Abstract
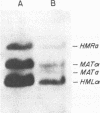
A mutation defective in the homothallic switching of mating type alleles, designated hml alpha-2, has previously been characterized. The mutation occurred in a cell having the HO MATa HML alpha HMRa genotype, and the mutant culture consisted of ca. 10% a mating type cells, 90% nonmater cells of haploid cell size, and 0.1% sporogenous diploid cells. Genetic analyses revealed that nonmater haploid cells have a defect in the alpha 2 cistron at the MAT locus. This defect was probably caused by transposition of a cassette originating from the hml alpha-2 allele by the process of the homothallic mating type switch. That the MAT locus of the nonmater cells is occupied by a DNA fragment indistinguishable from the Y alpha sequence in electrophoretic mobility was demonstrated by Southern hybridization of the EcoRI-HindIII fragment encoding the MAT locus with a cloned HML alpha gene as the probe. The hml alpha-2 mutation was revealed to be a one-base-pair deletion at the ninth base pair in the X region from the X and Y boundary of the HML locus. This mutation gave rise to a shift in the open reading frame of the alpha 2 cistron. A molecular mechanism for the mating type switch associated with the occurrence of sporogenous diploid cells in the mutant culture is discussed.
Full text
PDF
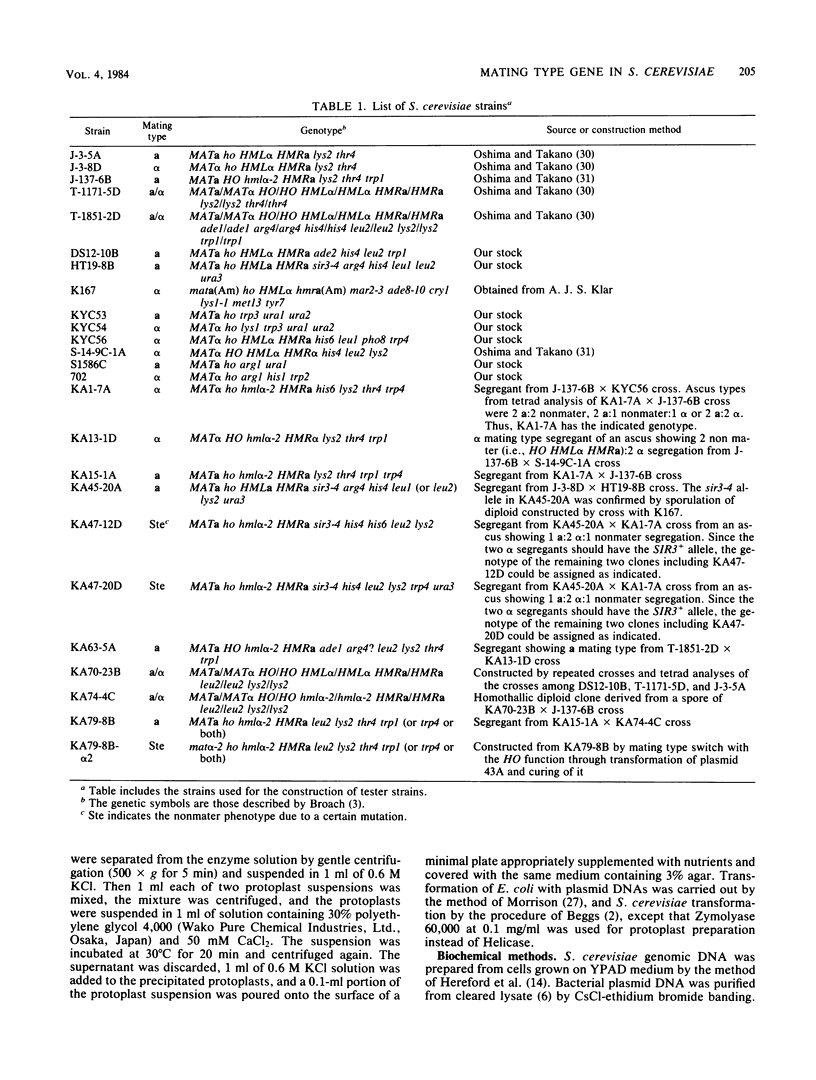



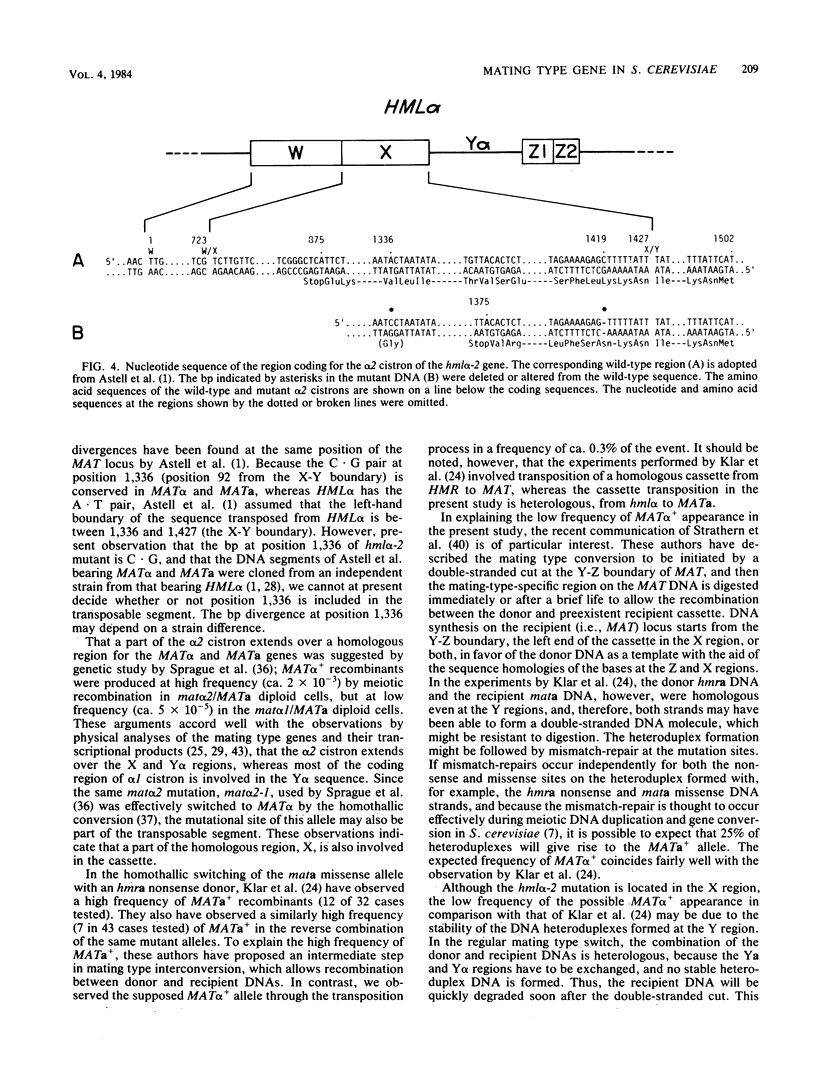


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Ahlstrom-Jonasson L., Smith M., Tatchell K., Nasmyth K. A., Hall B. D. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell. 1981 Nov;27(1 Pt 2):15–23. doi: 10.1016/0092-8674(81)90356-1. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTHORNE D. C. A DELETION IN YEAST AND ITS BEARING ON THE STRUCTURE OF THE MATING TYPE LOCUS. Genetics. 1963 Dec;48:1727–1729. doi: 10.1093/genetics/48.12.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., George J. P. A mutation that permits the expression of normally silent copies of mating-type information in Saccharomyces cerevisiae. Genetics. 1979 Sep;93(1):13–35. doi: 10.1093/genetics/93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Mascioli D. W., Rogers D. T. Illegal transposition of mating-type genes in yeast. Cell. 1980 Jun;20(2):519–528. doi: 10.1016/0092-8674(80)90638-8. [DOI] [PubMed] [Google Scholar]
- Harashima S., Nogi Y., Oshima Y. The genetic system controlling homothallism in Saccharomyces yeasts. Genetics. 1974 Aug;77(4):639–650. doi: 10.1093/genetics/77.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S., Oshima Y. Mapping of the homothallic genes, HM alpha and HMa, in Saccharomyces yeasts. Genetics. 1976 Nov;84(3):437–451. doi: 10.1093/genetics/84.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L., Fahrner K., Woolford J., Jr, Rosbash M., Kaback D. B. Isolation of yeast histone genes H2A and H2B. Cell. 1979 Dec;18(4):1261–1271. doi: 10.1016/0092-8674(79)90237-x. [DOI] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types I. Direct Observations of the Action of the Homothallism (HO) Gene. Genetics. 1976 Jun;83(2):245–258. doi: 10.1093/genetics/83.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types II. Restoration of Mating Ability to Sterile Mutants in Homothallic and Heterothallic Strains. Genetics. 1977 Mar;85(3):373–393. doi: 10.1093/genetics/85.3.373b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Hall B. D. Mutation of a heterothallic strain to homothallism. Genetics. 1975 May;80(1):77–85. doi: 10.1093/genetics/80.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON J. R., MORTIMER R. K. Use of snail digestive juice in isolation of yeast spore tetrads. J Bacteriol. 1959 Aug;78:292–292. doi: 10.1128/jb.78.2.292-292.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983 May;80(10):3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S., Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979 Sep;93(1):37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., McIndoo J., Hicks J. B., Strathern J. N. Precise mapping of the homothallism genes HML and HMR in Saccharomyces cerevisiae. Genetics. 1980 Oct;96(2):315–320. doi: 10.1093/genetics/96.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., McIndoo J., Strathern J. N., Hicks J. B. Evidence for a physical interaction between the transposed and the substituted sequences during mating type gene transposition in yeast. Cell. 1980 Nov;22(1 Pt 1):291–298. doi: 10.1016/0092-8674(80)90176-2. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Broach J. R., Hicks J. B. Regulation of transcription in expressed and unexpressed mating type cassettes of yeast. Nature. 1981 Jan 22;289(5795):239–244. doi: 10.1038/289239a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K., Hall B. D., Astell C., Smith M. A position effect in the control of transcription at yeast mating type loci. Nature. 1981 Jan 22;289(5795):244–250. doi: 10.1038/289244a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Oshima T., Takano I. Mating-type differentiation by transposition of controlling elements in Saccharomyces cerevisiae. Genetics. 1981 Mar-Apr;97(3-4):531–549. doi: 10.1093/genetics/97.3-4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T., Takano I. Mutants Showing Heterothallism from a Homothallic Strain of SACCHAROMYCES CEREVISIAE. Genetics. 1980 Apr;94(4):841–857. doi: 10.1093/genetics/94.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y., Takano I. Mating types in Saccharomyces: their convertibility and homothallism. Genetics. 1971 Mar;67(3):327–335. doi: 10.1093/genetics/67.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rine J., Strathern J. N., Hicks J. B., Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979 Dec;93(4):877–901. doi: 10.1093/genetics/93.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Rine J., Herskowitz I. Homology and non-homology at the yeast mating type locus. Nature. 1981 Jan 22;289(5795):250–252. doi: 10.1038/289250a0. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Blair L. C., Herskowitz I. Healing of mat mutations and control of mating type interconversion by the mating type locus in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3425–3429. doi: 10.1073/pnas.76.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Herskowitz I. Asymmetry and directionality in production of new cell types during clonal growth: the switching pattern of homothallic yeast. Cell. 1979 Jun;17(2):371–381. doi: 10.1016/0092-8674(79)90163-6. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Klar A. J., Hicks J. B., Abraham J. A., Ivy J. M., Nasmyth K. A., McGill C. Homothallic switching of yeast mating type cassettes is initiated by a double-stranded cut in the MAT locus. Cell. 1982 Nov;31(1):183–192. doi: 10.1016/0092-8674(82)90418-4. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Spatola E., McGill C., Hicks J. B. Structure and organization of transposable mating type cassettes in Saccharomyces yeasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2839–2843. doi: 10.1073/pnas.77.5.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J., Hicks J., Herskowitz I. Control of cell type in yeast by the mating type locus. The alpha 1-alpha 2 hypothesis. J Mol Biol. 1981 Apr 15;147(3):357–372. doi: 10.1016/0022-2836(81)90488-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Nasmyth K. A., Hall B. D., Astell C., Smith M. In vitro mutation analysis of the mating-type locus in yeast. Cell. 1981 Nov;27(1 Pt 2):25–35. doi: 10.1016/0092-8674(81)90357-3. [DOI] [PubMed] [Google Scholar]
- Toh-e A., Tada S., Oshima Y. 2-micrometers DNA-like plasmids in the osmophilic haploid yeast Saccharomyces rouxii. J Bacteriol. 1982 Sep;151(3):1380–1390. doi: 10.1128/jb.151.3.1380-1390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]