45:5336. [Google Scholar]; (b) DeSales J, Greenhouse R. J Org Chem. 1982;47:3668. [Google Scholar]; (c) Plate R, Nivard RJF, Ottenheijm HCJ. Tetrahedron. 1986;42:4503. [Google Scholar]; (d) Plate R, Ottenheijm HCJ. Tetrahedron. 1986;42:4511. [Google Scholar]; (e) Hamel P, Preville P. J Org Chem. 1996;61:1573. doi: 10.1021/jo951420n. [DOI] [PubMed] [Google Scholar]; (f) Hamel P. J Org Chem. 2002;67:2854. doi: 10.1021/jo0109220. [DOI] [PubMed] [Google Scholar]; (g) Hamel P. Tetrahedron Lett. 1997;38:8473. [Google Scholar]
- 19.(a) Xu F, Shi W, Wang J. J Org Chem. 2005;70:4191. doi: 10.1021/jo050109v. [DOI] [PubMed] [Google Scholar]; (b) Jiao L, Zhang Q, Liang Y, Zhang S, Xu J. J Org Chem. 2006;71:815. doi: 10.1021/jo052135z. [DOI] [PubMed] [Google Scholar]
- 20.For sulfur migration in carbohydrates, see:; (a) Smoliakova IP. Curr Org Chem. 2000;4:589. [Google Scholar]; (b) Johnston BD, Pinto BM. J Org Chem. 2000;65:4607. doi: 10.1021/jo000226k. [DOI] [PubMed] [Google Scholar]; (c) Yu B, Yang Z. Org Lett. 2001;3:377. doi: 10.1021/ol006894+. [DOI] [PubMed] [Google Scholar]; (d) Yang Z, Yu B. Carbohydrate Res. 2001;333:105. doi: 10.1016/s0008-6215(01)00124-0. [DOI] [PubMed] [Google Scholar]; (e) Yu B, Yang Z. Tetrahedron Lett. 2000;41:2961. [Google Scholar]; (f) Yang Z, Cao H, Hu J, Shan R, Yu B. Tetrahedron. 2003;59:249. [Google Scholar]; (g) Yu B, Wang P. Org Lett. 2002;4:1919. doi: 10.1021/ol0259286. [DOI] [PubMed] [Google Scholar]; (h) Viso A, Poopeiko N, Castillon S. Tetrahedron Lett. 2000;41:407. [Google Scholar]; (i) Nicolaou KC, Ladduwahetty T, Randall JL, Chucholowski A. J Am Chem Soc. 1986;108:2466. doi: 10.1021/ja00269a066. [DOI] [PubMed] [Google Scholar]; (j) Nicolaou KC, Fylaktakidou KC, Monenschein H, Li Y, Wetershausen B, Mitchell HJ, Wei H-x, Guntupalli P, Hepworth D, Sugita K. J Am Chem Soc. 2003;125:15433. doi: 10.1021/ja0304953. [DOI] [PubMed] [Google Scholar]; (k) Nicolaou KC, Rodriguez RM, Mitchell HJ, Suzuki H, Fylaktakidou KC, Baudoin O, van Delft FL. Chem–Eur J. 2000;6:3095. doi: 10.1002/1521-3765(20000901)6:17<3095::aid-chem3095>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]; (l) Pratt MR, Bertozzi CR. J Am Chem Soc. 2003;125:6149. doi: 10.1021/ja029346v. [DOI] [PubMed] [Google Scholar]; (m) Liptak A, Sajtos F, Janossy L, Gehle D, Szilagyi L. Org Lett. 2003;5:3671. doi: 10.1021/ol0353518. [DOI] [PubMed] [Google Scholar]; (n) Maiareanu C, Kanai A, Wiebel JM, Pale P. J Carb Chem. 2005;24:831. [Google Scholar]
- 21.For selenium migration in carbohydrates, see:; (a) Nicolaou KC, Mitchell HJ, Fylaktakidou KC, Suzuki H, Rodriguez RM. Angew Chem., Int Ed. 2000;39:1089. doi: 10.1002/(sici)1521-3773(20000317)39:6<1089::aid-anie1089>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Fylaktakidou KC, Mitchell HJ, van Delft FL, Rodriguez RM, Conley SR, Jin Z. Chem Eur J. 2000;6:3166. doi: 10.1002/1521-3765(20000901)6:17<3166::aid-chem3166>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (c) Poopeiko N, Fernandez R, Barrena MI, Castillon S. J Org Chem. 1999;64:1375. See also ref. 16h. [Google Scholar]
- 22.(a) Plate R, Nivard RJF, Ottenheijm HCJ. Tetrahedron. 1986;42:4503. [Google Scholar]; (b) Hamel P. J Org Chem. 2002;67:2854. doi: 10.1021/jo0109220. [DOI] [PubMed] [Google Scholar]; (c) Volontereo A, Zanda M, Bravo P, Fronza G, Cavicchio G, Crucianelli M. J Org Chem. 1997;62:8031. doi: 10.1021/jo970863j. [DOI] [PubMed] [Google Scholar]; (d) Peng L, Zhang X, Zhang S, Wang J. J Org Chem. 2007;72:1192. doi: 10.1021/jo0618674. [DOI] [PubMed] [Google Scholar]
- 23.For the base- or transition metal-base-assisted propargyl-allenyl isomer-ization, see, for example:; (a) Garratt PJ, Neoh SB. J Am Chem Soc. 1975;97:3255. [Google Scholar]; (b) Oku M, Arai S, Katayama K, Shioiri T. Synlett. 2000:493. [Google Scholar]; (c) Yeo SK, Shiro M, Kanematsu K. J Org Chem. 1994;59:1621. [Google Scholar]; (d) Watanabe S-i, Miura Y, Iwamura T, Nagasawa H, Kataoka T. Tetrahedron Lett. 2007;48:813. [Google Scholar]; (e) Ma S, Hao X, Meng X, Huang X. J Org Chem. 2004;69:5720. doi: 10.1021/jo049593c. [DOI] [PubMed] [Google Scholar]; (f) Wang Y, Burton DJ. Org Lett. 2006;8:5295. doi: 10.1021/ol0620850. [DOI] [PubMed] [Google Scholar]; (g) Lepore SD, Khoram A, Bromfield DC, Cohn P, Jairaj V, Silvestri MA. J Org Chem. 2005;70:7443. doi: 10.1021/jo051040u. [DOI] [PubMed] [Google Scholar]
- 24.For stable thiirenium ions, see for example:; (a) Lucchini V, Modena G, Valle G, Capozzi G. J Org Chem. 1981;46:4720. [Google Scholar]; (b) Lucchini V, Modena G, Pasquato L. Gazz Chim Ital. 1997;127:177. [Google Scholar]
- 25.(a) Lucchini V, Modena G, Pasquato L. J Am Chem Soc. 1993;115:4527. [Google Scholar]; (b) Destro R, Lucchini V, Modena G, Pasquato L. J Org Chem. 2000;65:3367. doi: 10.1021/jo991731o. [DOI] [PubMed] [Google Scholar]
- 26.For deprotection of N-Bu-t-group in pyrroles, see:; (a) Leroy J, Wakselman C. Tetrahedron Lett. 1994;35:8605. [Google Scholar]; (b) La Porta P, Capuzzi L, Bettarini F. Synthesis. 1994;3:287. [Google Scholar]
- 27.For deprotection of N-Tr-group in pyrroles, see:; Chadwick DJ, Hodgson ST. J Chem Soc Perkin Trans. 1983;1:93. [Google Scholar]
- 28.For deprotection of analogous group in pyrroles, see:; Roder E, Wiedenfeld H, Bourauel T. Liebigs Ann Chem. 1985:1708. [Google Scholar]
- 29.Abele E, Popelis J, Shestakova I, Domracheva I, Arsenyan P, Lukevics E. Chem Heterocycl Comp. 2004;40:742. [Google Scholar]
- 30.(a) Budisa N, Pal PP, Alefelder S, Birle P, Krywcun T, Rubini M, Wenger W, Bae JH, Steiner T. Biol Chem. 2004;385:191. doi: 10.1515/BC.2004.038. [DOI] [PubMed] [Google Scholar]; (b) Bae JH, Alefelder S, Kaiser JT, Friedrich R, Moroder L, Huber R, Budisa N. J Mol Biol. 2001;309:925. doi: 10.1006/jmbi.2001.4699. [DOI] [PubMed] [Google Scholar]; (c) Boles JO, Henderson J, Hatch D, Silks LA. Biochem Biophys Res Commun. 2002;298:257. doi: 10.1016/s0006-291x(02)02438-5. [DOI] [PubMed] [Google Scholar]; (d) Welch M, Phillips RS. Bioorg Med Chem Lett. 1999;9:637. doi: 10.1016/s0960-894x(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 31.(a) Nicolau KC. Tetrahedron. 1981;37:4097. [Google Scholar]; (b) Nicolaou KC, Seitz SP, Sipio WJ, Blount JF. J Am Chem Soc. 1979;101:3884. [Google Scholar]; (c) Clive DLJ, Chittattu G. J Chem Soc Chem Commun. 1977:484. [Google Scholar]; (d) Aprile C, Gruttadauria M, Amato ME, D’Anna F, Lo Meo P, Riela S, Noto R. Tetrahedron. 2003;59:2241. [Google Scholar]; (e) Tiecco M, Testaferri L, Temperini A, Bagnoli L, Marini F, Santi C. Synlett. 2003:655. [Google Scholar]; (f) Tiecco M, Testaferri L, Bagnoli L. Tetrahedron. 1996;52:6811. [Google Scholar]; (g) Van de Weghe P, Bourg S, Eustache J. Tetrahedron. 2003;59:7365. [Google Scholar]; (h) Denmark SE, Edwards MG. J Org Chem. 2006;71:7293. doi: 10.1021/jo0610457. [DOI] [PubMed] [Google Scholar]; (i) Back TG, Moussa Z, Parvez M. J Org Chem. 2002;67:499. doi: 10.1021/jo016061c. [DOI] [PubMed] [Google Scholar]; (j) Wang X, Houk KN, Spichty M, Wirth T. J Am Chem Soc. 1999;121:8567. [Google Scholar]; (k) Huang Q, Hunter JA, Larock RC. J Org Chem. 2002;67:3437. doi: 10.1021/jo020020e. [DOI] [PubMed] [Google Scholar]; Larock RC. J Org CHem. 2002;67:1905. doi: 10.1021/jo011016q. [DOI] [PubMed] [Google Scholar]; (l) Pannecoucke X, Outurquin F, Paulmier C. Eur J Org Chem. 2002:995. [Google Scholar]; (m) Berthe B, Outurquin F, Paulmier C. Tetrahedron Lett. 1997;38:1393. [Google Scholar]; (n) Jones AD, Knight DW, Redfern AL, Gilmore J. Tetrahedron Lett. 1999;40:3267. [Google Scholar]; (o) Jones AD, Redfern AL, Knight DW, Morgan IR, Williams AC. Tetrahedron. 2006;62:9247. [Google Scholar]
- 32.D’Onofrio F, Margarita R, Parlanti L, Pernazza D, Piancatelli G. Tetrahedron. 1997;53:15843. [Google Scholar]
- 33.(a) Hartke K, Wendebourg HH. Liebigs Ann Chem. 1989;5:415. [Google Scholar]; (b) Hartke K, Wendebourg HH. Heterocycles. 1988;27:639. [Google Scholar]
- 34.(a) Agenas LB, Lindgren B. Arkiv foer Kemi. 1968;29:479. [Google Scholar]; (b) Agenas LB, Lindgren B. Arkiv foer Kemi. 1967;28:145. [Google Scholar]; (c) Chierici L. Farm Ed Sci. 1953;8:156. [Google Scholar]; (d) Vvedensky V Yu, Brandsma L, Shtefan ED, Trofimov BA. Tetrahedron. 1997;53:13079. [Google Scholar]; (e) Vvedensky V Yu, Brandsma L, Shtefan ED, Trofimov BA. Tetrahedron. 1997;53:16783. See also ref 28. [Google Scholar]
- 35.It should be mentioned, however, that attempts to cycloisomerize primary propargyl selenides led to formation of detectable amounts of diphenyl diselenide only, probably due to the lower stability of the less substituted allene intermediate 19.
- 36.For scattered reports on non-selective halogen migration in unsaturated systems, see for example:; (a) Bunnett JF. Acc Chem Res. 1972;54:140. [Google Scholar]; (b) Morton HE, Leanna MR. Tetrahedron Lett. 1993;34:4481. [Google Scholar]; (c) Samuel SP, Nin T-q, Erickson KL. J Am Chem Soc. 1989;111:1429. [Google Scholar]
- 37.Miura T, Iwasawa N. J Am Chem Soc. 2002;124:518. doi: 10.1021/ja0113091. [DOI] [PubMed] [Google Scholar]
- 38.Mamane V, Hannen P, Fürstner A. Chem.–Eur J. 2004;10:4556. doi: 10.1002/chem.200400220. [DOI] [PubMed] [Google Scholar]
- 39.Shen HC, Pal S, Lian JJ, Liu RS. J Am Chem Soc. 2003;125:15762. doi: 10.1021/ja0379159. [DOI] [PubMed] [Google Scholar]
- 40.For examples on electrophilic halogenations of furans, see:; (a) Keegstra MA, Klomp AJA, Brandsma L. Synth Commun. 1990;20:3371. [Google Scholar]; (b) Verkruijsse HD, Keegstra MA, Brandsma L. Synth Commun. 1989;19:1047. [Google Scholar]; (c) Song ZZ, Wong HNC. Liebigs Ann Chem. 1994;1:29. [Google Scholar]; (d) Yang Y, Wong HNC. Tetrahedron. 1994;50:9583. [Google Scholar]; (e) Kondo Y, Shilai M, Uchiyama M, Sakamoto T. J Am Chem Soc. 1999;11:3539. [Google Scholar]; (f) Gilman H, Mallory HE, Wright GF. J Am Chem Soc. 1932;54:733. [Google Scholar]; (g) Dickman DA, Ku YY, Morton HE, Chemburkar SR, Patel HH, Thomas A, Plata DJ, Sawick DP. Tetrahedron: Asymm. 1997;8:1791. [Google Scholar]; (h) Aiello E, Dattolo G, Cirrincione G, Almerico AM, D’Asdia I. J Heterocycl Chem. 1982;19:977. [Google Scholar]; (i) Belen’kii LI, Gromova GP, YaL Gol’dfarb. Chem Heterocycl Comp. 1978:246. [Google Scholar]; (j) Oleinik AF, Solov’ena NP, Turchin KF, Sheinker YuN, Novitskii KYu, Smirnova EN, Dozorova EN. Chem Heterocycl Comp. 1980:334. [Google Scholar]; (k) Belen’kii LI, Gromova GT, Gol’dfarb Ya L. Chem Heterocycl Comp. 1975:1249. [Google Scholar]; (l) Nolan SM, Cohen T. J Org Chem. 1981;46:2473. [Google Scholar]; (m) Nazarova ZN, Babaev Yu A, Umanskaya LG. Chem Heterocycl Comp. 1969;5:17. [Google Scholar]; (n) Bures EJ, Keay BA. Tetrahedron Lett. 1988;29:1247. [Google Scholar]; (o) Bures E, Nieman JA, Yu S, Spinazze PG, Bontront JLJ, Hunt IR, Rauk A, Keay BA. J Org Chem. 1997;62:8750. [Google Scholar]; (p) Carpenter AJ, Chadwick DJ. J Org Chem. 1985;50:4362. [Google Scholar]; (q) Kondo Y, Shilai M, Uchiyama M, Sakamoto T. J Am Chem Soc. 1999;121:3539. [Google Scholar]
- 41.For halogen induced cyclizations, see:; (a) Bew SP, Knight DW. Chem Commun. 1996:1007. [Google Scholar]; (b) El-Taeb GMM, Evans AB, Jones S, Knight DW. Tetrahedron Lett. 2001;42:5945. [Google Scholar]; (c) Rao MS, Esho N, Sergeant C, Dembinski R. J Org Chem. 2003;68:6788. doi: 10.1021/jo0345648. [DOI] [PubMed] [Google Scholar]; (d) Sniady A, Wheeler KA, Dembinski R. Org Lett. 2005;7:1769. doi: 10.1021/ol050372i. [DOI] [PubMed] [Google Scholar]; (e) Schultz-Fademrecht C, Zimmerman M, Frohlich R, Hoppe D. Synlett. 2003:1969. [Google Scholar]; (f) Obrecht D. Helv Chim Acta. 1989;72:447. [Google Scholar]; (g) Reich HJ, Olson RE. J Org Chem. 1987;52:2315. [Google Scholar]; (h) Gorzynski M, Rewicki D. Liebigs Ann Chem. 1986:625. [Google Scholar]
- 42.For examples of cyclocondensation of halogen-containing precursors, see:; (a) Tanabe Y, Wakimura K, Nishii Y, Muroya Y. Synthesis. 1996:388. [Google Scholar]; (b) Mee SPH, Lee V, Baldwin JE, Cowley A. Tetrahedron. 2004;60:3695. [Google Scholar]; (c) Kraus GA, Wang X. Synth Commun. 1998;28:1093. [Google Scholar]; (d) Reich HD, Olson RE. J Org Chem. 1987;52:2315. [Google Scholar]
- 43.Bromoallene 20a contained trace to notable amounts of bromopropargyl ketone, from which it was obtained. Under reaction conditions bromopropargyl ketone underwent rapid isomerization to 20a. The same applies to iodoallenes 20i and 20k (see Table 6). Facile propargyl–allenyl isomerization of propargyl ketones in the presence of gold catalyst was previously observed; see ref 7i.
- 44.(a) See ref 7n.; Sromek AW, Kel’in AV, Gevorgyan V. Angew Chem., Int Ed. 2004;43:2280. doi: 10.1002/anie.200353535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.For recent reviews on Au-catalyzed reactions, see:; (a) Hoffmann-Röder A, Krause N. Org Biomol Chem. 2005;3:387. doi: 10.1039/b416516k. [DOI] [PubMed] [Google Scholar]; (b) Hashmi ASK. Gold Bull. 2004;37:51. [Google Scholar]; (c) Hashmi ASK. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]; (d) Hashmi ASK, Hutchings GJ. Angew Chem., Int Ed. 2006;45:7896. doi: 10.1002/anie.200602454. [DOI] [PubMed] [Google Scholar]; (e) Hashmi ASK. Angew Chem., Int Ed. 2005;44:6990. doi: 10.1002/anie.200502735. [DOI] [PubMed] [Google Scholar]; For selected examples of Au-catalyzed synthesis of heterocycles, see:; (f) Yao T, Zhang X, Larock RC. J Am Chem Soc. 2004;126:11164. doi: 10.1021/ja0466964. [DOI] [PubMed] [Google Scholar]; (g) Zhang L, Kozmin SA. J Am Chem Soc. 2005;127:6962. doi: 10.1021/ja051110e. [DOI] [PubMed] [Google Scholar]; (h) Hoffmann-Röder A, Krause N. Org Lett. 2001;3:2537. doi: 10.1021/ol016205+. [DOI] [PubMed] [Google Scholar]; For Au-catalyzed carbocyclizations, see for example:; (i) Fürstner A, Hannen P. Chem Comm. 2004:2546. doi: 10.1039/b412354a. [DOI] [PubMed] [Google Scholar]; (j) Shi X, Gorin DJ, Toste FD. J Am Chem Soc. 2005;127:5802. doi: 10.1021/ja051689g. [DOI] [PubMed] [Google Scholar]; (k) Luzung MR, Markham JP, Toste FD. J Am Chem Soc. 2004;126:10858. doi: 10.1021/ja046248w. [DOI] [PubMed] [Google Scholar]; (l) Zhang L, Kozmin SA. J Am Chem Soc. 2004;126:11806. doi: 10.1021/ja046112y. [DOI] [PubMed] [Google Scholar]; (m) Nieto-Oberhuber C, Lopez S, Echavarren AM. J Am Chem Soc. 2005;127:6178. doi: 10.1021/ja042257t. [DOI] [PubMed] [Google Scholar]; See also ref. 38.; (n) Gorin DJ, Toste FD. Nature. 2007;446:395. doi: 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]; (o) Fürstner A, Davies PW. Angew Chem., Int Ed. 2007;46:3410. doi: 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- 46.For examples of 1,2-shift in carbenoids, see:; (a) Xiao F, Wang J. J Org Chem. 2006;71:5789. doi: 10.1021/jo0605391. and references therein. [DOI] [PubMed] [Google Scholar]; (b) Markham JP, Staben ST, Toste FD. J Am Chem Soc. 2005;127:9708. doi: 10.1021/ja052831g. [DOI] [PubMed] [Google Scholar]; (c) Jiménez-Núñez E, Claverie CK, Nieto-Oberhuber C, Echavarren AM. Angew Chem., Int Ed. 2006;45:5452. doi: 10.1002/anie.200601575. [DOI] [PubMed] [Google Scholar]; (d) Kirsch SF, Binder JT, Crone B, Duschek A, Haug TT, Liébert C, Menz H. Angew Chem., Int Ed. 2007;46:2310. doi: 10.1002/anie.200604544. [DOI] [PubMed] [Google Scholar]
- 47.For general reviews, see:; (a) Ducrot PH. One or more CH and/or CC bond(s) formed by rearrangement. In: Katritzky AR, Taylor RJK, editors. Comprehensive Organic Functional Group Transformations II. Vol. 1. Elsevier; Oxford, UK: 2005. p. 375. [Google Scholar]; (b) Constantieux T, Rodriguez J. Synthesis by fragmentation and rearrangement. In: Cossy J, editor. Science of Synthesis. Vol. 26. Thieme; New York: 2005. p. 413. [Google Scholar]; (c) Pattenden G. Carbon-Carbon σ-Bond Formation: Rearrangement Reactions. In: Trost BM, Fleming I, editors. Comprehensive organic synthesis: selectivity, strategy, and efficiency in modern organic chemistry. Vol. 3. Pergamon Press; New York: 1991. p. 705. [Google Scholar]
- 48.For a synthesis of carbocycles involving 1,2-alkyl shift to carbenoid center in allenes, see:; (a) Funami H, Kusama H, Iwasawa N. Angew Chem., Int Ed. 2006;46:909. doi: 10.1002/anie.200603986. [DOI] [PubMed] [Google Scholar]; (b) Lee JH, Toste FD. Angew Chem., Int Ed. 2007;46:912. doi: 10.1002/anie.200604006. [DOI] [PubMed] [Google Scholar]
- 49.(a) For a synthesis of dehydrofuranones via 1,2-alkyl shift, analogous to a formal ketol rearrangement, see:; Kirsch SF, Binder JT, Liébert C, Menz H. Angew Chem., Int Ed. 2006;45:5878. doi: 10.1002/anie.200601836. and references therein. [DOI] [PubMed] [Google Scholar]; See, also:; (b) Crone B, Kirsch SF. J Org Chem. 2007;72:5435. doi: 10.1021/jo070695n. [DOI] [PubMed] [Google Scholar]
- 50.There has been a recent discussion on the role of Brønsted acids in homogenous transition metal-catalyzed reactions. For the most relevant references, see:; (a) Hashmi ASK. Catal Today. 2007;122:211. [Google Scholar]; (b) Li Z, Zhang J, Brouwer C, Yang CG, Reich NW, He C. Org Lett. 2006;8:4175. doi: 10.1021/ol0610035. [DOI] [PubMed] [Google Scholar]; (c) Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org Lett. 2006;8:4179. doi: 10.1021/ol061174+. [DOI] [PubMed] [Google Scholar]; (d) Rhee JU, Krische MJ. Org Lett. 2005;7:2493. doi: 10.1021/ol050838x. [DOI] [PubMed] [Google Scholar]
- 51.For, use of TTBP as a TfOH scavenger, see for example:; Crich D, Vinogradova O. J Org Chem. 2006;71:8473. doi: 10.1021/jo061417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.See mechanistic discussion below and refs 70 and 71.
- 53.For the most recent reviews on Nazarov cyclization and related transformations, see:; (a) Frontier AJ, Collison C. Tetrahedron. 2005;61:7577. and references therein. [Google Scholar]; (b) Pellissier H. Tetrahedron. 2005;61:6479. and references therein. [Google Scholar]; (c) Tius MA. Acc Chem Res. 2003;36:284. doi: 10.1021/ar0200394. [DOI] [PubMed] [Google Scholar]; (d) Tius MA. Eur J Org Chem. 2005:2193. [Google Scholar]; See also:; (e) Hashmi ASK, Bats JW, Choi J-H, Schwarz L. Tetrahedron Lett. 1998;39:7491. [Google Scholar]
- 54.For the cuprate-assisted transformation of propargylic thioacetals into allenyl copper species, see: ref 8c and d.
- 55.(a) Reich HJ, Shah SK, Gold PM, Olson RE. J Am Chem Soc. 1981;103:3112. [Google Scholar]; (b) Reich HJ, Shah SK. J Am Chem Soc. 1977;99:263. [Google Scholar]; (c) Reich HJ, Gold PM, Chow F. Tetrahedron Lett. 1979:4433. [Google Scholar]
- 56.The more facile propargyl-allenyl isomerization for selenoalkynones is well supported by notable cycloisomerization of 16a under thermal conditions in the absence of base and Cu-catalyst (Table 3, entry 4).
- 57.(a) Schmid GH, Garratt DG. Tetrahedron Lett. 1975:3991. [Google Scholar]; (b) Poleschner H, Seppelt K. J Chem Soc Perkin Trans 1. 2002;23:2668. [Google Scholar]
- 58.It should be noted that involvement of any possible ionization pathways during cycloisomerization of thioalkynones 12 was ruled out by the absence of scrambling for alkyl- and arylthio- groups in crossover experiment.
- 59.For halirenium species, see for example:; (a) Noguchi M, Okada H, Watanabe M, Okuda K, Nakamura O. Tetrahedron. 1996;52:6581. [Google Scholar]; (b) Lucchini V, Modena G, Pasquato L. J Am Chem Soc. 1995;117:2297. [Google Scholar]
- 60.Yamamoto Y. J Org Chem. 2007;72:7817. doi: 10.1021/jo070579k. [DOI] [PubMed] [Google Scholar]
- 61.This is in striking contrast to cycloisomerization of alkynyl imines and ketones, where significant loss of deuterium was observed; see refs 9 and 12.
- 62.Decrease in regioselectivity of deuterium vs bromine migration is explained by the isotope effect analogous to that observed by Hashmi in the Pd-catalyzed cycloisomerization of allenyl ketones; see ref 7s.
- 63.For 1,2-hydride shift in Au- and Pt-carbenoid intermediates, see for example:; (a) Fürstner A, Stelzer F, Szillat H. J Am Chem Soc. 2001;123:11863. doi: 10.1021/ja0109343. [DOI] [PubMed] [Google Scholar]; (b) Mamane V, Gress T, Krause H, Fürstner A. J Am Chem Soc. 2004;126:8654. doi: 10.1021/ja048094q. [DOI] [PubMed] [Google Scholar]; See also refs 8k, 45h, and 48a,b.
- 64.For activation of enone moiety by Lewis acids see, for example:; (a) Childs RF, Mulholland DL, Nixon A. Can J Chem. 1982;60:801. [Google Scholar]; (b) Schwier T, Gevorgyan V. Org Lett. 2005;7:5191. doi: 10.1021/ol0521026. [DOI] [PubMed] [Google Scholar]
- 65.For examples of 1,2-shift in vinyl cations, see:; (a) Capozzi G, Lucchini V, Marcuzzi F, Melloni G. Tetrahedron Lett. 1976;17:717. [Google Scholar]; (b) Jaeckel KP, Hanack M. Tetrahedron Lett. 1974;15:1637. [Google Scholar]
- 66.See also ref 7q.
- 67.(a) Miller B. J Am Chem Soc. 1970;92:432. [Google Scholar]; (b) Dolbier WR, Anapolle KE, McCullagh L, Matsui K, Riemann JM, Rolison D. J Org Chem. 1979;44:2845. [Google Scholar]; (c) Oda M, Breslow R. Tetrahedron Lett. 1973;14:2537. [Google Scholar]
- 68.A control experiment, where thioalkynone 8 was subjected to the prolonged cycloisomerization conditions and monitored by GC for changes in distribution of products 10 and 11, ruled out any routes involving intraannular 1,2-sulfur migration20 from 2-thio- to 3-thiofuran after assembly of the furan core.
- 69.For Pd-carbenoid species in the synthesis of furans, see ref 7s. For Pd-catalyzed rearrangement see:; (a) Rautenstrauch V, Burger U, Wirthner P. Chimia. 1985;39:225. [Google Scholar]; (b) Rautenstrauch V. J Org Chem. 1984;49:950. [Google Scholar]; (c) Kataoka H, Watanabe K, Goto K. Tetrahedron Lett. 1990;31:4181. [Google Scholar]; (d) Mahrwald R, Schick H. Angew Chem., Int Ed Engl. 1991;30:593. [Google Scholar]; (e) Kato K, Yamamoto Y, Akita YH. Tetrahedron Lett. 2002;43:6587. [Google Scholar]; For Pt-catalyzed rearrangment see:; (f) Harrak Y, Blaszykowski C, Bernard M, Cariou K, Mainetti E, Mouries V, Dhimane AL, Fensterbank L, Malacria M. J Am Chem Soc. 2004;126:8656. doi: 10.1021/ja0474695. [DOI] [PubMed] [Google Scholar]; For Au-catalyzed rearrangement, see:; (g) Mamane V, Gress T, Krause H, Fürstner A. J Am Chem Soc. 2004;126:8654. doi: 10.1021/ja048094q. [DOI] [PubMed] [Google Scholar]; See also refs 8k and 45.
- 70.Saunders WH, Paine RH. J Am Chem Soc. 1961;83:882. [Google Scholar]
- 71.The observed migratory aptitude trends (Ph vs. Et, and Ph vs. Me) do not correspond to those reported in literature for 1,2-alkyl migration to carbenoid center. See, for example:; (a) Philip H, Keating J. Tetrahedron Lett. 1961;2:523. [Google Scholar]; (b) Graf von der Schulenburg W, Hopf H, Walsh R. Angew Chem., Int Ed. 1999;38:1128. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1128::AID-ANIE1128>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


