Abstract
Background
Prior studies of adult post-traumatic stress disorder suggest abnormal functioning of prefrontal and limbic regions. Cumulative childhood and adult trauma exposures are major risk factors for developing adult PTSD, yet their contribution to neural dysfunction in PTSD remains poorly understood. This study aimed to examine the neural correlates of childhood and adult trauma exposure, and post-traumatic stress symptoms (PTSS) within a single model.
Methods
Medication-free male combat veterans (n=28, average 26.6 years) with a wide range of PTSS were recruited from the community between 2010 and 2011. Subjects completed an emotional face morphing task while undergoing fMRI. Clinical ratings included the Clinician-Administered PTSD Scale (CAPS), Childhood Trauma Questionnaire (CTQ), and Combat Exposure Scale (CES). A priori regions were examined via multivariate voxelwise regression in SPM8, using depressive symptoms and IQ as covariates.
Results
In the angry condition, CAPS scores positively correlated with activation in the medial prefrontal cortex (BA10, z=3.51), hippocampus (z=3.47), insula (z=3.62), and, in earlier blocks, the amygdala. CES and CTQ positively correlated with activation in adjacent areas of the dorsal anterior cingulate (BA32, z=3.70; BA24, z=3.88, respectively). In the happy condition, CAPS, CTQ, and CES were not significantly correlated with activation patterns.
Conclusions
Dorsal anterior cingulate activation observed in prior studies of PTSD may be attributable to the cumulative effects of childhood and adult trauma exposure. In contrast, insula, hippocampus, and amygdala activation may be specific to PTSS. The specificity of these results to threat, and not positive stimuli, is consistent with abnormalities in threat processing associated with PTSS.
Keywords: post-traumatic stress, neuroimaging, child maltreatment, fMRI, trauma, emotion regulation
Introduction
Neural models of adult post-traumatic stress disorder (PTSD) suggest a number of abnormalities in circuitry underlying emotion regulation. Relatively common findings have included increased activation of the amygdala, insula, and dorsal anterior cingulate cortex (dACC) in response to emotional stimuli, particularly negative or threatening stimuli (Rauch et al. 2006; Shin & Liberzon 2010). In contrast, the ventromedial prefrontal cortex (vmPFC) shows relatively decreased activation in PTSD (Rauch et al. 2006; Shin & Liberzon 2010). The hippocampus shows increased activation to encoding of negative stimuli, but impaired recruitment during fear extinction in subjects with PTSD (Milad et al. 2009; Brohawn et al. 2010). These findings have led to a neural model of PTSD in which there is heightened detection and encoding of emotional stimuli mediated by the amygdala and hippocampus, increased threat appraisal and expression of fear responses by the dACC, with impaired fear suppression by the vmPFC and hippocampus (Rauch et al. 2006; Quirk & Mueller 2008; Shin & Liberzon 2010). Abnormal functioning of this network may underlie symptoms of re-experiencing, avoidance, and hyperarousal that are characteristic of PTSD.
Currently, the contribution of cumulative trauma, particularly childhood maltreatment, to neural dysfunction in PTSD remains largely unexplored. A history of childhood maltreatment represents one of the largest risk factors for the development of post-traumatic stress disorder (PTSD) as an adult. In the military population, the risk is similar to that of the adult index trauma (effect size ~0.25) (Brewin et al. 2000). Structural brain studies of trauma-exposed children (without apparent psychiatric illness) suggest reduced volume of the prefrontal cortex (Hanson et al. 2010; Edmiston et al. 2011). Studies of adults with a history of childhood maltreatment have demonstrated reduced prefrontal cortex and hippocampal volumes, and increased amygdala activation to threat stimuli (Andersen et al. 2008; Lupien et al. 2009; Dannlowski et al. 2012). Thus, childhood maltreatment exposure may impact emotion regulation circuitry to create vulnerability for the development of post-traumatic stress symptoms following subsequent trauma in adulthood.
Adult trauma exposure may further impact emotion regulation circuitry to create vulnerability for PTSD symptoms. A study of combat veterans without PTSD or mental illness revealed that combat exposure itself was associated with increased amygdala and insula activation to emotional stimuli (van Wingen et al. 2011a). These changes were reported to normalize after 1.5 years, though changes in amygdala-dACC coupling related to perceived combat threat remained (van Wingen et al. 2011b). Together, these findings suggest that childhood and adult trauma exposure may act in a cumulative way to alter emotion regulation circuitry and precipitate the clinical symptoms of PTSD.
Here we examined the functional neural correlates of childhood maltreatment, combat exposure, and post-traumatic stress symptoms (PTSS) in young military veterans. We included all variables in a single model to examine their relative contributions to brain activation. In response to implicit threat stimuli, but not positive stimuli, we hypothesized that childhood maltreatment, combat exposure, and current PTSS would correlate with increased amygdala, insula, dACC, and hippocampal activation, and impaired vmPFC recruitment in an additive way. In line with the dimensional approaches proposed for DSM-V as well as the NIMH Research Domain Criteria (in particular, the threat processing domain) (Helzer & American Psychiatric Association 2008; Insel et al. 2010), we examined these variables in subjects with a wide range of PTSS, both above and below the PTSD threshold.
Method
Participants
Twenty-eight young male combat veterans from Operations Enduring and Iraqi Freedom were recruited from the Pittsburgh area through public media advertisements from October 2010 to November 2011. Written informed consent was obtained from all participants after procedures were explained according to University of Pittsburgh Institutional Review Board guidelines. DD Form 214 documentation of prior military service was obtained from all potential participants. Subjects were between the ages of 18–35, right handed, and medication-free. Exclusion criteria included active substance abuse (past month), suicidality, psychotic or bipolar disorder, MRI contraindication, or neurological disease. In addition, participants were not included if they were currently receiving treatment for traumatic brain injury or had concussive symptoms at the time of the study. Of the 28 participants in the current sample, 13 participants (46.4%) reported exposure to blast, fire or explosion. Nine participants (32.1%) reported a history of closed head injury, and two of them also endorsed a loss of consciousness during their combat deployment. Seven of these participants endorsed being dazed, confused or “seeing stars”, two participants endorsed not remembering the injury, and four participants endorsed having symptoms of concussion afterward. Symptoms included headache, dizziness and irritability. However, none had concussive symptoms or were being treated for any sequelae of traumatic brain injury at the time of participation.
Subjects were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al. 2002). Past month PTSS were assessed using the Clinician-Administered PTSD Scale (CAPS) (Blake et al. 1995). Eighteen subjects met full criteria for PTSD as identified by the CAPS, using the F1/I2 criteria (Weathers et al. 2001). Three subjects met criteria for current major depressive disorder. Subjects were free of other current comorbid psychiatric disorders as determined by the SCID. Past DSM-IV diagnoses included major depressive disorder (n=5), bulimia nervosa (n=1), alcohol abuse/dependence (n=15), cannabis abuse/dependence (n=4), and cocaine abuse/dependence (n=1). The prevalence of these diagnoses did not significantly differ between PTSD and non-PTSD subjects (Chi-squared test, p>0.1). Depressive symptom severity was measured using the Beck Depression Inventory (BDI) (Beck et al. 1961). Level of combat exposure was measured with the Combat Exposure Scale (CES) (Keane et al. 1989). Childhood maltreatment history was assessed using the Childhood Trauma Questionnaire (CTQ) (Scher et al. 2001). The total CTQ score was used for this study. Additional rating scales included the Dissociative Experiences Scale (DES) (Bernstein & Putnam 1986), and the National Adult Reading Test (NART) (Blair & Spreen 1989) to estimate IQ.
Demographic and clinical variables for the subjects are shown in Table 1. Across all subjects, Spearman’s rank correlation of clinical variables revealed that CAPS score was significantly (p<0.05) correlated with BDI (ρ=0.57), CES (ρ=0.44), DES (ρ=0.42), and CTQ (ρ=0.41). BDI scores were also correlated with DES (ρ=0.43), and CTQ (ρ=0.41), and CTQ was correlated with subject age (ρ=0.44). NART (IQ) scores were not significantly correlated with other variables.
Table 1.
Demographic and clinical variables for participants.
| Average | SD | Range | Spearman's Rank Correlation (ρ) |
|
|---|---|---|---|---|
| Age | 26.6 | 2.6 | 22.2–31.8 | CTQ (0.44) |
| CAPS Past Month (CAPS) | 40.9 | 20.9 | 2–82 | BDI (0.57), CES (0.44), DES (0.42), CTQ (0.41) |
| Combat Exposure Scale (CES) | 17.2 | 9.9 | 0–35 | CAPS (0.44) |
| Childhood Trauma Questionnaire (CTQ) | 36.9 | 14.5 | 25–86 | CAPS (0.41), BDI (0.41), Age (0.44) |
| Dissociative Experiences Scale (DES) | 8.4 | 7.2 | 0.7–30.4 | CAPS (0.42), BDI (0.43) |
| Beck Depression Inventory (BDI) | 7.0 | 5.4 | 0–23 | CAPS (0.57), DES (0.43), CTQ (0.41) |
| NART (IQ) | 106.5 | 8.0 | 91–120 | -- |
Variables are displayed across all subjects (n=28). Spearman’s rank correlation among these variables are displayed for p<0.05. NART=National Adult Reading Test.
Dynamic faces task
Subjects completed fMRI during which they performed a dynamic faces task. This paradigm is an implicit emotion processing task that elicits activation in the amygdala and medial PFC and has been previously described (Almeida et al. 2011). Briefly, participants were asked to identify the color of a semi-transparent foreground color flash that appears during the presentation of a dynamically-changing background face (neutral to emotional). Emotion conditions included: happy, sad, angry and fearful. Only angry and happy conditions were examined in our analyses (angry as the most threatening emotion condition, and happy as a positive, safe condition for comparison). Control trials consisted of a simple morphing shape with similar structural and movement characteristics to each face stimulus. Emotional and control blocks were presented in a pseudorandomized order so that no two blocks of any condition were presented sequentially.
MRI acquisition and image processing
Neuroimaging data were collected using a 3-T Siemens Trio MRI scanner at the Magnetic Resonance Research Center in the University of Pittsburgh Medical Center. Structural 3D axial MPRAGE images were acquired in the same session (TE: 3.29 ms, TR: 2200 ms, Flip angle 9°, FOV = 256 mm × 192 mm, Slice thickness: 1 mm, Matrix: 256 × 256, 192 continuous slices). Blood-oxygen-level dependent (BOLD) images were then acquired with a gradient echo EPI sequence during 13min covering 39 axial slices (3.1 mm thick, TR/TE = 2000/28 ms, FOV = 205 cm × 205 cm, matrix = 64 × 64; Flip angle 90°).
Analyses were conducted in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Structural MPRAGE images for each participant were manually realigned and segmented into native space. BOLD images were realigned and unwarped, coregistered with the anatomical grey matter image, and spatially normalized into standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model. Normalized images were resampled to 2 mm × 2 mm × 2 mm voxels and smoothed with a 6mm full-width at half-maximum Gaussian filter.
A first-level (within subject) fixed-effect model was constructed with four emotions (anger, fear, sad, and happy) and the control condition entered as separate regressors in the design matrix, including movement parameters as nuisance variables and the canonical HRF model. The second-level analysis included two separate, multivariate voxelwise regressions for the angry-shape and happy-shape contrasts within SPM8. Only angry and happy conditions were included in the final analysis as preliminary analyses suggested suboptimal task-induced activation in the fear and sad conditions, making them unsuitable for hypothesis testing in this group of subjects. Angry and happy conditions were not directly compared. Regressors of interest included the CAPS, CES, CTQ, and DES, and were covaried for depressive symptoms and estimated IQ. Dissociative symptoms were included in this model given evidence suggesting the opposite pattern of brain activation in dissociative subtypes of PTSD (e.g. increased vmPFC, decreased amygdala activation) (Lanius et al. 2010). Task-induced effects were first determined across all subjects for each emotion condition. Subsequently, task-related activation patterns for each regressor of interest were examined from the same model.
Functional regions of interest (ROIs) were defined as clusters of contiguous voxels exceeding the a priori statistical threshold in a manner similar to Milad et al. (Milad et al. 2009; Rougemont-Bücking et al. 2011). We focused our fMRI data analysis a priori on the anteromedial PFC, amygdala, hippocampus, and insula. For these areas we used a threshold of uncorrected, p<0.001, and a minimum spatial extent of 10 contiguous voxels. We used a more stringent threshold of p<0.0001 for other brain regions.
Results
Task performance
Overall subjects performed with high accuracy in identifying color flashes during the angry and happy trials (90% and 91% correct, respectively). Multivariate regression was performed in SPSS to include all clinical variables in a single model and determine whether they predicted differences in reaction times or accuracy for the angry or happy conditions relative to the shape condition. CAPS, CES, CTQ, DES, and NART scores showed no significant correlation with relative reaction times or accuracy. BDI score correlated positively with a longer reaction times to happy vs. shape (β=0.50, p=0.04), but not angry vs. shape. BDI was not correlated with relative accuracy for either emotion.
fMRI responses during the dynamic faces task
Angry versus shape
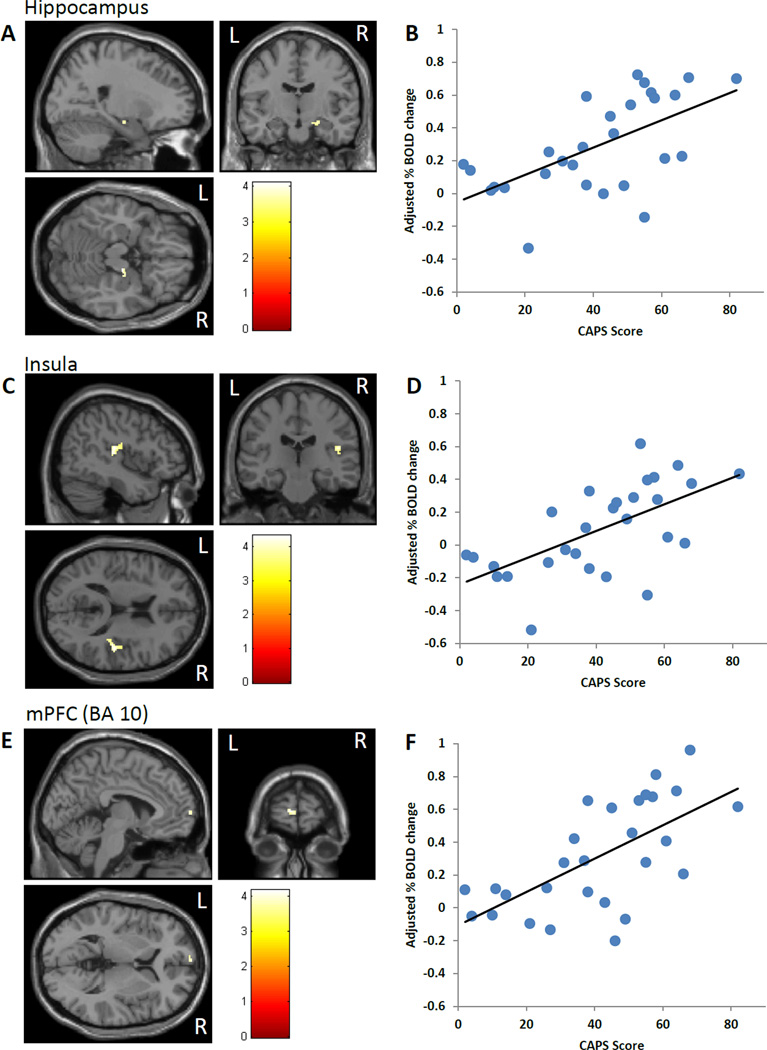
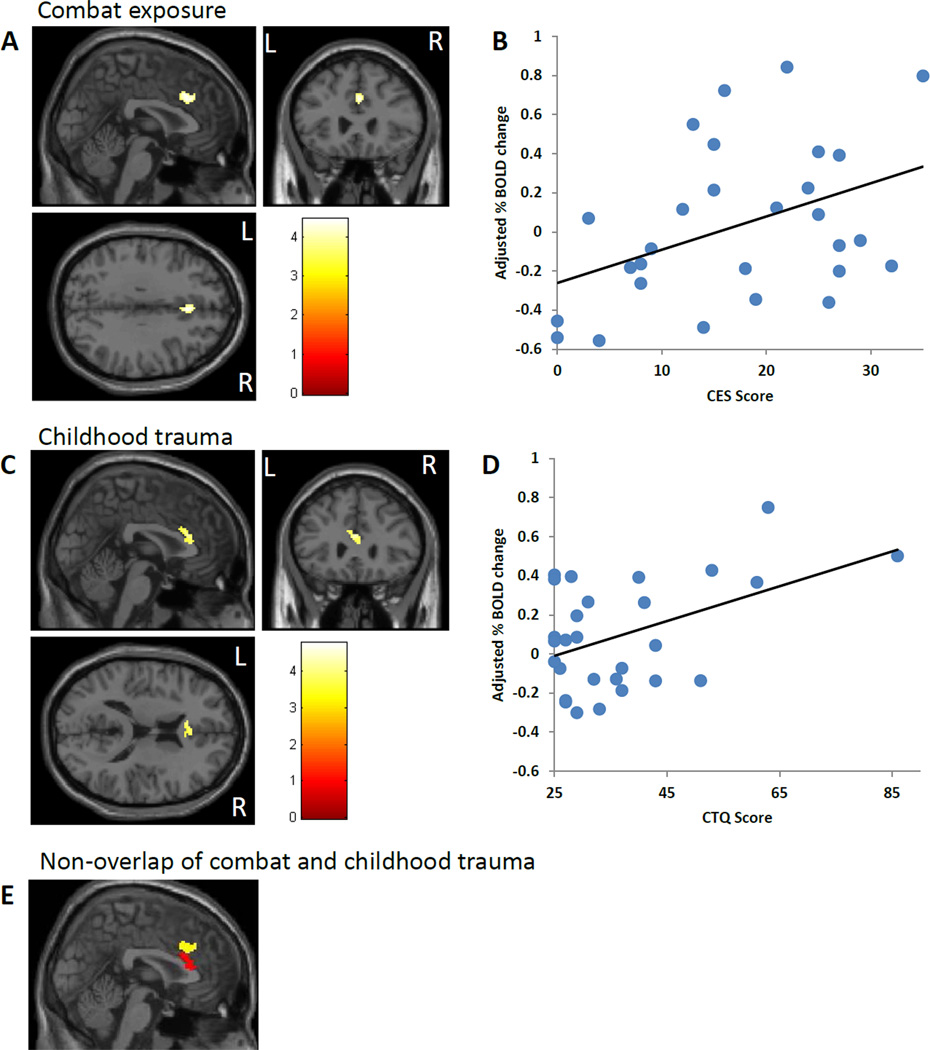
A summary of regional brain activation is shown in Table 2. Across all subjects, the angry trials (contrasted to shape) activated the medial and ventral PFC (BA 9, 10, 11, 32), the right hippocampus and amygdala, and the bilateral fusiform gyrus (Figure 1 A). In the multivariate regression, which included all clinical variables in a single model within SPM, CAPS scores positively correlated with activation in the right hippocampus (Fig. 2 A,B), right insula (BA 13, Fig. 2 C,D), and mPFC (BA 10, Fig. 2 E,F). CES scores positively correlated with dACC (BA 32) activation (Fig. 3 A,B). CTQ scores correlated positively with an adjacent area of the dACC (BA 24) (Fig. 3 C,D). Analysis of the CES- and CTQ-associated clusters in the dACC using ImCalc within SPM revealed that there was no spatial overlap (Fig. 3 E). In contrast to CES and CTQ, DES scores negatively correlated with dACC (BA 24, Table 2). None of the variables in the model were significantly correlated with amygdala activation.
Table 2.
fMRI responses during the dynamic faces task.
| Angry-Shape | ||||||
| Contrast | Region | Z-score | k | x | y | z |
| Task effects | Right Parahippocampal Gyrus, Hippocampus, Amygdala | 4.72 | 285 | 24 | −14 | −16 |
| mPFC (BA 10/11) | 4.41 | 117 | 0 | 54 | −12 | |
| mPFC (BA 9/10) | 4.62 | 517 | 0 | 62 | 14 | |
| Rostral ACC (BA 32) | 3.37 | 13 | −2 | 44 | 6 | |
| CAPS positive correlation | Right Hippocampus | 3.47 | 26 | 18 | −14 | −16 |
| Left mPFC (BA 10) | 3.51 | 21 | −8 | 66 | 6 | |
| Right Insula (BA 13) | 3.62 | 98 | 46 | −24 | 16 | |
| CES positive correlation | dACC (BA 24) | 3.70 | 85 | 4 | 24 | 34 |
| Left BA 6/43 | 4.00 | 16 | −62 | −10 | 12 | |
| CTQ positive correlation | dACC (BA 24) | 3.88 | 164 | −2 | 24 | 22 |
| Left Inferior Frontal Gyrus (BA 44) | 4.11 | 26 | −52 | 8 | 14 | |
| Right Inferior Frontal Gyrus (BA 6/44) | 4.51 | 26 | 54 | 2 | 20 | |
| DES negative correlation | dACC (BA 24) | 3.43 | 27 | 0 | 30 | 18 |
| Happy-Shape | ||||||
| Contrast | Region | Z-score | k | x | y | z |
| Task effects | Right Parahippocampal Gyrus, Hippocampus, Amygdala, vmPFC (BA 25/47) | 5.61 | 730 | −34 | 6 | −16 |
| vmPFC (BA 11) | 3.79 | 52 | −2 | 56 | −16 | |
| mPFC (BA 10) | 3.51 | 32 | −6 | 60 | 20 | |
| mPFC (BA 9/10) | 3.76 | 12 | −4 | 60 | 30 | |
| DES positive correlation | mPFC (BA 9) | 3.49 | 22 | 6 | 50 | 36 |
| Right Superior Temporal Gyrus | 4.39 | 27 | 66 | −6 | 4 | |
| Left Parietal Lobe (BA 3) | 4.44 | 22 | −50 | −24 | 58 | |
| Left Occipital Lobe (BA 18) | 4.10 | 20 | −18 | −96 | 10 | |
Multivariate regression was conducted in SPM8 to examine the neural correlates of CAPS, CES, CTQ, and DES in the angry-shape and happy-shape contrast conditions. For each emotion contrast, overall task effects are shown, followed by significant correlations with clinical variables. Analyses were controlled for BDI and IQ scores. A priori functional regions of interest (shown in regular type) were identified at p<0.001 uncorrected, with minimum cluster size k=10 voxels. Regions outside of a priori areas (shown in italics) were identified at the more stringent p<0.0001 threshold. CAPS=Clinician-administered PTSD Scale, CES=Combat Exposure Scale, CTQ=Childhood Trauma Questionnaire, BDI=Beck Depression Inventory. vmPFC=ventromedial prefrontal cortex, dACC=dorsal anterior cingulate cortex. k=cluster size. Peak activation coordinates (x, y, z) are based on the Montreal Neurological Institute atlas.
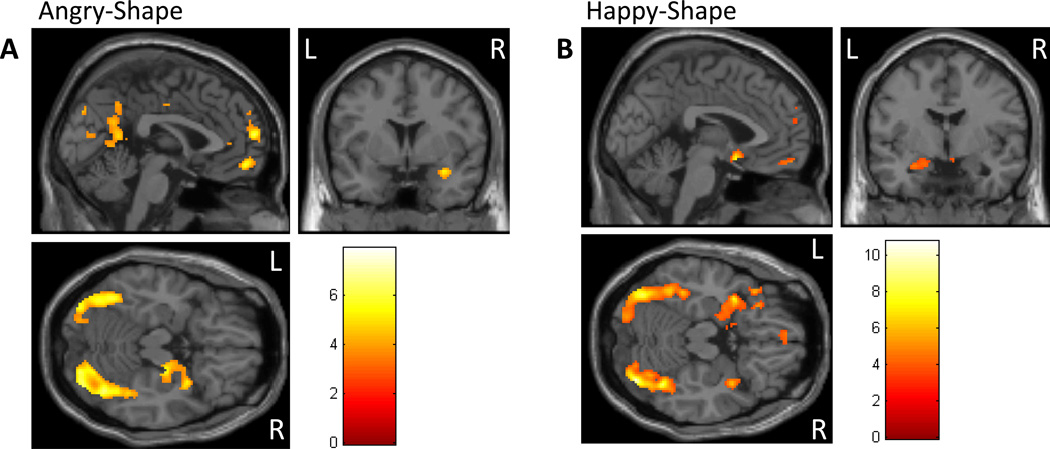
Figure 1.
Task effects. Activation patterns across all subjects are displayed at the p<0.001 uncorrected threshold. (A) The angry-shape contrast revealed activation in the vmPFC, amygdala, hippocampus, and the visual/face processing stream including the fusiform gyrus. (B) The happy-shape contrast revealed activation in the vmPFC, amygdala, hippocampus, and the visual/face processing stream including the fusiform gyrus. Results were covaried for depressive symptoms and IQ. vmPFC=ventromedial prefrontal cortex.
Figure 2.
CAPS correlations for angry-shape contrast. CAPS scores (past month) were positively correlated with activation in the hippocampus (A, B), insula (C, D), and mPFC (E, F). Activation images are shown for each brain region (A, C, E) along with partial plots of the extracted percent BOLD signal change for each cluster vs. CAPS scores (B, D, F). Results were covaried for depressive symptoms and IQ. CAPS=Clinician Administered PTSD Scale.
Figure 3.
Trauma exposure correlates for angry-shape contrast. CES (A, B) and CTQ (C, D) scores were positively correlated with activation in adjacent areas of the dACC (BA 32 and BA 24, respectively). Activation images (A, C) and partial plots of the extracted percent BOLD signal change vs. trauma exposure (B, D) are shown. (E) CES-(yellow) and CTQ- (red) associated dACC clusters were adjacent and without any spatial overlap. Results were covaried for depressive symptoms and IQ. CES=Combat Exposure Scale. CTQ=Childhood Trauma Questionnaire.
In regions outside of these a priori areas, CES positively correlated with activation in left BA 6/43. CTQ positively correlated with activation in bilateral BA 44 and another cluster including BA 2 and 40.
Given the above results, we performed two secondary analyses. First, we assessed for an interaction between CES and CTQ in the dACC by including an interaction term in the voxelwise multivariate regression. This analysis revealed no significant interaction in the dACC, while their independent associations with dACC activation remained. Second, the lack of a correlation between trauma exposure or PTSS and amygdala activation in this contrast is surprising and could be due to habituation of amygdala activation over repeated blocks. To examine this possibility, we extracted the task-induced amygdala activation for each block and performed further analysis within SPSS. Repeated measures ANOVA revealed a significant effect of block (F=11.84, p<0.001). This effect was driven by robust amygdala activation to angry faces in blocks 1 and 2, but no activation in block 3, suggesting habituation. In multivariate regressions for blocks 1 and 2, CAPS was positively correlated with amygdala activation (β=0.49, p=0.08; β=0.64, p=0.03 respectively). CES, CTQ, and DES were not correlated with amygdala activation in any of the blocks.
Happy versus shape
A summary of regional brain activation is shown in Table 2. Across all subjects, the happy trials (contrasted to shape) activated the medial and ventral PFC (BA 10, 11, 25, 47), the right hippocampus and amygdala, and the bilateral fusiform gyrus (Fig. 1 B). In the multivariate regression, which included all clinical variables in a single model within SPM, CAPS, CES, and CTQ scores were not significantly correlated with any brain activation or deactivation. DES scores correlated positively with dorsal mPFC (BA 9) activation (Table 2).
In regions outside of these a priori areas, DES also positively correlated with activation in the right superior temporal gyrus, and left parietal and occipital lobe.
Discussion
To our knowledge, this is one of the few studies to examine, within a single model, the contribution of childhood and adult trauma exposure, and PTSS to alterations in brain circuitry that have been associated with PTSD. We tested this circuitry using a dynamic faces task, which requires implicit emotion regulation and attentional processing of emotional cues. We focused on angry and happy emotional distractors in our analyses, with the prediction that angry faces are potent threat cues and would specifically elicit differences associated with childhood maltreatment, combat exposure, and PTSS. Both emotion conditions elicited activation of the vmPFC and amygdala, suggesting engagement of circuitry underlying the detection of emotional cues and implicit regulation of emotional responses.
In the angry, but not happy trials, PTSS (CAPS scores) were positively correlated with insula and hippocampus activation, and a small cluster in BA10. PTSS also correlated with amygdala activation in earlier angry blocks. Combat exposure and childhood maltreatment scores positively correlated with activation of adjacent areas of the anterior dACC. Dissociative symptoms, in contrast, negatively correlated with dACC activation.
Increased dACC activation has been observed in studies of PTSD, including studies of fear conditioning and extinction, emotional interference, and resting (Shin & Liberzon 2010). The dACC subserves the appraisal and expression of fear and anxiety, as well as conflict adaptation (Phillips et al. 2008; Etkin et al. 2011; Shackman et al. 2011). The dACC shows strong connectivity with the amygdala and is the likely homologue of the rat prelimbic cortex (PL) (Milad et al. 2006; Etkin et al. 2011). Deactivation, stimulation, and neuronal recording studies of the rat PL suggest that this region promotes the expression of fear responses, e.g. (Vidal-Gonzalez et al. 2006; Quirk & Mueller 2008; Burgos-Robles et al. 2009; Sierra-Mercado et al. 2011). As such, heightened activity in this structure could underlie, in part, the hypervigilance (heightened threat appraisal) and generalized fear responses associated with PTSS. A study of Vietnam veteran twin pairs revealed increased dACC activation in both the twin with PTSD and their non-PTSD co-twin, suggesting that increased dACC activation is a familial risk factor for developing PTSD (Shin et al. 2009, 2011). Our results suggest that one important familial risk factor may be childhood maltreatment exposure, which would be expected to be a common risk among siblings. Our findings further suggest that both childhood maltreatment and adult combat exposure independently correlate with dACC activation to threat. Clinically, both childhood maltreatment and combat exposure scores were significantly correlated with PTSS. Thus, trauma-associated dACC activation could represent one putative mechanism for the development of PTSS, by creating vulnerability at the brain level. A recent study in rats revealed that chronic stress exposure resulted in a failure to decrease prelimbic firing during fear extinction recall (Wilber et al. 2011). In humans, a longitudinal study of combat veterans revealed that perceived threat during combat may lead to a lasting, reduced negative coupling between the dACC and amygdala, even in the absence of PTSD (van Wingen et al. 2011b). In this context, our findings suggest that both childhood and adult trauma exposure may sensitize the dACC, which may promote the appraisal of threat and the expression of fear responses through its connections with the amygdala. PTSS could then emerge from this vulnerability with additional changes in brain circuitry as described below.
Consistent with studies of PTSD, our results demonstrate that PTSS are positively correlated with insula, amygdala, and hippocampus activation to threat stimuli. Furthermore, these associations were specific to PTSS, and not attributable to childhood maltreatment or combat exposure. Increased insula and amygdala activation have been relatively common findings in studies of PTSD, with hippocampal activation being more variable (Shin & Liberzon 2010). While our study is associational only, we speculate that altered activation in these areas could result from greater dACC activation related to trauma exposure. The dACC is known to have strong connections with both the amygdala and insula (Shackman et al. 2011). Sensitization of the dACC could, at least in vulnerable individuals, then lead to greater activation of the insula and amygdala-hippocampus complex, promoting anxiety and fear responses typical of PTSD. This would presumably occur in concert with vmPFC hypoactivation, though we did not detect associations with the vmPFC in our study (see below for discussion). Clearly, more longitudinal studies in traumatized populations, beginning in childhood, will be needed to examine these possibilities.
PTSS were also positively associated with dorsomedial PFC (BA10) activation in our study. This area has been implicated in the suppression of emotional responses, and in selecting choices or actions based on the affective value of an outcome (Phillips et al. 2008; Grabenhorst & Rolls 2011). In this context, increased BA10 activation may reflect a greater effort required in the presence of PTSS to suppress emotional responses and choose correct responses. Given the lack of differences in reaction times or accuracy, this could reflect a compensatory mechanism.
Our study did not reveal any significant correlations between trauma exposure or PTSS and vmPFC activation. While vmPFC hypoactivation is commonly reported in studies of PTSD, some studies have found no differences between PTSD and control subjects (Francati et al. 2007; Etkin & Wager 2007). There are several possible reasons for the lack of vmPFC associations in this study. First, though our study included subjects with a wide range of PTSS, we may not have had sufficiently high CAPS scores to detect an association within the vmPFC. When considering only subjects with PTSD, their past month CAPS scores were lower (average 53) than other studies of PTSD. Subjects were also younger than in most studies, with fewer comorbidities, and less chronicity of PTSD. Thus it is possible that vmPFC differences, which have also been shown to correlate with PTSD symptom severity (Shin & Liberzon 2010), are only more evident with more severe and chronic PTSD. Second, it is possible that alterations in vmPFC may have been present shortly after the end of deployment but have since normalized. Further longitudinal studies will be needed to test these possibilities.
Of note, we found that dissociative symptoms were negatively correlated with dACC activation in the threat, but not positive, emotion trials. Reduced dACC activation related to dissociative symptoms could reflect reduced appraisal and expression of fear and anxiety at the neural level (Lanius et al. 2010). It should be noted, however, that dissociative symptoms in this sample were quite low, with all but three individuals with DES scores less than 15. Thus comparisons to studies of dissociative subtypes of PTSD are limited.
There are several limitations to the current study. First, these findings are cross-sectional and correlational only and cannot be used to infer causation. It is thus difficult to tease out whether observed changes represent preexisting vulnerabilities, state-related changes, or compensatory changes. Second, these findings may not generalize to other populations (particularly women given the all-male sample), trauma types, or to individuals with more severe PTSD as discussed above. Third, while subjects were free of any current alcohol or substance abuse, this study did not have measures of cumulative, lifelong alcohol use which could affect the current results. However, the PTSD and non-PTSD subjects had similar frequencies of past alcohol abuse/dependence, which makes it less likely that this potential confound systematically influenced the results. Finally, this study enrolled a relatively small number of subjects and would merit replication with a larger sample.
In conclusion, our findings suggest that dorsal ACC activation observed in prior studies of PTSD may be attributable to childhood and adult trauma exposure. In contrast, insula, amygdala, and hippocampal activation may be specifically associated with the PTSD syndrome. The specificity of these results to threat, and not positive stimuli, is consistent with abnormalities in threat processing associated with PTSD. Taken together, the results of the current study begin to suggest putative mechanisms by which childhood and adult trauma exposure may create a vulnerable brain substrate for the development of PTSS, which itself may emerge with additional changes in limbic circuitry.
Acknowledgments
The authors would like to thank Benjamin Paul and Ashlee Filippone for their assistance with participant screening, MRI procedures, and data organization. The authors would also like to thank Michael and Morven Heller for their sponsorship.
This work was supported by the AACAP Pilot Research Award (RH), Department of Defense Congressionally Directed Medical Research Program PT073961 (AG), and the National Institute of Mental Health MH083035 (AG), MH076971 (MP), MH088913 (MP). Dr. Germain has served as a consultant for Concurrent Technologies Corporation.
Footnotes
Declaration of Interest: Drs. Herringa, Phillips, Fournier, and Kronhaus report no potential conflicts of interest.
References
- Almeida JRC, de, Kronhaus DM, Sibille EL, Langenecker SA, Versace A, Labarbara EJ, Phillips ML. Abnormal left-sided orbitomedial prefrontal cortical-amygdala connectivity during happy and fear face processing: a potential neural mechanism of female MDD. Frontiers in Psychiatry / Frontiers Research Foundation. 2011;2:69. doi: 10.3389/fpsyt.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of neuropsychiatry and clinical neurosciences. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. The Journal of Nervous and Mental Disease. 1986;174:727–735. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of traumatic stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of consulting and clinical psychology. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biological psychiatry. 2010;68:1023–1030. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics & Adolescent Medicine. 2011;165:1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depression and anxiety. 2007;24:202–218. doi: 10.1002/da.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE American Psychiatric Association. Dimensional approaches in diagnostic classification: refining the research agenda for DSM-V. Arlington, Va.: American Psychiatric Association; 2008. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Keane T, Fairbank J, Caddell J, Zimering R, Taylor K, Mora C. Clinical evaluation of a measure to assess combat exposure. Psychological assessment. 1989;1:53–55. [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. The American Journal of Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews.Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neuroscience & Therapeutics. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. Journal of traumatic stress. 2001;14:843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature reviews. Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman AJ, Rauch SL, Pitman RK. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Archives of general psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, Macklin ML, Gold AL, Karpf RD, Orr SP, Rauch SL, Pitman RK. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. The American Journal of Psychiatry. 2011;168:979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernández G. Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry. 2011a;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernandez G. The neural consequences of combat stress: long-term follow-up. Molecular Psychiatry. 2011b;17:116–118. doi: 10.1038/mp.2011.110. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & Memory (Cold Spring Harbor, N.Y.) 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depression and anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]