Abstract
Vestibular compensation is the process of behavioral recovery following peripheral vestibular lesion. In clinics, the histaminergic medicine is the most widely prescribed for the treatment of vertigo and motion sickness, however, the molecular mechanisms by which histamine modulates vestibular function remain unclear. During recovery from the lesion, the modulation of histamine receptors in the medial vestibular nucleus (MVN) and the flocculus may play an important role. Here with the means of quantitative real-time PCR, western blotting and immunohistochemistry, we studied the expression of histamine receptors (H1, H2, and H3) in the bilateral MVN and the flocculus of rats on the 1st, 3rd, and 7th day following unilateral labyrinthectomy (UL). Our results have shown that on the ipsi-lesional flocculus the H1, H2 and H3 receptors mRNA and the protein increased significantly on the 1st and 3rd day, with compare of sham controls and as well the contralateral side of UL. However, on the 7th day after UL, this expression returned to basal levels. Furthermore, elevated mRNA and protein levels of H1, H2 and H3 receptors were observed in the ipsi-lesional MVN on the 1st day after UL compared with sham controls and as well the contralateral side of UL. However, this asymmetric expression was absent by the 3rd post-UL. Our findings suggest that the upregulation of histamine receptors in the MVN and the flocculus may contribute to rebalancing the spontaneous discharge in bilateral MVN neurons during vestibular compensation.
Introduction
Unilateral labyrinthectomy (UL) induces characteristic postural and oculomotor asymmetry in rodents, many of the deficits subside within a week after UL, the so-called period of adaptive plasticity, which is described as vestibular compensation. [1] The neurophysiological basis of vestibular compensation is the asymmetrical mass discharge on bilateral neurons in vestibular nuclear complex after UL. [2], [3] However, it is still not clear about the molecular mechanisms of vestibular compensation. Because UL induced a permanent destroy of vestibular end organ, the compensatory process has been attributed to central nervous system plasticity. [4], [5] Many regions of the brain such as the medial vestibular nucleus (MVN) and the flocculus have been shown to be involved in the process of vestibular compensation [6].
In the process of vestibular compensation, it has been suggested that the flocculus plays a crucial role. For instance, in behaviour research it has been shown that vestibular compensation may be delayed dramatically by two ways. [2], [7] The one is ablation of the vestibule-cerebellum, particularly paraflocculus-flocculus, uvula and nodulus, and the other is disruption of climbing fiber inputs to the flocculus. Even after compensation, flocculectomy leads to the reappearance of UL-induced behavioral deficits. [8] Recently, some neuroanatomical data [9], [10] have indicated that the floccular target neurons are localized to the rostral MVN, and these neurons receive monosynaptic inhabitation from the flocculus. In electrophysiological experiments, it has been reported that both the inhibitory signals from the cerebellar Purkinje cells and the commissural inhibition system mediate the silencing of ipsi-lesional MVN neurons after UL. [8] Hence, the MVN and flocculus may be the both important areas in vestibular compensation, although the neurochemical mechanism involved in this process remains largely unrevealed.
Previous studies have indicated that many neurotransmitters, such as Gamma-amino-butyric acid (GABA), glutamate, glycine, histamine and so on are involved in the process of vestibular compensation. [11] Histamine acts mainly as a neuromodulator rather than a neurotransmitter in the central nervous system. [12], [13] Furthermore, H3 receptor mediate presynaptic inhibition of releasing other neurotransmitters including: noradrenaline, serotonin, dopamine, glutamate, and GABA. [14], [15] All of the above neurotransmitters are implicated in central vestibular neurotransmission. [16] In clinics, the histaminergic medicine is the most widely prescribed for the treatment of vertigo and motion sickness, although the molecular mechanisms by which histamine modulates vestibular function remain unclear. [17], [18] In behaviour research of cats, it has been shown that betahistine and thioperamide both strongly accelerated behavioral recovery following UL. The first one is an H3 receptor antagonist and a weak H1 receptor agonist, and the later one is a more selective H3 receptor antagonist, the influence on behaviour may be due to an increased release of histamine in the brain. [19] Some neuroanatomical studies have shown that the soma of histaminergic neurons are located in the tuberomammillary nucleus of the posterior hypothalamic region, these histaminergic neurons send axonal projections to the whole vestibular nuclei complex and cerebellum. [20], [21] Different histamine receptors distribute differently on the neurons of the MVN and the flocculus. The H1 and H2 receptors are located postsynaptically, while the H3 receptor is located presynaptically. It has been shown that the H3 autoreceptor is located on the afferent histaminergic fibers of the neurons in the MVN and the flocculus locates. The H1, H2 and H3 heteroreceptors may be located either on the perykaria [22] or on the non-histaminergic afferents fibers of the neurons in the MVN and the flocculus. [1], [22]–[24] Functionally, it has been reported that the histamine receptors have an excitatory effect on both the MVN and the cerebellar neurons. [23]–[26] Taken together, the histamine receptors in the MVN and the flocculus may play a crucial role in vestibular compensation.
However, previous studies in different species have provided inconsistent data on histamine receptors mRNA and protein expression in the MVN during vestibular compensation. For instance, Tighilet et al [27] reported that the levels of H3 receptor binding were downregulated in the MVN of cats following unilateral vestibular neurectomy. On the contrary, Lozada et al [28] found H3 receptor mRNA to be upregulated in the MVN of rats after UL. Therefore, it remains unclear how the histamine receptors are expressed indeed with vestibular compensation. How do the expression of H1, H2 and H3 receptors mRNA and the corresponding protein levels of the MVN and flocculus change after UL (for example, on the 1st, the 3rd and the 7th day)? Is the performance of gene expression activities in the ipsi-lesional MVN and flocculus different from that in the contra-lesional side, and from sham control as well? All these questions will be answered in the end of our studies.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Huazhong University of Science and Technology (Permit Number: S251). All surgery was performed under a mixture of ketamine and chlorpromazine anesthesia, and all efforts were made to minimize suffering.
Animal Model
Adult male Sprague-Dawley rats (200–250 g) were obtained from the experimental animal center of Tongji Medical College, Huazhong University of Science and Technology. In despite of poor visual acuity, [29], [30] the albino rats have well gained rebalance after UL, so vestibular compensation process of the albino rats is reliable and complete. Hence, the unilateral labyrinthectomy on albino rats is a mature and generally accepted animal model in vestibular compensation. Furthermore, some researches indicated that the albino rats strain (Sprague-Dawley rats) has better auditory acuity and excelling in skilled reaching compared to other rat strains. [31], [32] The animals had free access to food and water. A total number of 90 rats were divided into three groups, as follows: 36 rats for quantitative real-time PCR, 36 rats for western blotting and 18 rats for immunohistochemistry. Experimental animals in each group were randomly divided into sham controls and experimental groups on the 1st, 3rd and 7th day after UL. The post-surgery times were chosen based on previous reports in rats. [2], [33] The 1st day after UL represents the acute, uncompensated stage when spontaneous nystagmus is vigorous and postural asymmetry is severe. By the 3rd day, these symptoms have partly diminished, and by the 7th day, the static symptoms have substantially compensated but dynamic reflex deficits remain. After decapitation under anesthesia (ketamine-chlorpromazine mixture 10∶1, 2 ml/kg, intraperitoneal injection), a complete surgery was performed on the right side under an operating microscope according to the method reported by Kitahara et al. [34] Firstly, the tympanic bulla was exposed by a retroauricular approach and opened using the surgical electrodrill, and then the stapedial artery was exposed for coagulation. Secondly, after a small hole was made around the oval window using the surgical electrodrill, the membranous labyrinth was removed and aspirated with a suction pump. Thirdly, absolute alcohol was injected into the opening to ensure that the vestibule was completely destroyed. At the end of surgery, the wound was sutured. For a control of confounded effects of anesthesia and unilateral soft tissue injury, rats were submitted to a sham operation under the same anesthesia, the tympanic bulla on the right side was opened, but the membranous labyrinth was not destroyed before suture. It has been reported that alcohol can influence the histamine level in some regions of the brain by alcohol consumption (drinking) both in rodents and in humans. [35], [36] However, the change patterns of histamine receptors expression levels after UL using only the mechanical UL method in our preliminary experiment were well coincident with that by a combined UL method (Fig. S1).
Quantitative Real-time PCR
Quantitative analysis of the H1, H2 and H3 receptors mRNA in the MVN and the flocculus was performed by real-time PCR. On the 1st, 3rd and 7th day after UL or sham operation, 36 rats (n = 6 per group) were killed by cervical dislocation without anesthesia. Following the rat brain atlas of Paxinos and Watson, [37] the bilateral flocculus was carefully removed from each brain and the bilateral MVN was dissected under a microscopic using the detailed procedures as described previously by Horii et al. [6] Total RNA was extracted using an RNeasy mini kit (Axygen, USA) according to the manufacturer’s instructions. cDNA was reverse transcribed using a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Japan). The RNA and cDNA of each sample were analysed using a GeneQuant pro RNA Calculator to assess the concentrations and purity. Quantitative real-time PCR was performed with real-time SYBR Green PCR reagents and the 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Validated primers were designed for each target mRNA. The primer pairs for H1, H2, H3 and an internal standard (β-actin) were as follows: H1 forward, 5′-CTGGTCACAGTGGGCCTCAA-3′; H1 reverse, 5′-CTGCCACAGACAGGCT-GACAA-3′; H2 forward, 5′-ATGGAGCCCAATGGCACAG-3′; H2 reverse, 5′-GCCAGCAATGGTGATGAGGA-3′; H3 forward, 5′-CCTCGGTCTTCAACATC-GTACTCA-3′; H3 reverse, 5′-ACCCACACC ATGCCATCTTC-3′; β-actin forward, 5′-CCTGGAGAAGAGCTATGAGC-3′; β-actin reverse, 5′-ACAGGATTCCATACC-CAGG-3′. The amplification conditions were as follows: 1 min at 95°C, and then 40 cycles of 15 s at 95°C, 20 s at 60°C, and 35 s at 72°C. An internal standard was used to normalize the relative gene expression levels. A melting curve analysis was performed for each gene, and the specificity and integrity of the PCR products were confirmed by the presence of a single peak. The relative expression values were calculated from the differences in Ct of the values between the target mRNA and an internal standard (β-actin). The change in the corresponding mRNA levels between the experimental group and the control group was analyzed with the 2−ΔΔCt method, as the previous literature reported [38].
Western Blotting
The aim of this experiment is to quantify the protein expression of H1, H2 and H3 receptors in the MVN and the flocculus. Animals were selected on the 1st, 3rd and 7th day after UL, and similarly with sham operation as well. Totally 36 rats (n = 6 per group) were executed by cervical dislocation without anaesthesia. Following the rat brain atlas of Paxinos and Watson, [37] the bilateral flocculus was carefully removed from each brain and the bilateral MVN was dissected under a microscopic using the detailed procedures as described previously by Horii et al. [6] The total protein in the MVN and the flocculus was extracted using a RIPA Lysis Buffer (Beyotime, Haimen, China) according to the manufacturer’s instructions. Protein concentrations were determined with an Enhanced BCA Protein Assay Kit (Beyotime, Haimen, China). Twenty micrograms of each protein lysate was separated by 8% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated for 1 hour in a blocking solution of TBS containing 5% non-fat milk, washed briefly in TBS and incubated overnight at 4°C with the appropriate dilution of the following primary antibodies: anti-H1 (Santa Cruz, USA, diluted 1∶500), anti-H2 (Santa Cruz, USA, diluted 1∶250) or anti-H3 antibody (Santa Cruz, USA, diluted 1∶500). After washing the membranes to remove excess primary antibody, the membranes were incubated for 1 h at room temperature with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (Beyotime, Haimen, China, diluted 1∶5000). Membranes were visualized with BeyoECL Plus (Beyotime, Haimen, China). Quantitation of the detected bands was performed with the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., USA). β-actin was used as an internal control.
Immunohistochemistry
Following the completion of the quantitative real-time PCR and western blotting studies, immunohistochemistry was used to label and describe the distribution of the H1, H2 and H3 receptors on neurons of the flocculus and the MVN. On the 1st, 3rd and 7th day after UL or sham operation, 18 rats (n = 3 per group) were anesthetized deeply with a ketamine-chlorpromazine mixture (10∶1, 2 ml/kg, intraperitoneal injection), and perfused transcardially with a 0.9% normal saline wash followed by fixative (4% paraformaldehyde in 0.1 M phosphate buffer). After decapitating the bodies, the temporal bones were removed, and the brains were dissected out. The cerebellum and brain stem portion were fixed in the paraformaldehyde-phosphate buffer for 12 hours at 4°C, rinsed with distilled water for half an hour, dehydrated with a graded alcohol series, cleared in xylene, immersed in paraffin, and then embedded in paraffin. Finally, brainstems with flocculus were sectioned at 5 µm and collected on poly-L-lysine-coated glass slides.
After the sections were deparaffinized, they were incubated overnight at 4°C in anti-H1, anti-H2 or anti-H3 antibody (Santa Cruz, USA, diluted 1∶100). The sections were washed and incubated in a goat anti-rabbit secondary antibody (Beyotime, Haimen, China) for 60 minutes at room temperature. The nuclei were counter-stained with a DAPI staining solution (Beyotime, Haimen, China) for 5 min at room temperature. After washing with PBS, the sections were examined under a laser scanning confocal microscope (Nikon, Japan).
Neurons Counting
Three animals from each group were used for neurons counting analysis. Four sections from the rostral to the caudal level of MVN or flocculus (Bregma −9.96 mm - Bregma −12.00 mm) in each rat were selected for counting the H1, H2 and H3 receptors-positive neurons following the guidance of the atlas [37]. Two sections were selected from the rostral and the caudal part of the brain [39] (Bregma −9.96 mm - Bregma −12.00 mm) including the MVN or flocculus using the guidance of the atlas. [37] They represented the rostral and caudal zones of Purkinje cell/climbing fiber zones, which control the eye movement in all vertical planes from sagittal to transverse planes. Two sections at 50 µm intervals were selected from the middle part of the brain [40] (Bregma −9.96 mm - Bregma −12.00 mm) including the MVN or flocculus using the guidance of the atlas. [37] They represented the middle zone of Purkinje cell/climbing fiber zones, which controls the eye movement in the horizontal plane [41], [42].
The number of H1, H2 and H3 receptors-positive neurons was counted in each corresponding region of the visual system in the same area of the bilateral flocculus and the bilateral MVN, with the number of labeled neurons expressed per unit area (mm2). [43], [44] Neurons were counted only if their nuclei were completely within the margins of the visual system by using Image-Pro-Plus (6.0) software (Media Cybernetics, Inc., USA). All images were acquired using the same exposure time and illumination conditions. Histological quantification was conducted by an investigator blinded to the experimental status of the animals.
Statistical Analysis
Data are presented as means±SEM. The analysis was performed with SPSS 13.0 software (SPSS Inc., USA). All data were analyzed using one-way analysis of variance (ANOVA) followed by student Newman-Keuls multiple comparison tests. ANOVAs were performed only on data that were obtained from the same gels and 96-well plates. Because we ran tissue from the ipsi-lesional, sham and contra-lesional conditions on the one set of gels and 96-well plates, data were analysed separately for the 1st day, 3rd day and 7th day condition. This meant that comparisons between the ipsi-lesional, sham and contra-lesional conditions were not confounded by changes in the background density between different gels and 96-well plates. The significance level was set at 0.05 for all comparisons.
Results
The results are analysed and presented from three different aspects. 1) By real-time PCR we can analyse quantitatively the H1, H2 and H3 receptors mRNA expression in different stage after UL. 2) The corresponding H1, H2 and H3 receptors protein expression is quantified with the means of western blotting. 3) The location and distribution of H1, H2 and H3 receptors is analysed by immunostaining of the brain sections, particularly the MVN and the flocculus.
Behavior Observation after UL
Immediately after recovery from anaesthesia, rats that underwent UL manifested diverse behavioural symptoms: head tilting toward the operated side, limb extending on the intact side (Fig. 1A), barrel rolling (Fig. 1a–f), circling walk (Fig. 1g–n) and spontaneous nystagmus. These symptoms largely disappeared with the 1st day to the 7th day after UL.
Figure 1. Behavior observation after UL.
Behavioral asymmetries after UL on the right side. (A) The animal laid on the lesioned (right) side and showed hypotonia in the ipsilateral limbs, while the contralateral limbs were hypertonic, the head tilting towards to the operated side. (a–f) and (g–n) show the barrel rolling and circling walk, respectively.
UL Increases the mRNA Levels of H1, H2 and H3 Receptors in the Ipsi-lesional Flocculus and MVN
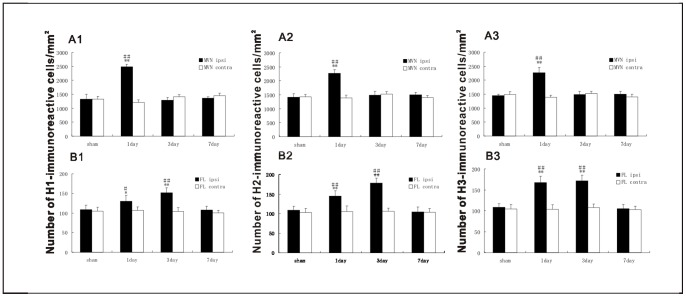
On the 1st and the 3rd day after UL, the mRNA expression of H1 (Fig. 2B1), H2 (Fig. 2B2) and H3 (Fig. 2B3) receptors on the ipsi-lesional flocculus was significantly increased, compared with that on the contra-lesional flocculus and with that of the control group as well. However, on the 7th day, the differences between them became minimal. Please note, the contra-lesional side and the sham controls have no related changes of the mRNA expression (Fig. 2B1–3). Moreover, in the ipsi-lesional MVN, the mRNA levels of the H1 (Fig. 2A1), H2 (Fig. 2A2) and H3 (Fig. 2A3) receptors underwent a large and significant increase on the 1st day after UL compared with either the contra-lesional side or the sham controls. On the other side, on the 3rd and the 7th day after UL, the difference between them vanished. Similarly, the contra-lesional side and the sham controls did not show any obvious change in the data (Fig. 2A1–3).
Figure 2. The mRNA expression of H1, H2 and H3 receptors.
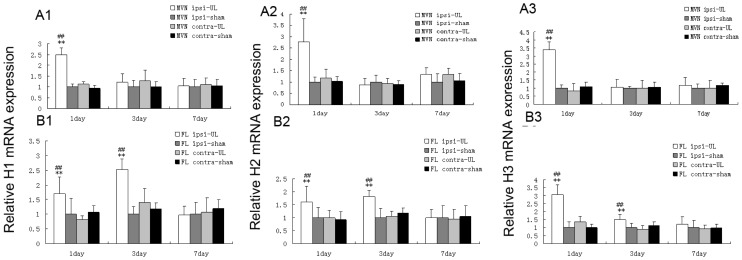
Quantitative analysis of the H1, H2 and H3 receptors mRNA in the MVN (A1–3) and the flocculus (B1–3) on the 1st, 3rd and 7th day following UL or sham operation. The H1, H2 and H3 receptors mRNA levels significantly increased in the ipsi-lesional MVN on the 1st day following UL, and H1, H2 and H3 receptors mRNA levels increased in ipsi-lesional flocculus on the 1st and 3rd day following UL as well. Columns represent means±SEM of 6 rats per group. **P<0.01 vs. sham; ## P<0.01 vs. contra. FL, flocculus; MVN, medial vestibular nucleus.
UL Increases the Protein Expression of H1, H2 and H3 Receptors in the Ipsi-lesional Flocculus and MVN
Similar to mRNA expression of H1, H2 and H3 receptors, the expression of corresponding H1 (Fig. 3A2–3 and Fig.4 B1), H2 (Fig. 3B2–3 and Fig. 4B2) and H3 (Fig. 3C2–3 and Fig. 4B3) receptors proteins in the ipsi-lesional flocculus was increased significantly, compared with both that on the contra-lesional side and in the control group on the 1st and the 3rd day after UL. On the 7th day, the difference between them was minimal, while the contra-lesional side and the sham controls have no related changes of the protein expression (Fig. 4B1–3). Additionally, in the ipsi-lesional MVN, the protein levels of the H1 (Fig. 3A1 and Fig. 4A1), H2 (Fig. 3B1 and Fig. 4A2) and H3 (Fig. 3C1 and Fig. 4A3) receptors were significantly increased on the 1st day after UL compared with both that on the contra-lesional side and in the control group, while on the 3rd and 7th day after UL, the difference was minimal. Similarly, the contra-lesional side and the sham controls did not show any obvious change in the data (Fig. 4A1–3).
Figure 3. Immunoblot of histamine receptors in the flocculus and the MVN.
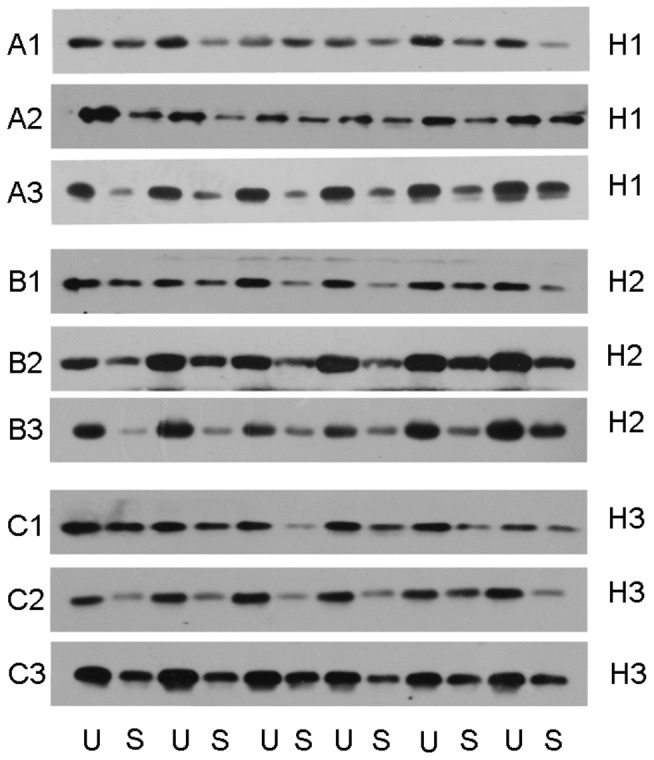
Samples assayed are shown as follows: H1 (A1, 1 day post-UL in the MVN; A2, 1 day post-UL in the flocculus; A3 3 day post-UL in the flocculus) H2 (B1, 1 day post-UL in the MVN; B2 1 day post-UL in the flocculus; B3 3 day post-UL in the flocculus) H3 (C1, 1 day post-UL in the MVN; C2 1 day post-UL in the flocculus; C3 3 day post-UL in the flocculus). U, unilateral labyrinthectomy; S, Sham operation.
Figure 4. H1, H2 and H3 receptors protein expression.
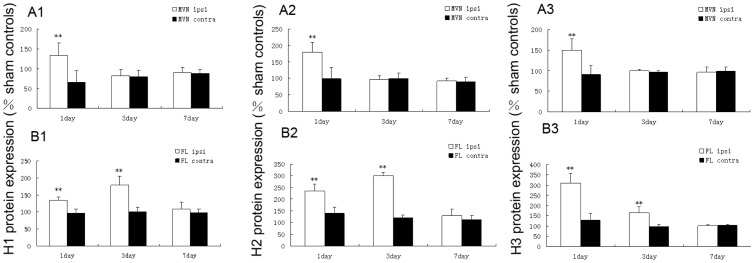
The expression is expressed as a percentage of sham controls, in the MVN (A1–3) and flocculus (B1–3) on the 1st, 3rd and 7th day following UL. The protein levels of the H1, H2 and H3 receptors significantly increased in the ipsi-lesional MVN on the 1st day following UL, and protein levels of the H1, H2 and H3 receptors increased in ipsi-lesional flocculus on the 1st and 3rd day following UL as well. Columns represent means±SEM of 6 rats per group. **P<0.01 vs. sham. ipsi, ipsi-lesional side, contra, contra-lesional side.
UL Induces the Asymmetrical Distribution of H1, H2 and H3 Receptors in Bilateral Flocculus and MVN
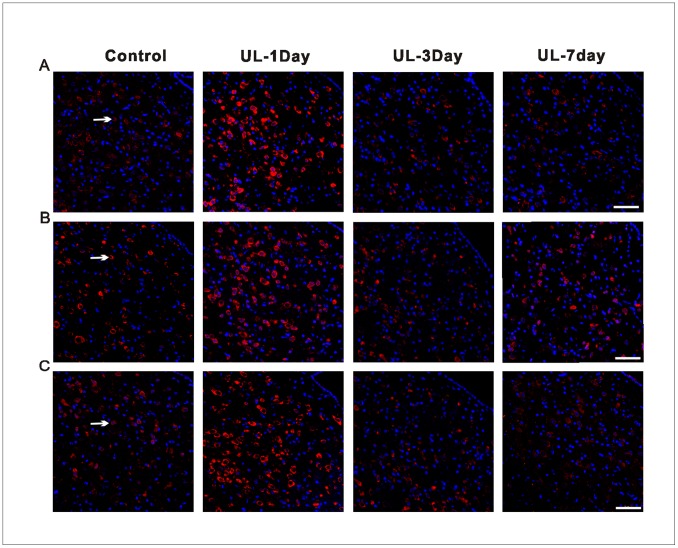
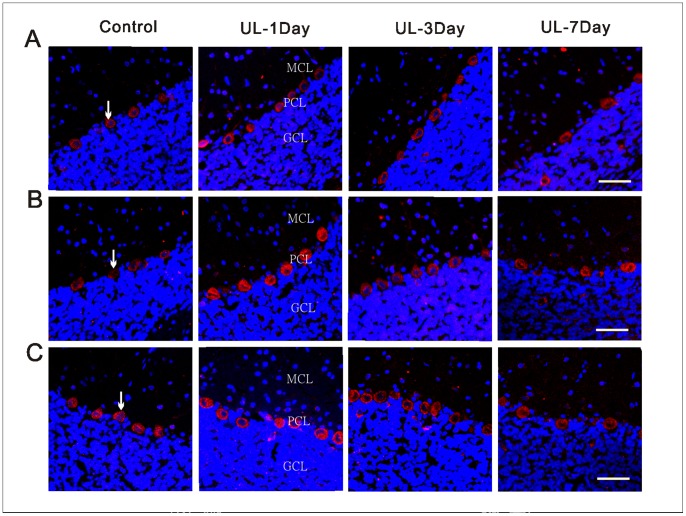
The H1, H2 and H3 receptors were detected by the immunohistochemistry technique on both the MVN and the flocculus. The H1 (Fig. 5A), H2 (Fig. 5B) and H3 (Fig. 5C) receptors of the MVN neurons were immunostained in the membranes and cytoplasm. In the control groups, there were no significant differences in the number of H1, H2 and H3 receptors-positive neurons in the MVN and flocculus between the four sections (Fig. S2). The H1, H2 and H3 receptors-positive neurons were symmetrically distributed between bilateral MVN. With the UL groups, at the 1st day, the H1, H2 and H3 receptors-positive neurons distributed asymmetrically between bilateral MVN. That is, the number of H1 (Fig. 6A1), H2 (Fig. 6A2) and H3 (Fig. 6A3) receptors-positive neurons was significantly increased in the ipsi-lesional MVN compared with either that in the contra-lesional side or that in the control groups. While at the 3rd and 7th day the asymmetrical distribution is minimal. In the flocculus, the H1 (Fig. 7A), H2 (Fig. 7B) and H3 (Fig. 7C) receptors-positive neurons were concentrated in the molecular and Purkinje cell layers, especially in the Purkinje cell layers. The H1, H2 and H3 receptors-positive neurons were symmetrically distributed between bilateral flocculus in control groups. At the 1st and 3rd day after UL, the H1, H2 and H3 receptors-positive neurons distributed asymmetrically between the bilateral flocculus. That is, the number of H1 (Fig. 6B1), H2 (Fig. 6B2) and H3 (Fig. 6B3) receptors-positive neurons was significantly increased in the ipsi-lesional flocculus compared with either that in the contra-lesional side or that in the control groups. While at the 7th day, this asymmetrical distribution disappeared again.
Figure 5. Localization and distribution of histamine receptors in the MVN.
Representative confocal images showing the localization and distribution of H1 (A), H2 (B) and H3 (C) receptors in the same area of the ipsi-lesional MVN following UL or sham operation at different postoperative days. Arrows show the H1-, H2- and H3 receptors -positive neurons. UL-1day, 1 day after UL; UL-3day, 3 day after UL, UL-7day, 7 day after UL; Control, with intact labyrinths; Calibration bar = 50 µm.
Figure 6. Changes in the number of H1, H2 and H3 receptors-positive neurons in the MVN and the flocculus after UL or sham operation.
ipsi, ipsi-lesional side, contra, contra-lesional side. Number of H1, H2 and H3 receptors-positive neurons in each corresponding region of the visual system was expressed per unit area (mm2). **P<0.01 vs. sham; ## P<0.01 vs. contra; *P<0.05 vs. sham; # P<0.05 vs. contra.
Figure 7. Localization and distribution of histamine receptors in the flocculus.
Representative confocal images showing the expression and localization of the H1 (A), H2 (B) and H3 (C) receptors in the same area of the ipsi-lesional flocculus following UL or sham operation at different post-operative days. Arrows show the H1-, H2- and H3 receptors -positive neurons. UL-1day, 1 day after UL; UL-3day, 3 day after UL; UL-7day, 7 day after UL; Control, with intact labyrinths; GCL, granule cell layer; PCL, Purkinje cell layer; MCL, molecular cell layer. Calibration bar = 40 µm.
Discussion
Here we demonstrated the spatiotemporal dynamics of H1, H2 and H3 receptors induction in the MVN and the flocculus on the 1st, 3rd and 7th day after UL. The present study provides the quantitative and systematic data regarding H1, H2 and H3 receptors mRNA and protein levels in the cerebello-vestibular pathway during the process of vestibular compensation. After the UL treatment with rats, there is an increase in both mRNA and protein levels of H1, H2 and H3 receptors in the ipsi-lesional flocculus and the MVN. To the best of our knowledge, this study is the first molecular investigation of histamine receptors expression in the cerebello-vestibular pathway following UL in rats.
Compared with either the contra-lesional side or with the sham controls, the ipsi-lesional flocculus had significantly higher expression of the H1, H2 and H3 receptors on the 1st and 3rd day after UL, while such enhancement disappeared at the 7th day after UL. The cerebello-vestibular pathway, originated from Purkinje cells of the anterior cerebellar vermis, mediates monosynaptic inhibition of the vestibular nucleus neurons. [11], [45] As the major inhibitory neurons, Purkinje cells release GABA, and GABA could be one of the inhibitory neurotransmitters in the cerebello-vestibular pathway. [46] During the process of vestibular compensation, MVN neurons on both sides of the brainstem develop the rebalance of their resting activity, and the flocculus plays an important role in the process. It has been proposed that ipsi-lesional flocculectomy prevented the compensatory increase of intrinsic excitability in the de-afferented MVN neurons after UL [47], [48].
In addition to changes in the intrinsic membrane excitability of bilateral MVN neurons, there is a concurrent, rapid downregulation of the functional efficacy of GABA and glycine receptor expression in the ipsi-lesional MVN after UL. [49]–[52] Previous studies have indicated that histaminergic could inhibit the release of GABA at Purkinje cell synapses in two ways: 1) through a direct action on presynaptic H3 receptor (presumably located on GABAergic terminals), and 2) through a novel, indirect pathway that involved the increased releasing of glycine by activation of postsynaptic H1/H2 receptor (presumably on glycinergic neurons). [12] In our study, the upregulation of H1, H2 and H3 receptors in ipsi-lesional flocculus might abate the inhibitory GABAergic signals from the ipsi-lesional flocculus to the ipsi-lesional MVN neurons, which consequently rebalanced the resting activity in bilateral MVN neurons after UL.
The expression of H1, H2 and H3 receptors in the ipsi-lesional MVN was increased on the 1st day after UL but returned to the level of the control groups on the 3rd and the 7th day after UL. The changes in histamine receptors expression observed in our study are consistent with the temporal changes in the early static vestibular manifestations and the changes in MVN neurons metabolic activity during vestibular compensation. [2], [33] These results are also consistent with Lozada’s data, [28] who found an increase in the mRNA levels of H3 receptor isoforms in MVN 24 h after UL by performing in situ hybridization in rats. While on the 2nd and 7th day post-UL, the mRNA expression returned to control levels. However, using autoradiography, Tighilet et al [27] reported that H3 receptor binding levels were downregulated in the MVN at the 7th day following unilateral vestibular neurectomy in cats, such discrepancy between the different data might be due to differences in experimental performances, operation methods and the animal species tested.
Immediately after UL, the resting activity of ipsi-lesional MVN neurons is largely abolished, while contra-lesional MVN neurons are hyperactive. [33] Ipsi-lesional MVN neurons silencing is mediated by the commissural inhibition system, which links each MVN to its contralateral counterpart. [52] The recovery of resting discharge in the ipsi-lesional MVN neurons and the rebalancing of the resting activity of the bilateral MVN neurons after UL may be achieved by compensatory change in the gains of the commissural inhibition system. [52] This compensatory change after UL is believed to be accompanied and exacerbated by an imbalance in GABA receptor interactions across the bilateral MVN neurons. [53] Therefore, the dynamic regulation of GABAergic inhibitory efficacy potentially rebalances the bilateral excitability of MVN neurons after UL [54].
It has been shown that histamine inhibits the stimulus-evoked release of GABA in the MVN, both through a direct activation of pre-synaptic H3 receptor on GABAergic synaptic terminals, and through an indirect pathway that involves the increased release of glycine by H1/H2 receptor. [12] In response to the compensatory change of commissural inhibition, there is a marked down-regulation of GABA receptor efficacy in the ipsi-lesional MVN neurons after UL during vestibular compensation in vivo. [52] Thus it is possible that endogenous-release of H1, H2 and H3 receptors in the ipsi-lesional MVN may modulate the GABA release in the commissural system after UL. As a result, this over expression of histamine receptors promotes the disinhibition of neurons in the ipsi-lesional MVN neurons, and induces the rebalance of the resting activity of bilateral MVN neurons.
In our study, the expression of histamine receptors in bilateral MVN were compared with each other and with the sham operation controls, and that in bilateral flocculus were compared with each other and with the sham operation controls as well. These experiments were performed in the same situation during the same time for each run. Therefore, these results were stable and confirmable. While the histamine receptors in the contra-lesional MVN and contra-lesional flocculus do not change overtime. It is possible that function of histamine receptors in the contra-lesional MVN and flocculus may change during vestibular compensation as a result of modifications in affinity or efficacy.
In conclusion, we investigated the changes of both the mRNA and the protein expression of H1, H2 and H3 receptors in the MVN and the flocculus at the 1st, the 3rd and the 7th day after UL. Our results have demonstrated that the H1, H2 and H3 receptors are upregulated in both the ipsi-lesional MVN and the ipsi-lesional flocculus in the early stage (within three days) during vestibular compensation, which indicated that histamine receptors in the MVN and flocculus may play roles in the early stage (within three days) of vestibular compensation. In addition, other neurotransmitters in the central nervous system, or histamine receptors in other regions of the central nervous system may participate in the later stage of vestibular compensation. Upregulation of histamine receptors in ipsi-lesional MVN and ipsi-lesional flocculus may reduce the inhibitory effects of flocculus and commissural inhibition system by regulation GABA, so as to rebalance the resting activity of bilateral MVN neurons (Fig. 8C). The histamine play an important role as a neurotransmitter and neuromodulator in the cerebello-vestibular pathway during the process of vestibular compensation. Histaminergic modulation of neurotransmitter release in the cerebello-vestibular pathway is important in vestibular synaptic plasticity and behavioural recovery after UL. However, such aspect is beyond the scope of this study and should be further explored.
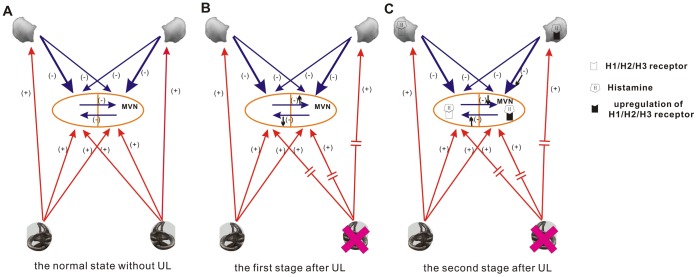
Figure 8. Schematic diagram of the changes in histamine receptors expression in the flocclar-MVN pathways following UL.
A. the normal state without UL: The resting activity of bilateral MVN neurons remains balance because of the inhibitory effects of flocculus and commissural inhibition system and the excitable effects of periphery vestibular organs. B The first stage after UL: Immediately after UL, due to the inhibitory effects of flocculus and commissural inhibition system, the resting activity of ipsi-lesional MVN neurons is largely abolished, while contra-lesional MVN neurons are hyperactive. C The second stage after UL: Upregulation of histamine receptors (H1, H2, H3) in ipsi-lesional MVN and flocculus may reduce the inhibitory effects of flocculus and commissural inhibition system by regulation GABA, so as to rebalance the resting activity of bilateral MVN neurons. MVN, medial vestibular nucleus; UL, unilateral labyrinthectomy.
Supporting Information
Immunoblot of histamine receptors in the MVN and the flocculus after mechanical UL. Samples assayed are shown as follows: A, 1 day post-mechanical UL in the MVN; B, 1 day post-mechanical UL in the flocculus; C, 3 day post- mechanical UL in the flocculus U, unilateral labyrinthectomy; S, sham operation. The protein levels of the H1, H2 and H3 receptors significantly increased in the ipsi-lesional MVN on the 1st day following the mechanical UL, and protein levels of the H1, H2 and H3 receptors increased in ipsi-lesional flocculus on the 1st and 3rd day following mechanical UL as well.
(TIF)
Histograms of the neurons counting of H1, H2 and H3 receptors-positive neurons in the rostral, middle and caudal part of the MVN and the flocculus in control groups. Number of H1, H2 and H3 receptors-positive neurons in each corresponding region of the visual system was expressed per unit area (mm2).
(TIF)
Funding Statement
This work was supported by the National twelfth-Five Year Research Program of China (No. 2012BAI12B02), a grant from National Natural Science Foundation of China (No. 30872865 and No. 30672309) and the Key Project of the National Eleventh-Five Year Research Program of China (No. 2007BAI18B13). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Airaksinen MS, Panula P (1988) The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol 273: 163–186. [DOI] [PubMed] [Google Scholar]
- 2. Darlington CL, Smith PF (2000) Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Prog Neurobiol 62: 313–325. [DOI] [PubMed] [Google Scholar]
- 3. De Zeeuw CI, Berrebi AS (1995) Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. Eur J Neurosci 7: 2322–2333. [DOI] [PubMed] [Google Scholar]
- 4. Galiana HL, Flohr H, Jones GM (1984) A reevaluation of intervestibular nuclear coupling: its role in vestibular compensation. J Neurophysiol 51: 242–259. [DOI] [PubMed] [Google Scholar]
- 5. Ris L, de Waele C, Serafin M, Vidal PP, Godaux E (1995) Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol 74: 2087–2099. [DOI] [PubMed] [Google Scholar]
- 6. Horii A, Smith PF, Darlington CL (2001) Quantitative changes in gene expression of glutamate receptor subunits/subtypes in the vestibular nucleus, inferior olive and flocculus before and following unilateral labyrinthectomy in the rat: real-time quantitative PCR method. Exp Brain Res 139: 188–200. [DOI] [PubMed] [Google Scholar]
- 7. Courjon JH, Flandrin JM, Jeannerod M, Schmid R (1982) The role of the flocculus in vestibular compensation after hemilabyrinthectomy. Brain Res 239: 251–257. [DOI] [PubMed] [Google Scholar]
- 8. Kitahara T, Takeda N, Saika T, Kubo T, Kiyama H (1997) Role of the flocculus in the development of vestibular compensation: immunohistochemical studies with retrograde tracing and flocculectomy using Fos expression as a marker in the rat brainstem. Neuroscience 76: 571–580. [DOI] [PubMed] [Google Scholar]
- 9. Babalian AL, Vidal PP (2000) Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: An in vitro whole brain study. J Neurophysiol 84: 2514–2528. [DOI] [PubMed] [Google Scholar]
- 10. Shin M, Moghadam SH, Sekirnjak C, Bagnall MW, Kolkman KE, et al. (2011) Multiple types of cerebellar target neurons and their circuitry in the vestibulo-ocular reflex. J Neurosci 31: 10776–10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Waele C, Muhlethaler M, Vidal PP (1995) Neurochemistry of the central vestibular pathways. Brain Res Brain Res Rev 20: 24–46. [DOI] [PubMed] [Google Scholar]
- 12. Bergquist F, Ruthven A, Ludwig M, Dutia MB (2006) Histaminergic and glycinergic modulation of GABA release in the vestibular nuclei of normal and labyrinthectomised rats. J Physiol 577: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Z, Hatton GI (2000) Histamine suppresses non-NMDA excitatory synaptic currents in rat supraoptic nucleus neurons. J Neurophysiol 83: 2616–2625. [DOI] [PubMed] [Google Scholar]
- 14. Schlicker E, Malinowska B, Kathmann M, Gothert M (1994) Modulation of neurotransmitter release via histamine H3 heteroreceptors. Fundam Clin Pharmacol 8: 128–137. [DOI] [PubMed] [Google Scholar]
- 15. Brown RE, Haas HL (1999) On the mechanism of histaminergic inhibition of glutamate release in the rat dentate gyrus. J Physiol 515 (Pt 3): 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith PF, Darlington CL (1996) Recent advances in the pharmacology of the vestibulo-ocular reflex system. Trends Pharmacol Sci 17: 421–427. [DOI] [PubMed] [Google Scholar]
- 17.Fischer AJ (1991) Histamine in the treatment of vertigo. Acta Otolaryngol Suppl 479: 24–28. [DOI] [PubMed]
- 18. Lacour M, Sterkers O (2001) Histamine and betahistine in the treatment of vertigo: elucidation of mechanisms of action. CNS Drugs 15: 853–870. [DOI] [PubMed] [Google Scholar]
- 19. Tighilet B, Mourre C, Trottier S, Lacour M (2007) Histaminergic ligands improve vestibular compensation in the cat: behavioural, neurochemical and molecular evidence. Eur J Pharmacol 568: 149–163. [DOI] [PubMed] [Google Scholar]
- 20. Panula P, Yang HY, Costa E (1984) Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A 81: 2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dietrichs E, Haines DE, Roste GK, Roste LS (1994) Hypothalamocerebellar and cerebellohypothalamic projections–circuits for regulating nonsomatic cerebellar activity? Histol Histopathol 9: 603–614. [PubMed] [Google Scholar]
- 22. Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, et al. (2002) A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience 114: 173–193. [DOI] [PubMed] [Google Scholar]
- 23. Pollard H, Moreau J, Arrang JM, Schwartz JC (1993) A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience 52: 169–189. [DOI] [PubMed] [Google Scholar]
- 24. Arrang JM, Drutel G, Garbarg M, Ruat M, Traiffort E, et al. (1995) Molecular and functional diversity of histamine receptor subtypes. Ann N Y Acad Sci 757: 314–323. [DOI] [PubMed] [Google Scholar]
- 25. Wang JJ, Dutia MB (1995) Effects of histamine and betahistine on rat medial vestibular nucleus neurones: possible mechanism of action of anti-histaminergic drugs in vertigo and motion sickness. Exp Brain Res 105: 18–24. [DOI] [PubMed] [Google Scholar]
- 26. Vizuete ML, Traiffort E, Bouthenet ML, Ruat M, Souil E, et al. (1997) Detailed mapping of the histamine H2 receptor and its gene transcripts in guinea-pig brain. Neuroscience 80: 321–343. [DOI] [PubMed] [Google Scholar]
- 27. Tighilet B, Trottier S, Mourre C, Lacour M (2006) Changes in the histaminergic system during vestibular compensation in the cat. J Physiol 573: 723–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lozada AF, Aarnisalo AA, Karlstedt K, Stark H, Panula P (2004) Plasticity of histamine H3 receptor expression and binding in the vestibular nuclei after labyrinthectomy in rat. BMC Neurosci 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Redfern WS, Storey S, Tse K, Hussain Q, Maung KP, et al. (2011) Evaluation of a convenient method of assessing rodent visual function in safety pharmacology studies: effects of sodium iodate on visual acuity and retinal morphology in albino and pigmented rats and mice. J Pharmacol Toxicol Methods 63: 102–114. [DOI] [PubMed] [Google Scholar]
- 30. Heiduschka P, Schraermeyer U (2008) Comparison of visual function in pigmented and albino rats by electroretinography and visual evoked potentials. Graefes Arch Clin Exp Ophthalmol 246: 1559–1573. [DOI] [PubMed] [Google Scholar]
- 31. Overbeck GW, Church MW (1992) Effects of tone burst frequency and intensity on the auditory brainstem response (ABR) from albino and pigmented rats. Hear Res 59: 129–137. [DOI] [PubMed] [Google Scholar]
- 32. Nikkhah G, Rosenthal C, Hedrich HJ, Samii M (1998) Differences in acquisition and full performance in skilled forelimb use as measured by the 'staircase test' in five rat strains. Behav Brain Res 92: 85–95. [DOI] [PubMed] [Google Scholar]
- 33. Curthoys IS, Halmagyi GM (1999) Vestibular compensation. Adv Otorhinolaryngol 55: 82–110. [DOI] [PubMed] [Google Scholar]
- 34. Kitahara T, Takeda N, Emson PC, Kubo T, Kiyama H (1997) Changes in nitric oxide synthase-like immunoreactivities in unipolar brush cells in the rat cerebellar flocculus after unilateral labyrinthectomy. Brain Res 765: 1–6. [DOI] [PubMed] [Google Scholar]
- 35. Lintunen M, Hyytia P, Sallmen T, Karlstedt K, Tuomisto L, et al. (2001) Increased brain histamine in an alcohol-preferring rat line and modulation of ethanol consumption by H(3) receptor mechanisms. FASEB J 15: 1074–1076. [DOI] [PubMed] [Google Scholar]
- 36. Lozeva V, Tuomisto L, Tarhanen J, Butterworth RF (2003) Increased concentrations of histamine and its metabolite, tele-methylhistamine and down-regulation of histamine H3 receptor sites in autopsied brain tissue from cirrhotic patients who died in hepatic coma. J Hepatol 39: 522–527. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G WC (2007) The Rat Brain in Stereotaxic Coordinates, 6th edition.: Academic Press, New York.
- 38. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 39. Mizukoshi A, Kitama T, Omata T, Ueno T, Kawato M, et al. (2000) Motor dynamics encoding in the rostral zone of the cat cerebellar flocculus during vertical optokinetic eye movements. Exp Brain Res 132: 260–268. [DOI] [PubMed] [Google Scholar]
- 40. Kitama T, Omata T, Mizukoshi A, Ueno T, Sato Y (1999) Motor dynamics encoding in cat cerebellar flocculus middle zone during optokinetic eye movements. J Neurophysiol 82: 2235–2248. [DOI] [PubMed] [Google Scholar]
- 41. Sato Y, Kawasaki T (1991) Identification of the Purkinje cell/climbing fiber zone and its target neurons responsible for eye-movement control by the cerebellar flocculus. Brain Res Brain Res Rev 16: 39–64. [DOI] [PubMed] [Google Scholar]
- 42.Sato Y, Kawasaki T, Mizukoshi K (1991) Eye movement control by Purkinje cell/climbing fiber zones of cerebellar flocculus in cat. Acta Otolaryngol Suppl 481: 237–241. [DOI] [PubMed]
- 43. Hong SM, Cha CI, Park BR (2009) Changes in calbindin expression within the flocculus after unilateral labyrinthectomy in rats. Neurosci Lett 460: 52–55. [DOI] [PubMed] [Google Scholar]
- 44. Fan GR, Yin ZD, Sun Y, Chen S, Zhang WJ, et al. (2013) Reversible neurotoxicity of kanamycin on dorsal cochlear nucleus. Brain Res 1502: 30–46. [DOI] [PubMed] [Google Scholar]
- 45. Ito M, Kawai N, Udo M (1968) The origin of cerebellar-induced inhibition of deiters neurones. 3. Localization of the inhibitory zone. Exp Brain Res 4: 310–320. [DOI] [PubMed] [Google Scholar]
- 46. Obata K, Takeda K (1969) Release of gamma-aminobutyric acid into the fourth ventricle induced by stimulation of the cat's cerebellum. J Neurochem 16: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 47.Kitahara T, Takeda N, Kiyama H, Kubo T (1998) Molecular mechanisms of vestibular compensation in the central vestibular system–review. Acta Otolaryngol Suppl 539: 19–27. [PubMed]
- 48. Johnston AR, Seckl JR, Dutia MB (2002) Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiol 545: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB (2005) Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76: 349–392. [DOI] [PubMed] [Google Scholar]
- 50. Smith PF, Curthoys IS (1989) Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev 14: 155–180. [DOI] [PubMed] [Google Scholar]
- 51. Ris L, Godaux E (1998) Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J Neurophysiol 80: 2352–2367. [DOI] [PubMed] [Google Scholar]
- 52. Yamanaka T, Him A, Cameron SA, Dutia MB (2000) Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol 523 Pt 2: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gliddon CM, Darlington CL, Smith PF (2005) GABAergic systems in the vestibular nucleus and their contribution to vestibular compensation. Prog Neurobiol 75: 53–81. [DOI] [PubMed] [Google Scholar]
- 54. Precht W, Shimazu H, Markham CH (1966) A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J Neurophysiol 29: 996–1010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunoblot of histamine receptors in the MVN and the flocculus after mechanical UL. Samples assayed are shown as follows: A, 1 day post-mechanical UL in the MVN; B, 1 day post-mechanical UL in the flocculus; C, 3 day post- mechanical UL in the flocculus U, unilateral labyrinthectomy; S, sham operation. The protein levels of the H1, H2 and H3 receptors significantly increased in the ipsi-lesional MVN on the 1st day following the mechanical UL, and protein levels of the H1, H2 and H3 receptors increased in ipsi-lesional flocculus on the 1st and 3rd day following mechanical UL as well.
(TIF)
Histograms of the neurons counting of H1, H2 and H3 receptors-positive neurons in the rostral, middle and caudal part of the MVN and the flocculus in control groups. Number of H1, H2 and H3 receptors-positive neurons in each corresponding region of the visual system was expressed per unit area (mm2).
(TIF)