Abstract
Many studies have shown that the systemic administration of cytokines or vaccination with cytokine-secreting tumors augments an antitumor immune response that can result in eradication of tumors. However, these approaches are hampered by the risk of systemic toxicity induced by soluble cytokines. In this study, we have evaluated the efficacy of 4TO7, a highly tumorigenic murine mammary tumor cell line, expressing glycosyl phosphatidylinositol (GPI)-anchored form of cytokine molecules alone or in combination with the costimulatory molecule B7-1 as a model for potential cell or membrane-based breast cancer vaccines. We observed that the GPI-anchored cytokines expressed on the surface of tumor cells greatly reduced the overall tumorigenicity of the 4TO7 tumor cells following direct live cell challenge as evidenced by transient tumor growth and complete regression within 30 days post challenge. Tumors co-expressing B7-1 and GPI-IL-12 grew the least and for the shortest duration, suggesting that this combination of immunostimulatory molecules is most potent. Protective immune responses were also observed following secondary tumor challenge. Further, the 4TO7-B7-1/GPI-IL-2 and 4TO7-B7-1/GPI-IL-12 transfectants were capable of inducing regression of a wild-type tumor growing at a distant site in a concomitant tumor challenge model, suggesting the tumor immunity elicited by the transfectants can act systemically and inhibit the tumor growth at a distant site. Additionally, when used as irradiated whole cell vaccines, 4TO7-B7-1/GPI-IL-12 led to a significant inhibition in tumor growth of day 7 established tumors. Lastly, we observed a significant decrease in the prevalence of myeloid-derived suppressor cells and regulatory T-cells in the tumor microenvironment on day 7 post challenge with 4TO7-B7-1/GPI-IL-12 cells, which provides mechanistic insight into antitumor efficacy of the tumor-cell membrane expressed IL-12. These studies have implications in designing membrane-based therapeutic vaccines with GPI-anchored cytokines for breast cancer.
Keywords: breast cancer, membrane anchored cytokines, antitumor immunity, tumor microenvironment
1. Introduction
Breast cancer is among the leading types of cancer among women in the United States, with an estimated 229,060 new cases in 2012 alone [1] and devising new strategies of breast cancer therapy remains a priority in medical research. While there have been numerous preclinical studies that have evaluated different methods and approaches to enhance the overall immunogenicity of tumor cells, few have been capable of inducing clinically relevant responses. One such approach includes the genetic modification of tumor cells to secrete cytokines including IL-12 and GM-CSF [2-4], or using GM-CSF secreting tumor vaccines with or without concomitant chemotherapy [5, 6].
In order for a cancer immunotherapy to be effective in a clinical therapeutic setting, the immune suppressive nature of the tumor microenvironment must be overcome [7, 8]. Tumor cells have been shown to up-regulate the expression of the inhibitory molecules PD-L1 and CTLA-4 [9-11]. Moreover, tumors also produce inhibitory cytokines and factors such as IL-10, vascular endothelial growth factor (VEGF), prostaglandins and transforming growth factor beta (TGF-β) that can induce immune tolerance by preventing dendritic cell (DC) maturation [12, 13] and promoting the differentiation and maturation of regulatory T cells (Tregs) [14, 15] and myeloid derived suppressor cells (MDSCs) [16].
To potentially address this issue, we have established glycosyl phosphatidylinositol (GPI)-anchored forms of the cytokines IL-2 and IL-12 for stable surface expression onto tumor cells along with the costimulatory molecule B7-1. The expression of these molecules allows for immune activation to take place locally at the vaccination site rather than systemic activation, which could potentially be toxic to patients [17-19]. This localized immune activation can effectively manipulate or skew the tumor microenvironment towards being less immune suppressive. Additionally, direct targeting of tumor antigens to antigen presenting cells is more likely to occur following engagement of the modified tumor cells expressing IL-2 and IL-12 with their cognate receptors found on DCs.
Herein, we report for the first time the direct effects of membrane-anchored cytokines such as IL-2 and IL-12 on the tumorigenicity of a highly tumorigenic mouse mammary cancer model. Additionally, our studies provide insight into the potential mechanisms underlying the reduced tumorigenicity of these genetically modified cells as evidenced by a significant reduction in the local and peripheral immune suppressive microenvironment of tumor-bearing hosts.
2. Materials and Methods
2.1. Cell culture and animals
4TO7 tumor cells, a kind gift of Fred Miller (Wayne State University), were cultured in DMEM media (Cellgro) with 10% FBS at 37°C. cDNA encoding GPI-anchored forms of murine IL-2, and IL-12 were constructed in our laboratory by attaching a GPI-anchor signal sequence as previously described [20, 21]. cDNA encoding murine B7-1 was kindly provided by Gordan Freeman (Boston, MA). The cDNAs were subcloned into the pUB6A expression vector (Invitrogen Corp). Cells were transfected using FuGene6 transfection reagent (Roche Molecular Biochemicals) and selected with blasticidin (10μg/ml). In order to select the population of cells expressing the GPI-molecules, the cells were subjected sequentially to a) magnetic activated cell sorting (MACS) (Dynal Biotech Dynabeads, Invitrogen), b) panning [22] and c) fluorescence activated cell sorting (FACS). The wild-type 4TO7 (4TO7-WT) cell population was later subjected to four rounds of in vivo passage following subcutaneous (s.c.) injection into BALB/c mice to yield more reproducible, aggressive tumor growth with palpable tumor development within 6 days (4TO7RG).
Female BALB/C mice 6-8 weeks of age were purchased from Jackson Laboratories and were maintained in accordance with IACUC approved institutional guidelines and protocols.
2.2. Characterization of tumor cells
Flow Cytometry
Surface expression of B7-1, IL-2 and IL-12 was determined by flow cytometry analysis. Briefly, cells were incubated for 30mins at 4°C with directly-conjugated antibodies as follows: IL-2-PE (clone S4B6), IL-12-PE (clone 17.8), and B7-1-FITC (clone 1G10) (BD Biosciences). The cells were then washed, formalin-fixed and analyzed using a FACSCaliber cytometer and analyzed with FlowJo software.
ELISA and Western blot analysis
Cell transfectants (2×105/well) were seeded in 24-well plates for 48h. After which, culture supernatant was collected, cells were washed and lysed using 2% octyl-β-glucoside, 50mM Tris-HCl pH8, 2mM PMSF, 5mM EDTA and protease inhibitor cocktail (1:100, Sigma). IL-2 and IL-12 in the cell lysate and culture supernatant was detected by sandwich ELISA according to the manufacturer’s instructions (eBioscience) and western blotting techniques as previously described [23].
PIPLC (phosphatidylinositol phospholipase-C) treatment
Cell transfectants were treated with a 1:1000 dilution of the PIPLC enzyme (Glyko Prozyme, San Leandro, CA) in PBS/0.1% Ovalbumin and incubated for 45mins in a 37°C water bath with slight agitation every 10mins. At the end of the incubation, the cells were centrifuged and washed with FACS buffer (PBS/1%CCS/1%EDTA) and stained for FACS analysis.
CFSE dilution
CFSE staining was used to determine the growth rate of tumor cells using adapted methods as previously described [24]. Cells were then washed with FACS buffer and either analyzed immediately to verify CFSE incorporation or cultured for FACS analysis at the specified time points.
2.3. Tumor challenge studies
Direct challenge
Mice (n=5/group) were challenged subcutaneously (s.c.) in the rear hind flank with 4TO7-WT or transfected 4TO7 cells (2×105). Tumor size (mm2) was measured using Vernier calipers every 2-3 days with 2×2 perpendicular measurements. Tumor-free mice were subjected to a secondary challenge with 4TO7-WT cells (2×105) 30-33 days later on the opposite hind flank. Mice were monitored weekly for tumor growth. Mice were euthanized when the tumor size reached >2 cm2.
Concomitant Immunity
Mice (n=5/group) were challenged with 4TO7RG cells (2×105) on the right hind flank and simultaneously challenged with each of the 4TO7 transfectants (2×105) on the opposite hind flank (s.c.). Mice were monitored as mentioned previously.
Therapeutic whole cell vaccination studies
Prior to vaccination, tumor cells were exposed to 80Gy of gamma irradiation. Mice were challenged subcutaneously with 5×104 4TO7RG cells on the right hind flank and vaccinated with 2×105 irradiated cells on the opposite hind flank seven days later. Mice were monitored as previously mentioned.
2.4. Cellular phenotyping of immune infiltrates and Immunohistochemistry staining (IHC)
Tumor cells (2×105) were mixed in a 1:1 ratio with 250μL of Matrigel™ (BD Falcon) and injected into the hind flank of BALB/c mice (s.c.). Seven days post inoculation, the spleens, tumor-draining lymph nodes (TDLNs) and Matrigel were harvested. The Matrigels were either digested using collagenase type III (Sigma) for 1h or formalin-fixed for IHC staining. Single cell suspensions were prepared from the digested Matrigels and red blood cells were lysed. Cells were then washed, Fc blocked (clone 2.4G2) and stained with directly conjugated antibodies (eBioscience) for 25mins at 4°C to detect T cells (CD4+ and CD8+), B cells (B220+), DCs (CD11b+CD11c+), Tregs (CD4+CD25+FoxP3+) and MDSCs (CD11b+Gr1+). Samples were analyzed as described previously. Formalin-fixed Matrigels were embedded in paraffin blocks, sectioned and stained with hematoxylin and eosin (H&E). Blood vessels were visualized by staining endothelial cells with a CD31 primary antibody at 1:200 dilution (Abcam).
2.5. Statistical analysis
Differences between tumor growth curves and spleen sizes groups were analyzed using ANOVA or the Student t test, respectively. Values of p < 0.05 were considered significant. For survival studies, Kaplan-Meier survival curves were plotted and analyzed. All graphs and statistical calculations were analyzed using Prism software (GraphPad).
3. Results
3.1. Establishment of 4TO7 murine breast cancer cells expressing GPI-anchored IL-2 and IL-12
4TO7 murine mammary tumor cells were transfected to express transmembrane B7-1, GPI-IL-2, GPI-IL-12, B7-1 and GPI-IL-2, or B7-1 and GPI-IL-12. The GPI-linkage of the cytokine molecules on the surface of the cells was verified using the PIPLC enzyme, which specifically cleaves the glycosyl phosphatidylinositol lipid tail of the GPI-anchored molecule and leads to a decrease in surface molecule expression. After PIPLC treatment, the percentage of cells positive for B7-1 did not decrease as the B7-1 construct was not GPI-linked (Figure 1A-B). However, expression of IL-2 and IL-12 decreased following PIPLC treatment, verifying that the cytokines were anchored to the cell membrane by a GPI-linkage. The IL-12 GPI-linkage appears to be more susceptible to PIPLC cleavage, as its expression decreased by 94.5% while IL-2 expression decreased by 68.4% for the single transfectants (Figure 1A) and 96.7% and 77.5% for the double transfectants (Figure 1B), respectively. The sensitivity of the GPI-anchor to cleavage has been shown to vary depending on the molecule that it anchors and the cell type in which it is expressed [25].
Figure 1. IL-2 and IL-12 are expressed on the surface of 4TO7 via a GPI-anchor.
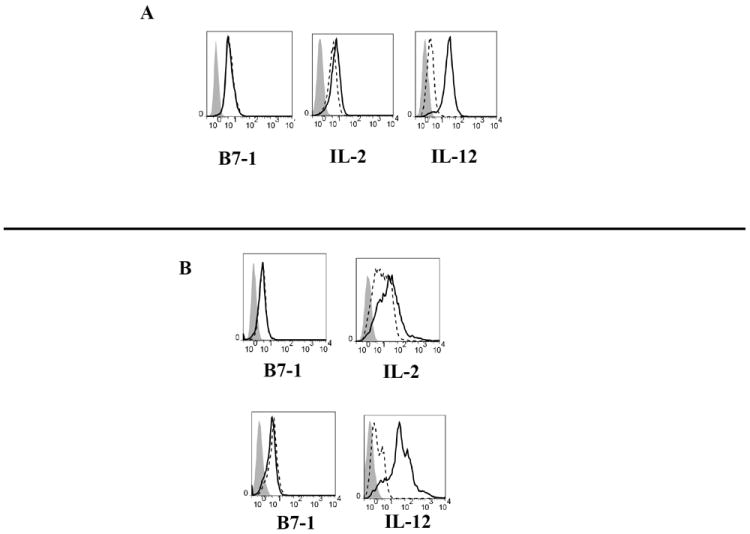
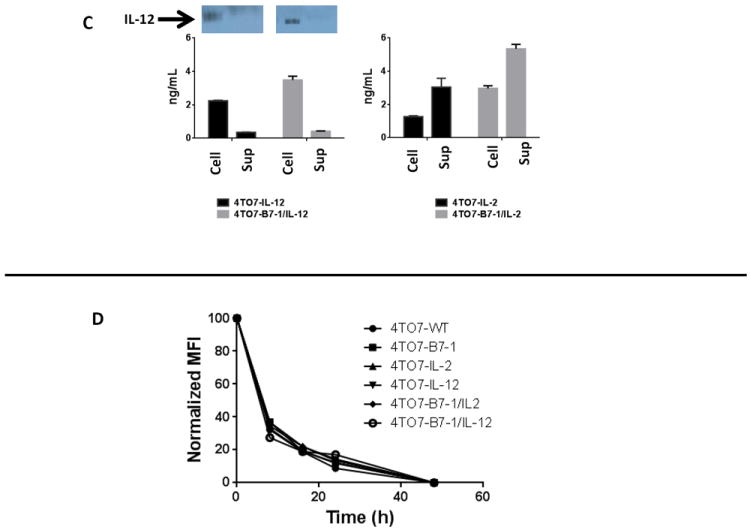
The enzyme PIPLC was used to verify the GPI-linkage of the membrane-bound B7, IL-2 and IL-12 molecules in the A) single and B) double transfectant cell lines. The shaded histogram represents staining with an isotype control antibody. The dashed line represents protein expression at baseline and the solid line represents protein expression following PIPLC treatment. C) Western blot analysis of IL-12 and ELISA of IL-2 and IL-12 in the cell lysate (Cell) and culture supernatant (Sup) of 2×105 4TO7 transfectants after 48 h culture. D) 4TO7 tumor cells were labeled with CFSE and analyzed using flow cytometry at 0, 8, 16, 24, and 48 h post culture. The mean fluorescence intensity (MFI) of each cell line was normalized relative to its initial (0hr) MFI. 100% was normalized as the 0hr time point for each sample.
Lastly, to determine whether GPI-IL-12 and GPI-IL-2 is found in the supernatant of 4TO7 transfectants, we analyzed cells and supernatants after culturing tumor cells for 48h. No measurable IL-12 was detected in the supernatant of transfected cells by Western blot, suggesting that GPI-IL-12 remains cell-associated and is minimally shed (Figure 1C). This is further supported by ELISA which indicates that 2.24ng/mL and 3.49ng/mL is cell-associated whereas only 0.35ng/mL and 0.411ng/mL is found in the supernatant of 4TO7-IL-12 and 4TO7-B7-1/IL-12, respectively. However, despite not being able to detect IL-2 by Western blot, we observed substantial amounts of IL-2 in supernatants of 4TO7-IL-2 cells by ELISA suggesting that IL-2 is expressed both as membrane-bound and secreted forms.
3.2. Membrane expression of IL-12 along with B7-1 leads to minimal tumor growth and rapid tumor rejection following direct challenge
While the expression of IL-2, IL-12 or B7-1 alone or in combination did not alter the in vitro growth properties of the 4TO7 tumor cells as indicated by CFSE dilution (Figure 1D), upon direct in vivo challenge, 4TO7-WT tumors grew progressively whereas the tumors from the transfected cell lines all completely regressed (Figure 2A). Thirty days post injection, none of the mice challenged with transfected cells had tumors while 100% of the mice challenged with wild-type cells had developed tumors. The onset of tumor formation of the 4TO7B7-1/IL-12 tumors was the slowest to appear and the smallest in size. This data indicates that the stable surface expression of B7-1 alone or in combination with IL-2 or IL-12 enhances the overall immunogenicity of 4TO7-WT tumor cells.
Figure 2. Expression of B7-1 alone or in combination with GPI-IL-2 and GPI-IL-12 reduces the overall tumorigenicity of 4TO7 tumor cells.
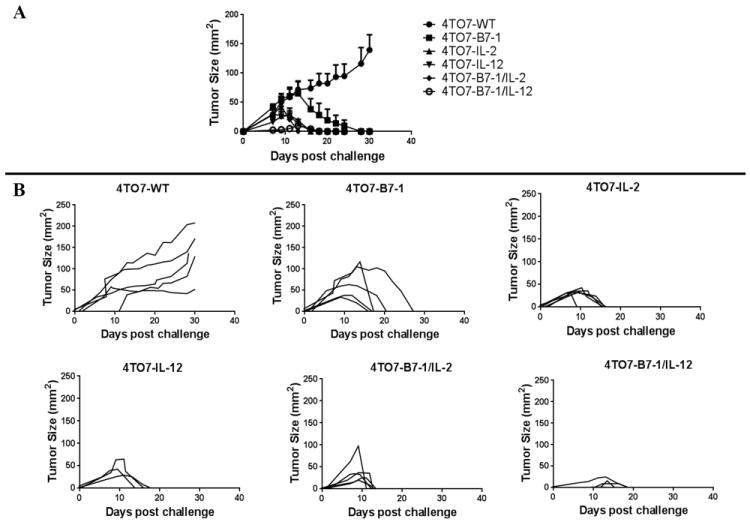
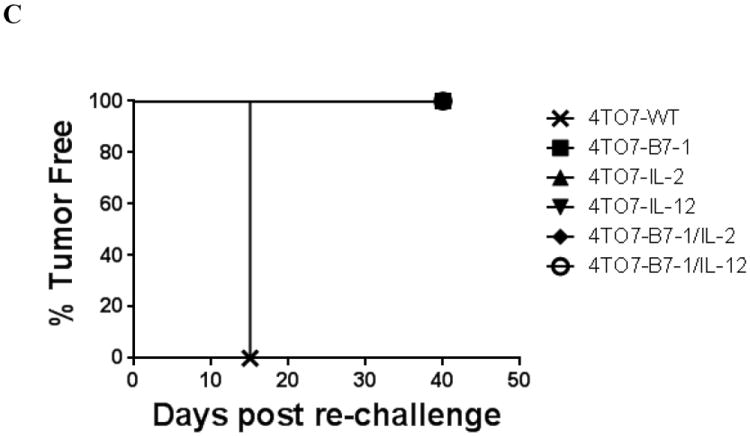
BALB/C mice (n=5/group) were challenged s.c. in the hind flank with 2×105 cells and tumor growth was monitored. A) Mean tumor size was calculated as the average of the five tumor measurements per group. B) Each data line represents an individual mouse per group. C) Tumor-free mice were re-challenged with 2×105 wild-type cells to determine the induction of protective immune responses. Mean ± SEM is depicted.
3.3. Transfected 4TO7 tumor cells are capable of inducing protective immune responses
All of the mice previously challenged with transfected 4TO7 cells were tumor-free 30 days after initial direct challenge (Figure 2B) and were then re-challenged with 2×105 4TO7-WT cells. All of the mice remained tumor-free, whereas all of the mice in the previously unchallenged group developed progressively growing tumors and had to be sacrificed within 30 days post challenge due to large tumor burden (Figure 2C). Taken together, protective antitumor immunity was induced by the transfected 4TO7 tumor cells and was sustained for up to 150 days of monitoring following re-challenge with 4TO7-WT cells (data not shown).
3.4. Splenomegaly correlates with tumor burden and MDSC
Throughout our studies, control mice challenged with 4TO7 cells displayed highly significant splenomegaly (p<0.0001) relative to naïve or tumor-free mice and overall tumor burden correlated with the spleen weight, R2= 0.943 (Figure 3A). Consistent with previous reports that splenomegaly is associated with an increased infiltration of MDSCs [26], we also observed a significant increase in MDSCs from an average of 4.02% in tumor-free mice to 32.5% in tumor-positive mice with a corresponding percent decrease in other cell types including T and B cells (Figure 3B). There was no significant difference observed in spleen weight or splenocyte cell populations between naïve mice and mice that were tumor-free at the end of the aforementioned studies (data not shown).
Figure 3. Significant increase in myeloid derived suppressor cell (MDSC) population correlates with observed splenomegaly.
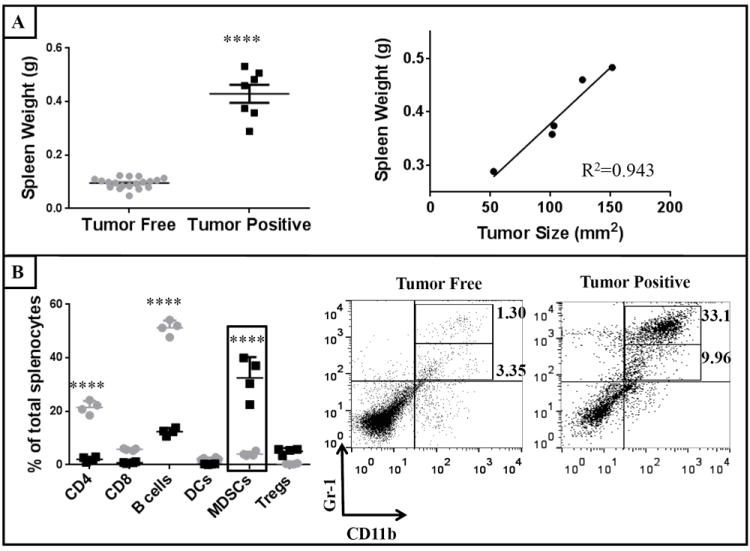
Spleens from naïve, tumor-free and 4TO7 challenged mice were weighed prior to single cell suspensions being prepared for flow cytometry analysis of splenic cell populations. A) Spleen weights of tumor positive mice were significantly higher than tumor-free mice and correlated with overall tumor burden. B) A significant increase in splenic MDSCs in tumor-positive (
 ) relative to tumor-free (
) relative to tumor-free (
 ) mice. An unpaired Student’s t test and ANOVA analysis was conducted to determine statistical significance among spleen weights and splenic populations, respectively. (****p<0.0001)
) mice. An unpaired Student’s t test and ANOVA analysis was conducted to determine statistical significance among spleen weights and splenic populations, respectively. (****p<0.0001)
3.5. The co-expression of B7-1 and GPI-IL-12 alters the tumor microenvironment of live 4TO7 cells
To gain insight into the early immune factors mediating the reduced tumorigenicity of 4TO7 B7-1/IL-12, tumor cells were mixed in a 1:1 ratio with Matrigel and transplanted subcutaneously into BALB/c mice. Staining for H&E and CD31, an endothelial cell marker, was used to visualize the angiogenesis in the Matrigel plugs. Reduced vasculature was observed with the co-expression of B7-1 and IL-12 relative to wild-type (Figure 4A). Among the cellular infiltrates found within the Matrigel, we observed that the co-expression of B7-1/IL-12 led to a significant decrease in MDSCs relative to wild-type challenged mice at the tumor site (13.5% to 5.2%) and in the spleen (17.0% to 5.98%) and a marked decrease in the TDLNs (9.9% to 6.5%) (Figure 4B). This reduction of MDSCs is somewhat surprising given that 4TO7 cells have been shown to secrete granulocyte-colony stimulating factor (G-CSF), a factor that can promote the recruitment and mobilization of MDSCs to the tumor site as well as distant organs to establish pre-metastatic niches [27]. Additionally, we observed that within the Matrigel, 15.3% of total cells from wild-type challenged mice were Tregs whereas in 4TO7B7-1/IL-12 challenged mice there was a significant decrease in Tregs to 7.28% (Figure 4B). We also saw a notable decrease in Tregs in the TDLNs as well as in the spleen. The co-expression of B7-1 and IL-12 also led to an increased infiltration of CD8+ T cells and DCs (Figure 4B, lower panels), as well as CD4+ T cells and B cells (data not shown) relative to wild-type challenged mice. These observations suggest that the reduced tumorigenicity observed with the co-expression of B7-1 and IL-12 is based on a duality of inhibiting components of active immune suppression as well as increasing the infiltration of effector immune cell populations.
Figure 4. Significant reduction in MDSCs and regulatory T cells (Tregs) in mice challenged with 4TO7-B7-1/IL-12 cells.
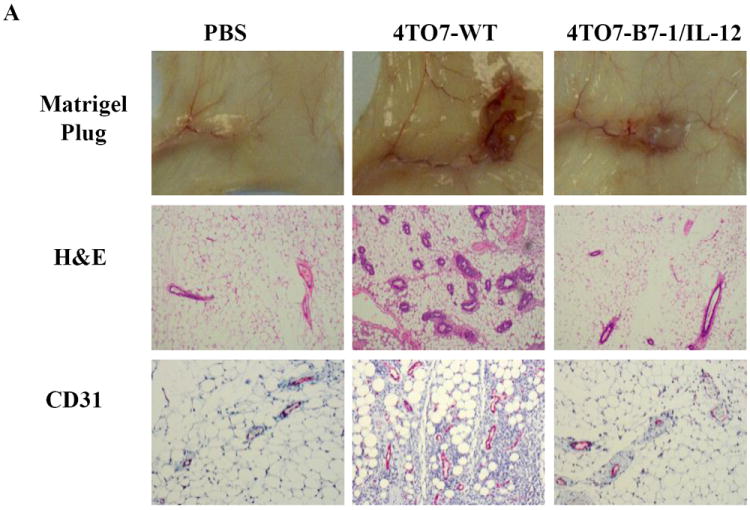
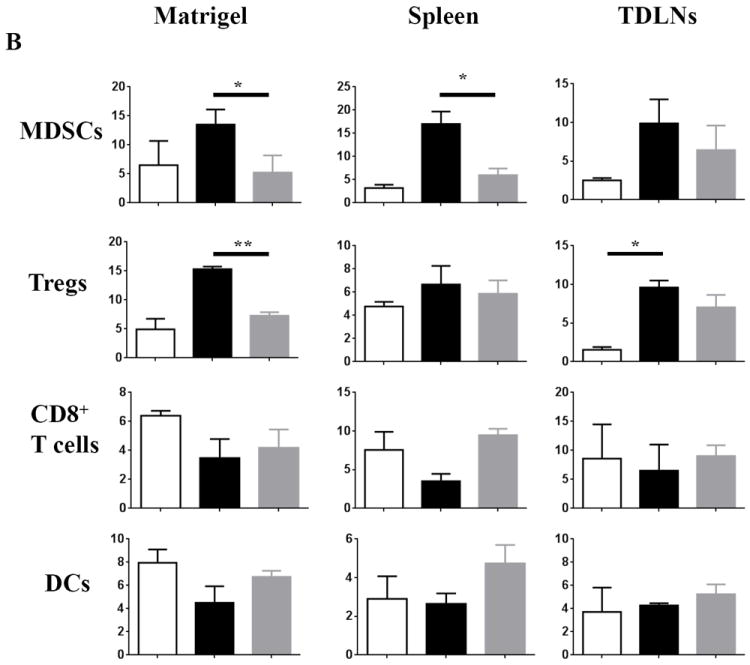
Tumor cells were mixed in a 1:1 ratio with Matrigel and inoculated s.c. into the hind flank of mice. Seven days later, the matrigels, spleens and tumor draining lymph nodes (TDLNs) were harvested. A) Reduced angiogenesis is present at 4TO7-B7-1/IL-12 tumor site relative to 4TO7-WT. Representative matrigels from two separate experiments are shown with H&E (10×) and CD31 endothelial cell staining (20× magnification). B) Co-expression of B7-1 and GPI-IL-12 decreased the prevalence of MDSCs (CD11b+Gr1+) and Tregs (CD4+CD25+FoxP3+) locally as well as in the periphery, while increasing the prevalence of CD8+ T cells and DCs (CD11c+CD11b+). White bar: PBS, Black bar: 4TO7-WT, Gray: 4TO7B7-1/IL-12. (*p<0.05, **p<0.01). Mean ± SEM is depicted.
3.6. Simultaneous challenge with transfected 4TO7 tumor cells causes regression of a tumor growing at a distant site
Next, we wanted to determine whether the antitumor immune response elicited by the transfected 4TO7 cells is capable of acting systemically and induce regression of a 4TO7RG tumor, a highly aggressive variant of 4TO7, growing at a distant site. In this concomitant immunity model, we observed that the growth of the 4TO7RG tumor on the right flank was generally slower and less progressive in the mice challenged concomitantly with the 4TO7 transfectants (Figure 5A). The mice challenged with the double transfectants 4TO7-B7-1/IL-2 and 4TO7-B7-1/IL-12 showed a delay in overall growth kinetics and had the smallest average 4TO7RG tumor growth, with both groups remaining <10mm2. Of the single 4TO7 transfectants, 4TO7-IL-12 was the most effective at reducing overall tumor burden with an average tumor size of ~30mm2 by day 27 post challenge. In contrast, 4TO7-B7-1 and 4TO7-IL-2 challenged mice developed wild-type tumors similar in size as the control mice. These findings indicate that the stable co-expression of B7-1 along with Th1 skewing cytokines IL-2 or IL-12 on the surface of 4TO7 tumor cells can overcome the immune suppression mediated by a developing, highly aggressive tumor.
Figure 5. Co-expression of B7-1 with GPI-IL-2 or GPI-IL-12 induces concomitant immune responses and inhibits tumor growth in a therapeutic setting.
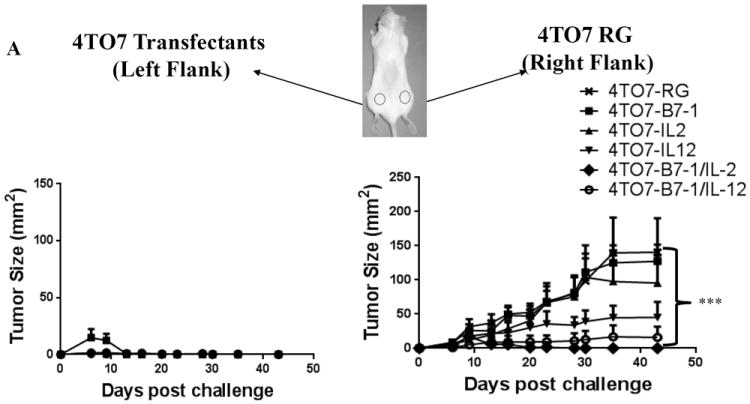
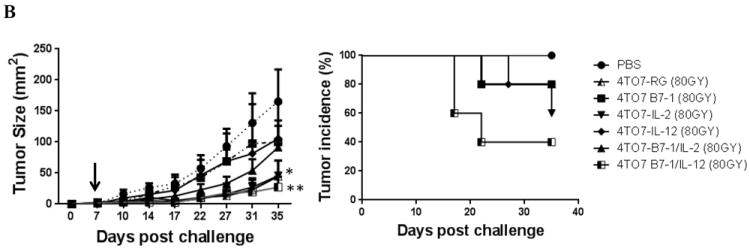
A) Groups of mice (n=5) were challenged with 4TO7RG cells (2×105) on the right hind flank and simultaneously challenged subcutaneously with each of the 4TO7 transfectants (2×105) on the opposite hind flank. Mice challenged with 4TO7 cells expressing B7-1/IL-2 or B7-1/IL-12 led to a significant inhibition of 4TO7-WT tumor growth at a distant site. B) Mice were challenged with 5×104 4TO7RG cells and vaccinated subcutaneously with irradiated (80Gy) 4TO7 transfectants 7 days later. Vaccination with 4TO7B7-1/IL-12 cells induced significant tumor inhibition. Statistical significance was determined using ANOVA analysis. (*p<0.05, *** p<0.001). Mean ± SEM is depicted.
3.7. Therapeutic vaccination with irradiated 4TO7 cells expressing cells B7-1 and GPI-IL-12 significantly reduces overall tumor burden
To assess the therapeutic efficacy of expressing GPI-anchored cytokines on the surface of breast cancer cells in a clinically relevant model, mice were vaccinated with irradiated 4TO7 transfectants on day 7 post challenge with 4TO7RG cells. We observed that after only one vaccination, mice treated with irradiated 4TO7B7-1/GPI-IL-12 cells had significantly lower tumor burden than unvaccinated mice with an average tumor size of 27.2mm2 as compared to 165.3mm2 (Figure 5B). Additionally, the tumor incidence was decreased to 40%. These results indicate that the co-expression B7-1 and GPI-IL-12 is capable of inducing antitumor immune responses against a highly aggressive tumor variant in a therapeutic setting.
4. Discussion
The 4TO7 murine mammary tumor cell line was transfected to express GPI-anchored forms of cytokines IL-2 and IL-12 alone or in combination with the costimulatory molecule B7-1. Our challenge studies demonstrated that the transfectants had decreased tumorigenicity, induced long-term protective immunity and growth inhibition of a distant, concomitant tumor as well as significantly reduced overall tumor burden in a therapeutic setting. Interestingly, we also observed that irradiated 4TO7RG cells significantly inhibited 4TO7RG growth comparable to 4TO7 transfectants. The 4TO7 transfectants were established from 4TO7-WT cells and not the more aggressive, in vivo passaged variant 4TO7RG. For our concomitant and therapeutic vaccination studies we chose to challenge with 4TO7RG to test the stringency of the antitumor immune response that would be induced by the GPI-molecules. Due to the likely intratumor heterogeneity [28] of the 4TO7RG cells, transfected clones may no longer be representative of the total antigenic population that must be presented to induce more complete tumor inhibition of 4TO7RG tumors. These findings have potential clinical implications such that clonally selected cell lines developed from a patient’s highly heterogeneous tumor tissue that are modified with B7-1/GPI-IL-12 could be effective against the heterogeneous tumor.
Previous studies have evaluated the role of co-expressing B7-1 and secretory IL-12 [29-31]. Direct challenge of mice with A20 murine B cell lymphoma expressing B7-1 alone delayed onset of tumor appearance, whereas challenge with A20-IL-12 or A20-B7-1/IL-12 led to complete rejection [30]. Our challenge studies suggest that, like soluble IL-12, GPI-anchored IL-12 is also capable of synergizing with B7-1 in inducing antitumor immunity, but without the potential risks of systemic toxicities associated with soluble cytokines [17-19]. Pan et al reported that a single chain murine IL-12 expressed as a fusion protein with the transmembrane domain and cytosolic tail of murine B7-1 is capable of reducing the tumorigenicity of CT26 murine colon cancer cells and upon intratumoral administration via an adenoviral vector is effective in a therapeutic setting [32]. Our approach is unique in that it modifies soluble cytokines with a GPI-anchor, which we have previously shown can spontaneously incorporate onto the lipid bilayers of cell membranes [21, 23].
The reduced tumorigenicity of 4TO7 B7-1/IL-12 tumor cells appears to correlate with a significant reduction in immune suppressive cell populations, namely MDSCs and FoxP3+ Tregs. Furthermore, in human breast cancer, decreased Tregs has been associated with increased complete pathologic response [33] and improved overall survival [34] after neoadjuvant therapy. Similarly, it has been reported that among breast and colon cancer patients the presence of Lin-CD33+HLA-DR-/low MDSCs is associated with a poor overall survival [35]. Thus, effective targeting of the development [36] or suppressive activities [37] of these cell populations, would be of great therapeutic benefit and have been investigated in several cancers [38].
Our results indicate that cytokine molecules expressed on the surface of 4TO7 murine cancer cells can induce protective immunity, inhibit progression of a distant tumor and effectively overcome the immune suppressive tumor microenvironment. Taken together these findings suggest that a cell or membrane-based vaccine containing GPI-anchored cytokines could be a viable immunotherapeutic approach to treat breast cancer.
Highlights.
Surface expression of cytokines (GPI-cytokines) reduced 4TO7 tumorigenicity
GPI-cytokines may reduce systemic toxicity associated with soluble cytokines
GPI-IL-2 or GPI-IL-12 expressed with B7-1 induce protective & concomitant immunity
GPI-cytokines effectively overcome the immune suppressive tumor microenvironment
A vaccine with GPI-cytokines may be a viable immunotherapy to treat breast cancer
Acknowledgments
This work was supported by NIH Funding: 1 R01 CA138993-01A1 to PS and 1 F31 CA165897-01 to ENB and 11SDG5710004 to RS from American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erica N. Bozeman, Email: ebozema@emory.edu.
Ashley Cimino-Mathews, Email: acimino1@jhmi.edu.
Deepa K. Machiah, Email: dkodand@emory.edu.
Jaina Patel, Email: jmpate2@emory.edu.
Arun Krishnamoothy, Email: arun.krishnamoorthy@duke.edu.
Linda Tien, Email: ltien@uchicago.edu.
Rangaiah Shashidharamurthy, Email: rangaiahsh@pcom.edu.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Mach N, Dranoff G. Cytokine-secreting tumor cell vaccines. Current opinion in immunology. 2000 Oct;12(5):571–5. doi: 10.1016/s0952-7915(00)00144-8. [DOI] [PubMed] [Google Scholar]
- 3.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 1993 Apr 15;90(8):3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinchieri G, Scott P. Interleukin-12: basic principles and clinical applications. Current topics in microbiology and immunology. 1999;238:57–78. doi: 10.1007/978-3-662-09709-0_4. [DOI] [PubMed] [Google Scholar]
- 5.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer research. 2005 Sep 15;65(18):8059–64. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 6.Emens LA. GM-CSF-secreting vaccines for solid tumors. Curr Opin Investig Drugs. 2009 Dec;10(12):1315–24. [PubMed] [Google Scholar]
- 7.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011 Nov 23;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Emens LA, Silverstein SC, Khleif S, Marincola FM, Galon J. Toward integrative cancer immunotherapy: targeting the tumor microenvironment. Journal of translational medicine. 2012;10:70. doi: 10.1186/1479-5876-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009 Feb 1;15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002 Aug;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 11.Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Fala F, Fabbi M, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. International journal of cancer Journal international du cancer. 2005 Nov 20;117(4):538–50. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature reviews Immunology. 2004 Dec;4(12):941–52. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 13.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. The American journal of pathology. 2004 Dec;165(6):1853–63. doi: 10.1016/S0002-9440(10)63238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K, et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer research. 2009 Mar;29(3):881–8. [PubMed] [Google Scholar]
- 15.Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004 Oct 1;173(7):4352–9. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annual review of immunology. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO, Seipp CA, et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–75. [PubMed] [Google Scholar]
- 18.Siegel JP, Puri RK. Interleukin-2 toxicity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991 Apr;9(4):694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- 19.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997 Oct 1;90(7):2541–8. [PubMed] [Google Scholar]
- 20.McHugh RS, Ahmed SN, Wang YC, Sell KW, Selvaraj P. Construction, purification, and functional incorporation on tumor cells of glycolipid-anchored human B7-1 (CD80) Proceedings of the National Academy of Sciences of the United States of America. 1995 Aug 15;92(17):8059–63. doi: 10.1073/pnas.92.17.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagarajan S, Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer research. 2002 May 15;62(10):2869–74. [PubMed] [Google Scholar]
- 22.Wysocki LJ, Sato VL. “Panning” for lymphocytes: a method for cell selection. Proceedings of the National Academy of Sciences of the United States of America. 1978 Jun;75(6):2844–8. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagarajan S, Selvaraj P. Human tumor membrane vesicles modified to express glycolipid-anchored IL-12 by protein transfer induce T cell proliferation in vitro: a potential approach for local delivery of cytokines during vaccination. Vaccine. 2006 Mar 20;24(13):2264–74. doi: 10.1016/j.vaccine.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 24.Chen JC, Chang ML, Muench MO. A kinetic study of the murine mixed lymphocyte reaction by 5,6-carboxyfluorescein diacetate succinimidyl ester labeling. Journal of immunological methods. 2003 Aug;279(1-2):123–33. doi: 10.1016/s0022-1759(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 25.Ratnoff WD, Knez JJ, Prince GM, Okada H, Lachmann PJ, Medof ME. Structural properties of the glycoplasmanylinositol anchor phospholipid of the complement membrane attack complex inhibitor CD59. Clinical and experimental immunology. 1992 Mar;87(3):415–21. doi: 10.1111/j.1365-2249.1992.tb03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuPre SA, Hunter KW., Jr Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Experimental and molecular pathology. 2007 Feb;82(1):12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proceedings of the National Academy of Sciences of the United States of America. 2010 Dec 14;107(50):21248–55. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer research. 2012 Oct 1;72(19):4875–82. doi: 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen XY, Mandelbaum S, Li ZH, Hitt M, Graham FL, Hawley TS, et al. Tricistronic viral vectors co-expressing interleukin-12 (1L-12) and CD80 (B7-1) for the immunotherapy of cancer: preclinical studies in myeloma. Cancer gene therapy. 2001 May;8(5):361–70. doi: 10.1038/sj.cgt.7700321. [DOI] [PubMed] [Google Scholar]
- 30.Pizzoferrato E, Chu NR, Hawley TS, Lieu FH, Barber BH, Hawley RG, et al. Enhanced immunogenicity of B cell lymphoma genetically engineered to express both B7-1 and interleukin-12. Human gene therapy. 1997 Dec 10;8(18):2217–28. doi: 10.1089/hum.1997.8.18-2217. [DOI] [PubMed] [Google Scholar]
- 31.Putzer BM, Hitt M, Muller WJ, Emtage P, Gauldie J, Graham FL. Interleukin 12 and B7-1 costimulatory molecule expressed by an adenovirus vector act synergistically to facilitate tumor regression. Proceedings of the National Academy of Sciences of the United States of America. 1997 Sep 30;94(20):10889–94. doi: 10.1073/pnas.94.20.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan WY, Lo CH, Chen CC, Wu PY, Roffler SR, Shyue SK, et al. Cancer immunotherapy using a membrane-bound interleukin-12 with B7-1 transmembrane and cytoplasmic domains. Molecular therapy : the journal of the American Society of Gene Therapy. 2012 May;20(5):927–37. doi: 10.1038/mt.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008 Apr 15;14(8):2413–20. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 34.Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. The Journal of pathology. 2011 Jul;224(3):389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 35.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011 Aug 25;118(8):2254–65. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer research. 2012 Mar 15;72(6):1373–83. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- 37.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. International immunopharmacology. 2007 Feb;7(2):140–51. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012 Jun 20;144(3):250–68. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]


