Abstract
Allopurinol is a purine analogue that inhibits xanthine oxidase. It is mainly used for the treatment of hyperuricemia in patients with gout or tumor lysis syndrome. Experience with allopurinol in pregnancy is scarce. In 2011, Kozenko et al. reported on a child with multiple malformations after maternal treatment with allopurinol throughout pregnancy. Possible teratogenicity of allopurinol was proposed due to the similarity of the pattern of malformations in children with mycophenolate embryopathy. A possible common mechanism of both drugs, i.e. disruption of purine synthesis, was discussed. We report on the outcome of 31 prospectively ascertained pregnancies with allopurinol exposure at least during first trimester. Pregnancy outcomes were 2 spontaneous abortions, 2 elective terminations of pregnancy and 27 live born children. The overall rate of major malformations (3.7%) and of spontaneous abortions (cumulative incidence 11%, 95%-CI 3–40) were both within the normal range. However, there was one child with severe malformations including microphthalmia, cleft lip and palate, renal hypoplasia, low-set ears, hearing deficit, bilateral cryptorchidism, and micropenis. The striking similarity of the anomalies in this child and the case described by Kozenko et al. might be considered as a signal for teratogenicity. Thus, we would recommend caution with allopurinol treatment in the first trimester, until further data are available.
Introduction
Allopurinol is a purin analogue that inhibits xanthine oxidase. It is mainly used for the treatment of hyperuricemia in patients with gout or tumor lysis syndrome. As these conditions are infrequent in women of childbearing age allopurinol use in pregnancy has rarely been observed. The possible antioxidant function of allopurinol has recently led to an increasing discussion on extending treatment indications to cardiovascular disease[1] and pre-eclampsia [2]. In addition, allopurinol is used as adjunct therapy in order to increase efficacy of thiopurines in inflammatory bowel disease, a condition frequently affecting women of childbearing age [3], [4].
A recent publication reported on normal pregnancy outcome after allopurinol treatment throughout pregnancy [5]. On the other hand, possible teratogenicity of allopurinol had been proposed after observation of a child with multiple malformations after maternal treatment throughout pregnancy [6]. So far, there are no prospective data on allopurinol use in pregnancy. Therefore, counseling of allopurinol is difficult. We report the first case series of prospectively followed pregnancies exposed to allopurinol.
Materials and Methods
Ascertainment of cases and follow-up procedure
The Berlin Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy offers risk assessment to physicians, other health care providers (HCP) and pregnant women. Patient data are documented through the risk consultation. Usually, data are ascertained prospectively, i.e. neither the outcome of pregnancy nor the results of prenatal diagnostics are primarily known, but are ascertained at a later stage. In retrospectively reported cases, the outcome of pregnancy is already known and has usually prompted the initial contact to our institute. Using structured questionnaires, all relevant data with respect to medications, exposures to other agents and/or diseases and course and outcome of pregnancy are documented with informed consent of the patient. The following information is obtained: obstetric and medical history, family history, patient's profession and education, treatment indications and details of exposures (timing in pregnancy, duration, dose and concomitant medication, vitamins, in particular folic acid), assisted conception, inadvertent use of hormonal contraceptives during pregnancy, social or illicit drugs and smoking. About 8 weeks after the expected date of delivery, information about complications during pregnancy (i.e. infections, gestational diabetes, preeclampsia, etc.), details in case of pregnancy loss, gestational age at birth, sex, birth weight, length, head circumference, pH, and Apgar scores and if applicable details of congenital anomalies and postnatal disorders during neonatal period are obtained. For further details on the methodology see [7].
Ethics
The study is based on observational data and was approved by the Ethics Committee of the Charité-Universitätsmedizin Berlin, Germany. All patients were informed that their medical information will be stored and used for future scientific research. In case they have contacted us directly they have provided us with a written consent. In case their HCP have contacted us, the HCP confirm that their patients have agreed. All our correspondences to patients and HCP contain information about our data handling and the patient's rights.
Study design
Prospectively ascertained pregnancies with maternal allopurinol exposure were identified from 1991 until June 2012. Since the vulnerable phase for birth defects induced by teratogens is limited to the 1st trimester, only pregnancies with exposure to allopurinol at least between last menstrual period (LMP) and gestational week 12 were included in the study. Cases were included independent of treatment indication and exposure interval within the 1st trimester. Exposure to allopurinol may have lasted longer than week 12.
The primary goal of the study was to determine the rate of major birth defects, defined as structural abnormalities of medical, surgical or cosmetic relevance induced during embryogenesis. Special emphasis was to further evaluate the teratogenic potential of allopurinol as proposed by Kozenko et al. [6]. All birth defects were classified according to Merks [8] and Rasmussen [9]. Secondary endpoints of our study were the rates of miscarriage, stillbirth and preterm delivery (<37 weeks). Weeks of pregnancy were defined by LMP. Birth weight was adjusted to gestational age at birth and sex. Percentile categories were calculated according to Voigt et al. [10].
Statistical analysis
The birth defect rate was calculated using live births plus pregnancy losses with pathology. For calculating rates of major birth defects, genetic or chromosomal disorders were excluded. Because of the limited number of exposed cases we did not use a control group.
Since crude rates of pregnancy outcome based on observational data are biased we calculated cumulative incidences of spontaneous abortions, elective terminations of pregnancy (ETOP) and live births. For further details of this method see Meister et al. [11].
Results
Maternal characteristics
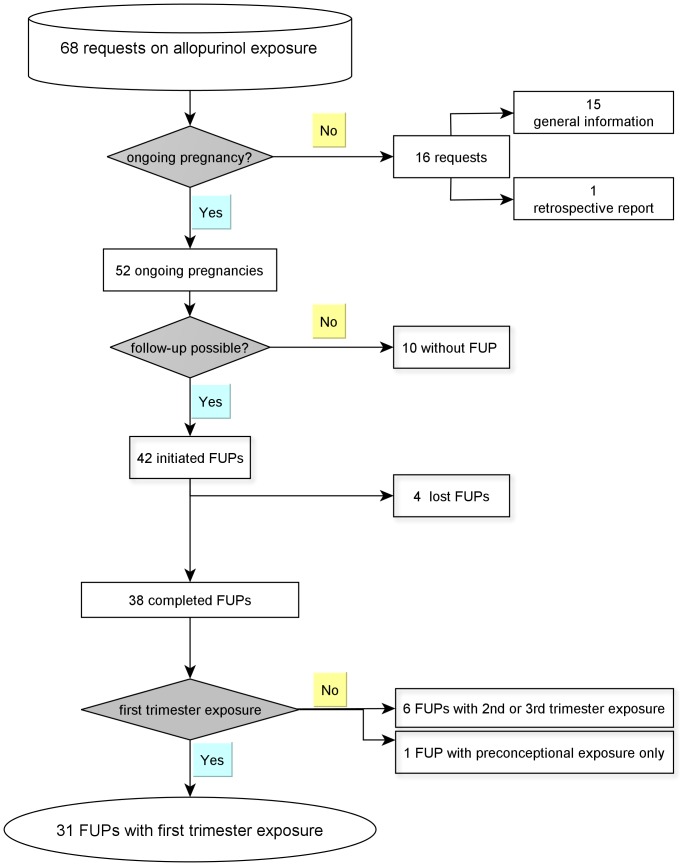
From January 1991 until June 2012 a total of 38 pregnancies with maternal allopurinol exposure and complete follow-up were identified (Fig. 1). Thirty-one pregnancies with first trimester exposure fulfilled the criteria of being prospectively ascertained and were included in this study for evaluation of pregnancy outcome. One pregnancy with uneventful outcome after long term allopurinol exposure was reported retrospectively and was therefore not considered for relative risk calculation.
Figure 1. Flow chart on cases of allopurinol exposure and pregnancy (FUP = Follow-up).
Treatment indications were hyperuricemia (n = 18), gout (n = 4), renal diseases (n = 4), rheumatoid arthritis (n = 2), and systemic lupus erythematosus, chronic myeloic leucemia, hyperoxaluria (each n = 1).
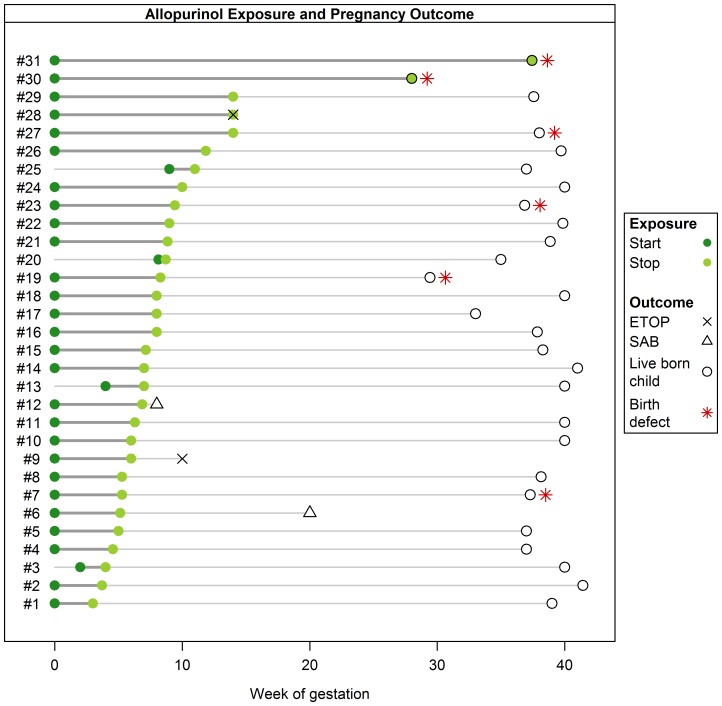
Median dosage of allopurinol was 100 mg (range 50–600 mg/d, interquartile range (IQR) 100–300 mg/d). Treatment with allopurinol was initiated before pregnancy in 27 women. Four patients started allopurinol during 1st trimester. 15/31 patients stopped therapy before week 8 after LMP, only 2 women continued treatment with allopurinol throughout pregnancy (pregnancies #30 and #31) (see Fig. 2).
Figure 2. Diagram summarizing pregnancy interval of allopurinol exposure, week at first contact and outcome of 31 prospectively ascertained pregnancies.
Except for one mother treated with mycophenolate until week 6+6 after kidney transplantation (pregnancy #12) and one mother with cyclophosphamide (single dose at week 7+3, pregnancy #26) there were no patients with teratogenic co-medication.
On the other hand only two women were exclusively exposed to allopurinol (pregnancies #3 and #24), indicating the high co-morbidity of our patients. Twelve women were treated for hypertension, nine of whom needed two or more antihypertensives, including methyldopa (n = 5), ACE-Inhibitors or AT II receptor inhibitors (n = 7), ß-blockers (n = 7), diuretics (n = 8), monoxidine (n = 2), dihydralazine (n = 1), and clonidine (n = 1). In addition, three women used diuretics for treatment of edema. There were three women with type 2 diabetes, two women developed gestational diabetes. Interestingly, three women were on tuberculostatic therapy including pyrazinamide, well known to reduce excretion of uric acid. Further details of maternal characteristics are summarized in Table 1. Information on BMI was available from 2005 onwards. In seven of 14 patients the BMI was >35 (see also Table 1).
Table 1. Maternal characteristics and obstetrical history of allopurinol exposed women.
| Age (yrs) | 32 (28–37) (22–42) | n = 30/31 | |
| BMI 1 | 33.5 (23–38) (20–50) | n = 14/31 | |
| Educational level | 9 years exam | 2 (18%) | n = 11/31 |
| 10/11 years exam | 6 (55%) | ||
| secondary school exam | 1 (9%) | ||
| academic study | 2 (18%) | ||
| Smoking | No | 26 (90%) | n = 29/31 |
| < = 5 cig/day | 1 (3%) | ||
| >5 cig/day | 2 (7%) | ||
| Alcohol | No | 30 (100%) | n = 30/31 |
| Previous pregnancies | 0 | 13 (43%) | n = 30/31 |
| 1 | 5 (17%) | ||
| 2 | 4 (13%) | ||
| 3 or more | 8 (27%) | ||
| Previous parities | 0 | 13 (43%) | n = 30/31 |
| 1 | 7 (23%) | ||
| 2 | 6 (20) | ||
| 3 or more | 4 (13%) | ||
| Previous miscarriages | 0 | 26 (90%) | n = 29/31 |
| 1 | 3 (10%) | ||
| 2 or more | 0 (0%) | ||
| Previous children with birth defect | 0 | 27 (93%) | n = 29/31 |
| 1 | 2 (7%) | ||
| 2 or more | 0 (0%) | ||
| Week at first TIS contact | 8.9 (6.9–12.8) (3.1–20) | n = 31/31 |
For age, BMI, and week at first TIS contact, median, interquartile range, and min/max are presented.
BMI was only available for cases ascertained after 2004.
First contacts to our institute were initiated via gynaecologists (n = 17), clinical geneticists (n = 6), other physicians (n = 4) and patients (n = 4).
Pregnancy outcome
Of the 31 pregnancies exposed to allopurinol, there were 2 spontaneous abortions, 2 elective terminations of pregnancy and 27 live births (see Fig. 2).
One spontaneous abortion (pregnancy #12) occurred in a patient after renal transplantation receiving several co-medications including mycophenolate, ciclosporine and various antihypertensives. Another spontaneous abortion occurred at week 20 in a patient with hypertension (pregnancy #6) treated with valsartan and hydrochlorothiazide. Chorioamnionitis was diagnosed. No malformations were found in the fetus. Two pregnancies were terminated, both for personal reasons (pregnancy #9 and #28, see Fig. 2).
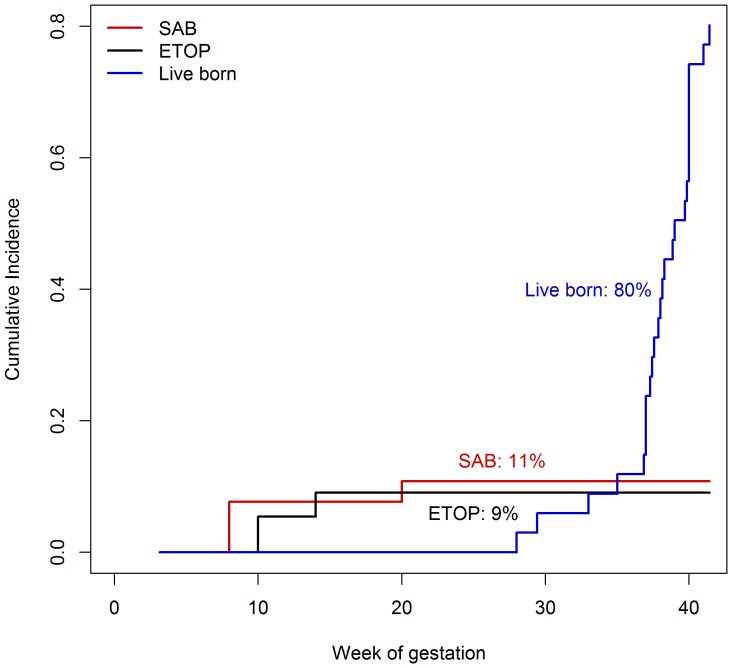
Cumulative incidences were 11% (95%-CI 3–40) for spontaneous abortions and 9% (95%-CI 2–32) for ETOPs (Fig. 3).
Figure 3. Estimation of cumulative incidences using survival analysis technique.
Probability of spontaneous abortion was 11% (95%-confidence interval (CI) 3–40), ETOP 9% (95%-CI 2–32), and live birth 80% (95%-CI 60–85).
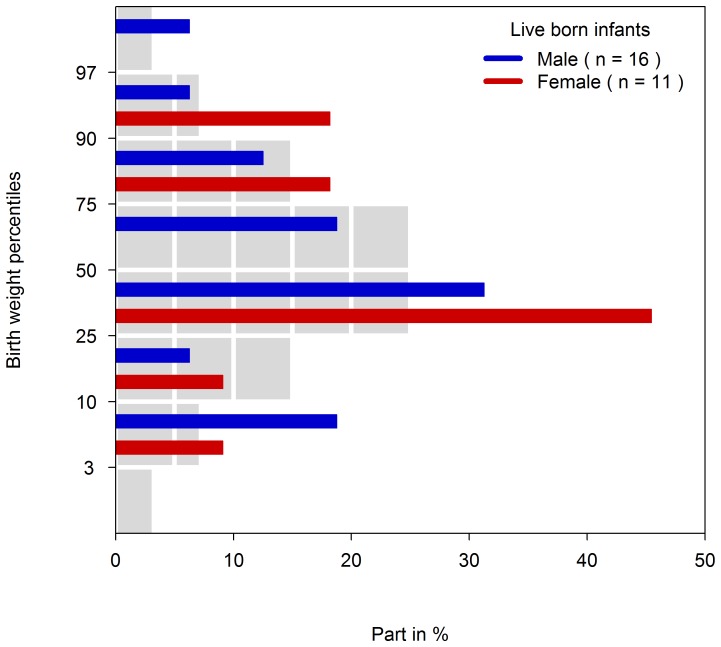
The cumulative incidence for live births was 80% (95%-CI 60–94) (Fig. 3). There were 16 males and 11 females born alive. Five children (19%) were born premature (gestational age at birth <37 weeks). Caesarean section was performed in nearly half of the live births (13/27). Birth weights were within normal range after correction for sex and gestational age (see Table 2 and Fig. 4). Pre-eclampsia had been diagnosed in five pregnancies all exposed during 1st trimester until latest week 14 and a HELLP-syndrome in one exposed throughout pregnancy (#30).
Table 2. Child characteristics.
| Gestational week at birth | 38.1 (37–40) (28–41.4) | n = 27/27 |
| Preterm birth | 5 (19%) | n = 27/27 |
| Weight (g) | 3340 (2850–3628) (990–4690) | n = 27/27 |
| Length (cm) | 50 (48–52) (36–55) | n = 26/27 |
| Head circumference (cm) | 35 (33.5–36) (26–38) | n = 21/27 |
For gestational week at birth, weight, length and head circumference, median, interquartile range, and min/max are presented.
Figure 4. Birth weight percentiles.
Bars in colors give the proportions of newborns by percentiles. Grey bars represent the proportion of newborn in the German Perinatal Project general population [10] in each percentile category.
There were 6 infants (5 boys and 1 girl) with congenital anomalies (Table 3). Minor malformations were diagnosed in four (#7, #19, #27, #30). In patient #23 congenital hypoparathyroidism was diagnosed after birth, an autosomal-dominant condition already known in the mother.
Table 3. Summary of congenital anomalies after first trimester exposure with allopurinol.
| Nr. | Gestational age at call (weeks) | Allopurinol exposure (weeks after LMP) and dose (mg/d) | Treatment indication | Comedication | Gestational age at birth | Congenital anomalies | Classification of anomalies |
| #7 | 5+2 | 0–5+2 (100) | Hyperuricemia | Ramipril, metoprolol, alpha-methyldopa, hydrochlorothiazide, amlodipine, simvastatin, metformin, venlafaxin, tilidine, naloxone, paracetamol, acetylsalicylic acid, insulin, insulin lispro | 37+2 | Patent foramen ovale, pulmonary artery stenosis (hemodynamically not relevant) | Minor |
| #19 | 11+3 | 0–8+2 (100) | Glomerulonephritis | Amlodipine, valsartan, metoprolol, hydrochlorothiazide, alpha-methyldopa, desloratadin, | 29+2 | Small patent ductus arteriosus, mild ptosis right eye, umbilical hernia | Minor |
| #23 | 9+3 | 0–9+3 (50) | Hyperuricemia | Calcitriol | 36+6 | Congenital hypoparathyroidism (autosomal-dominant) | Genetic |
| #27 | 15 | 0–14 (300) | Gout | Furosemide, pravastatin, cerivastatin | 38 | Hemangioma | Minor |
| #30 | 12+4 | 0–28 (100) | Hemolytic uremic syndrome | Alpha-methyldopa, metoprolol, moxonidine, furosemide clonidine, darbepoetin alfa, danaparoid, enoxaparine, corticosteroids, colecalciferol, alfacalcidol, | 28 | Persistent ductus arteriosus, patent foramen ovale, umbilical hernia | Minor |
| #31 | 5 | 0–37+3 (100) | Hyperoxaluria type I | Hydrochlorothiazide, sodiumcarbonate, pyridoxine | 37+3 | Multiple malformations (see also Table 4) | Major |
One child (#31) with allopurinol exposure throughout pregnancy had multiple malformations (further details are given in Tables 3 and 4). The mother had hyperoxaluria type 1, a rare metabolic disease, and was concomitantly treated with hydrochlorothiazide (12,5 mg/d), pyridoxine (100 mg/d) and sodium carbonate.
Table 4. Clinical features of the patient described by Kozenko et al. [6] and our patient # 31.
| Patient from Kozenko et al. | Patient #31 from our case series | |
| Gestational age of birth | 41 weeks | 37+3 weeks |
| Allopurinol exposure throughout pregnancy | 300 mg/d | 100 mg/d |
| Orofacial anomalies | Cleft lip and palate (right), unilateral microtia, EACA1, Micrognathia | Cleft lip and palate (left), low-set ears, conductive deafness, retrognathia |
| Ophthalmological anomalies | Microphthalmia, optic nerve hypoplasia, coloboma upper eyelid | Microphthalmia |
| Gastrointestinal anomalies | Diaphragmatic hernia Pulmonary agenesis (left) | Hepatosplenomegaly/cholestasis |
| Urogenital anomalies | Unilateral renal agenesis, bilateral cryptorchidism | Renal hypoplasia, bilateral cryptorchidism, micropenis |
| CNS | Hydrocephaly, hypoplasia of corpus callosum | Enlargement of ventricles |
| Further anomalies | Osteopenia | |
| Cardiovascular defects | - | - |
| Karyotype | 46,XY | 46,XY |
External auditory canal atresia.
The rate of major malformations in our cohort was 3.7% (1/27).
Discussion
Gout, hyperuricemia and other treatment indications for allopurinol are rare in women of reproductive age. Gout usually improves in pregnancy and attacks recommence after birth, due to the decreased estrogen levels [12]. During 2nd and 3rd trimester allopurinol has been described for tumor lysis syndrome [13]. Gülmezoğlu et al. [14] reported its safe use in a trial including 27 women in the 3rd trimester to evaluate the antioxidant potency of allopurinol for the prevention of pre-eclampsia.
To our knowledge, first trimester exposure of allopurinol has only been reported in a few case reports including 4 normal outcomes [15], [16] and a recent report [6] on a child with multiple malformations including microphthalmia, cleft lip and palate, microtia and diaphragmatic hernia after maternal allopurinol exposure throughout pregnancy (summary of features in Table 4). The authors proposed a possible teratogenic effect and noted the similarities to the mycophenolate embryopathy based on similar pathways, i.e. the interruption of purine biosynthesis. Data from animal studies indicate teratogenic effects (facial clefts and minor skeletal defects) of allopurinol at high doses in mice [17], but not in rats and other rodents [18].
We present the first prospective case series covering 31 pregnancies with 1st trimester allopurinol exposure.
The rate of spontaneous abortions in our case series was in the expected range of 13–21% [19] as was the rate of major malformations (3.7%) based on one infant with multiple malformations. The infant's phenotype resembles the case report of Kozenko et al. [6]. In our infant a Fraser syndrome had been suspected initially but was not confirmed. The karyotype was normal, but further genetic analysis like array-CGH was declined by the parents. Therefore, we cannot exclude a genetic origin of this rare malformation syndrome.
The mother of our patient had hyperoxaluria, a severe renal disease. In addition to allopurinol, she was treated with hydrochlorothiazide and pyridoxine, both drugs not considered as teratogens based on animal studies and human data [20]. Pyridoxine is widely used for the treatment of hyperemesis in early pregnancy [21].
The mother of the patient reported by Kozenko et al. [6] received multivitamins and methyldopa, the latter being the first line therapy for hypertension in pregnancy and not suspected as a teratogen. Thus, our patient and the patient reported by Kozenko were not exposed to any teratogenic co-medication in pregnancy that might explain the observed malformations. Furthermore, both co-medications are not directly involved in purine metabolism.
Our case series represents women with either multiple risk factors or severe illness, known to be associated with an increased risk of poor pregnancy outcomes. The high rate of nearly 20% prematurity in our case series may be explained by maternal co-morbidity. To our surprise we did not observe lower birth weights after adjustment for sex and gestational age, which might have been expected in cases of hypertension or autoimmune diseases in pregnancy. The high number of obese women might have compensated this effect, as new-borns of obese mothers tend to be heavier than new-borns of normal weight women [22].
Obesity and hypertension were present in the majority of our women. These conditions are risk factors for hyperuricemia or gout in premenopausal women [23]. So far, no substantial malformation risk has been ascribed to the underlying maternal diseases in our cohort such as hypertension, systemic lupus erythematosus or other autoimmune diseases [24] [25]. In contrast to other metabolic diseases, such as phenylketonuria, hyperoxaluria has not been suspected to cause teratogenic effects. There are several reports on normal pregnancy outcomes in women with hyperoxaluria [26]. However, a high BMI is associated with an increased malformation risk [27], [28].
Although being the largest case series so far, our study is still limited by the small sample size. With only 2 women exposed in the 2nd and 3rd trimester, we are not able to examine any effect on pregnancy outcome of allopurinol exposure after the first trimester. The surprisingly high rate of 6/31 patients with pre-eclampsia or HELLP syndrome in our cohort may be explained by the underlying conditions of the women, A protective effect of allopurinol on pre-eclampsia as postulated by Gülmezoğlu et al. [14] cannot be confirmed by our data.
The important strength and advantage of our study is the prospective character of data ascertainment, allowing relative risk calculations for adverse pregnancy outcomces.
The similarity of rare malformations in the infants reported by Kozenko [6] and our study (case #31) requires further exploration by case-control studies to confirm or reject the hypothesis of allopurinol teratogenicity [29] [30].
Conclusion
Allopurinol has been used for over 40 years without being suspected for teratogenicity in humans. This is corroborated by the overall malformation rate in our case series lying in the expected range. However, the presence of two infants with a similar rare combination of malformations including microphthalmia, cleft lip and palate, and microtia after first trimester exposure to allopurinol is striking and might be considered as a signal for teratogenicity [29]. Thus, we would recommend caution with allopurinol in the first trimester, unless further data are available. In case of inadvertent exposure during 1st trimester high resolution ultrasound is recommended to confirm normal fetal development.
Acknowledgments
We would like to thank all contributing physicians, their patients and families as well as our colleagues in the Berlin TIS.
Funding Statement
The study has been performed with financial support from the German Federal Institute for Drugs and Medical Devices. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Struthers A, Shearer F (2012) Allopurinol: novel indications in cardiovascular disease. Heart 98: 1543–1545 heartjnl-2012-302249 [pii];10.1136/heartjnl-2012-302249 [doi] [DOI] [PubMed] [Google Scholar]
- 2. Miller SL, Wallace EM, Walker DW (2012) Antioxidant therapies: a potential role in perinatal medicine. Neuroendocrinology 96: 13–23 000336378 [pii];10.1159/000336378 [doi] [DOI] [PubMed] [Google Scholar]
- 3.Hoentjen F, Seinen ML, Hanauer SB, de Boer NK, Rubin DT, et al.. (2012) Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 10.1002/ibd.23021 [doi]. [DOI] [PubMed]
- 4. Seinen ML, de Boer NK, Smid K, van Asseldonk DP, Bouma G, et al. (2011) Allopurinol enhances the activity of hypoxanthine-guanine phosphoribosyltransferase in inflammatory bowel disease patients during low-dose thiopurine therapy: preliminary data of an ongoing series. Nucleosides Nucleotides Nucleic Acids 30: 1085–1090 10.1080/15257770.2011.597371 [doi] [DOI] [PubMed] [Google Scholar]
- 5.Seinen ML, de Boer NK, van Hoorn ME, van Bodegraven AA, Bouma G (2012) Safe use of allopurinol and low-dose mercaptopurine therapy during pregnancy in an ulcerative colitis patient. Inflamm Bowel Dis. 10.1002/ibd.22945 [doi]. [DOI] [PubMed]
- 6. Kozenko M, Grynspan D, Oluyomi-Obi T, Sitar D, Elliott AM, et al. (2011) Potential teratogenic effects of allopurinol: a case report. Am J Med Genet A 155A: 2247–2252 10.1002/ajmg.a.34139 [doi] [DOI] [PubMed] [Google Scholar]
- 7. Schaefer C, Ornoy A, Clementi M, Meister R, Weber-Schoendorfer C (2008) Using observational cohort data for studying drug effects on pregnancy outcome–methodological considerations. Reprod Toxicol 26: 36–41. [DOI] [PubMed] [Google Scholar]
- 8. Merks JH, van Karnebeek CD, Caron HN, Hennekam RC (2003) Phenotypic abnormalities: terminology and classification. Am J Med Genet A 123A: 211–230. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, et al. (2003) Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol 67: 193–201. [DOI] [PubMed] [Google Scholar]
- 10. Voigt M, Rochow N, Hesse V, Olbertz D, Schneider KT, et al. (2010) Kurzmitteilung zu den Perzentilenwerten für die Körpermaße der Neugeborenen. Z Geburtshilfe Neonatol 214: 24–29. [DOI] [PubMed] [Google Scholar]
- 11. Meister R, Schaefer C (2008) Statistical methods for estimating the probability of spontaneous abortion in observational studies–analyzing pregnancies exposed to coumarin derivatives. Reprod Toxicol 26: 31–35. [DOI] [PubMed] [Google Scholar]
- 12.Coddington CC, Albrecht RC, Cefalo RC (1979) Gouty nephropathy and pregnancy. Am J Obstet Gynecol 133: 107–108. 0002-9378(79)90420-4 [pii]. [DOI] [PubMed]
- 13. Brown ML, Strauss B, Glasser SR (2001) Chemotherapy in treatment of non-hodgkin's lymphoma in pregnancy (Abstract). Obstet Gynecol 97: S39. [Google Scholar]
- 14. Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MM (1997) Antioxidants in the treatment of severe pre-eclampsia: an explanatory randomised controlled trial. Br J Obstet Gynaecol 104: 689–696. [DOI] [PubMed] [Google Scholar]
- 15. Farber M, Knuppel RA, Binkiewicz A, Kennison RD (1976) Pregnancy and von Gierke's disease. Obstet Gynecol 47: 226–228. [PubMed] [Google Scholar]
- 16. Ali R, Ozkalemkas F, Kimya Y, Koksal N, Ozkocaman V, et al. (2009) Pregnancy in chronic lymphocytic leukemia: experience with fetal exposure to chlorambucil. Leuk Res 33: 567–569 S0145-2126(08)00273-7 [pii];10.1016/j.leukres.2008.05.019 [doi] [DOI] [PubMed] [Google Scholar]
- 17. Fujii T, Nishimura H (1972) Comparison of teratogenic action of substances related to purine metabolism in mouse embryos. Jpn J Pharmacol 22: 201–206. [DOI] [PubMed] [Google Scholar]
- 18. Bragonier JR, Roesky N, Carver MJ (1964) Teratogenesis: Effects of substituted purines and the influence of the 4 hydroxypyrazolonpyrimidine in the rat. Proc Soc Exp Biol Med 116: 685–688. [DOI] [PubMed] [Google Scholar]
- 19. Hoeltzenbein M, Elefant E, Vial T, Finkel-Pekarsky V, Stephens S, et al. (2012) Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 158A: 588–596 10.1002/ajmg.a.35223 [doi] [DOI] [PubMed] [Google Scholar]
- 20.Briggs GG, Freeman RK, and Yaffe SJ (2011) Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams&Wilkins.
- 21. Anderka M, Mitchell AA, Louik C, Werler MM, Hernandez-Diaz S, et al. (2012) Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol 94: 22–30 10.1002/bdra.22865 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stuebe AM, Landon MB, Lai Y, Spong CY, Carpenter MW, et al. (2012) Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. Am J Obstet Gynecol 207: 62–67 S0002-9378(12)00482-6 [pii];10.1016/j.ajog.2012.04.035 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhole V, de VM, Rahman MM, Krishnan E, Choi H (2010) Epidemiology of gout in women: Fifty-two-year followup of a prospective cohort. Arthritis Rheum 62: 1069–1076 10.1002/art.27338 [doi] [DOI] [PubMed] [Google Scholar]
- 24. Tendron A, Gouyon JB, Decramer S (2002) In utero exposure to immunosuppressive drugs: experimental and clinical studies. Pediatr Nephrol 17: 121–130. [DOI] [PubMed] [Google Scholar]
- 25. Smyth A, Oliveira GH, Lahr BD, Bailey KR, Norby SM, et al. (2010) A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 5: 2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norby SM, Milliner DS (2004) Outcomes and complications of pregnancy in women with primary hyperoxaluria. Am J Kidney Dis 43: : 277–285. S0272638603014896 [pii]. [DOI] [PubMed] [Google Scholar]
- 27. Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, et al. (2007) Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med 161: 745–750 161/8/745 [pii];10.1001/archpedi.161.8.745 [doi] [DOI] [PubMed] [Google Scholar]
- 28. Carmichael SL, Rasmussen SA, Shaw GM (2010) Prepregnancy obesity: a complex risk factor for selected birth defects. Birth Defects Res A Clin Mol Teratol 88: 804–810 10.1002/bdra.20679 [doi] [DOI] [PubMed] [Google Scholar]
- 29. Carey JC, Martinez L, Balken E, Leen-Mitchell M, Robertson J (2009) Determination of human teratogenicity by the astute clinician method: review of illustrative agents and a proposal of guidelines. Birth Defects Res A Clin Mol Teratol 85: 63–68 10.1002/bdra.20533 [doi] [DOI] [PubMed] [Google Scholar]
- 30. Jones KL, Carey JC (2011) The importance of dysmorphology in the identification of new human teratogens. Am J Med Genet C Semin Med Genet 157: 188–194 10.1002/ajmg.c.30311 [doi] [DOI] [PubMed] [Google Scholar]