Abstract
Autoimmune connective tissue diseases (ACTDs) are a family of consistent systemic autoimmune inflammatory disorders, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc) and Sjögren’s syndrome (SS). Toll-like receptors (TLRs) are located on various cellular membranes and sense exogenous and endogenous danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), playing a critical role in innate immune responses. During the past decade, the investigation of TLRs in inflammation autoimmune diseases has been fruitful. In this report, we review the significant biochemical, physiological and pathological studies of the key functions of TLRs in ACTDs. Several proteins in the TLR signaling pathways (e.g., IKK-2 and MyD88) have been identified as potential therapeutic targets for the treatment of ACTDs. Antibodies, oligodeoxyribonucleotides (ODNs) and small molecular inhibitors (SMIs) have been tested to modulate TLR signaling. Some drug-like SMIs of TLR signaling, such as RDP58, ST2825, ML120B and PHA-408, have demonstrated remarkable potential, with promising safety and efficacy profiles, which should warrant further clinical investigation. Nonetheless, one should bear in mind that all TLRs exert both protective and pathogenic functions; the function of TLR4 in inflammatory bowel disease represents such an example. Therefore, an important aspect of TLR modulator development involves the identification of a balance between the suppression of disease-inducing inflammation, while retaining the beneficiary host immune response.
Keywords: Toll-like receptor, autoimmune diseases, inflammation, small molecule modulator, drug discovery
1. Introduction
Autoimmune connective tissue diseases (ACTDs) are characterized by the spontaneous stimulation of the immune system with the production of autoantibodies, which are specific for self-components in the nucleus and cytoplasm, often macromolecular complexes of proteins and nucleic acids. ACTDs can affect any connective tissue of the human body via inflammation or destruction. Possible causes of ACTDs include genetic (Chai, Phipps, & Chua, 2012; Romano, et al., 2011), hormonal (Jacobson, Gange, Rose, & Graham, 1997; Luppi, 2003) and environmental factors (Arnson, Shoenfeld, & Amital, 2010); genetic factors may predispose an individual to the development of ACTDs. The classic ACTDs include systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), Sjögren’s syndrome (SS) and mixed connective tissue disease (MCTD) (Diamond & Lipsky, 2008).
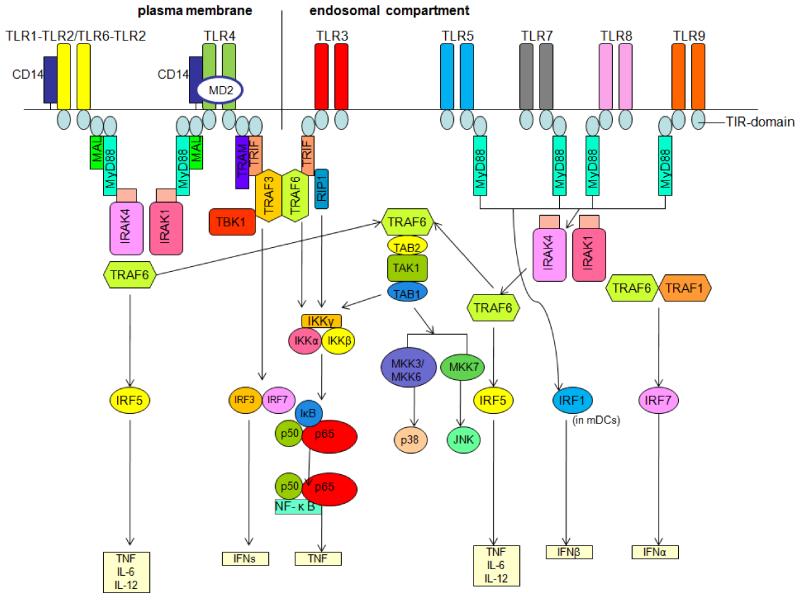
Toll-like receptors (TLRs) are a family of evolutionarily conserved innate immune receptors that play a crucial role in the first-line defense against foreign agents. These protein receptors are characterized by their ability to respond to invading pathogens promptly by recognizing particular TLR ligands, including flagellin and lipopolysaccharide (LPS) of bacteria, nucleic acids derived from viruses and zymosan of fungi (Takeuchi, et al., 2002). These ligands can activate dendritic cells (DCs), macrophages, B cells, T cells and other antigen-presenting cells (APCs). These immunocompetent cells express different subsets of TLRs (Table 1) and TLR activation allows for the effective presentation of microbial antigens to cells of the adaptive immune system. However, recent findings have also revealed that TLRs recognize and respond to endogenous ligands produced during infection or damage (Asea, et al., 2002; Brentano, Schorr, Gay, Gay, & Kyburz, 2005; Okamura, et al., 2001; Park, et al., 2004; Smiley, King, & Hancock, 2001; Termeer, et al., 2002; Vabulas, et al., 2002; Vollmer, et al., 2005; Yasuda, et al., 2009) (Table 2). The identification and characterization of endogenous ligands of TLRs provides a novel perspective for exploring the etiology of autoimmune diseases. After ligands bind to TLRs or their accessory protein, such as myeloid differentiation protein 2 (MD-2) for TLR4, TLRs dimerize (hetero- or homodimerize) and undergo a conformational change that in turn leads to the recruitment of downstream signaling molecules. A family of five adaptor proteins known as myeloid differentiation primary response gene 88 (MyD88), TIR domain-containing adaptor protein (TIRAP)/MyD88 adaptor-like protein (MAL), TIR-domain-containing adapter-inducing interferon-β (TRIF), TRIF-related adaptor molecule (TRAM) and sterile α- and armadillo motif-containing protein (SARM) are involved in the downstream signaling pathways of TLRs (O’Neill & Bowie, 2007; O’Neill, Fitzgerald, & Bowie, 2003; Roelofs, Abdollahi-Roodsaz, Joosten, van den Berg, & Radstake, 2008). These downstream pathways also involve many kinases (IRAKs, TAK1, MAPK, PI3K, etc.), IRFs and NF-κB (for a recent review, see (Akira & Takeda, 2004)), which leads to the production of pro-inflammatory factors (IFN-α, IFN-β, IFN-γ, IL-6, etc.), perpetuating inflammation (Figure 1).
Table 1.
Expression profile of TLRs among different immunocompetent cells
| Cell type | TLRs expressed | Ref. |
|---|---|---|
| Macrophage | TLR1-9 | (McCoy & O’Neill, 2008) |
| B cell | TLR1, TLR2, TLR3, TLR4, TLR6, TLR7 and TLR9, but not TLRs TLR5 and TLR8 |
(Gururajan, Jacob, & Pulendran, 2007) |
| T cell | TLR1-9 | (Babu, Blauvelt, Kumaraswami, & Nutman, 2006; Tabiasco, et al., 2006) |
| Dendritic cell | TLR1-9 | (Reis e Sousa, 2004) |
Note: TLR expression levels show a high degree of variation among individuals. In mice, there may be strain-specific differences in TLR expression.
Table 2.
Exogenous and endogenous ligands of TLRs
| TLR | Exogenous ligands | Origin of ligands | Ref. | Endogenous ligands |
Ref. |
|---|---|---|---|---|---|
| TLR1 | Triacyl lipopeptides | bacteria and mycobacteria | (Takeuchi, et al., 2002) | ||
| Soluble factors | Neisseria meningitidis | (Wyllie, et al., 2000) | |||
| TLR2 | Lipoprotein/lipopeptides | various pathogens | (Aliprantis, et al., 1999) | Heat-shock protein 70 |
(Asea, et al., 2002) |
| Peptidoglycan | gram-positive bacteria | (Schwandner, Dziarski, Wesche, Rothe, & Kirschning, 1999; Takeuchi,et al., 1999) | HGMB1 | (Park, et al., 2004) | |
| Lipoteichoic acid | gram-positive bacteria | (Schwandner, et al., 1999) | |||
| Lipoarabinomannan | mycobacteria | (Means, et al., 1999) | |||
| Phenol-soluble modulin |
Staphylococcus
epidermidis |
(Hajjar, et al., 2001) | |||
| Glyco inositol phospholipids |
Trypanosoma cruzi | (Coelho, et al., 2002) | |||
| Glycolipids | Treponema maltophilum | (Opitz, et al., 2001) | |||
| Porins | Neisseria | (Massari, et al., 2002) | |||
| Atypical lipopolysaccharide | Leptospira interrogans | (Werts, et al., 2001) | |||
| Atypical lipopolysaccharide | Porphyromanas gingivalis | (Hirschfeld, et al., 2001) | |||
| Zymosan | fungi | (Underhill, et al., 1999) | |||
| TLR3 | Double-stranded RNA | viruses | (Alexopoulou, Holt, Medzhitov, & Flavell, 2001) | Double- stranded DNA |
(Brentano, et al., 2005) |
| TLR4 | Lipopolysaccharide | gram-negative bacteria | (Poltorak, et al., 1998) | Heat-shock protein 70 |
(Vabulas, et al., 2002) |
| Fusion protein | respiratory syncytial virus | (Kurt-Jones, et al., 2000) | Type III repeat extra domain A of fibronectin |
(Okamura, et al., 2001) | |
| envelope protein | mouse mammary-tumor virus |
(Rassa, Meyers, Zhang, Kudaravalli, & Ross, 2002) | Oligosacchari des of hyaluronic acid |
(Termeer, et al., 2002) | |
| Heat-shock protein 60 | Chlamydia pneumoniae | (Bulut, et al., 2002; Ohashi, Burkart, Flohe, & Kolb, 2000) | Polysaccharid e fragment of heparan sulfate |
(Johnson, Brunn, Kodaira, & Platt, 2002) | |
| Fibrinogen | (Smiley, et al., 2001) | ||||
| TLR5 | Flagellin | bacteria | (Hayashi, et al., 2001) | ||
| TLR6 | Diacyl lipopeptides | Mycoplasma | (Takeuchi, et al., 2001) | ||
| Lipoteichoic acid | gram-negative bacteria | (Schwandner, et al., 1999) | |||
| Zymosan | fungi | (Ozinsky, et al., 2000) | |||
| TLR7 | Single-stranded RNA | viruses | (Diebold, Kaisho, Hemmi, Akira, & Reis e Sousa, 2004; Heil, et al., 2004) |
Single- stranded RNA |
(Vollmer, et al., 2005) |
| Imiquimod (R837) | synthetic compound (FDA approved drug) |
(Hemmi, et al., 2002) | |||
| TLR8 | Single-stranded RNA | viruses | (Heil, et al., 2004) | Single- stranded RNA |
(Vollmer, et al., 2005) |
| TLR9 | CpG-containing DNA | bacteria and viruses | (Hemmi, et al., 2000) | DNA | (Yasuda, et al., 2009) |
| TLR10 | Not Determined | not determined | |||
| TLR11 | profilin | uropathogenic pathogen, toxoplasma |
(Kucera, et al., 2010; Zhang, et al., 2004) | ||
| TLR13 | 23s rRNA | gram-negative/positive bacteria |
(Li & Chen, 2012; Oldenburg, et al., 2012) |
Figure 1. Toll-like receptor (TLR) signaling pathways.
Myeloid differentiation primary response gene 88 (MyD88) is the key signaling adaptor for TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, TLR8 and TLR9. Only TLR3 and TLR4 signal via TIR-domain-containing adapter-inducing interferon-β (TRIF). Each adaptor of the respective receptor complex positively regulates transcription factor activation, with the exception of, the sterile α- and armadillo motif-containing protein (SARM, not shown in this figure), which inhibits TRIF-mediated transcription factor activation. MAL, MyD88 adaptor-like protein; TRAM, TRIF-related adaptor molecule; TBK1, tumor necrosis factor receptor-associated factor (TRAF) family member-associated NF-κB activator (TANK)-binding kinase 1; RIP1, receptor-interacting protein 1; IRAK, IL-1R-associated kinase; TAK1, transforming growth factor-β-activated kinase; TAB, TAK1-binding protein; IKK, inhibitor of NF-κB kinase; IκB, inhibitor of NF-κB; NF-κB, nuclear factor-κB; TNF, tumor necrosis factor; IRF, interferon regulatory factor; IFN, interferon; mDCs, myeloid dendritic cells; MKK, mitogen-activated protein kinase kinase; JNK, JUN N-terminal kinase.
Increasing evidence suggests that innate and adaptive immune responses that mediate autoimmune diseases are, at least in part, driven by the binding of PAMPs and DAMPs to TLRs (Mills, 2011). TLR activation induces the production of pro-inflammatory factors and type I interferons, which contributes to the development and/or progression of systemic autoimmune diseases (Marshak-Rothstein, 2006). Therefore, targeting TLRs and modulating TLR signaling have emerged as an important strategy for the treatment of ACTDs.
2. Systemic lupus erythematosus and TLRs
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that involves almost every organ of the human body, including the skin, kidney, blood cells, blood vessels, heart, pleura, central and peripheral nervous systems, muscles and joints (Tassiulas & T. Boumpas, 2008). Approximately 40% of SLE patients exhibit defects in the clearance of apoptotic cells, which are removed rapidly by macrophages in healthy individuals (Kruse, et al., 2010). An SLE murine model also shows a defect in the clearance of cellular debris (Herrmann, et al., 1998). The inefficient clearance of cellular debris leads to an increased release of host DNA and RNA, which induces the production of autoantibodies (Kruse, et al., 2010). A number of studies have provided data consistent with the idea that TLRs recognize the host DNA/RNA-containing immune complex and promote the inflammation and activation of immune cells, leading to the production of pathogenic autoantibodies and the development of clinical features of autoimmunity.
2.1 TLR3
TLR3 in SLE patients may act on mesangial cells in kidneys directly. The TLR3 ligand polyinosinic/polycytidylic acid (poly(I:C)) worsens glomerulonephritis in MRLlpr/lpr mice without an increased titer of anti-dsDNA antibodies (Patole, et al., 2005). Nonetheless, deactivation of TLR3 does not affect the production of autoantibodies against either RNA- or DNA-containing antigens or the severity of glomerulonephritis (Christensen, et al., 2005).
2.2 TLR4
The function of TLR4 is associated with the production of autoantibodies and glomerulonephritis in SLE. Repeated injection of low-dose LPS into lupus-prone mice (MRL/n, BXSB, or NZW) accelerates the development of lupus, increases the production of autoantibodies and worsens renal injury (Hang, et al., 1983). Activation of TLR4 results in the production of anti-dsDNA antibodies and the development of immune complex-mediated glomerulonephritis in transgenic mice (B. Liu, et al., 2006). Inhibition of TLR4 signaling by Chaperonin 10 has been found to suppress cutaneous lupus and lupus nephritis (Kulkarni, et al., 2012). These results suggest that enhanced TLR4 signaling alone is a sufficient and a potent trigger to induce SLE. Renal injury is reduced and anti-nuclear, anti-dsDNA and anti-cardiolipin antibodies titers are decreased in TLR4-deficient C57BL/6lpr/lpr mice compared with TLR4-producing C57BL/6lpr/lpr mice (Lartigue, et al., 2009). TLR4 deficiency in tlr4−/− C57BL/6lpr/lpr mice also results in reduced levels of cytokines involved in the development of SLE, i.e., IFN-γ and IL-6 (Lartigue, et al., 2009). No TLR4 polymorphism had been found to influence the susceptibility to SLE until recently. A polymorphism in MyD88 adaptor-like (MAL) protein was found to be associated with reduced susceptibility to SLE. Reduced SLE susceptibility modulates intracellular signaling triggered by TLR2 and TLR4 activation (Castiblanco, et al., 2008).
2.3 TLR5
The chromosomal region lq41-42 is known to contain susceptibility genes of SLE (Graham, et al., 2001). The TLR5 gene maps to chromosome lq41 and contains a common stop codon polymorphism (allele C1174T), which abolishes the signaling of TLR5. Populations with this stop codon produce reduced levels of pro-inflammatory cytokines compared with the wild type control, suggesting that the TLR5 stop codon polymorphism is associated with protection from the development of SLE; however, it may increase the risk of infection (Hawn, et al., 2005).
2.4 TLR7/TLR9
TLR7 and TLR9 are associated with the production of IFN-α and the stimulation of B cells in SLE. The nucleic acid-containing immune complexes engulfed by plasmacytoid dendritic cells are translocated to the endosome and stimulate TLR7 or TLR9, resulting in a massive release of IFN-α (Means, et al., 2005; Savarese, et al., 2006). Inhibitors of TLR7 abolish plasmacytoid dendritic cell IFN-α production stimulated by the immune complexes derived from SLE patient sera (Barrat, et al., 2005). Delivery of the RNA-containing immune complex to TLR7 is mediated by Fc γ receptors (FcγRs). TLR7 binding with RNA results in dimerization, activation and internalization (Vollmer, et al., 2005). Myeloid dendritic cells express FcγRs and respond to TLR7/8 ligands, which leads to the secretion of IL-12p70 and the induction of IFNγ-producing type1 T helper (Th1) cell proliferation (Napolitani, Rinaldi, Bertoni, Sallusto, & Lanzavecchia, 2005; Roelofs, et al., 2009). Murine tlr7−/− plasmacytoid dendritic cells stimulated with an antibody binding U1snRNP produce markedly reduced levels of IFN-α and IL-6 compared with wild type cells (Savarese, et al., 2006). The immune complex can stimulate B cells via association with the B cell receptor (BCR). Stimulation of B cells results in elevated proliferation and production of autoantibodies (Leadbetter, et al., 2002). An extended study by Marshak-Rothstein and co-workers has shown that murine tlr7−/− or tlr9−/− B cells are not stimulated by the immune complex and the inhibitors of TLR7 or TLR9 eradicates the stimulation of B cells (Lau, et al., 2005; Leadbetter, et al., 2002).
TLR9 is involved in the production of type I IFN (i.e., IFN-α and IFN-γ) and TNF-α in SLE. The increased IFN-α levels in serum and type I IFN-regulated genes in peripheral blood mononuclear cells (PBMCs) derived from SLE patients has been suggested to be responsible for failed apoptosis (Baechler, et al., 2003; Bengtsson, et al., 2000). In the presence of GM-CSF, DNA-containing immune complexes activate CD16 and TLR9 in dendritic cells. The activation of CD16 and TLR9 results in the upregulation of TNF-α. Inhibitors of TLR9 abolish the production of TNF-α and the tlr9−/− myeloid dendritic cells reduce the effect of inhibition (Boule, et al., 2004). The immune complex co-localizes with CD32 and TLR9 in the endosome and stimulates plasmacytoid dendritic cells to produce IFN-α (Means, et al., 2005). Interferon regulatory factor 7 (IRF7) is a transcription factor required for IFN-α production. IRF7 interacts with and is activated by MyD88 (an adaptor of TLR9). In conventional dendritic cells, A/D-type CpG oligodeoxynucleotide (CpG-A, an IFN-α-inducing TLR9 ligand) is rapidly transferred to the lysosome. CpG-A is retained in the endosome of plasmacytoid dendritic cells together with the MyD88/IRF7 complex (Honda, et al., 2005). Immune complexes containing nucleic acid stimulate plasmacytoid dendritic cells and myeloid dendritic cells, which induce the proliferation of Th1 cells and the release of IFN-γ. Auto-reactive Th1 cells promote the production of autoantibodies in B cells. The key function of TLR7 and TLR9 in SLE has been also demonstrated in animal models. Lupus-prone mice lacking TLR7 do not produce anti-Sm antibody (a specific auto-antibody of SLE) and display ameliorated disease manifestation, decreased lymphocyte activation and decreased serum IgG levels (Christensen, et al., 2006). In contrast, overexpression of TLR7 aggravates systemic autoimmunity (Subramanian, et al., 2006). TLR9 exhibits opposite functions in different murine models, which are reviewed in (W. U. Kim, Sreih, & Bucala, 2009). TLR9 exerts a possible protective function against SLE, as demonstrated in various murine models of lupus (Lartigue, et al., 2006; Wu & Peng, 2006; P. Yu, et al., 2006). However, the function of TLR9 in humans remains unknown. It has been shown that two alleles downregulate TLR9 expression and one of them predisposes human to an increased risk of developing SLE, especially in the Japanese population (Tao, et al., 2007).
3. Rheumatoid arthritis and TLRs
Rheumatoid arthritis (RA) is primarily a chronic inflammatory disease of the synovial joints and the surrounding connective tissue (Firestein, 2008). Sometimes, RA presents as systemic vasculitides that affects several organs (e.g., muscles, eyes, lungs, kidneys and meninx) (Harris Jr. & Firestein, 2008). Many results have shown that various TLRs participate in the development and maintenance of RA inflammation. Untreated RA can cause cartilage destruction, bone erosion and tendon fracture, leading to the deformation and dysfunction of joints (Genovese, 2008). Autoantibodies are found in RA patients, in whom rheumatic factor (directed against to the Fc portion of IgG molecules) and antibodies that target citrullinated peptides display the highest prevalence and diagnostic value. The production of autoantibodies involves the recognition of auto-antigens via TLR signaling pathways.
The overexpression of a variety of TLRs (TLR2, TLR3, TLR5, TLR6, TLR7 and TLR9) has been observed in RA synovium patients compared with healthy controls or osteoarthritis patients (Radstake, et al., 2004; Roelofs, et al., 2005; Tamaki, et al., 2011). There are several hypotheses regarding what triggers the overexpression of TLRs in RA joints. The microbial pathogens or minor trauma that causes tissue damage can induce the release of endogenous TLR ligands, leading to inflammation via TLR activation. Several microbes have been suggested to participate in the pathogenesis of RA, including mycobacteria, mycoplasma, Escherichia coli, Proteus mirabilis, Epstein-Barr virus and human parvovirus 19 (Rashid & Ebringer, 2007). Nonetheless, conclusive evidence is still lacking. A recent study also suggested that porphyromonas gingivalis is a potential source of RA pathogenesis (Lundberg, Wegner, Yucel-Lindberg, & Venables, 2010). Stimulation and activation of synovial fibroblasts via TLR2 leads to the production of multiple inflammatory chemokines in RA joints, which causes chronic inflammation (Pierer, et al., 2004; Seibl, et al., 2003). Compared with healthy controls, dendritic cells derived from RA patients have shown elevated levels of inflammatory cytokines, such as TNF-α and IL-6, mediated by TLR2 and TLR4 (Radstake, et al., 2004).
Multiple animal models have illustrated the important function of TLRs in the development of arthritis (Huang & Pope, 2009). Intra-articular injection of streptococcal cell wall has been shown to induce arthritis via TLR2 and MyD88 in mice (Joosten, et al., 2003). Another TLR2 ligand peptidoglycan induces arthritis through the same pathway (Z. Q. Liu, Deng, Foster, & Tarkowski, 2001). Necrotic cells release intracellular citrullinated proteins and activate peptidyl arginine deiminase (PAD), which citrullinate fibrinogen and α-enolase in RA synovium (Foulquier, et al., 2007). Citrullinated peptides are detected by APCs and presented to T cells (Ireland & Unanue, 2011). B cells are also activated and produce anti-citrullinated peptide antibodies (ACPAs). The RA-specific citrullinated fibrinogen-containing immune complex co-stimulates macrophages via TLR4 and FcγR (Sokolove, Zhao, Chandra, & Robinson, 2011). Although TLR2 and TLR4 expressed on the cell surface are the primary targets of endogenous ligands in RA, endosomal TLR3 upregulated in macrophages may also play a potential role in the initiation and maintenance of arthritis in animal models (Meng, et al., 2010). Another endosomal TLR, TLR8, has been suggested to contribute independently to the production of TNF-α in rheumatoid synovial membrane cell cultures (Abdollahi-Roodsaz, et al., 2008; Sacre, et al., 2008). In the IL-1 receptor antagonist knockout murine model IL1rn−/−, different TLR knockouts show opposite effects. While tlr4−/− mice are protected against severe arthritis, tlr2−/− mice show much more severe arthritis characterized by reduced suppressive function of regulatory T cells and increased IFN-γ production by T effector cells (Abdollahi-Roodsaz, et al., 2008). Specific inhibition of TLR4 reduces the severity of animal model arthritis and results in lower IL-1 expression levels in arthritic joints (Abdollahi-Roodsaz, et al., 2007). IL-1 is an important cytokine in promoting damage associated with RA. Decreased expression of IL-1 results in a reduction in joint inflammation (Furst, 2004). Therefore, TLR4 has been suggested to be a potential target for RA treatment. Chaperonin 10, a TLR4 inhibitor, has been shown to be well-tolerated and effective in the treatment of RA in a double-blind randomized trial (Vanags, et al., 2006).
4. Systemic sclerosis and TLRs
Systemic sclerosis (SSc) is characterized by the over-production and deposition of collagen in the skin, kidneys, heart, lungs, gastrointestinal tract and blood vessel endothelium (Varga & Denton, 2008). These abnormalities have been suggested to result from autoimmune dysfunctions involving cytokines, immune cells and fibroblasts (Varga & Denton, 2008). Meanwhile, many TLRs participate in the development of autoimmune dysfunction.
Several TLRs are implicated in the production of cytokines in SSc patients. Stimulation of dendritic cells derived from SSc patients with the ligands of TLR2, TLR3 or TLR4 results in increased secretion of IL-1, IL-6 and TNF-α compared with those isolated from patients in the late stages of the disease or healthy controls (van Bon, et al., 2010). The levels of IL-12 produced by dendritic cells are low upon stimulation with TLR ligands in most SSc patients, whereas the levels of IL-10 secreted by dendritic cells are particularly elevated in patients with early diffused SSc (van Bon, et al., 2010). TLR4 has been suggested to mediate the stimulation of monocytes and dendritic cells via LPS. TLR4-stimulated dendritic cells secrete increased levels of IL-10 and to in turn increase the serum levels of the profibrotic chemokine CCL18, which attracts T-cells, in SSc (van Lieshout, et al., 2009). The TLR3 ligand poly(I:C) substantially enhances the expression of both IFN and TGF-β responsive genes in fibroblasts (Farina, et al., 2010). Serum HMGB-1 and soluble RAGE levels in SSc patients are elevated, suggesting a correlation between disease severity and immunological abnormalities (Yoshizaki, et al., 2009). The above results support the hypothesis that autoantibodies stimulate T cells via TLRs and in turn promote the production of autoantibodies from B cells in SSc patients in a similar way as observed in SLE. IFN-α production in cultured normal PBMCs is significantly increased when induced by anti-topoisomerase I antibody-positive SSc patient sera. Plasmacytoid dendritic cell activation induces IFN-α production (D. Kim, et al., 2008). The production of IFN-α also requires immune complexes containing CpG-rich DNA or single-stranded RNA (and associated proteins, e.g., autoantibodies against DNA, RNA, or DNA/RNA binding proteins) derived from dying cells and FcγRII (Boule, et al., 2004; D. Kim, et al., 2008; Ronnblom, Eloranta, & Alm, 2006).
Recent studies have shown that SSc may share the same range of IFN-mediated diseases with SLE. Some SSc patients manifest a “lupus-like” high IFN-inducible gene expression pattern that correlates with the presence of anti-topoisomerase and anti-U1RNP antibodies (Assassi, et al., 2010). Increased expression of interferon-responsive genes (IRGs) may be mediated by DNA/RNA-containing immune complexes (Lafyatis & York, 2009). IFN-α production is induced by the RNA-containing immune complexes in plasmacytoid dendritic cells regulated by interactions with monocytes, NK cells and plasmacytoid dendritic cells involving several pro-inflammatory and anti-inflammatory cytokines (Eloranta, et al., 2009). These results support the hypothesis that the endosomal TLRs, such as TLR7 and TLR9, may participate in the pathogenesis of SSc, such as in SLE.
5. Sjögren’s syndrome and TLRs
Sjögren’s syndrome (SS) is a systemic autoimmune disease in which immune cells mistakenly attack and destroy exocrine glands (Carsons, 2008). This dysfunction of the immune cells also involves TLRs. The salivary and lacrimal glands are heavily targeted; therefore, typical symptoms of SS include dryness of the mouth and eyes. However, SS may also affect other organs, including the kidneys, lungs, liver, pancreas, peripheral nervous system and brain, with infiltration and destruction by immune cells (Carsons, 2008).
The expression profile of TLRs in SS patients varies greatly from that of healthy individuals. TLR7 and TLR9 mRNA are upregulated in the PBMCs of primary Sjögren’s syndrome (pSS) patients compared with controls (Zheng, Zhang, Yu, & Yang, 2010). Some cells in the epithelial islands, lymphocytes and ductal epithelial cells of the parotid gland in pSS patients are positive for TLR7 and TLR9, while TLR7-positive or TLR9-positive cells are rarely found in the ductal epithelial cells of controls (Zheng, et al., 2010). Cultured salivary gland epithelial cells (SGECs) are found to express functional TLR2, TLR3 and TLR4 following treatment with the respective ligands (Spachidou, et al., 2007). The constitutive expression of TLR1, TLR2 and TLR4 mRNA is significantly higher in SS-SGECs than in control SGECs (Spachidou, et al., 2007). TLR2, TLR3, TLR4 and MyD88 are overexpressed in the labial salivary glands of pSS patients compared with controls. Expression of TLR2, TLR3, TLR4 and MyD88 has also been observed in infiltrating mononuclear cells, acinar cells and ductal epithelial cells. The TLR expression pattern is similar in cultured human salivary gland cells (Kawakami, et al., 2007). The ligands of TLRs stimulate the expression of CD54 and the production of IL-6 and then induce the phosphorylation of ERK, JNK and p38, without the phosphorylation of Akt or the activation of NF-κB p65 (Kawakami, et al., 2007). Activation of TLR2 by peptidoglycan has been suggested to induce the production of IL-17 and IL-23 in the PBMCs of SS patients via pathways including IL-6, signal transducer and activator of transcription 3 (STAT3) and NF-κB (Kwok, et al., 2012). The TLR3 ligand poly(I:C) not only stimulates innate immune responses, but it is also involved in the activation of programmed cell death via anoikis in SS by upregulation of pro-apoptotic Bmf, BimEL and Bax and the downregulation of pro-survival Bcl-2 (Manoussakis, Spachidou, & Maratheftis, 2010).
TLR7 and TLR9 are involved in the proliferation, differentiation and transition of B cells. Most TLRs are expressed at very low or undetectable levels in human naive B cells, while the expression levels of TLR7 and TLR9 are rapidly induced by BCR activation. In addition to producing antibodies, B cells can present antigens, secrete cytokines and regulate T cell functions (Lanzavecchia & Sallusto, 2007; Meyer-Bahlburg & Rawlings, 2008). B cells expressing specific BCRs of nucleic acids can internalize auto-antibody- and DNA-containing immune complexes, resulting in the activation of endosomal TLR7 and TLR9 (Leadbetter, et al., 2002). TLR7/9 activation leads to the proliferation and differentiation of human memory B cells into plasma cells. Plasma cells are responsible for the secretion of cytokines and the upregulation of activation markers for antigen presentation to T cells (Meyer-Bahlburg & Rawlings, 2008). The coupling of TLR9 and BCR in the absence of T cells enables naive B cells to be selectively activated by microbial stimuli, which renders the specificity of the human immune system. TLRs are constitutively expressed in memory B cells, which ensure permanent antibody production of all memory specificities sustaining the serological memory (Lanzavecchia & Sallusto, 2007; Meyer-Bahlburg & Rawlings, 2008; Shlomchik, 2009). TLR9 has been suggested to deliver sufficient signaling to keep B cells alive and confer auto-reactive B cells with a marginal zone-like phenotype. As clusters of transitional type II B cells in the salivary glands of SS patients express mRNAs for Notch-2, Blimp-1 and TLR9 but not Pax-5, Bcl-6 and activation-induced cytidine deaminase (AID) (Guerrier, et al., 2012).
SS patients also display their own “IFN signature”. Immune complexes containing U1snRNP and hY1RNA induce the production of IFN-α in SLE and SS patients (Lovgren, et al., 2006). The expression profile of 23 genes in the IFN pathways (including TLR8 and TLR9) varies significantly between SS patients and controls (Gottenberg, et al., 2006). Engagement of TLR3 in salivary glands results in the loss of glandular function associated with the production of IFN-α, IFN-β and inflammatory cytokines (i.e., IL-6 and TNF-α) (Deshmukh, Nandula, Thimmalapura, Scindia, & Bagavant, 2009). Increased expression of the IFN-inducible genes BAFF and IFN-induced transmembrane protein 1 in ocular epithelial cells has also been demonstrated via real-time PCR (Gottenberg, et al., 2006). Analysis of mRNA isolated from the peripheral blood of SS patients has consistently revealed the overexpression of IFN-induced genes (Emamian, et al., 2009).
6. TLR modulators as potential therapeutics of ACTDs
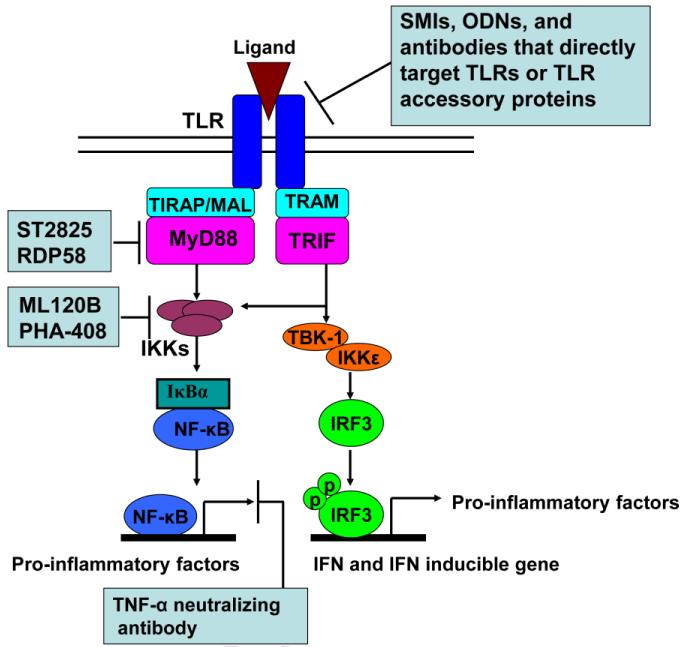
Blocking ligands binding to TLRs effectively suppress downstream inflammation activities. This approach has proven to be successful for various diseases. Antibodies against TLRs (Elass, et al., 2005; Erridge, Spickett, & Webb, 2007; Kumar, Nagineni, Chin, Hooks, & Detrick, 2004; Roger, et al., 2009; Ungaro, et al., 2009; M. Yu, et al., 2006) and oligodeoxyribonucleotides (ODNs) (Barrat, et al., 2005; Dong, Ito, Ishii, & Klinman, 2004, 2005; Latz, et al., 2007; Ranjith-Kumar, et al., 2008; Zeuner, et al., 2002) have been used to inhibit TLR signaling (Figure 2). Additionally, small molecule inhibitors (SMIs) of TLR downstream signaling have shown promise for targeting ACTDs (Figure 2) (Capolunghi, et al., 2010; DeVry, et al., 2004; Dorner, 2010; Kawamoto, Ii, Kitazaki, Iizawa, & Kimura, 2008; Kishore, et al., 2003; Kyburz, Brentano, & Gay, 2006; Mbalaviele, et al., 2009; Nakamura, et al., 2007; Schopf, et al., 2006; Tidswell, et al., 2010; Vanags, et al., 2006).
Figure 2. Diverse TLR modulators block TLR signaling pathways at different stages.
Some SMIs, ODNs and TLR-neutralized antibodies directly block TLRs and TLR accessory proteins. RDP58, which inhibits the interaction of MyD88 with IRAK4 and TRAF6, ST2825, which inhibits MyD88 dimerization, and IKK-2 inhibitors (ML120B and PHA-408) are promising drug-like SMIs for ACTD treatment. TNF-α-neutralized antibodies have been widely applied in the treatment of ACTDs (such as RA and ankylosing spondylitis).
6.1 Antibodies against TLRs
Several antibodies have been designed to block the interaction between TLRs and their respective ligands (Nelson, et al., 2000). Antibodies against TLR1, TLR2 and TLR4 have been proven to be functional in vitro (Elass, et al., 2005; Erridge, et al., 2007; M. Yu, et al., 2006). Matrix metalloproteinase-9 (MMP-9) is a critical factor of the host defense mechanism, which functions by facilitating leukocyte extravasation in infected tissues. Mycobacterial lipomannans (ML) induce MMP-9 gene expression in human macrophage-like, differentiated THP-1 cells. Pretreatment with anti-TLR1 (IgG1κ clone GD2.F4), anti-TLR2 (IgG2a clone TL2-1) and anti-CD14 (IgG1 clone MEM-18) antibodies inhibits MMP-9 gene expression in cultured THP-1 cells (Elass, et al., 2005). In human coronary artery endothelial cells, pre-incubation with anti-TLR2 (clone TL2.5) antibody inhibits E-selectin expression induced by non-enterobacterial LPS and the established TLR2 ligand Pam3CSK4 (Erridge, et al., 2007). Neutralizing anti-TLR2 antibody inhibits HMGB1-induced IL-8 release in HEK/TLR2 overexpressing cells in a dose-dependent manner (M. Yu, et al., 2006). OPN-305, a humanized IgG4 monoclonal antibody (MAb) against TLR2 developed by Opsona Therapeutics, is under development as a treatment for the prevention of delayed graft function following renal transplantation (Arslan, et al., 2012). As TLR2 signaling also contributes to the development of ACTDs, OPN-305 might also be used to treat ACTDs. Similarly, anti-TLR4 antibody inhibits HMGB1-mediated IL-8 release in whole blood and isolated primary macrophages derived from healthy volunteers in a dose-dependent manner (M. Yu, et al., 2006).
However, the current in vivo results of TLRs antibodies are less clear. A novel TLR4 antagonist antibody ameliorates inflammation but impairs mucosal healing in two murine inflammatory bowel disease (IBD) models (Ungaro, et al., 2009). However, the repression of inflammation demonstrates that overexpressed TLR4 in the intestinal mucosa of IBD patients is not only a contributing factor to the development of inflammation but also an important mediator of mucosal repair. This study also highlights the difference between the therapeutic effect of anti-TLR4 antibodies in chronic inflammation and acute sepsis (Roger, et al., 2009). Furthermore, in addition to the inconvenience of repeated injections and the high costs, antibodies of TLRs only target cell surface TLRs, as they cannot cross the cell membrane, limiting their application with endosomal TLRs.
6.2 Oligodeoxyribonucleotides (ODNs)
As shown in Figure 1 and Table 2, endosomal TLRs recognize different types of nucleic acids. TLR3 recognizes double-stranded RNAs (Alexopoulou, et al., 2001; Brentano, et al., 2005), TLR7 and TLR8 recognize single-stranded RNAs (Diebold, et al., 2004; Heil, et al., 2004; Vollmer, et al., 2005), and TLR9 recognizes single-stranded DNA molecules containing hypomethylated CpG motifs (Hemmi, et al., 2000; Yasuda, et al., 2009). Endosomal TLRs may come into contact with nucleic acid directly via their ectodomain (Latz, et al., 2007). This finding has led to the development of single-stranded oligodeoxyribonucleotides (ODNs) designed to inhibit endosomal TLR activity (Table 3). Some ODNs have demonstrated the ability to inhibit the activation of TLRs in recent studies (TLR3 (Ranjith-Kumar, et al., 2008), and TLR7 and TLR9 (Barrat, et al., 2005)). ODNs have been developed into medication for inflammatory diseases, as they have been effective in murine models of arthritis (Dong, et al., 2004; Zeuner, et al., 2002) and SLE (Dong, et al., 2005). Suppressive ODNs are hypothesized to interact directly with endosomal TLRs competing with ligands and preventing signaling (Latz, et al., 2007). The sequence, length and resistance to DNase of ODNs are essential for inhibition (Barrat, et al., 2005).
Table 3.
Oligodeoxyribonucleotides (ODNs) associated with endosomal TLRs
| Endosomal TLR Targets | Sequences of ODNs | Ref. |
|---|---|---|
| TLR3 | TCGTCGTTTGTCGTTTTGTCGTT | (Ranjith-Kumar, et al., 2008) |
| TLR7 | TCCTGGAGGGGTTGT | (Barrat, et al., 2005) |
| TLR9 | TGCTTGCAAGCTTGCAAGCA | (Barrat, et al., 2005) |
| TLR7 and TLR9 | TGCTCCTGGAGGGGTTGT | (Barrat, et al., 2005) |
6.3 Small molecule modulators
Small molecule inhibitors (SMIs) can be taken orally and are designed to penetrate the cell membrane and therefore target endosomal TLRs with low costs and convenience of use. Based on evidence that malfunction of innate immune TLR signaling contributes to autoimmune diseases, SMIs have been designed to inhibit innate immune signaling and treat inflammation and autoimmune diseases.
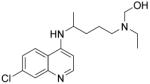
Chloroquine and its derivatives have had moderate effects in the treatment of RA (Kyburz, et al., 2006) and SLE (Dorner, 2010) for decades, although the mechanism of their therapeutic effect is not precisely known (Abarientos, et al., 2011). Chloroquine derivatives are thought to reduce TLR signaling by inhibiting the acidification of endosomes (Hacker, et al., 1998), which is a prerequisite for activation of TLR3, TLR7, TLR8 and TLR9 (de Bouteiller, et al., 2005; Gibbard, Morley, & Gay, 2006; Lee, et al., 2003; Rutz, et al., 2004). However, significant off-target toxicity at higher doses limits their use.
CPG-52364, a specific SMI of TLR7/8/9 developed by Pfizer, interferes at an early stage of the immune cascade by blocking inappropriate immune activation. Clinically, CPG-52364 has been reported to be well tolerated in healthy volunteers. CPG-52364 inhibits disease development in SLE and other autoimmune disorders, such as RA and psoriasis, without causing general suppression of immune function. Additionally, preclinical data have shown that the combination of CPG-52364 with hydroxychloroquine delivers enhanced efficacy, suggesting that CPG-52364 could be used clinically either in combination with hydroxychloroquine or as a replacement therapy for hydroxychloroquine in the first-line treatment of SLE.
RDP58, a protease resistant decapeptide (2HN-Arg-Nle-Nle-Nle-Arg-Nle-Nle-Nle-Gly-Tyr-CONH2. Nle=norleucine) developed by a computer-assisted rational design based on human leukocyte antigen (HLA)-derived peptides, has been found to both inhibit the interaction of MyD88 with IRAK4 and TRAF6 and be effective in treating different types of autoimmune inflammatory disorders (DeVry, et al., 2004; W. Liu, Deyoung, Chen, Evanoff, & Luo, 2008; Travis, et al., 2005). DeVry and co-workers have shown that RDP58 treatment reduces cellular infiltration within the spinal cord and TNF-α expression levels in an acute experimental autoimmune encephalomyelitis rat model (DeVry, et al., 2004). Liu and co-workers have further found that RDP58 reduces TNF-α, nerve growth factor and substance P expression and markedly ameliorates histopathology in an autoimmune cystitis mouse model. Clinical trials have shown that RDP58, administered at a dose of 200 mg or 300 mg, is effective in treating mild-to-moderate ulcerative colitis (Travis, et al., 2005). Additionally, oral administration of RDP58 conjugated to the cholera toxin B subunit has been found to significantly improve survival rates and histopathology manifestation of allograft kidney tissue (Yu, et al., 2012).
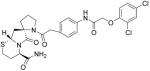
ST2825 (Table 4), a synthetic peptido-mimetic that inhibits MyD88 dimerization, interferes with recruitment of IRAK1 and IRAK4 via MyD88 and suppresses pro-inflammatory factor over-production. Capolunghi and co-workers have shown that ST2825 inhibits TLR9 activation and blocks autoantibody production in human B cells derived from SLE patients (Capolunghi, et al., 2010).
Table 4.
Small molecule inhibitors (SMIs) of TLR signaling pathways applied for the treatment of autoimmune diseases
| Compounds | Targets | Chemical Structures | Ref. |
|---|---|---|---|
| Hydroxychloro quine |
unknown |
|
(Dorner, 2010) |
|
| |||
| ST2825 | MyD88 |
|
(Capolunghi, et al., 2010) |
| ML120B | IKK2 |
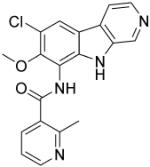
|
(Schopf, et al., 2006) |
| PHA-408 | IKK2 |
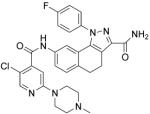
|
(Mbalaviele, et al., 2009) |
IKK-2 is a protein subunit of IκB kinase, which is a critical component of TLR innate immune signaling. IKK-2 activity causes activation of NF-κB and pro-inflammation factor over-production (Baldwin, 2012; Kanarek & Ben-Neriah, 2012). Therefore, IKK-2 is also an important drug target for ACTDs due to TLR signaling over-activation. ML120B (Table 4) is a potent, selective, reversible and ATP-competitive inhibitor of IKK-2 with an IC50 of 60 nM. Newton and co-workers have shown that ML120B is effective to prevent activation of NF-κB in pulmonary epithelial cells and results in the inhibition of pro-inflammatory factors (Newton, et al., 2007). ML120B has therapeutic inhibitory effects on joint destruction in a rat adjuvant-induced arthritis model (Schopf, et al., 2006). Additionally, ML120B has been found to inhibit the inflammation of joints in a murine antibody-induced arthritis model (Izmailova, et al., 2007). PHA-408 (Table 4), another ATP-competitive IKK-2 inhibitor (IC50, 40 nM), developed by Pfizer, inhibits TNF-α production, joint swelling and bone destruction in a streptococcal cell wall-induced arthritis rat model and is well-tolerated at maximally efficacious doses (Mbalaviele, et al., 2009).
7. Perspectives
The human innate immune system is geared to sense endogenous/exogenous danger-associated molecular patterns (DAMPs)/pathogen-associated molecular patterns (PAMPs). Increasing experimental evidence suggests that the malfunction of TLR signaling significantly contributes to the development of ACTDs; therefore, components of TLR signaling pathways are highly relevant drug targets for the treatment of autoimmune diseases. Several TLR modulators have been developed, which are currently being tested in clinical trials. The challenge is to modulate immune signaling without over-suppressing innate immune signaling and deregulating other signaling pathways. Therefore, it is important to find a balance between the suppression of disease-inducing inflammation while retaining the beneficiary host immune response. The future development of TLR modulators for ACTD therapeutics shall focus on this goal.
Acknowledgement
The U.S. National Institutes of Health (Grant Numbers: GM101279, GM103843) and the Peking Union Medical College Hospital financially supported this work. We thank Dr. Peter Brown and Dr. Kui Cheng for proofreading.
Abbreviations
- ACTDs
autoimmune connective tissue diseases
- SLE
systemic lupus erythematosus
- RA
rheumatoid arthritis
- SSc
systemic sclerosis
- SS
Sjögren’s syndrome
- MCTD
mixed connective tissue disease
- TLRs
Toll-like receptors
- DAMPs
danger-associated molecular patterns
- PAMPs
pathogen-associated molecular patterns
- MyD88
myeloid differentiation primary response gene 88
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- SARM
sterile α- and armadillo-motif-containing protein
- TIRAP
TIR domain-containing adaptor protein
- MAL
MyD88 adaptor-like protein
- TRAM
TRIF-related adaptor molecule
- TRAF
tumor necrosis factor receptor-associated factor
- TBK1
TRAF family member-associated NF-κB activator (TANK)-binding kinase 1
- RIP1
receptor-interacting protein 1
- IRAK
IL-1R-associated kinase
- TAK1
transforming growth factor β-activated kinase 1
- TAB
TAK1-binding protein
- NF-κB
nuclear factor-κB
- IKK
inhibitor of NF-κB kinase
- IκB
inhibitor of NF-κB
- TNF
tumor necrosis factor
- IRF
interferon regulatory factor
- IFN
interferon
- mDCs
myeloid dendritic cells
- MKK
mitogen-activated protein kinase kinase
- JNK
JUN N-terminal kinase
- LPS
lipopolysaccharide
- MD-2
myeloid differentiation protein 2
- HMGB1
high mobility group box 1
- FcγRs
Fc γ receptors
- RAGE
receptor for advanced glycation end products
- APCs
antigen-presenting cells
- dsDNA
double-stranded DNA
- poly(I:C)
polyinosinic/polycytidylic acid
- BCR
B cell receptor
- PBMCs
peripheral blood mononuclear cells
- IRF7
interferon regulatory factor 7
- PAD
peptidyl arginine deiminase
- ACPA
anti-citrullinated peptide antibodies
- IRGs
interferon responsive genes
- SGECs
salivary gland epithelial cells
- SMIs
small molecule inhibitors
- STAT3
signal transducer and activator of transcription 3
- AID
activation-induced cytidine deaminase;
- ODNs
oligodeoxyribonucleotides
- MMP-9
matrix metalloproteinase-9
- ML
mycobacterial lipomannans
- IBD
inflammatory bowel disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- Abarientos C, Sperber K, Shapiro DL, Aronow WS, Chao CP, Ash JY. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin Drug Saf. 2011;10:705–714. doi: 10.1517/14740338.2011.566555. [DOI] [PubMed] [Google Scholar]
- Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, Heuvelmans-Jacobs M, Akira S, Nicklin MJ, Ribeiro-Dias F, van den Berg WB. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, van der Meer JW, Netea MG, van den Berg WB. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–2967. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Arslan F, Houtgraaf JH, Keogh B, Kazemi K, de Jong R, McCormack WJ, O’Neill LA, McGuirk P, Timmers L, Smeets MB, Akeroyd L, Reilly M, Pasterkamp G, de Kleijn DP. Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ Cardiovasc Interv. 2012;5:279–287. doi: 10.1161/CIRCINTERVENTIONS.111.967596. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Assassi S, Mayes MD, Arnett FC, Gourh P, Agarwal SK, McNearney TA, Chaussabel D, Oommen N, Fischbach M, Shah KR, Charles J, Pascual V, Reveille JD, Tan FK. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 2010;62:589–598. doi: 10.1002/art.27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J Immunol. 2006;176:3885–3889. doi: 10.4049/jimmunol.176.7.3885. [DOI] [PubMed] [Google Scholar]
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS. Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunol Rev. 2012;246:327–345. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, Ronnblom L. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52:2656–2665. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435–1440. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- Capolunghi F, Rosado MM, Cascioli S, Girolami E, Bordasco S, Vivarelli M, Ruggiero B, Cortis E, Insalaco A, Fanto N, Gallo G, Nucera E, Loiarro M, Sette C, De Santis R, Carsetti R, Ruggiero V. Pharmacological inhibition of TLR9 activation blocks autoantibody production in human B cells from SLE patients. Rheumatology (Oxford) 2010;49:2281–2289. doi: 10.1093/rheumatology/keq226. [DOI] [PubMed] [Google Scholar]
- Carsons S. Sjögren’s Syndrome. In: Firestein GS, Budd RC, Harris ED Jr., McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology, 8th ed. 8th ed. 2008. [Google Scholar]
- Castiblanco J, Varela DC, Castano-Rodriguez N, Rojas-Villarraga A, Hincapie ME, Anaya JM. TIRAP (MAL) S180L polymorphism is a common protective factor against developing tuberculosis and systemic lupus erythematosus. Infect Genet Evol. 2008;8:541–544. doi: 10.1016/j.meegid.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Chai HC, Phipps ME, Chua KH. Genetic risk factors of systemic lupus erythematosus in the Malaysian population: a minireview. Clin Dev Immunol, 2012. 2012:963730. doi: 10.1155/2012/963730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Coelho PS, Klein A, Talvani A, Coutinho SF, Takeuchi O, Akira S, Silva JS, Canizzaro H, Gazzinelli RT, Teixeira MM. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes induce in vivo leukocyte recruitment dependent on MCP-1 production by IFN-gamma-primed-macrophages. J Leukoc Biol. 2002;71:837–844. [PubMed] [Google Scholar]
- de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, Bates EE, Caux C. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem. 2005;280:38133–38145. doi: 10.1074/jbc.M507163200. [DOI] [PubMed] [Google Scholar]
- Deshmukh US, Nandula SR, Thimmalapura PR, Scindia YM, Bagavant H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 2009;38:42–47. doi: 10.1111/j.1600-0714.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVry CG, Valdez M, Gao L, Wang J, Kotsch K, Volk HD, Bechmann I, Buelow R, Iyer S. RDP58, a novel immunomodulatory peptide, ameliorates clinical signs of disease in the Lewis rat model of acute experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;152:33–43. doi: 10.1016/j.jneuroim.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Diamond B, Lipsky PE. Harrison’s Principles of Internal Medicine. In: Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson JL, Loscalzo J, editors. Autoimmunity and Autoimmune Diseases. 17th edition. 2008. Chapter 312. [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligonucleotides protect against collagen-induced arthritis in mice. Arthritis Rheum. 2004;50:1686–1689. doi: 10.1002/art.20263. [DOI] [PubMed] [Google Scholar]
- Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB x NZW mice. Arthritis Rheum. 2005;52:651–658. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- Dorner T. Therapy: Hydroxychloroquine in SLE: old drug, new perspectives. Nat Rev Rheumatol. 2010;6:10–11. doi: 10.1038/nrrheum.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elass E, Aubry L, Masson M, Denys A, Guerardel Y, Maes E, Legrand D, Mazurier J, Kremer L. Mycobacterial lipomannan induces matrix metalloproteinase-9 expression in human macrophagic cells through a Toll-like receptor 1 (TLR1)/TLR2- and CD14-dependent mechanism. Infect Immun. 2005;73:7064–7068. doi: 10.1128/IAI.73.10.7064-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta ML, Lovgren T, Finke D, Mathsson L, Ronnelid J, Kastner B, Alm GV, Ronnblom L. Regulation of the interferon-alpha production induced by RNA-containing immune complexes in plasmacytoid dendritic cells. Arthritis Rheum. 2009;60:2418–2427. doi: 10.1002/art.24686. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Peripheral blood gene expression profiling in Sjogren’s syndrome. Genes Immun. 2009;10:285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C, Spickett CM, Webb DJ. Non-enterobacterial endotoxins stimulate human coronary artery but not venous endothelial cell activation via Toll-like receptor 2. Cardiovasc Res. 2007;73:181–189. doi: 10.1016/j.cardiores.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Farina GA, York MR, Di Marzio M, Collins CA, Meller S, Homey B, Rifkin IR, Marshak-Rothstein A, Radstake TR, Lafyatis R. Poly(I:C) drives type I IFN- and TGFbeta-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;130:2583–2593. doi: 10.1038/jid.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS. Etiology and Pathogenesis of Rheumatoid Arthritis. In: Firestein GS, Budd RC, Harris ED Jr., McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology, 8th ed. 8th ed. 2008. [Google Scholar]
- Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Mechin MC, Vincent C, Nachat R, Yamada M, Takahara H, Simon M, Guerrin M, Serre G. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–3553. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- Furst DE. Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clin Ther. 2004;26:1960–1975. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Genovese MC. Treatment of Rheumatoid Arthritis. In: Firestein GS, Budd RC, Harris ED Jr., McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology, 8th ed. 8th ed. 2008. [Google Scholar]
- Gibbard RJ, Morley PJ, Gay NJ. Conserved features in the extracellular domain of human toll-like receptor 8 are essential for pH-dependent signaling. J Biol Chem. 2006;281:27503–27511. doi: 10.1074/jbc.M605003200. [DOI] [PubMed] [Google Scholar]
- Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RR, Langefeld CD, Gaffney PM, Ortmann WA, Selby SA, Baechler EC, Shark KB, Ockenden TC, Rohlf KE, Moser KL, Brown WM, Gabriel SE, Messner RP, King RA, Horak P, Elder JT, Stuart PE, Rich SS, Behrens TW. Genetic linkage and transmission disequilibrium of marker haplotypes at chromosome 1q41 in human systemic lupus erythematosus. Arthritis Res. 2001;3:299–305. doi: 10.1186/ar319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier T, Le Pottier L, Devauchelle V, Pers JO, Jamin C, Youinou P. Role of toll-like receptors in primary Sjogren’s syndrome with a special emphasis on B-cell maturation within exocrine tissues. J Autoimmun. 2012 doi: 10.1016/j.jaut.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, Heeg K, Lipford GB, Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, O’Mahony DS, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- Hang L, Slack JH, Amundson C, Izui S, Theofilopoulos AN, Dixon FJ. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med. 1983;157:874–883. doi: 10.1084/jem.157.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ED, Jr., Firestein GS. Clinical Features of Rheumatoid Arthritis. In: Firestein GS, Budd RC, Harris ED Jr., McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology, 8th ed. 8th ed. 2008. [Google Scholar]
- Hawn TR, Wu H, Grossman JM, Hahn BH, Tsao BP, Aderem A. A stop codon polymorphism of Toll-like receptor 5 is associated with resistance to systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2005;102:10593–10597. doi: 10.1073/pnas.0501165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland JM, Unanue ER. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J Exp Med. 2011;208:2625–2632. doi: 10.1084/jem.20110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmailova ES, Paz N, Alencar H, Chun M, Schopf L, Hepperle M, Lane JH, Harriman G, Xu Y, Ocain T, Weissleder R, Mahmood U, Healy AM, Jaffee B. Use of molecular imaging to quantify response to IKK-2 inhibitor treatment in murine arthritis. Arthritis Rheum. 2007;56:117–128. doi: 10.1002/art.22303. [DOI] [PubMed] [Google Scholar]
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Koenders MI, Smeets RL, Heuvelmans-Jacobs M, Helsen MM, Takeda K, Akira S, Lubberts E, van de Loo FA, van den Berg WB. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- Kanarek N, Ben-Neriah Y. Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunol Rev. 2012;246:77–94. doi: 10.1111/j.1600-065X.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nakashima K, Tamai M, Nakamura H, Iwanaga N, Fujikawa K, Aramaki T, Arima K, Iwamoto N, Ichinose K, Kamachi M, Ida H, Origuchi T, Eguchi K. Toll-like receptor in salivary glands from patients with Sjogren’s syndrome: functional analysis by human salivary gland cell line. J Rheumatol. 2007;34:1019–1026. [PubMed] [Google Scholar]
- Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Kim D, Peck A, Santer D, Patole P, Schwartz SM, Molitor JA, Arnett FC, Elkon KB. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008;58:2163–2173. doi: 10.1002/art.23486. [DOI] [PubMed] [Google Scholar]
- Kim WU, Sreih A, Bucala R. Toll-like receptors in systemic lupus erythematosus; prospects for therapeutic intervention. Autoimmun Rev. 2009;8:204–208. doi: 10.1016/j.autrev.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- Kruse K, Janko C, Urbonaviciute V, Mierke CT, Winkler TH, Voll RE, Schett G, Munoz LE, Herrmann M. Inefficient clearance of dying cells in patients with SLE: anti-dsDNA autoantibodies, MFG-E8, HMGB-1 and other players. Apoptosis. 2010;15:1098–1113. doi: 10.1007/s10495-010-0478-8. [DOI] [PubMed] [Google Scholar]
- Kucera K, Koblansky AA, Saunders LP, Frederick KB, De La Cruz EM, Ghosh S, Modis Y. Structure-based analysis of Toxoplasma gondii profilin: a parasite-specific motif is required for recognition by Toll-like receptor 11. J Mol Biol. 2010;403:616–629. doi: 10.1016/j.jmb.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni OP, Ryu M, Kantner C, Sardy M, Naylor D, Lambert D, Brown R, Anders HJ. Recombinant chaperonin 10 suppresses cutaneous lupus and lupus nephritis in MRL-(Fas)lpr mice. Nephrol Dial Transplant. 2012;27:1358–1367. doi: 10.1093/ndt/gfr544. [DOI] [PubMed] [Google Scholar]
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Kwok SK, Cho ML, Her YM, Oh HJ, Park MK, Lee SY, Woo YJ, Ju JH, Park KS, Kim HY, Park SH. TLR2 ligation induces the production of IL-23/IL-17 via IL-6, STAT3 and NF-kB pathway in patients with primary Sjogren’s syndrome. Arthritis Res Ther. 2012;14:R64. doi: 10.1186/ar3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2006;2:458–459. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]
- Lafyatis R, York M. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol. 2009;21:617–622. doi: 10.1097/BOR.0b013e32832fd69e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Lartigue A, Colliou N, Calbo S, Francois A, Jacquot S, Arnoult C, Tron F, Gilbert D, Musette P. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]
- Lartigue A, Courville P, Auquit I, Francois A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Chen ZJ. Sequence specific detection of bacterial 23S ribosomal RNA by TLR13. elife. 2012;1:e00102. doi: 10.7554/eLife.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Yang Y, Dai J, Medzhitov R, Freudenberg MA, Zhang PL, Li Z. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol. 2006;177:6880–6888. doi: 10.4049/jimmunol.177.10.6880. [DOI] [PubMed] [Google Scholar]
- Liu W, Deyoung BR, Chen X, Evanoff DP, Luo Y. RDP58 inhibits T cell-mediated bladder inflammation in an autoimmune cystitis model. J Autoimmun. 2008;30:257–265. doi: 10.1016/j.jaut.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZQ, Deng GM, Foster S, Tarkowski A. Staphylococcal peptidoglycans induce arthritis. Arthritis Res. 2001;3:375–380. doi: 10.1186/ar330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Ronnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjogren’s syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nat Rev Rheumatol. 2010;6:727–730. doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21:3352–3357. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- Manoussakis MN, Spachidou MP, Maratheftis CI. Salivary epithelial cells from Sjogren’s syndrome patients are highly sensitive to anoikis induced by TLR-3 ligation. J Autoimmun. 2010;35:212–218. doi: 10.1016/j.jaut.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Mariette X, Gottenberg JE. Pathogenesis of Sjogren’s syndrome and therapeutic consequences. Curr Opin Rheumatol. 2010;22:471–477. doi: 10.1097/BOR.0b013e32833c36c5. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Sommers CD, Bonar SL, Mathialagan S, Schindler JF, Guzova JA, Shaffer AF, Melton MA, Christine LJ, Tripp CS, Chiang PC, Thompson DC, Hu Y, Kishore N. A novel, highly selective, tight binding IkappaB kinase-2 (IKK-2) inhibitor: a tool to correlate IKK-2 activity to the fate and functions of the components of the nuclear factor-kappaB pathway in arthritis-relevant cells and animal models. J Pharmacol Exp Ther. 2009;329:14–25. doi: 10.1124/jpet.108.143800. [DOI] [PubMed] [Google Scholar]
- McCoy CE, O’Neill LA. The role of toll-like receptors in macrophages. Front Biosci. 2008;13:62–70. doi: 10.2741/2660. [DOI] [PubMed] [Google Scholar]
- Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- Meng L, Zhu W, Jiang C, He X, Hou W, Zheng F, Holmdahl R, Lu S. Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats. Arthritis Res Ther. 2010;12:R103. doi: 10.1186/ar3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Rawlings DJ. B cell autonomous TLR signaling and autoimmunity. Autoimmun Rev. 2008;7:313–316. doi: 10.1016/j.autrev.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KH. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Shimizu Y, Sato Y, Miyazaki Y, Satoh T, Mizuno M, Kato Y, Hosaka Y, Furusako S. Toll-like receptor 4 signal transduction inhibitor, M62812, suppresses endothelial cell and leukocyte activation and prevents lethal septic shock in mice. Eur J Pharmacol. 2007;569:237–243. doi: 10.1016/j.ejphar.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PN, Reynolds GM, Waldron EE, Ward E, Giannopoulos K, Murray PG. Monoclonal antibodies. Mol Pathol. 2000;53:111–117. doi: 10.1136/mp.53.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R, Holden NS, Catley MC, Oyelusi W, Leigh R, Proud D, Barnes PJ. Repression of inflammatory gene expression in human pulmonary epithelial cells by small-molecule IkappaB kinase inhibitors. J Pharmacol Exp Ther. 2007;321:734–742. doi: 10.1124/jpet.106.118125. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–290. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Oldenburg M, Kruger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, Bathke B, Lauterbach H, Suter M, Dreher S, Koedel U, Akira S, Kawai T, Buer J, Wagner H, Bauer S, Hochrein H, Kirschning CJ. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337:1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W, Zahringer U, Hartung T, Gobel UB, Schumann RR. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J Biol Chem. 2001;276:22041–22047. doi: 10.1074/jbc.M010481200. [DOI] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Patole PS, Grone HJ, Segerer S, Ciubar R, Belemezova E, Henger A, Kretzler M, Schlondorff D, Anders HJ. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol. 2005;16:1326–1338. doi: 10.1681/ASN.2004100820. [DOI] [PubMed] [Google Scholar]
- Pierer M, Rethage J, Seibl R, Lauener R, Brentano F, Wagner U, Hantzschel H, Michel BA, Gay RE, Gay S, Kyburz D. Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol. 2004;172:1256–1265. doi: 10.4049/jimmunol.172.2.1256. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, Joosten LA, van den Berg WB. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Duffy KE, Jordan JL, Eaton-Bassiri A, Vaughan R, Hoose SA, Lamb RJ, Sarisky RT, Kao CC. Single-stranded oligonucleotides can inhibit cytokine production induced by human toll-like receptor 3. Mol Cell Biol. 2008;28:4507–4519. doi: 10.1128/MCB.00308-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid T, Ebringer A. Rheumatoid arthritis is linked to Proteus--the evidence. Clin Rheumatol. 2007;26:1036–1043. doi: 10.1007/s10067-006-0491-z. [DOI] [PubMed] [Google Scholar]
- Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Roelofs MF, Abdollahi-Roodsaz S, Joosten LA, van den Berg WB, Radstake TR. The orchestra of Toll-like receptors and their potential role in frequently occurring rheumatic conditions. Arthritis Rheum. 2008;58:338–348. doi: 10.1002/art.23217. [DOI] [PubMed] [Google Scholar]
- Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, van Lieshout AW, Sprong T, van den Hoogen FH, van den Berg WB, Radstake TR. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- Roelofs MF, Wenink MH, Brentano F, Abdollahi-Roodsaz S, Oppers-Walgreen B, Barrera P, van Riel PL, Joosten LA, Kyburz D, van den Berg WB, Radstake TR. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA) Ann Rheum Dis. 2009;68:1486–1493. doi: 10.1136/ard.2007.086421. [DOI] [PubMed] [Google Scholar]
- Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106:2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E, Manetti M, Guiducci S, Ceccarelli C, Allanore Y, Matucci-Cerinic M. The genetics of systemic sclerosis: an update. Clin Exp Rheumatol. 2011;29:S75–86. [PubMed] [Google Scholar]
- Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- Rutz M, Metzger J, Gellert T, Luppa P, Lipford GB, Wagner H, Bauer S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur J Immunol. 2004;34:2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- Sacre SM, Lo A, Gregory B, Simmonds RE, Williams L, Feldmann M, Brennan FM, Foxwell BM. Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J Immunol. 2008;181:8002–8009. doi: 10.4049/jimmunol.181.11.8002. [DOI] [PubMed] [Google Scholar]
- Savarese E, Chae OW, Trowitzsch S, Weber G, Kastner B, Akira S, Wagner H, Schmid RM, Bauer S, Krug A. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- Schopf L, Savinainen A, Anderson K, Kujawa J, DuPont M, Silva M, Siebert E, Chandra S, Morgan J, Gangurde P, Wen D, Lane J, Xu Y, Hepperle M, Harriman G, Ocain T, Jaffee B. IKKbeta inhibition protects against bone and cartilage destruction in a rat model of rheumatoid arthritis. Arthritis Rheum. 2006;54:3163–3173. doi: 10.1002/art.22081. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, Michel BA, Seger RA, Gay S, Lauener RP. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spachidou MP, Bourazopoulou E, Maratheftis CI, Kapsogeorgou EK, Moutsopoulos HM, Tzioufas AG, Manoussakis MN. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren’s syndrome. Clin Exp Immunol. 2007;147:497–503. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabiasco J, Devevre E, Rufer N, Salaun B, Cerottini JC, Speiser D, Romero P. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- Tamaki Y, Takakubo Y, Hirayama T, Konttinen YT, Goodman SB, Yamakawa M, Takagi M. Expression of Toll-like receptors and their signaling pathways in rheumatoid synovitis. J Rheumatol. 2011;38:810–820. doi: 10.3899/jrheum.100732. [DOI] [PubMed] [Google Scholar]
- Tao K, Fujii M, Tsukumo S, Maekawa Y, Kishihara K, Kimoto Y, Horiuchi T, Hisaeda H, Akira S, Kagami S, Yasutomo K. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann Rheum Dis. 2007;66:905–909. doi: 10.1136/ard.2006.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassiulas IO, T. Boumpas DT. Clinical Features and Treatment of Systemic Lupus Erythematosus. In: Firestein GS, Budd RC, Harris ED Jr., McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology, 8th ed. 8th ed. 2008. [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, Opal SM. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med. 2010;38:72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]
- Travis S, Yap LM, Hawkey C, Warren B, Lazarov M, Fong T, Tesi RJ. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm Bowel Dis. 2005;11:713–719. doi: 10.1097/01.mib.0000172807.26748.16. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Ungaro R, Fukata M, Hsu D, Hernandez Y, Breglio K, Chen A, Xu R, Sotolongo J, Espana C, Zaias J, Elson G, Mayer L, Kosco-Vilbois M, Abreu MT. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1167–1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- van Bon L, Popa C, Huijbens R, Vonk M, York M, Simms R, Hesselstrand R, Wuttge DM, Lafyatis R, Radstake TR. Distinct evolution of TLR-mediated dendritic cell cytokine secretion in patients with limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2010;69:1539–1547. doi: 10.1136/ard.2009.128207. [DOI] [PubMed] [Google Scholar]
- van Lieshout AW, Vonk MC, Bredie SJ, Joosten LB, Netea MG, van Riel PL, Lafyatis R, van den Hoogen FH, Radstake TR. Enhanced interleukin-10 production by dendritic cells upon stimulation with Toll-like receptor 4 agonists in systemic sclerosis that is possibly implicated in CCL18 secretion. Scand J Rheumatol. 2009;38:282–290. doi: 10.1080/03009740802572467. [DOI] [PubMed] [Google Scholar]
- Vanags D, Williams B, Johnson B, Hall S, Nash P, Taylor A, Weiss J, Feeney D. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet. 2006;368:855–863. doi: 10.1016/S0140-6736(06)69210-6. [DOI] [PubMed] [Google Scholar]
- Varga J, Denton CP. Firestein GS, Budd RC, Harris ED Jr., McInnes IB, Ruddy S, Sergent JS, editors. Systemic Sclerosis and the Scleroderma-Spectrum Disorders. Kelley’s Textbook of Rheumatology, 8th ed. (8th ed.) 2008 [Google Scholar]
- Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, Underhill DM, Kirschning CJ, Wagner H, Aderem A, Tobias PS, Ulevitch RJ. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- Wyllie DH, Kiss-Toth E, Visintin A, Smith SC, Boussouf S, Segal DM, Duff GW, Dower SK. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J Immunol. 2000;165:7125–7132. doi: 10.4049/jimmunol.165.12.7125. [DOI] [PubMed] [Google Scholar]
- Yamashita TS, Khan MA, Kushner I. Genetic analysis of families with multiple cases of rheumatoid arthritis. Dis Markers. 1986;4:113–119. [PubMed] [Google Scholar]
- Yasuda K, Richez C, Uccellini MB, Richards RJ, Bonegio RG, Akira S, Monestier M, Corley RB, Viglianti GA, Marshak-Rothstein A, Rifkin IR. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J Immunol. 2009;183:3109–3117. doi: 10.4049/jimmunol.0900399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki A, Komura K, Iwata Y, Ogawa F, Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Sato S. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: association with disease severity. J Clin Immunol. 2009;29:180–189. doi: 10.1007/s10875-008-9252-x. [DOI] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Yu P, Wellmann U, Kunder S, Quintanilla-Martinez L, Jennen L, Dear N, Amann K, Bauer S, Winkler TH, Wagner H. Toll-like receptor 9-independent aggravation of glomerulonephritis in a novel model of SLE. Int Immunol. 2006;18:1211–1219. doi: 10.1093/intimm/dxl067. [DOI] [PubMed] [Google Scholar]
- Yu X, Song B, Huang C, Xiao Y, Fang M, Feng J, Wang P, Zhang G. Prolonged survival time of allografts by the oral administration of RDP58 linked to the cholera toxin B subunit. Transpl Immunol. 2012 doi: 10.1016/j.trim.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Zeuner RA, Ishii KJ, Lizak MJ, Gursel I, Yamada H, Klinman DM, Verthelyi D. Reduction of CpG-induced arthritis by suppressive oligodeoxynucleotides. Arthritis Rheum. 2002;46:2219–2224. doi: 10.1002/art.10423. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zhang Z, Yu C, Yang C. Expression of Toll-like receptors 7, 8, and 9 in primary Sjogren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:844–850. doi: 10.1016/j.tripleo.2010.01.006. [DOI] [PubMed] [Google Scholar]