Abstract
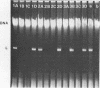
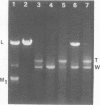
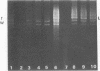
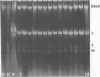
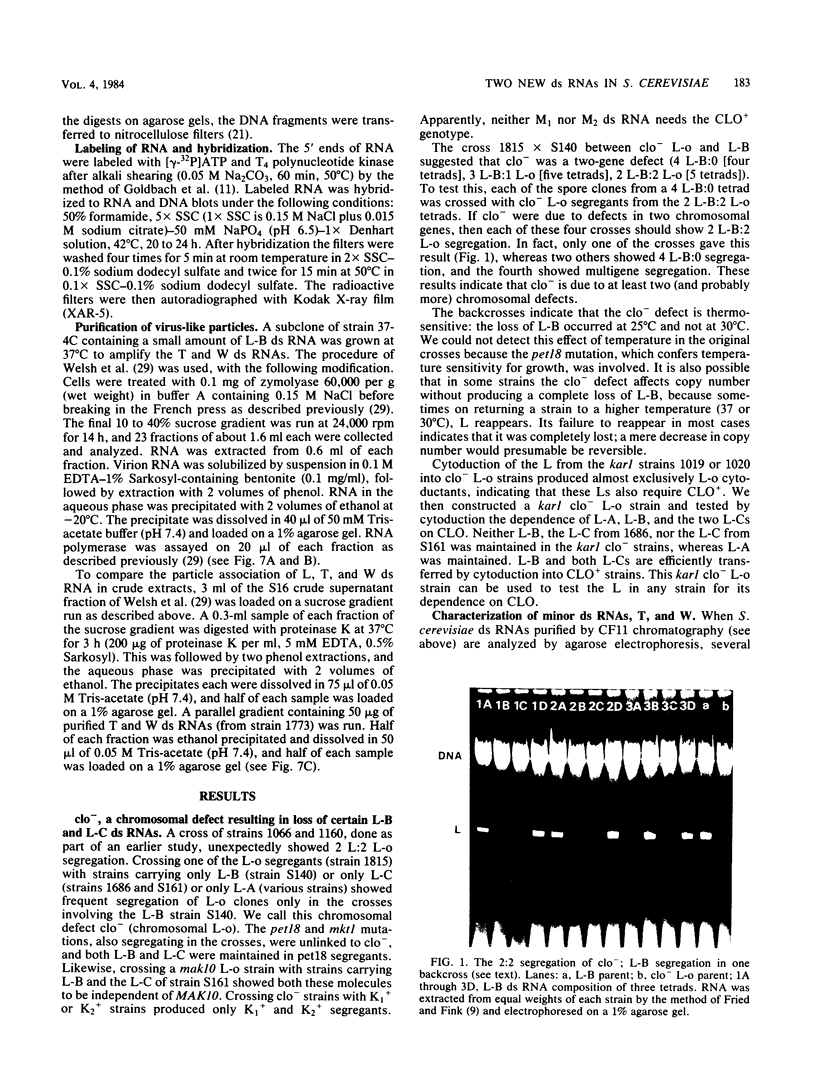
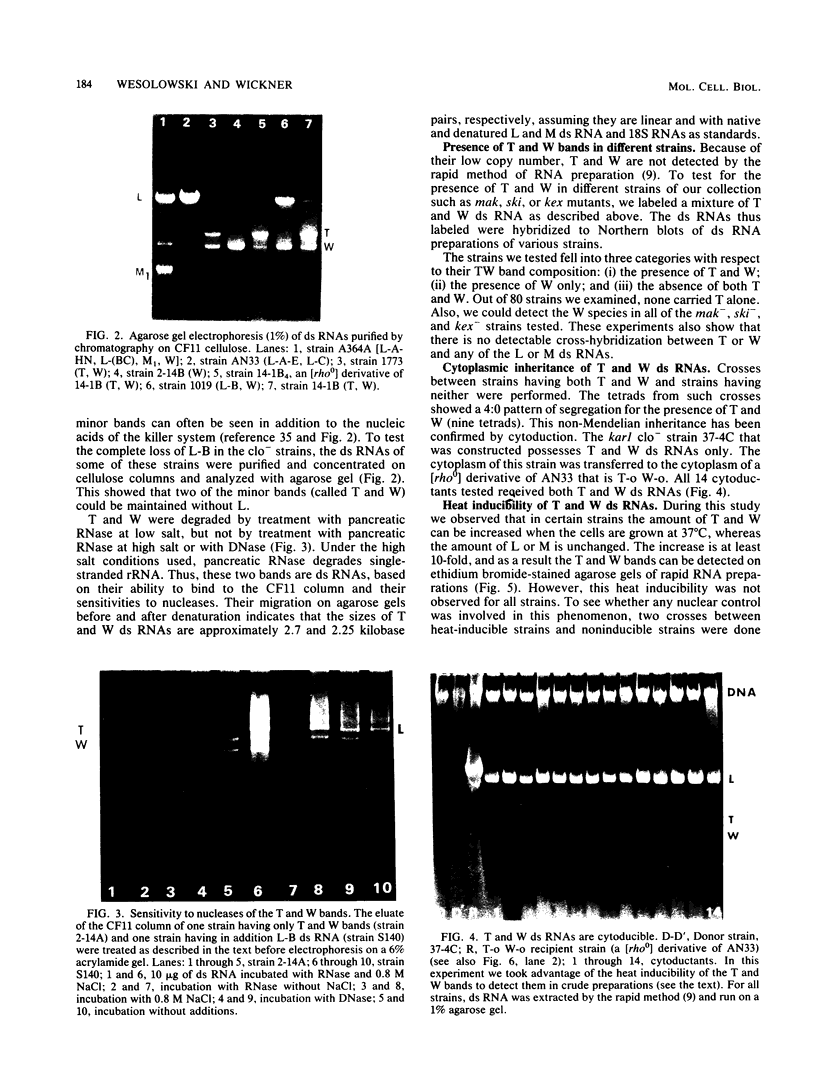
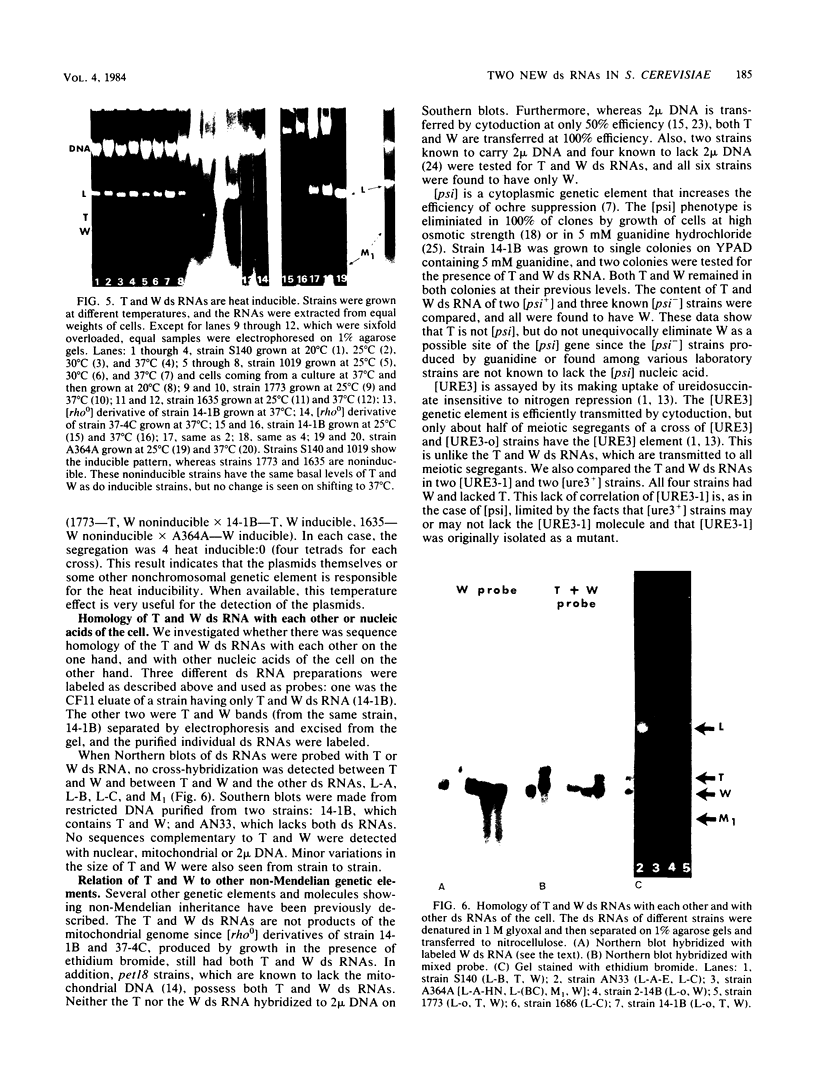
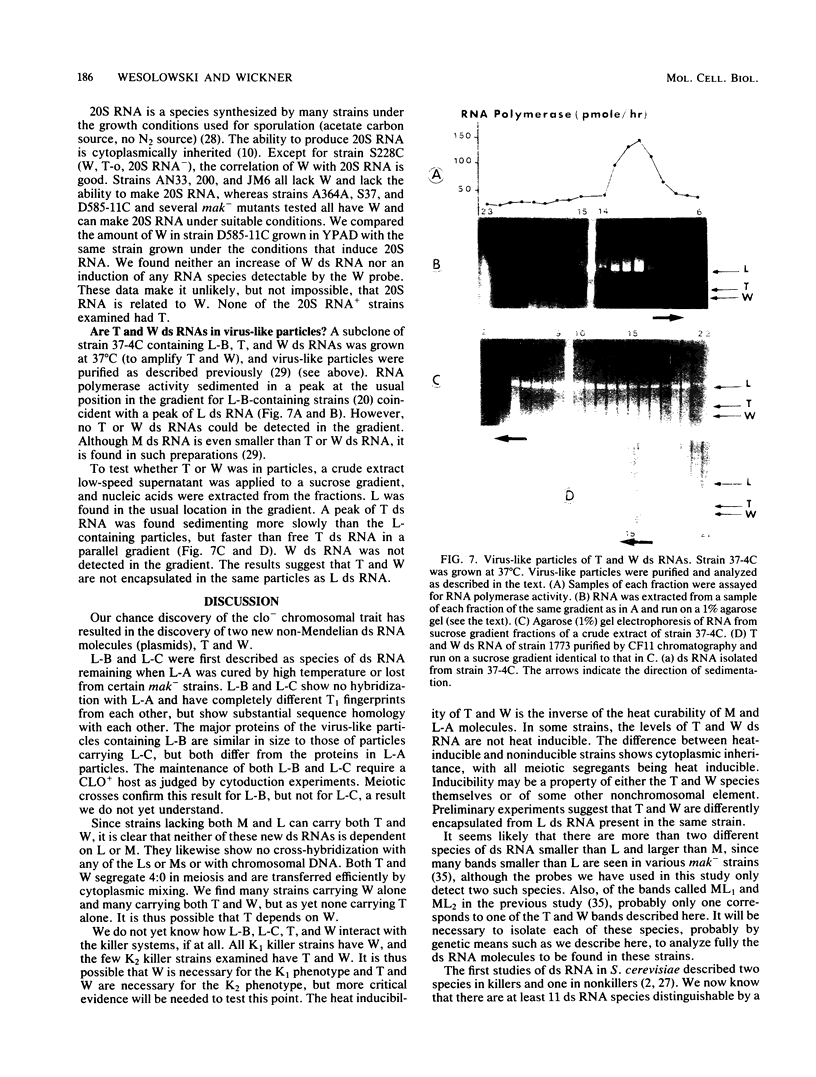
Certain strains of Saccharomyces cerevisiae were found to have a complex nuclear defect (designated clo-) that makes cells unable to maintain some L-B and some L-C double-stranded RNAs at 25 degrees C. The clo- strains were not defective in maintenance of L-A, M1, or M2 double-stranded RNAs. Most clo-strains lacking L and M carry small amounts of two double-stranded RNA species intermediate in size between L and M and denoted T (2.7 kilobase pairs) and W (2.25 kilobase pairs). Some strains carry both T and W, some carry neither, and some carry only W; no strains carrying only T have been found. Both T and W show 4+:0 segregation in meiosis and efficient transmission by cytoplasmic mixing (cytoduction), indicating that they are non-Mendelian genetic elements. T and W do not cross-hybridize with each other or with L-A, L-B, L-C, M1, M2, or chromosomal DNA. T and W are apparently distinct from other known non-Mendelian genetic elements (2mu DNA, [rho], [psi], 20S RNA, [URE3]). In most strains the copy number of both T and W is increased about 10-fold by the growth of cells at 37 degrees C. This heat inducibility of T and W is under control of a cytoplasmic gene. T and W double-stranded RNAs are not found in a purified L-containing virus-like particle preparation from a strain containing L-B, T, and W double-stranded RNAs. The role, if any, of T or W in the killer systems is not known.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigle M., Lacroute F. Genetical aspects of [URE3], a non-mitochondrial, cytoplasmically inherited mutation in yeast. Mol Gen Genet. 1975;136(4):327–335. doi: 10.1007/BF00341717. [DOI] [PubMed] [Google Scholar]
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Brennan V. E., Field L., Cizdziel P., Bruenn J. A. Sequences at the 3' ends of yeast viral dsRNAs: proposed transcriptase and replicase initiation sites. Nucleic Acids Res. 1981 Aug 25;9(16):4007–4021. doi: 10.1093/nar/9.16.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H. Physiology of killer factor in yeast. Adv Microb Physiol. 1981;22:93–122. doi: 10.1016/s0065-2911(08)60326-4. [DOI] [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvik B., Haber J. E. New cytoplasmic genetic element that controls 20S RNA synthesis during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):261–269. doi: 10.1128/jb.134.1.261-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Lacroute F. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J Bacteriol. 1971 May;106(2):519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. J., Wickner R. B. Pet18: a chromosomal gene required for cell growth and for the maintenance of mitochondrial DNA and the killer plasmid of yeast. Mol Gen Genet. 1978 Oct 4;165(2):115–121. doi: 10.1007/BF00269899. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Klein H. L. Deoxyribonucleic acid sequence organization of a yeast plasmid. J Bacteriol. 1977 Jan;129(1):472–481. doi: 10.1128/jb.129.1.472-481.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in yeast. Methods Cell Biol. 1975;11:221–233. doi: 10.1016/s0091-679x(08)60325-8. [DOI] [PubMed] [Google Scholar]
- Singh A., Helms C., Sherman F. Mutation of the non-Mendelian suppressor, Psi, in yeast by hypertonic media. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1952–1956. doi: 10.1073/pnas.76.4.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Co-curing of plasmids affecting killer double-stranded RNAs of Saccharomyces cerevisiae: [HOK], [NEX], and the abundance of L are related and further evidence that M1 requires L. J Bacteriol. 1982 May;150(2):545–551. doi: 10.1128/jb.150.2.545-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982 Dec;31(2 Pt 1):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Guerry-Kopecko P., Wickner R. B. A stable plasmid carrying the yeast Leu2 gene and containing only yeast deoxyribonucleic acid. J Bacteriol. 1980 Jan;141(1):413–416. doi: 10.1128/jb.141.1.413-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Wickner R. B. Curing of the 2 mu DNA plasmid from Saccharomyces cerevisiae. J Bacteriol. 1981 Mar;145(3):1421–1424. doi: 10.1128/jb.145.3.1421-1424.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite M. F., Mundy C. R., Cox B. S. Agents that cause a high frequency of genetic change from [psi+] to [psi-] in Saccharomyces cerevisiae. Genetics. 1981 Aug;98(4):691–711. doi: 10.1093/genetics/98.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. Induction of yeast killer factor mutations. J Bacteriol. 1977 Oct;132(1):346–348. doi: 10.1128/jb.132.1.346-348.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejksnora P. J., Haber J. E. Ribonucleoprotein particle appearing during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):246–260. doi: 10.1128/jb.134.1.246-260.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J., Wickner R. B. Virion DNA-independent RNA polymerase from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Jun 11;8(11):2349–2363. doi: 10.1093/nar/8.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Genetic control of replication of the double-stranded RNA segments of the killer systems in Saccharomyces cerevisiae. Arch Biochem Biophys. 1983 Apr 1;222(1):1–11. doi: 10.1016/0003-9861(83)90496-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Killer systems in Saccharomyces cerevisiae: three distinct modes of exclusion of M2 double-stranded RNA by three species of double-stranded RNA, M1, L-A-E, and L-A-HN. Mol Cell Biol. 1983 Apr;3(4):654–661. doi: 10.1128/mcb.3.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980 Aug;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Toh-e A. [HOK], a new yeast non-Mendelian trait, enables a replication-defective killer plasmid to be maintained. Genetics. 1982 Feb;100(2):159–174. doi: 10.1093/genetics/100.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. W., Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Van Leeuwenhoek. 1978;44(1):59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]