Abstract
Fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) in basement membranes and interstitial tissues, resulting from increased synthesis or decreased degradation of ECM or both. The plasminogen activator/plasmin system plays an important role in ECM degradation, whereas the plasminogen activator inhibitor 1 (PAI-1) is a physiologic inhibitor of plasminogen activators. PAI-1 expression is increased in the lung fibrotic diseases and in experimental fibrosis models. The deletion of the PAI-1 gene reduces, whereas the overexpression of PAI-1 enhances, the susceptibility of animals to lung fibrosis induced by different stimuli, indicating an important role of PAI-1 in the development of lung fibrosis. Many growth factors, including transforming growth factor beta (TGF-β) and tumor necrosis factor alpha (TNF-α), as well as other chemicals/agents, induce PAI-1 expression in cultured cells and in vivo. Reactive oxygen and nitrogen species (ROS/RNS) have been shown to mediate the induction of PAI-1 by many of these stimuli. This review summarizes some recent findings that help us to understand the role of PAI-1 in the development of lung fibrosis and ROS/RNS in the regulation of PAI-1 expression during fibrogenesis.
INTRODUCTION
Fibrosis is a characteristic feature and terminal stage of many lung disorders, including idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis, drug- and radiation-induced lung injury, sarcoidosis, silicosis, asbestosis, cystic fibrosis (CF), and acute respiratory distress syndrome (ARDS). Despite the severe outcome, no efficacious treatment is known for these devastating fibrotic diseases because of a poor understanding of the complex pathologic processes. Fibrosis, characterized by excessive accumulation of extracellular matrix (ECM) in basement membranes and interstitial tissues, results from increased synthesis or decreased degradation of ECM (or both). Although intensive studies have been conducted, the molecular mechanism underlying the development of fibrosis is still not well understood. ECM degradation is mediated mainly by the matrix metalloproteinases (MMPs) and plasmin (4, 45, 129). Plasmin is converted from zymogen plasminogen by tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA). The activities of tPA and uPA, under physiologic conditions, are controlled by plasminogen activator inhibitor 1 (PAI-1). Therefore, PAI-1 plays an important role in the regulation of ECM degradation. PAI-1 expression is increased in many fibrotic diseases and in experimental fibrosis models. Genetic modification of PAI-1 expression alters the sensitivity of animals to the fibrogenesis induced by various stimuli. These data suggest an important role of PAI-1 in the development of fibrosis, although the mechanism underlying the induction of PAI-1 during fibrogenesis and whereby PAI-1 promotes fibrosis remains to be further characterized. Emerging evidence indicates that reactive oxygen and nitrogen species (ROS/RNS) contribute importantly to the development of fibrosis under many pathologic conditions. The molecular mechanism whereby ROS/RNS mediate the fibrogenic effect, however, is largely unknown. This review focuses on the role of PAI-1 in the development of fibrosis and the redox regulation of PAI-1 gene expression during fibrogenesis.
PLASMINOGEN ACTIVATOR INHIBITOR-1 AND FIBROSIS
Plasminogen activation system and collagen degradation
ECM degradation is mediated mainly by two proteolytic systems, the fibrinolytic (plasminogen/plasmin) system and the MMP system. Although MMPs play a major role in the degradation of the ECM, increasing evidence indicates that plasmin is also important in ECM degradation. Plasmin can directly degrade various types of ECM proteins including fibronectin, laminin, proteoglycan, fibrin, denatured collagens, and type IV collagen (4, 5, 14, 59, 99, 148, 231). Most MMPs are synthesized and secreted as inactive proenzymes. The activities of MMPs are regulated at different levels, including gene expression, activation of proenzymes, and inhibition of active enzymes by tissue inhibitors of metalloproteinases (TIMPs) (106). Activation of promatrix metalloproteinases by sequential proteolysis of the propeptide that blocks the active-site cleft is regarded as one of the key levels of regulation of the activities for these proteinases. Although various agents/stimuli, including trypsin, ROS, and organomercurials, can activate MMPs in vitro (49, 80, 176, 177), a relatively well characterized mechanism for in vivo activation of MMP proenzymes involves the plasminogen activator–plasmin cascade (134, 143, 166, 175, 240). Therefore, in addition to degrading ECM proteins directly, plasmin can also activate MMPs, including MMP-2, MMP-3, MMP-9, MMP-12, and MMP-13 (106, 134, 143, 161, 166), which in turn degrade collagens and other ECM proteins.
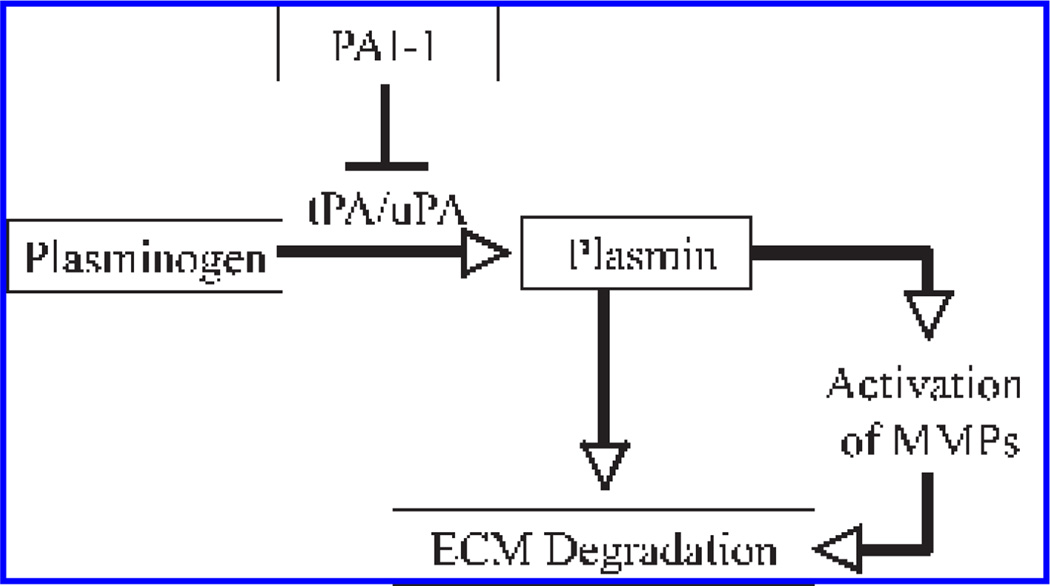
Circulating plasminogen originates mainly from the liver, but many other tissues/organs, including kidney, lung, heart, brain, and spleen, also produce plasminogen locally (251). Inactive proenzyme plasminogen is converted to plasmin, a serine protease, by enzymatic cleavage of a 19-amino acid peptide, catalyzed by tPA and uPA. Plasminogen activation takes place in solutions, such as plasma, and on the cell surface. Many types of cells express plasminogen receptor on their surfaces, allowing localized activation of plasminogen (31). Binding to cell-surface receptors also substantially increases the rate of plasminogen activation, as compared with the reaction in solutions (58, 67, 159). In general, tPA is involved mainly in the activation of circulated plasminogen (fibrin clots), whereas uPA is involved in the activation of cell surface–bound plasminogen (67, 159). Under physiologic conditions, the activities of tPA and uPA are controlled by plasminogen activator inhibitors (PAIs), which inhibit plasminogen activator activity and therefore the activation of plasminogen, leading to ECM accumulation (Fig. 1).
FIG. 1. Plasminogen activation system and extracellular matrix (ECM) degradation.
Plasminogen is converted to plasmin by tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). Plasmin then degrades ECM components directly or indirectly by activating matrix metallo-proteinases (MMPs). Under physiologic conditions, the activities of tPA and uPA are controlled by plasminogen activator inhibitor 1 (PAI-1).
Three PAIs have been identified, with type 1 plasminogen activator inhibitor (PAI-1) being the primary physiologic inhibitor of tPA and uPA in vivo (51). PAI-1 is a single-chain glycoprotein with a molecular mass of 50,000 Da, and it belongs to the serpin gene family (51). PAI-1 reacts very rapidly with single-chain and two-chain t-PA as well as with two-chain u-PA, with a second-order inhibition rate constant of 107 M/sec, but it does not react with single-chain u-PA (123). In addition to inhibiting t-PA/u-PA activity directly, PAI-1 can also block t-PA–mediated clot lysis by binding to fibrin and can inactivate u-PA via internalizing the u-PA and u-PA receptor (u-PAR) complex (43, 171, 196). PAI-1 is synthesized and secreted as an active form, which is unstable in solution and spontaneously converts into the inactive (latent) form with a half-life ~1–2 h at 37°C (136). Almost all of active PAI-1 in circulation binds to vitronectin, which extends its half-life by twofold to 10-fold. By binding to vitronectin, PAI-1 competes with u-PAR-de-pendent or integrin-dependent binding of cells to the ECM. Thereby, PAI-1 also plays an important role in cell adhesion and/or migration via a mechanism independent of its antiproteolytic activity (53, 135).
Increased PAI-1 expression in human lung fibrotic diseases
Fibrosis is a terminal stage of many lung disorders including IPF, hypersensitivity pneumonitis, drug- and radiation-induced lung injury, sarcoidosis, silicosis, asbestosis, CF, and ARDS. It has been reported that the fibrinolytic activity is decreased in broncheoalveolar lavage fluid (BALF) from patients with ARDS (13, 83, 100), IPF or interstitial lung diseases (33, 75, 84, 121), and sarcoidosis (33, 89). Such decreased fibrinolytic activity is associated with an increase in PAI-1 expression (75, 83, 121), suggesting an important role of PAI-1 in the development of the lung fibrotic diseases (13, 83, 84, 100).
IPF is a progressive and lethal fibrotic lung disease with unknown etiology and poor survival. It was speculated in the past that lung fibrosis results from an unremitting inflammatory response to an exogenous insult, which led to fibroblast activation/proliferation and eventually culminated in progressive fibrosis. Therefore, antiinflammatory agents alone or in combination with cytotoxic drugs have been used in clinics as a standard therapeutic regimen for the treatment of IPF. However, little evidence indicates that these agents alter the natural history of the disease or improve survival of the patients, suggesting that inflammation may not play a major role in the development of pulmonary fibrosis. Nevertheless, although the mechanism underlying the development of IPF is unclear, emerging evidence indicates that increased expression of PAI-1 may contribute importantly to the pathogenesis of IPF. Nakstad et al. (167) reported that plasminogen activator activity was significantly lower, associated with high levels of antifibrinolytic activity, in IPF patients as compared with controls, suggesting that an imbalance between fibrinolytic and antifibrinolytic systems plays a role in the pathogenesis of IPF. Kotani et al. (121) also reported that PAI-1 levels in BAL supernatant fluids and PAI-2 levels in BAL cell lysates were significantly higher in IPF patients than those in normal subjects. These observations further suggest a critical role of PAI-1 in the development of IPF.
Several polymorphisms in the PAI-1 gene promoter, including the deletion/insertion polymorphism (4G/5G), have been reported and are associated with increased plasma levels of PAI-1 (152). In vitro experiments have shown that the 4G allele produces 6 times more PAI-1 RNA than the 5G allele in response to IL-1b (46). Individuals homozygous for the 4G allele have higher basal and inducible concentrations of PAI-1 than do those with one or two copies of the 5G allele (46). Interestingly, Kim et al. (116) reported that the patients with idiopathic interstitial pneumonia were more likely than the control population to have the promoter genotype of 4G/4G. These data provide direct evidence that IPF is genetically linked to high levels of PAI-1.
Sarcoidosis is a granulomatous disease associated with inflammation. Ninety percent of sarcoidosis cases are found in the lungs and, in many cases, will progress to pulmonary fibrosis. Chapman (33) reported that procoagulant activity in the macrophages extracted by lavage from patients with sarcoidosis was increased as compared with those from controls. Hasday et al. (89) further showed that mean procoagulant activity in the sarcoidosis group was significantly elevated, whereas plasminogen activator activity tended to be lower as compared with a control group. Therefore, it was concluded that in pulmonary sarcoidosis, abnormal expression of procoagulant and plasminogen activator activities in alveolar fluid may favor accumulation of fibrin matrix at inflammatory foci (89).
Cystic fibrosis is a genetic disease caused by mutations of the CF transmembrane conductance regulator (CFTR) gene (62, 239), which leads to a defect in CFTR proteins, a cAMP-dependent chloride channel. The major clinical problems associated with CFTR gene mutations are mucus accumulation, Pseudomonas aeruginosa infection, and chronic inflammation, with a consequence of remodeling and derangement of lung structure. Although the CFTR defect plays a dominant role in the development of CF, other factors may also contribute to the pathogenesis of the disease. Klinger et al. (118) reported that the PAI-1 gene, located at region q21.3-q22 of chromosome 7, is genetically linked with CF (118). Xiao et al. (241), furthermore showed that the PAI-1 level was elevated in induced sputum in patients with CF, which negatively correlated with pulmonary function. These observations indicate that PAI-1 may play a role in CF pathology.
Acute respiratory distress syndrome (ARDS) arises from a variety of local and systemic insults and is a major cause of acute respiratory failure. ARDS confers a high morbidity rate, in part due to the excessive deposition of collagen during the development of pulmonary fibrosis in the late-phase ARDS healing response. Fibrin deposition in the airspaces and lung microvasculature in ARDS results from the activation of the coagulation cascade and the impairment of fibrinolysis. Idell et al. (101) reported that procoagulant activity was increased in BAL and plasma 3 days after the onset of ARDS, whereas fibrinolytic activity was undetectable in BAL at 3 days after ARDS and remained depressed for up to 14 days. Such depressed fibrolytic activity was accompanied by an increase in PAI-1 activity (101). It has also been reported that alveolar PAI-1 antigen levels were more than 5 times higher in the patients with aspiration pneumonitis who progressed to ARDS than in those with uncomplicated aspiration pneumonitis, although plasma levels of PAI-1 antigen were not significantly different between the two groups (61). Several types of lung cells, including alveolar macrophages, endothelial cell, and epithelial cells, produce PAI-1 or PAs or both. Unstimulated alveolar macrophages usually express PA and are profibrinolytic; however, when stimulated, alveolar macrophages increased PAI-1 production and become antifibrolytic (34, 35). Chapman et al. (35) reported that alveolar macrophages from ADRS patients have increased PAI-1 mRNA, whereas Grau et al. (81) reported that lung microvascular endothelial cells isolated from ADRS patients expressed more PAI-1 than did controls and had a lower fibrinolytic potential, as measured by the PA/PAI-1 ratio. All these observations suggest that increased PAI-1 expression or decreased PA activity or both contribute importantly to the pathogenesis of ARDS.
Small-airway remodeling is a pathologic feature of asthma and contributes significantly to airflow obstruction. One of the major components of airway remodeling is excessive deposition of ECM proteins in bronchiolar walls (subepithelial fibrosis). Cho et al. (39) reported that mast cells from a patient with an asthma attack produced an increased amount of PAI-1. Buckova et al. (24) further showed that the 4G/5G polymorphism of the PAI-1 promoter is associated with an increased risk of asthma in the Czech population. These observations suggest a potential role of PAI-1 in asthmatic airway pathology (24, 39, 95).
PAI-1 and lung fibrosis: animal models
The central role of PAI-1 in the development of fibrosis has been well documented in several animal models. Bleomycin is a chemotherapy drug used for the treatment of some types of cancers. One of side effects associated with bleomycin treatment is lung fibrosis. The bleomycin-induced lung fibrosis model, therefore, has been widely used to study the pathogenesis of pulmonary fibrosis as well as the antifibrotic effects of potential therapeutic agents. It has been reported that intratracheal instillation of bleomycin to mice or rats increased PAI-1 activity in BALF and PAI-1 mRNA in the lung tissue, which was associated with a decrease in uPA activity (178, 253). It has also been shown that mice with homozygous deletion of PAI-1 were relatively protected from bleomycin-induced pulmonary fibrosis, whereas overexpression of PAI-1 enhanced bleomycin-induced lung fibrosis (40, 60, 90). Importantly, Hattori (90) showed that tranexamic acid, a plasminogen activation inhibitor, reversed the accelerated fibrin clearance observed in PAI-1–deficient mice, suggesting that increased plasminogen activation due to PAI-1 deficiency underlies the accelerated fibrin clearance observed in PAI-1–deficient mice. To further test the hypothesis that PAI-1 deficiency limits scarring through unopposed plasminogen activation, a tetracycline-inducible uPA transgenic mouse model was established (213). After doxycycline administration, these transgenic animals expressed increased levels of uPA in their bronchoalveolar lavage fluid and reduced lung collagen accumulation as well as mortality as compared with control mice after bleomycin treatment (213). Swaisgood et al. (222) further showed that bleomycintreated mice deficient for plasminogen or uPA had enhanced pulmonary fibrosis as compared with wild-type mice. By using adenovirus-mediated gene-transfer technique, Sisson et al. (214) showed that administration of human uPA–expressing adenovirus significantly reduced the amount of lung hydroxyproline and attenuated the bleomycin-induced lung collagen deposition. Taken together, the data suggest that the plasminogen system plays an essential role in ECM degradation and that increased PAI-1 expression promotes ECM deposition in bleomycin-treated mice probably by inhibiting plasminogen activation.
A potential role of PAI-1 in the development of lung fibrosis has also been studied in lung-fibrosis models induced by other stimuli. Silicosis is an occupational lung disease caused by inhalation of crystalline silica dust and features inflammation and scarring in the form of nodular lesions in the upper lobes of the lungs. Lardot et al. (128) reported that a single intratracheal administration of a fibrosing dose of crystalline silica in mice increased PAI-1 activity and protein levels in macrophages and neutrophils from BALF, associated with an induction of PAI-1 and PAI-2 mRNA in lung tissue. Sustained upregulation of PAI-1 and PAI- 2 mRNAs was still noted in lung tissue of these animals 1 month after silica treatment. These findings support the critical role of PAIs in the lung-remodeling process induced by silica. In an asthma model, Oh et al. (172) demonstrated that collagen deposition was twofold less, fibrin deposition was fourfold less, and MMP-9 activity was threefold higher in PAI-1 knockout mice than in wild-type mice after ovalbumin challenge, although the degree of airway inflammation was similar between PAI-1 knockout and wild-type mice. These results confirm a critical role of PAI-1 in ECM deposition in the airways of asthmatic patients (24, 39, 95).
Potential mechanisms whereby PAI-1 promotes ECM deposition
Although controversy still exists, three potential mechanisms whereby PAI-1 promotes ECM deposition have been proposed. The first and the most popular hypothesis is that PAI-1 promotes ECM deposition by inhibiting the activities of tPA and uPA, which leads to an inhibition of plasminogen activation and thus a decrease in ECM degradation. The observations from human diseases (13, 24, 33, 39, 46, 61, 75, 83, 84, 95, 100, 101, 116, 118, 121, 167, 242) and from experimental fibrosis models (40, 60, 90, 128, 172, 178, 253) strongly suggest that the abnormal induction of PAI-1 during tissue repair leads to the deposition of ECM by decreasing PA activity and the extent of plasmin formed; thus, the degradation of the ECM. In previous studies, we showed that transforming growth factor-beta (TGF-β), a most potent and ubiquitous profibrogenic cytokine, induced PAI-1 expression and inhibited the activities of tPA and plasmin as well as collagen degradation in cultured murine embryo fibroblasts (NIH3T3 cells) (234). Blockade of cell-surface binding of plasminogen/plasminogen activation with tranexamic acid or inhibition of plasmin activity with aprotinin significantly reduced the basal level of collagen degradation, both in the presence and absence of exogenous plasminogen (235). These data provide direct evidence that the plasminogen/plasmin system plays a critical role in collagen degradation and suggest that TGF- β inhibits collagen degradation probably by inducing PAI-1 expression and thus inhibiting plasminogen activation (235).
In addition to inhibiting ECM degradation, increasing evidence suggests other possible scenarios for PAI-1 effects. Matsuo (154) reported that at least four fibrogenic pathways were differentially expressed in PAI-1–overexpressing mice: (a) interstitial macrophage recruitment was more intense; (b) more interstitial myofibroblasts were found; (c) TGF- β and collagen I mRNA expression was higher; and (d) uPA activity was lower in PAI-1–overexpressing mice than in wild type mice (154). These results indicate that, in addition to potentially decreased fibrinolytic activity (decreased uPA activity), other mechanisms also contribute to the development of fibrosis observed in these PAI-1–overexpressing mice. As PAI-1 has been shown to regulate the adhesion and migration of a variety of cells in vitro and in vivo (6, 32, 51), it has been proposed that the elevated PAI-1 may promote ECM deposition by stimulating the migration of inflammatory or collagen-producing cells or both into the damaged tissue (140). Krag et al. (122) reported that although PAI-1 deficiency attenuated TGF-β–induced mesangial expansion, collagen accumulation, and basement-membrane thickening, no difference in protease activity was found between PAI-1–deficient and nondeficient mice. These data further support the notion that PAI-1 may promote ECM deposition through other mechanisms than inhibiting protease activity.
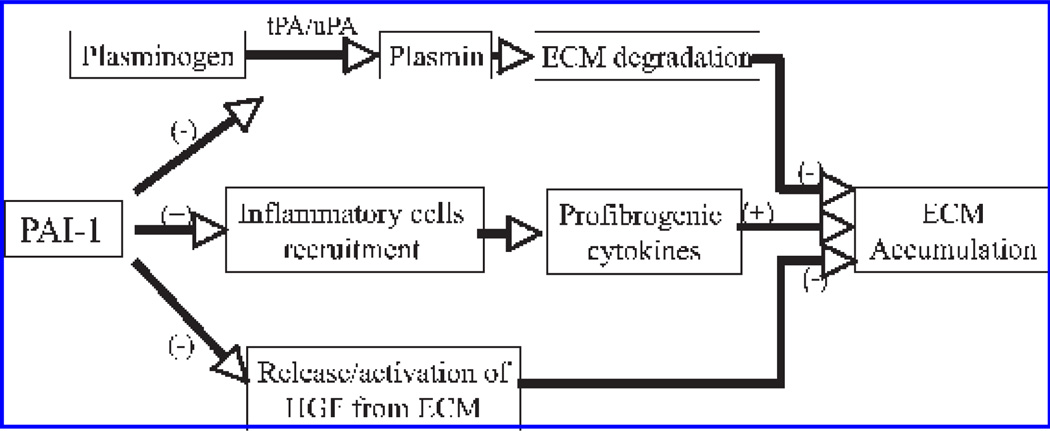
The third potential mechanism whereby PAI-1 promotes ECM deposition highlights the role of the plasminogen system in the release/activation of the antifibrotic growth factor, hepatocyte growth factor (HGF), from its sequestered sites, the extracellular matrix (69, 70, 91, 155, 160). Mizuno et al. (160) reported that administration of recombinant HGF to bleomycintreated mice increased lung MMP activities, induced myofibroblast apoptosis, and stimulated ECM degradation, whereas injection with MMI270, a broad-spectrum MMP inhibitor, blocked HGF-induced MMP activation and myofibroblast apoptosis. These data suggest that HGF is a potent antifibrotic agent that blocks bleomycin-induced lung fibrosis by activating MMPs. Matsuoka et al. (155) further showed that plasminogen addition to NIH3T3 cells or mouse lung fibroblasts increased conversion of pro-HGF to its active form. They also showed that the plasminogen effect was lost when lung fibroblasts from uPA-deficient mice were used, and was increased by fibroblasts from PAI-1–deficient mice (155), indicating that release of ECM-bound HGF by NIH3T3 cells and mouse lung fibroblasts is plasminogen dependent (155). Most interestingly, it was reported that HGF protein levels in BALF from PAI-1 knockout mice were higher than those from wild-type mice after bleomycin administration (91). Blocking plasminogen activation with tranexanic acid reversed such an increase in HGF in the BALF from PAI-1 knockout mice, whereas administration of an anti-HGF neutralizing antibody markedly increased collagen accumulation in the lungs of bleomycin-treated PAI-1 knockout mice (91). These results strongly suggest that, in addition to inhibiting tPA/uPA and ECM degradation, PAI-1 may promote ECM deposition by blocking HGF release/activation. A schematic diagram elucidating the potential mechanisms whereby PAI-1 promotes ECM deposition is presented in Fig. 2.
FIG. 2. Potential mechanisms whereby PAI-1 promotes ECM deposition.
PAI-1 promotes ECM deposition by (a) inhibiting tPA and uPA activities and thereby plasminogen activation and ECM degradation; (b) recruiting inflammatory cells to the sites and therefore increasing profibrogenic cytokines; and (c) suppressing the release of antifibrogenic growth factors from the sequestered sites on the ECM.
OXIDATIVE STRESS AND LUNG FIBROSIS
Fibrogenesis is a complex process and involves different types of cells as well as various cytokines, chemokines, and growth factors. Fibrogenesis occurs in most organs and can be induced by different stimuli, suggesting that common pathways or mechanisms may mediate this response (140). Although intensive studies have been conducted in the past decade, the molecular mechanism underlying this complex pathologic process is still not well understood. Oxidative stress occurs when exposure to oxidizing agents such as ozone and nitrogen dioxide, two important environmental pollutants, or the production of ROS/RNS is increased, or when the antioxidant capacity is decreased, or with a combination of these. Increasing evidence indicates that ROS/RNS play important roles in the development of fibrosis under various pathologic conditions such as liver fibrosis/cirrhosis caused by virus infection or alcohol consumption (8, 139, 182, 184, 186, 192, 212, 243), renal fibrosis associated with diabetics, hypertension, urinary tract obstruction (1, 55, 126, 168), and lung fibrosis (25, 42, 56, 72, 77, 150, 181, 185, 191, 194, 195, 199, 217) [for review see (117, 193)].
Increased oxidant production and oxidative damage in lung fibrotic diseases
The lung is a major target for oxidant damage because of its direct exposure to the atmosphere. Accumulating evidence suggests that oxidants generated endogenously or inhaled from the environment play an essential role in the pathogenesis of various pulmonary fibrotic disorders such as IPF, drug- and radiation-induced lung fibrosis, silicosis, asbestosis, CF, and ARDS (25, 42, 56, 72, 77, 117, 150, 181, 185, 187, 191, 193–195, 198–200, 217). It has been reported that inflammatory cells in BALF from IPF patients release increased amounts of reactive oxygen and nitrogen species (28, 191, 219, 220). Lipid and protein oxidation products, such as 8-isoprostane, have also been identified in the BALF or lung tissue in IPF patients (150, 163, 187). In addition to increased production of oxidants, numerous studies have shown that the levels of antioxidants including glutathione (GSH) (9, 10, 19, 21, 157, 162, 194) and the enzymes involved in the antioxidant defense such as MnSOD, catalase, and thioredoxin (127, 183, 228) are decreased in the lung-lining fluid of IPF patients. Increased oxidant production (56, 187, 195, 199, 200, 217) and decreased antioxidant capacity (30, 47, 181, 200, 201) have also been well documented in the lung tissue or BALF in CF patients. Such an imbalance between oxidants and antioxidants is most likely to contribute to the pathogenesis of IPF and CF, as antioxidant treatment has been shown to be able to improve the clinical manifestations (15, 52, 82, 88, 230).
Silicosis
Silicosis, one of the oldest occupational diseases, still kills thousands of people every year world-wide. It is an incurable lung disease caused by inhalation of dust containing free crystalline silica, which causes inflammation and scarring of the lung tissue. Exposure to particulate silica causes a persistent inflammation sustained by the release of oxidants in the alveolar space. ROS, which include hydroxyl radical, superoxide anion, hydrogen peroxide, and singlet oxygen, are generated not only at the silica particle surface but also by phagocytic cells attempting to digest the silica particle (16, 17, 72, 120, 233). The importance of ROS/RNS in silica-induced fibrosis has been well documented by studying the temporal relation between these events and by altering the fibrotic response with antioxidants. Porter et al. (188) reported that silica exposure induced pulmonary inflammation and damage in the rat lung tissue, which progressed to lung fibrosis, even after silica exposure ended. They further showed that silica-exposed rat lungs were in a state of oxidative stress and that silica-induced pulmonary NO and ROS production persisted even after silica exposure ended and the lung silica burden declined (189). These results suggest that ROS/RNS generated by silica itself or released by activated macrophages are responsible for the development of fibrosis in these silica-exposed rats (189). Gossart et al. (78) demonstrated that intratracheal instillation of silica in rats led to fibrosis characterized by cellular interstitial infiltrates with granulomas. Alveolar macrophages isolated from these rats showed an early and continuous increase in ROS production as well as TNF-α expression (78). Pretreatment of rats with a free radical scavenger, N-tert-butyl-α-phenylnitron, reversed lung histopathologic changes and decreased ROS production and TNF-α expression (78). Srivastava et al. (216) further showed that silica exposure resulted in lung inflammation, macrophage apoptosis, and significantly larger and more numerous silicotic lesions in wild-type mice than in inducible nitric oxide synthase (iNOS) –knockout mice, suggesting a role of NO in silica-induced lung injury. Taken together, these observations indicate that ROS/RNS mediate silica-induced lung fibrosis.
Radiation-induced lung fibrosis
Progressive irreversible fibrosis is one of the most clinically significant sequences of ionizing radiation. Three successive clinical and histopathologic phases can be distinguished for radiation-induced fibrosis: the prefibrotic inflammatory phase, the constitutive fibrotic cellular phase, and the matrix densification/remodeling phase. Growing evidence indicates that ROS play a key role in radiation-induced lung fibrosis, although the molecular mechanism whereby ROS mediate fibrogenic effects of radiation is not well understood (12, 50, 63, 64, 130, 147, 197, 236). Hydroxyl radicals are generated by ionizing radiation, either directly by oxidation of water, or indirectly by the formation of secondary ROS, which can be subsequently converted to hydroxyl radicals by further reduction by metabolic processes in the cell. Therefore, secondary radiation injury is influenced by the cellular antioxidant status and the activating mechanisms. Numerous studies have shown that antioxidants reduce the radiation-induced fibrotic disorders, both in clinical practice and in animal experiments (50, 63, 64, 130, 147, 236), further suggesting an important role of ROS in radiation-induced lung fibrosis.
Bleomycin-induced lung fibrosis
Lung fibrosis is one of the major side effects caused by bleomycin, a chemotherapy drug used for the cancer treatment. ROS/RNS have been shown to play important roles in bleomycin-induced lung fibrosis. Inghilleri et al. (102) reported that bleomycin treatment increased ROS production in both phagocytes and in type II alveolar epithelial cells in rats. Manoury et al. (151) further showed that BAL cells from bleomycin-treated wild-type mice had enhanced ROS production, whereas no increase was observed with BAL cells from the mice deficient in p47phox, a component of NADPH oxidase. Most important, no collagen deposition was found in the lungs of the p47phox-knockout mice after bleomycin treatment, suggesting that NADPH-oxidase–derived ROS are essential to the development of pulmonary fibrosis in bleomycin-treated mice. Extracellular superoxide dismutase (EC-SOD) is highly expressed in the ECM of the lung. Localization of EC-SOD to the matrix of the lung may protect against oxidative tissue damage that leads to pulmonary fibrosis. Fattman et al. (66) showed that the severity of lung fibrosis after bleomycin treatment was significantly increased in EC-SOD null mice as compared with wild-type mice.
The importance of ROS/RNS in the development of bleomycin-induced lung fibrosis has also been well elucidated in numerous studies with antioxidants. Chemicals with antioxidant properties or the enzymes involved in antioxidant defense have been shown to effectively ameliorate bleomycin-induced lung fibrosis in different animal models (3, 20, 137, 215, 245, 246). These data further suggest that ROS/RNS are essential in bleomycin-induced lung fibrosis.
Glutathione and lung fibrosis
Strong evidence that ROS/RNS are involved in the development of lung fibrosis comes from the studies using antioxidants. GSH, a tripeptide, is the most abundant intracellular free thiol and an important antioxidant. GSH participates in diverse biologic processes such as the synthesis of DNA and the metabolism of endogenous and exogenous compounds. However, the most important function of GSH is to detoxify oxidants. GSH concentration in the lung lining fluid has been reported to be 100-fold higher than that in plasma (29), indicating a critical role of GSH in lung antioxidant defense. It has been reported that the GSH concentration is decreased in experimental fibrosis models induced by various stimuli (3, 26, 144, 170) and in the lung-lining fluid in patients with pulmonary fibrotic disorders such as IPF, asbestosis, CF, and ARDS (9–11, 19, 21, 27, 149, 157, 158, 162, 194). The mechanism underlying GSH depletion in fibrotic diseases is unclear. Glutamate cysteine ligase (GCL) is the rate-limiting enzyme in de novo GSH synthesis. It has been reported that TGF-β, a most potent profibrogenic cytokine, suppresses the GCL promoter activity and inhibits endogenous GCL mRNA and protein expression, associated with a decline in the intracellular GSH level and an increase in cellular reactive oxygen species in different types of cells (2, 7). Tiitto et al. (229) further showed that GCL expression is low in the fibrotic areas of IPF disease. These data suggest that decreased GSH biosynthesis capacity may underlie the decrease in GSH concentrations observed in these diseases.
Although the mechanism and biologic significance of GSH depletion in fibrotic diseases has not been fully elucidated, GSH and N-acetylcysteine (NAC), a precursor of GSH, have been used clinically for the treatment of fibrotic diseases (10, 15, 22, 52, 82, 88, 157, 230). It has been reported that aerosol administration of GSH or NAC restored GSH concentration in lung-lining fluid and slowed the deterioration of lung functions in IPF and CF patients (15, 52, 82, 88, 230), indicating a potential therapeutic value of GSH/NAC in the treatment of lung fibrotic diseases. Several studies using bleomycin-induced lung fibrosis models also showed that NAC, when given orally or by aerosol inhalation, attenuated bleomycin-induced lung fibrosis in mice and rats (41, 87, 153, 246). These data further suggest that oxidative stress resulted from increased ROS/RNS production and that decreased GSH concentration contributes importantly to the development of fibrosis.
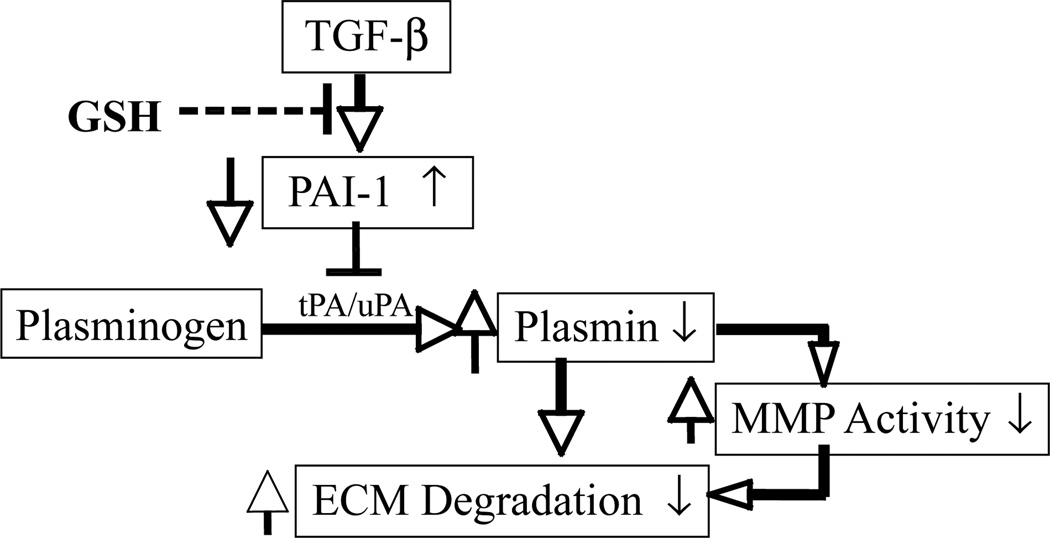
The mechanism underlying therapeutic/antifibrotic effects of GSH/NAC remains to be further explored. In previous studies, we showed that TGF-β decreased GSH and stimulated ROS production in murine embryo fibroblasts (NIH3T3 cells), whereas exogenous GSH abrogated TGF-β–induced collagen accumulation (138). Furthermore, we demonstrated that exogenous GSH or GSH ester selectively inhibited TGF-β–in-duced PAI-1 expression and restored tPA and plasmin activities, as well as the collagen degradation rate in TGF-β–treated fibroblasts (235). Most important, we showed that inhibition of plasminogen activation with tranexamic acid or the addition of active PAI-1 to a culture medium almost completely eliminates the restorative effects of GSH on collagen degradation in TGF-β–treated cells. As TGF-β is a most potent and ubiquitous profibrogenic cytokine and its expression is increased in almost all fibrotic diseases, our results suggest that GSH or NAC may exert antifibrotic/therapeutic effects by blocking TGF-β signaling, inhibiting PAI-1 expression, and stimulating collagen degradation (Fig. 3).
FIG. 3. Potential mechanism whereby GSH inhibits TGF-β–induced collagen accumulation in NIH3T3 cells.
GSH inhibits TGF-β-–induced PAI-1 expression and therefore increases plasmin formation and collagen degradation.
REDOX REGULATION OF PLASMINOGEN ACTIVATOR INHIBITOR 1 GENE EXPRESSION
Regulation of PAI-1 activity at protein level
Active PAI-1 has a very short half-life, and plasma PAI-1 concentration is low (6–80 ng/ml) under physiologic conditions; however, PAI-1 expression is rapidly induced upon stimulation, suggesting a high synthesis rate and tight control of PAI-1 gene expression. Many types of cells, including endothelial cells, adipocytes, fibroblasts, hepatocytes, smooth muscle cells, and epithelial cells, can synthesize PAI-1. The production of PAI-1 by these and other cells can be induced by various growth factors such as TGF-β and basic fibroblast growth factor, insulin-like growth factor, by cytokines such as interleukin-1 (IL-1) and interleukin-6 (IL-6), and by hormones such as corticosteroids (51, 54, 57, 141, 142). PAI-1 is synthesized and secreted by cells as an active form; however, two different activity states, active and latent, have been detected in vivo (51, 92, 133). The active inhibitory form of PAI-1 spontaneously converts to a latent conformation that can be partially reactivated by denaturing agents (133, 165). In addition to converting to latent PAI-1, which can be reactivated, the active inhibitory form of PAI-1 can be converted to the substrate form, which is irreversibly degraded by its target proteinases (76, 135). The biologic functions of latent and substrate forms of PAI-1 are unclear.
Although factors controlling the conversion of different forms of PAI-1 under physiologic conditions are unknown, it has been reported that oxidants can inactivate PAI-1 activity in vitro (131, 218). Lawrence et al. (131) showed that the rapidly acting PAI purified from cultured bovine aortic endothelial cells was inactivated by chloramine T under conditions that specifically oxidize methionine and cysteine residues. Both the PAI inhibitory activity and the ability of the PAI to form complexes with tPA were decreased in a dose-dependent manner by chloramine T. Incubation of the chloramine T–inactivated PAI with methionine sulfoxide peptide reductase in the presence of dithiothreitol (DTT) restored >90% of the PAI activity, although little activity was restored by either the reductase or DTT alone, indicating that the oxidation of at least one critical methionine residue is responsible for the loss of PAI activity (131). Strandberg et al. (218) showed, however, that mutant proteins of PAI-1, in which Met347 and either of two other methionines, Met266 or Met354, have been replaced with oxidation-resistant valine residues, were equally sensitive to oxidation as wild-type PAI-1, suggesting that a specific oxidation of the methionine residues is not responsible for the inactivation (218). They further showed that when PAI-1 was oxidized, it underwent a rapid conformational change that correlated to the loss of inhibitory activity, suggesting that the increased sensitivity to oxidation may be caused by a conformational change in the inhibitor molecule (218). Nonetheless, to uncover the molecular mechanism whereby PAI-1 activity is regulated under physiologic and pathologic conditions will greatly benefit the development of the therapeutic agents for the treatment of fibrotic diseases in which PAI-1 plays a pivotal role.
Redox regulation of PAI-1 gene expression
Although the PAI-1 activity can be modified at the protein level, the regulation of PAI-1 is achieved mainly by alteration in the rate of PAI-1 gene expression (103). The human PAI-1 gene is ~12.2 kb in length, composed of nine exons and eight introns. A similar structure has been found with rat and mouse PAI-1 genes (23, 190). The 5′-flanking region of the human PAI-1 gene contains a perfect “TATA” box and several transcription factor binding sites, including binding sites for Smads, activator protein 1 (AP-1), SP-1, hypoxia-induced factor–responsible element (HREs), and NF- κB (38, 97, 115, 164, 237). The transcription factor binding sites identified in the rat PAI-1 promoter show a high degree of sequence similarity to sequences in the mouse (190) and human (23, 36, 110) PAI-1 promoter, suggesting that they are regulated by similar mechanisms. PAI-1 is involved in many physiologic and pathologic processes, and its gene expression is induced by numerous stimuli. In this section, we focus only on the redox regulation of PAI-1 gene expression by several important growth factors/cytokines/agents that are involved in the development of fibrosis.
Transforming growth factor beta (TGF-β)
Although various cytokines, chemokines, and growth factors have been shown to play important roles in the development of fibrosis, TGF-β is considered to be the most potent and ubiquitous profibrogenic cytokine. TGF-β induces PAI-1 gene expression in various types of cells (37, 44, 85, 94, 125, 204, 244); an elevated PAI-1 level is also associated with increased TGF-β expression and ECM deposition under diverse pathologic conditions (48, 65, 79, 90, 145, 209, 232), indicating a critical role of PAI-1 in TGF-β fibrogenesis. TGF-β induces its target genes mainly through the Smad signaling pathway. Induction of PAI-1 by TGF-β through the Smad pathway has been well described in the past decade. On the binding of TGF-β to the membrane receptor, TGF-β signaling is transduced to the nucleus by a series of events involving phosphorylation of Smad 2/3 and nuclear translocation of Smad 2/3 and Smad 4 complex. Binding of the Smad2/3 and Smad 4 complex to Smad-binding sites in the promoter of the PAI-1 gene initiates the gene transcription.
Importantly, it has been reported that TGF-β increases ROS production (74, 93, 96, 105, 111, 169, 227), decreases GSH concentration (2, 7, 71, 107, 205), and suppresses the activities of antioxidant enzymes such as catalase, superoxide dismutase (SOD), and glutathione peroxidase (105, 114) in various types of cells and in vivo. Many of the effects of TGF-β (68, 74, 109, 111, 132, 205, 223, 255), including induction of PAI-1 (109, 207), are indeed mediated by ROS, although the underlying molecular mechanism is still not completely understood. In addition to the Smad pathway, several other pathways, including the mitogen-activated protein kinase (MAPK) pathway, also play a role in TGF-β induction of PAI-1 expression in various types of cells (73, 85, 94, 125, 204, 241, 244). As MAPK pathways are sensitive to the redox state of cells, it is hypothesized that ROS/RNS may mediate TGF-β induction of PAI-1 expression through activating MAPKs. Nevertheless, although it has been well documented that TGF-β increases ROS production, activates MAPK pathways, and induces PAI-1, whether ROS mediate the induction of PAI-1 by TGF-β through activating MAPKs and which component(s) in MAPK pathways is (are) the redox sensor, however, are unclear.
In an effort to elucidate the potential link between ROS production, MAPK activation, and the PAI-1 induction by TGF-β, we systematically studied the effects of the specific inhibitors and dominant negative mutants for JNK, p38, ERK MAPKs on TGF-β–induced PAI-1 expression, as well as the effecs of ROS scavengers, including GSH on TGF-β–induced MAPK activation and PAI-1 expression in NIH3T3 cells. Our results show that TGF-β increases ROS production, activates JNK and p38 pathways, but not ERK pathway, in NIH3T3 cells, associated with an induction of PAI-1 expression (234). JNK/p38 pathway inhibitor or dominant negative mutant significantly reduces TGF-β–induced PAI-1 expression at both mRNA and protein levels (234). Most importantly, inhibition of NADPH oxidase, which is responsible for the production of ROS in TGF-β–treated cells, or treatment of the cells with exogenous GSH, almost completely blocks TGF-β–induced JNK and p38 phosphorylation and partially inhibits TGF-β–induced PAI-1 expression (234). These results support the notion that MAPK pathways are involved in the induction of PAI-1 by TGF-β through a redox-sensitive mechanism.
The human PAI-1 promoter region close to the TATA box contains two TRE-like elements. The c-Jun homodimers and c-Jun/c-Fos heterodimers bind to these elements to mediate TGF-β responses (115). It has been reported that the transcriptional activation by Smad is mediated through the AP-1 transcription factor complex (85, 247). It has also been shown that ATF-2, a downstream substrate of both JNK and p38, participates in transcription complexes in association with Smad proteins (206). Two SP-1 binding site–like sequences are also identified at −72 to −67 and −45 to −40 in the proximal promoter region of the PAI-1 gene (38). It has been reported that TGF-β upregulates PAI-1 gene expression by activating SP-1–dependent transcription through the induction of Smad/SP-1 complex formation (44, 98). These data suggest that oxidants may mediate TGF-β–induced PAI-1 expression by activating MAPK pathways, which then facilitate the binding of Smad proteins to the PAI-1 promoter and activate PAI-1 gene expression. This notion is supported by our recent findings that, although GSH has no effect on TGF-β–induced Smad 2/3 phosphorylation or pSmad 2/3 and Smad 4 nuclear translocation, it blocks the binding of the transcription factors to not only the AP-1 and SP-1 but also Smad cis-elements in the PAI-1 promoter (234). Blocking the transcription factors AP-1, SP-1, and Smad ODNs with decoy oligonucleotides, on the other hand, abrogates the inhibitory effect of GSH on TGF-β–induced PAI-1 promoter activity (234).
Tumor necrosis factor alpha (TNF-α)
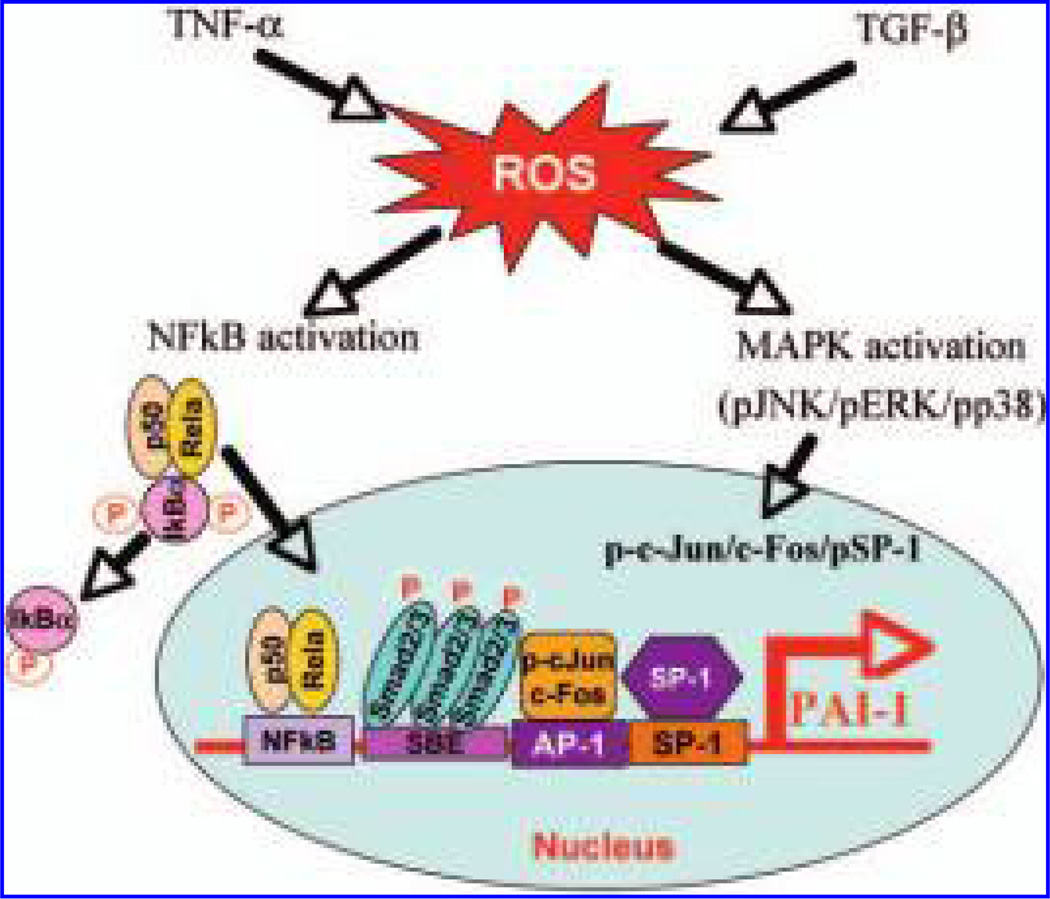
TNF-α is another important growth factor involved in the development of fibrosis under many pathologic conditions. The expression of TNF-α is increased in various lung fibrotic diseases (18, 179, 250, 252), and importantly, the elevation of TNF-α level in many fibrotic diseases is companied by an increase in PAI-1 expression (202, 225, 242). In vitro studies further show that TNF-α induces PAI-1 in various types of cells (37, 97, 146, 180, 203, 223, 224, 226) and ROS mediate such induction (174, 203, 223). Swiatkowska et al. (223) reported that PAI-1 expression in endothelial cells is upregulated by TNF-α or H2O2, which was reversed when NAC was added to the culture medium. NAC also blocked the PAI-1 promoter activity stimulated by TNF-α (223). Although it is not clear which signaling pathway(s) mediates TNF-α induction of PAI-1 expression through a redox-sensitive mechanism, several studies have suggested that NF-κB may be involved. Swiatkowska et al. (224) showed that TNF-α induced PAI-1 gene expression through a regulatory region present in segment −664/−680 of the PAI-1 promoter, which interacts with NF-κB, and that ROS mediate such induction (224). Hou et al. (97) also showed that a TNF-α–responsive enhancer located 15 kb upstream of the transcription start site in the PAI-1 gene, which contains a conserved NF-κB-binding site, mediated TNF-α response in bovine aortic endothelial cells. Conversely, Takeshita et al. (226) showed that protein kinase C, MAPK, protein tyrosine kinase, and NF-κB pathways are all involved in the induction of PAI-1in TNF-α–treated nonmalignant human hepatocyte cells. As the NF-κB pathway is sensitive to ROS, these data suggest that ROS/RNS mediate the induction of PAI-1 by TNF-α, probably by altering the NF-κB pathway signaling (Fig. 4).
FIG. 4. Potential signaling pathways mediating ROS induction of PAI-1 by TGF-β and TNF-α.
TGF-β and TNF-α increase ROS production, which, by activating the MAPK pathways and/or the NF-κB pathway, increase the transcription rate of the PAI-1 gene.
Angiotensin II (Ang II)
Ang II has numerous biologic functions and is involved in many pathologic conditions, including the development of fibrosis (104, 108, 112, 113, 119, 211, 221). It has been reported that Ang II induces ECM accumulation by a mechanism independent of its vasopressor effect (156), probably by stimulating PAI-1 expression and therefore inhibiting ECM degradation (108, 112, 113, 119, 173, 238). Oikawa et al. (173) reported that angiotensin-converting enzyme inhibitors (ACEIs; captopril or enalapril) or angiotensin II receptor antagonist (AIIRA, L158,809) markedly attenuated increased PAI-1 mRNA expression and reduced glomerular lesions (thrombosis, mesangiolysis, and sclerosis index) in a radiation-induced kidney fibrosis model. The data suggest that inhibition of the renin–angiotensin system may ameliorate radiation-induced injury by suppressing PAI-1 expression and thus accelerating ECM degradation. Jesmin et al. (108) further reported that the expression of PAI-1 in coronary vessels and the perivascular area was increased in diabetic hearts, which was associated with reduced activities of MMP-2 and membrane type-1 MMP (MT1-MMP) and increased deposition of collagen type I and III as well as fibrin. Such an increase in PAI-1 expression and ECM deposition was reversed to nondiabetic levels by the angiotensin II type 1 receptor blocker can-desartan (108). These results suggest that increased production of Ang II causes coronary matrix remodeling in insulin-resistant diabetes at least in part by increasing PAI-1 expression and thus decreasing MMP-2 and MT1-MMP activities (108). Although the molecular mechanism whereby Ang II induces PAI-1 expression has not been completely elucidated, several studies have shown that Ang II increases ROS/RNS production, which mediates the induction of PAI-1 by Ang II in different types of cells (124, 248, 249). It has been reported that Ang II increased ROS production in adipose tissue/adipocytes (124) and in rat aorta endothelial cells (248, 249), whereas olmasartan, an angiotensin II (Ang II) type-1 receptor blocker (124), and NAC (248, 249) suppressed Ang II–stimulated ROS production and PAI-1 expression.
Radiation
Although the underlying mechanism is still not well understood, it has been proposed that free radicals produced by radiation contribute importantly to radiation-associated fibrosis, in part by inducing PAI-1 expression. Zhao et al. (254, 255) showed that irradiating rat kidney tubule epithelial cells (NRK52E) with 1 to 20 Gy gamma-rays led to dose-dependent increases in steady-state levels of PAI-1 mRNA and protein within 24 and 48 h, respectively, whereas enhancement of intracellular soluble thiol pools with NAC abolished the radiation-induced upregulation of PAI-1. Overexpression of catalase also inhibited radiation-induced PAI-1 expression, suggesting a mechanistic role for hydrogen peroxide in the induction of PAI-1 expression during radiation exposure (255). Hageman et al. (86) demonstrated that irradiation of HepG2 cells leads to a significant increase in PAI-1 mRNA levels, whereas TGF-β shows strong cooperative effects with radiation in activating the PAI-1 gene (86). Schultze-Mosgau et al. (208) further showed that radiation induced PAI-1 expression in the skin of Wistar rats by increasing TGF-β expression as anti-TGF-β antibody blocked radiation-induced PAI-1 expression and skin fibrosis. Damage to the skin ECM is the hallmark of long-term exposure to solar UV radiation. Seite et al. (210) showed that a single minimal erythemal dose of UV significantly enhanced IL-1α, IL-1β, and PAI-1 mRNA levels in human skin in vivo (p < 0.05), indicating that increased PAI-1 expression is involved in UV-light–induced skin pathology. Nevertheless, as in many other situations, the signaling pathway(s) mediating ROS induction of PAI-1 expression in radiation-treated cells or animals remain largely unknown.
CONCLUSIONS
PAI-1 plays a pivotal role in the development of lung fibrosis. Various growth factors, cytokines, and chemical/physical agents can induce PAI-1 expression, and ROS/RNS mediate the induction of PAI-1 by many of these stimuli. Although MAPK and NF- κB pathways have been shown to be redox sensitive and may be the downstream effectors of ROS/RNS in the induction of PAI-1 by different stimuli, the molecular mechanism underlying PAI-1 induction by ROS/RNS remains largely unknown. As PAI-1 plays a critical role in the development of fibrosis, not only in the lung but also in many other organs, and increasing ROS/RNS production is a common feature for many fibrogenic agents, to uncover the mechanism underlying the induction of PAI-1 by ROS/RNS will provide important information not only for understanding the pathogenesis of these fibrotic diseases but also for the development of effective therapeutic agents for the treatment of these devastating diseases.
ACKNOWLEDGMENTS
The work was supported by a grant from National Institute of Environmental Health Sciences (NIH ES011831) and a grant from the American Lung Association (CI-1190-N).
ABBREVIATIONS
- ACE
angiotensin-converting enzyme
- AP-1
activator protein 1
- Ang II
angiotensin II
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- CF
cystic fibrosis
- DTT
dithiothreitol
- ECM
extracellular matrix
- ELF
epithelial lining fluid
- ERK
extracellular signal regulated kinase
- GSH
glutathione
- HGF
hepatocyte growth factor
- IPF
idiopathic pulmonary fibrosis
- JNK
c-Jun NH2-terninal kinase
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- NAC
N-acetylcysteine
- NF-κB
nuclear factor κB
- PA
plasminogen activator
- PAI-1
plasminogen activator inhibitor 1
- ROS/RNS
reactive oxygen/nitrogen species
- SBE
Smad binding element
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- tPA
tissue-type plasminogen activator
- uPA
urokinase-type plasminogen activator
REFERENCES
- 1.Agarwal R. Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am J Physiol Renal Physiol. 2003;284:F863–F869. doi: 10.1152/ajprenal.00385.2002. [DOI] [PubMed] [Google Scholar]
- 2.Arsalane K, Dubois CM, Muanza T, Begin R, Boudreau F, Asselin C, Cantin AM. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 3.Arslan SO, Zerin M, Vural H, Coskun A. The effect of melatonin on bleomycin-induced pulmonary fibrosis in rats. J Pineal Res. 2002;32:21–25. doi: 10.1034/j.1600-079x.2002.10796.x. [DOI] [PubMed] [Google Scholar]
- 4.Arthur MJ. Degradation of matrix proteins in liver fibrosis. Pathol Res Pract. 1994;190:825–833. doi: 10.1016/S0344-0338(11)80985-4. [DOI] [PubMed] [Google Scholar]
- 5.Arthur MJ. Fibrosis and altered matrix degradation. Digestion. 1998;59:376–380. doi: 10.1159/000007492. [DOI] [PubMed] [Google Scholar]
- 6.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 7.Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, Meredith MJ, Arteaga CL, Freeman ML. Smad3-ATF3 signaling mediates TGF-[beta] suppression of genes encoding Phase II detoxifying proteins. Free Radic Biol Med. 2005;38:375–387. doi: 10.1016/j.freeradbiomed.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Paradis V. Approaches for treatment of liver fibrosis in chronic hepatitis C. Clin Liver Dis. 2003;7:195–210. doi: 10.1016/s1089-3261(02)00076-4. [DOI] [PubMed] [Google Scholar]
- 9.Beeh KM, Beier J, Haas IC, Kornmann O, Micke P, Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:1119–1123. doi: 10.1183/09031936.02.00262402. [DOI] [PubMed] [Google Scholar]
- 10.Behr J, Degenkolb B, Krombach F, Vogelmeier C. Intracellular glutathione and bronchoalveolar cells in fibrosing alveolitis: effects of N-acetylcysteine. Eur Respir J. 2002;19:906–911. doi: 10.1183/09031936.02.00204902. [DOI] [PubMed] [Google Scholar]
- 11.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis: adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156:1897–1901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 12.Beinert T, Binder D, Stuschke M, Jorres RA, Oehm C, Fleischhacker M, Sezer O, Mergenthaler HG, Werner T, Possinger K. Oxidant-induced lung injury in anticancer therapy. Eur J Med Res. 1999;4:43–53. [PubMed] [Google Scholar]
- 13.Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, Chapman HA., Jr Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990;322:890–897. doi: 10.1056/NEJM199003293221304. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand Y, Demeule M, Rivard G-E, Beliveau R. Stimulation of tPA-dependent provisional extracellular fibrin matrix degradation by human recombinant soluble melanotransferrin. Biochim Biophys Acta (BBA) Mol Cell Res. 2006;1763:1024–1030. doi: 10.1016/j.bbamcr.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest. 2005;127:308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- 16.Blackford JA, Jr, Antonini JM, Castranova V, Dey RD. Intratracheal instillation of silica up-regulates inducible nitric oxide synthase gene expression and increases nitric oxide production in alveolar macrophages and neutrophils. Am J Respir Cell Mol Biol. 1994;11:426–431. doi: 10.1165/ajrcmb.11.4.7522485. [DOI] [PubMed] [Google Scholar]
- 17.Blackford JA, Jr, Jones W, Dey RD, Castranova V. Comparison of inducible nitric oxide synthase gene expression and lung inflammation following intratracheal instillation of silica, coal, carbonyl iron, or titanium dioxide in rats. J Toxicol Environ Health. 1997;51:203–218. doi: 10.1080/00984109708984022. [DOI] [PubMed] [Google Scholar]
- 18.Borm PJ, Palmen N, Engelen JJ, Buurman WA. Spontaneous and stimulated release of tumor necrosis factor-alpha (TNF) from blood monocytes of miners with coal workers’ pneumoconiosis. Am Rev Respir Dis. 1988;138:1589–1594. doi: 10.1164/ajrccm/138.6.1589. [DOI] [PubMed] [Google Scholar]
- 19.Borok Z, Grimes GJ, Buhl R, Bokser A, Hubbard RC, Holroyd K, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338:215–216. doi: 10.1016/0140-6736(91)90350-x. [DOI] [PubMed] [Google Scholar]
- 20.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282:L719–L726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- 21.Brown DM, Beswick PH, Bell KS, Donaldson K. Depletion of glutathione and ascorbate in lung lining fluid by respirable fibres. Ann Occup Hyg. 2000;44:101–108. [PubMed] [Google Scholar]
- 22.Brown KK, Raghu G. Medical treatment for pulmonary fibrosis: current trends, concepts, and prospects. Clin Chest Med. 2004;25:759–772. doi: 10.1016/j.ccm.2004.08.003. vii. [DOI] [PubMed] [Google Scholar]
- 23.Bruzdzinski CJ, Riordan-Johnson M, Nordby EC, Suter SM, Gelehrter TD. Isolation and characterization of the rat plasminogen activator inhibitor-1 gene. J Biol Chem. 1990;265:2078–2085. [PubMed] [Google Scholar]
- 24.Buckova D, Izakovicova Holla L, Vacha J. Polymorphism 4G/5G in the plasminogen activator inhibitor-1 (PAI-1) gene is associated with IgE-mediated allergic diseases and asthma in the Czech population. Allergy. 2002;57:446–448. doi: 10.1034/j.1398-9995.2002.03582.x. [DOI] [PubMed] [Google Scholar]
- 25.Bunnell E, Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;148:1174–1178. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- 26.Candan F, Alagozlu H. Captopril inhibits the pulmonary toxicity of paraquat in rats. Hum Exp Toxicol. 2001;20:637–641. doi: 10.1191/096032701718890540. [DOI] [PubMed] [Google Scholar]
- 27.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 28.Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987;79:1665–1673. doi: 10.1172/JCI113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol Respir Environ Exerc Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 30.Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis: conclusions from the CF Antioxidant Workshop, Bethesda, Maryland, November 11- 12, 2003. Free Radic Biol Med. 2007;42:15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–654. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- 32.Chapman HA. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714–724. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- 33.Chapman HA, Allen CL, Stone OL. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis. 1986;133:437–443. doi: 10.1164/arrd.1986.133.3.437. [DOI] [PubMed] [Google Scholar]
- 34.Chapman HA, Jr, Stone OL. A fibrinolytic inhibitor of human alveolar macrophages. Induction with endotoxin. Am Rev Respir Dis. 1985;132:569–575. doi: 10.1164/arrd.1985.132.3.569. [DOI] [PubMed] [Google Scholar]
- 35.Chapman HA, Yang XL, Sailor LZ, Sugarbaker DJ. Developmental expression of plasminogen activator inhibitor type 1 by human alveolar macrophages: possible role in lung injury. J Immunol. 1990;145:3398–3405. [PubMed] [Google Scholar]
- 36.Chen HC, Feener EP. MEK1,2 response element mediates angiotensin II-stimulated plasminogen activator inhibitor-1 promoter activation. Blood. 2004;103:2636–2644. doi: 10.1182/blood-2003-05-1737. [DOI] [PubMed] [Google Scholar]
- 37.Chen YQ, Sloan-Lancaster J, Berg DT, Richardson MA, Grinnell B, Tseng-Crank J. Differential mechanisms of plasminogen activator inhibitor-1 gene activation by transforming growth factor-beta and tumor necrosis factor-alpha in endothelial cells. Thromb Haemost. 2001;86:1563–1572. [PubMed] [Google Scholar]
- 38.Chen YQ, Su M, Walia RR, Hao Q, Covington JW, Vaughan DE. Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem. 1998;273:8225–8231. doi: 10.1074/jbc.273.14.8225. [DOI] [PubMed] [Google Scholar]
- 39.Cho SH, Tam SW, Demissie-Sanders S, Filler SA, Oh CK. Production of plasminogen activator inhibitor-1 by human mast cells and its possible role in asthma. J Immunol. 2000;165:3154–3161. doi: 10.4049/jimmunol.165.6.3154. [DOI] [PubMed] [Google Scholar]
- 40.Chuang-Tsai S, Sisson TH, Hattori N, Tsai CG, Subbotina NM, Hanson KE, Simon RH. Reduction in fibrotic tissue formation in mice genetically deficient in plasminogen activator inhibitor-1. Am J Pathol. 2003;163:445–452. doi: 10.1016/S0002-9440(10)63674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, Esteras A, Llombart-Bosch A, Morcillo EJ. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J. 2001;17:1228–1235. doi: 10.1183/09031936.01.00049701. [DOI] [PubMed] [Google Scholar]
- 42.Cross CE, Frei B, Louie S. The adult respiratory distress syndrome (ARDS) and oxidative stress: therapeutic implications. Adv Exp Med Biol. 1990;264:435–448. doi: 10.1007/978-1-4684-5730-8_69. [DOI] [PubMed] [Google Scholar]
- 43.Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Mol Biol Cell. 2001;12:1467–1479. doi: 10.1091/mbc.12.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta-induced physical and functional interactions between Smads and Sp1. J Biol Chem. 2000;275:40014–40019. doi: 10.1074/jbc.C000508200. [DOI] [PubMed] [Google Scholar]
- 45.Davidson JM. Biochemistry and turnover of lung interstitium. Eur Respir J. 1990;3:1048–1063. [PubMed] [Google Scholar]
- 46.Dawson SJ, Wiman B, Hamsten A, Green F, Humphries S, Henney AM. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem. 1993;268:10739–10745. [PubMed] [Google Scholar]
- 47.Day BJ. Glutathione: a radical treatment for cystic fibrosis lung disease? Chest. 2005;127:12–14. doi: 10.1378/chest.127.1.12. [DOI] [PubMed] [Google Scholar]
- 48.de Gouville A-C, Boullay V, Krysa G, Pilot J, Brusq J-M, Loriolle F, Gauthier J-M, Papworth SA, Laroze A, Gellibert F, Huet S. Inhibition of TGF-[beta] signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br J Pharmacol. 2005;145:166–177. doi: 10.1038/sj.bjp.0706172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 2004;104:2385–2393. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delanian S, Balla-Mekias S, Lefaix J-L. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17:3283–3290. doi: 10.1200/JCO.1999.17.10.3283. [DOI] [PubMed] [Google Scholar]
- 51.Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005;93:631–640. doi: 10.1160/TH05-01-0033. [DOI] [PubMed] [Google Scholar]
- 52.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. Highdose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 53.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dimova EY, Moller U, Herzig S, Fink T, Zachar V, Ebbesen P, Kietzmann T. Transcriptional regulation of plasminogen activator inhibitor-1 expression by insulin-like growth factor-1 via MAP kinases and hypoxia-inducible factor-1 in HepG2 cells. Thromb Haemost. 2005;93:1176–1184. doi: 10.1160/TH04-11-0761. [DOI] [PubMed] [Google Scholar]
- 55.Djamali A. Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol. 2007;293:F445–F455. doi: 10.1152/ajprenal.00037.2007. [DOI] [PubMed] [Google Scholar]
- 56.Dominguez C, Gartner S, Linan S, Cobos N, Moreno A. Enhanced oxidative damage in cystic fibrosis patients. Biofactors. 1998;8:149–153. doi: 10.1002/biof.5520080125. [DOI] [PubMed] [Google Scholar]
- 57.Dong J, Fujii S, Imagawa S, Matsumoto S, Matsushita M, Todo S, Tsutsui H, Sobel BE. IL-1 and IL-6 induce hepatocyte plasminogen activator inhibitor-1 expression through independent signaling pathways converging on C/EBP. Am J Physiol Cell Physiol. 2007;292:C209–C215. doi: 10.1152/ajpcell.00157.2006. [DOI] [PubMed] [Google Scholar]
- 58.Duval-Jobe C, Parmely MJ. Regulation of plasminogen activation by human U937 promonocytic cells. J Biol Chem. 1994;269:21353–21357. [PubMed] [Google Scholar]
- 59.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 60.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Solh AA, Bhora M, Pineda L, Aquilina A, Abbetessa L, Berbary E. Alveolar plasminogen activator inhibitor-1 predicts ARDS in aspiration pneumonitis. Intensive Care Med. 2006;32:110–115. doi: 10.1007/s00134-005-2847-2. [DOI] [PubMed] [Google Scholar]
- 62.Elborn JS, Shale DJ. Cystic fibrosis: 2. Lung injury in cystic fibrosis. Thorax. 1990;45:970–973. doi: 10.1136/thx.45.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 64.Epperly MW, Travis EL, Sikora C, Greenberger JS. Manganese [correction of Magnesium] superoxide dismutase (Mn-SOD) plasmid/liposome pulmonary radioprotective gene therapy: modulation of irradiation-induced mRNA for IL-I, TNF-alpha, and TGF-beta correlates with delay of organizing alveolitis/fibrosis. Biol Blood Marrow Transplant. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 65.Falk P, Ma C, Chegini N, Holmdahl L. Differential regulation of mesothelial cell fibrinolysis by transforming growth factor beta 1. Scand J Clin Lab Invest. 2000;60:439–447. doi: 10.1080/003655100448419. [DOI] [PubMed] [Google Scholar]
- 66.Fattman CL, Chang L-Y, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2003;35:763–771. doi: 10.1016/s0891-5849(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 67.Felez J, Miles LA, Fabregas P, Jardi M, Plow EF, Lijnen RH. Characterization of cellular binding sites and interactive regions within reactants required for enhancement of plasminogen activation by tPA on the surface of leukocytic cells. Thromb Haemost. 1996;76:577–584. [PubMed] [Google Scholar]
- 68.Ferroni P, Guagnano MT, Manigrasso MR, Ciabattoni G, Davi G. Increased plasminogen activator inhibitor-1 levels in android obesity: correlation with oxidative stress. J Thromb Haemost. 2005;3:1086–1087. doi: 10.1111/j.1538-7836.2005.01395.x. [DOI] [PubMed] [Google Scholar]
- 69.Flaumenhaft R, Rifkin DB. Extracellular matrix regulation of growth factor and protease activity. Curr Opin Cell Biol. 1991;3:817–823. doi: 10.1016/0955-0674(91)90055-4. [DOI] [PubMed] [Google Scholar]
- 70.Flaumenhaft R, Rifkin DB. The extracellular regulation of growth factor action. Mol Biol Cell. 1992;3:1057–1065. doi: 10.1091/mbc.3.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franklin CC, Rosenfeld-Franklin ME, White C, Kavanagh TJ, Fausto N. TGFbeta1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent post-translational and caspase-independent transcriptional regulatory mechanisms. FASEB J. 2003;17:1535–1537. doi: 10.1096/fj.02-0867fje. [DOI] [PubMed] [Google Scholar]
- 72.Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med. 2003;34:1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 73.Fukiko Furukawa KM, Shigeo Mori, Yoshiya Tahashi, Katsunori Yoshida, Yasushi Sugano, Hideo Yamagata, Masanori Matsushita, Toshihito Seki, Yutaka Inagaki, Mikio Nishizawa, Junichi Fujisawa, Kyoichi Inoue. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Trevijano ER, Iraburu MJ, Fontana L, Dominguez-Rosales JA, Auster A, Covarrubias-Pinedo A, Rojkind M. Transforming growth factor beta1 induces the expression of alpha1(I) procollagen mRNA by a hydrogen peroxide-C/EBPbeta-dependent mechanism in rat hepatic stellate cells. Hepatology. 1999;29:960–970. doi: 10.1002/hep.510290346. [DOI] [PubMed] [Google Scholar]
- 75.Geiser T. Idiopathic pulmonary fibrosis: a disorder of alveolar wound repair? Swiss Med Wkly. 2003;133:405–411. doi: 10.4414/smw.2003.09986. [DOI] [PubMed] [Google Scholar]
- 76.Gils A, Declerck PJ. The structural basis for the pathophysiological relevance of PAI-I in cardiovascular diseases and the development of potential PAI-I inhibitors. Thromb Haemost. 2004;91:425–437. doi: 10.1160/TH03-12-0764. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP. Role of oxidant stress in the adult respiratory distress syndrome: evaluation of a novel antioxidant strategy in a porcine model of endotoxin-induced acute lung injury. Shock. 1996;6(suppl 1):S23–S26. [PubMed] [Google Scholar]
- 78.Gossart S, Cambon C, Orfila C, Seguelas MH, Lepert JC, Rami J, Carre P, Pipy B. Reactive oxygen intermediates as regulators of TNF-alpha production in rat lung inflammation induced by silica. J Immunol. 1996;156:1540–1548. [PubMed] [Google Scholar]
- 79.Grandaliano G, Di Paolo S, Monno R, Stallone G, Ranieri E, Pontrelli P, Gesualdo L, Schena FP. Protease-activated receptor 1 and plasminogen activator inhibitor 1 expression in chronic allograft nephropathy: the role of coagulation and fibrinolysis in renal graft fibrosis. Transplantation. 2001;72:1437–1443. doi: 10.1097/00007890-200110270-00018. [DOI] [PubMed] [Google Scholar]
- 80.Grant GA, Goldberg GI, Wilhelm SM, He C, Eisen AZ. Activation of extracellular matrix metalloproteases by proteases and organomercurials. Matrix. 1992;1(suppl 1):217–223. [PubMed] [Google Scholar]
- 81.Grau GE, de Moerloose P, Bulla O, Lou J, Lei Z, Reber G, Mili N, Ricou B, Morel DR, Suter PM. Haemostatic properties of human pulmonary and cerebral microvascular endothelial cells. Thromb Haemost. 1997;77:585–590. [PubMed] [Google Scholar]
- 82.Griese M, Ramakers J, Krasselt A, Starosta V, Van Konings-bruggen S, Fischer R, Ratjen F, Mullinger B, Huber RM, Maier K, Rietschel E, Scheuch G. Improvement of alveolar glutathione and lung function but not oxidative state in cystic fibrosis. Am J Respir Crit Care Med. 2004;169:822–828. doi: 10.1164/rccm.200308-1104OC. [DOI] [PubMed] [Google Scholar]
- 83.Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, Grimminger F, Walmrath D, Temmesfeld-Wollbruck B, Seeger W. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia: comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:454–462. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- 84.Gunther A, Mosavi P, Ruppert C, Heinemann S, Temmesfeld B, Velcovsky HG, Morr H, Grimminger F, Walmrath D, Seeger W. Enhanced tissue factor pathway activity and fibrin turnover in the alveolar compartment of patients with interstitial lung disease. Thromb Haemost. 2000;83:853–860. [PubMed] [Google Scholar]
- 85.Guo B, Inoki K, Isono M, Mori H, Kanasaki K, Sugimoto T, Akiba S, Sato T, Yang B, Kikkawa R, Kashiwagi A, Haneda M, Koya D. MAPK/AP-1-dependent regulation of PAI-1 gene expression by TGF-[beta] in rat mesangial cells. Kidney Int. 2005;68:972–984. doi: 10.1111/j.1523-1755.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 86.Hageman J, Eggen BJ, Rozema T, Damman K, Kampinga HH, Coppes RP. Radiation and transforming growth factor-{beta} cooperate in transcriptional activation of the profibrotic plasminogen activator inhibitor-1 gene. Clin Cancer Res. 2005;11:5956–5964. doi: 10.1158/1078-0432.CCR-05-0427. [DOI] [PubMed] [Google Scholar]
- 87.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 88.Hartl D, Starosta V, Maier K, Beck-Speier I, Rebhan C, Becker BF, Latzin P, Fischer R, Ratjen F, Huber RM, Rietschel E, Krauss-Etschmann S, Griese M. Inhaled glutathione decreases PGE2 and increases lymphocytes in cystic fibrosis lungs. Free Radic Biol Med. 2005;39:463–472. doi: 10.1016/j.freeradbiomed.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 89.Hasday JD, Bachwich PR, Lynch JP, 3rd, Sitrin RG. Procoagulant and plasminogen activator activities of bronchoalveolar fluid in patients with pulmonary sarcoidosis. Exp Lung Res. 1988;14:261–278. doi: 10.3109/01902148809115128. [DOI] [PubMed] [Google Scholar]
- 90.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hattori N, Mizuno S, Yoshida Y, Chin K, Mishima M, Sisson TH, Simon RH, Nakamura T, Miyake M. The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism. Am J Pathol. 2004;164:1091–1098. doi: 10.1016/S0002-9440(10)63196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hekman CM, Loskutoff DJ. Bovine plasminogen activator inhibitor 1: specificity determinations and comparison of the active, latent, and guanidine-activated forms. Biochemistry. 1988;27:2911–2918. doi: 10.1021/bi00408a037. [DOI] [PubMed] [Google Scholar]
- 93.Herrera B, Murillo MM, Alvarez-Barrientos A, Beltran J, Fernandez M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-[beta] in fetal rat hepatocytes. Free Radic Biol Med. 2004;36:16–26. doi: 10.1016/j.freeradbiomed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 94.Hirashima Y, Kobayashi H, Suzuki M, Tanaka Y, Kanayama N, Terao T. Transforming growth factor-beta1 produced by ovarian cancer cell line HRA stimulates attachment and invasion through an up-regulation of plasminogen activator inhibitor type-1 in human peritoneal mesothelial cells. J Biol Chem. 2003;278:26793–26802. doi: 10.1074/jbc.M212187200. [DOI] [PubMed] [Google Scholar]
- 95.Hizawa N, Maeda Y, Konno S, Fukui Y, Takahashi D, Nishimura M. Genetic polymorphisms at FCER1B and PAI-1 and asthma susceptibility. Clin Exp Allergy. 2006;36:872–876. doi: 10.1111/j.1365-2222.2006.02413.x. [DOI] [PubMed] [Google Scholar]
- 96.Hong YH, Peng HB, La Fata V, Liao JK. Hydrogen peroxide-mediated transcriptional induction of macrophage colony-stimulating factor by TGF-beta1. J Immunol. 1997;159:2418–2423. [PubMed] [Google Scholar]
- 97.Hou B, Eren M, Painter CA, Covington JW, Dixon JD, Schoenhard JA, Vaughan DE. Tumor necrosis factor {alpha} activates the human plasminogen activator inhibitor-1 gene through a distal nuclear factor {kappa}B site. J Biol Chem. 2004;279:18127–18136. doi: 10.1074/jbc.M310438200. [DOI] [PubMed] [Google Scholar]
- 98.Hua X, Liu X, Ansari DO, Lodish HF. Synergistic cooperation of TFE3 and Smad proteins in TGF-beta-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev. 1998;12:3084–3095. doi: 10.1101/gad.12.19.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Y, Border WA, Lawrence DA, Noble NA. Noninhibitory PAI-1 enhances plasmin-mediated matrix degradation both in vitro and in experimental nephritis. Kidney Int. 2006;70:515–522. doi: 10.1038/sj.ki.5000353. [DOI] [PubMed] [Google Scholar]
- 100.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84:695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Idell S, Koenig KB, Fair DS, Martin TR, McLarty J, Maunder RJ. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 1991;261:L240–L248. doi: 10.1152/ajplung.1991.261.4.L240. [DOI] [PubMed] [Google Scholar]
- 102.Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol. 2006;125:661–669. doi: 10.1007/s00418-005-0116-7. [DOI] [PubMed] [Google Scholar]
- 103.Irigoyen JP, Munoz-Canoves P, Montero L, Koziczak M, Nagamine Y. The plasminogen activator system: biology and regulation. Cell Mol Life Sci. 1999;56:104–132. doi: 10.1007/PL00000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishikawa A, Tanaka M, Ohta N, Ozono S, Kitamura T. Prevention of interstitial fibrosis of renal allograft by angiotensin ii blockade. Transplant Proc. 2006;38:3498–3501. doi: 10.1016/j.transproceed.2006.10.085. [DOI] [PubMed] [Google Scholar]
- 105.Islam KN, Kayanoki Y, Kaneto H, Suzuki K, Asahi M, Fujii J, Taniguchi N. TGF-beta1 triggers oxidative modifications and enhances apoptosis in HIT cells through accumulation of reactive oxygen species by suppression of catalase and glutathione peroxidase. Free Radic Biol Med. 1997;22:1007–1017. doi: 10.1016/s0891-5849(96)00493-5. [DOI] [PubMed] [Google Scholar]
- 106.Janssens S, Lijnen HR. What has been learned about the cardiovascular effects of matrix metalloproteinases from mouse models? Cardiovasc Res. 2006;69:585–594. doi: 10.1016/j.cardiores.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 107.Jardine H, MacNee W, Donaldson K, Rahman I. Molecular mechanism of transforming growth factor (TGF)-beta1-induced glutathione depletion in alveolar epithelial cells: involvement of AP- 1/ARE and Fra-1. J Biol Chem. 2002;277:21158–21166. doi: 10.1074/jbc.M112145200. [DOI] [PubMed] [Google Scholar]
- 108.Jesmin S, Sakuma I, Hattori Y, Kitabatake A. Role of angiotensin ii in altered expression of molecules responsible for coronary matrix remodeling in insulin-resistant diabetic rats. Arterioscler Thromb Vasc Biol. 2003;23:2021–2026. doi: 10.1161/01.ATV.0000094235.78783.D1. [DOI] [PubMed] [Google Scholar]
- 109.Jiang Z, Seo JY, Ha H, Lee EA, Kim YS, Han DC, Uh ST, Park CS, Lee HB. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem Biophys Res Commun. 2003;309:961–966. doi: 10.1016/j.bbrc.2003.08.102. [DOI] [PubMed] [Google Scholar]
- 110.Johnson MR, Bruzdzinski CJ, Winograd SS, Gelehrter TD. Regulatory sequences and protein-binding sites involved in the expression of the rat plasminogen activator inhibitor-1 gene. J Biol Chem. 1992;267:12202–12210. [PubMed] [Google Scholar]
- 111.Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, Rhee SG, Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol. 2000;165:2190–2197. doi: 10.4049/jimmunol.165.4.2190. [DOI] [PubMed] [Google Scholar]
- 112.Kamada Y, Tamura S, Kiso S, Fukui K, Doi Y, Ito N, Terui Y, Saito T, Watanabe H, Togashi H, Kawata S, Matsuzawa Y. Angiotensin II stimulates the nuclear translocation of Smad2 and induces PAI-1 mRNA in rat hepatic stellate cells. Hepatology Res. 2003;25:296–305. doi: 10.1016/s1386-6346(02)00306-6. [DOI] [PubMed] [Google Scholar]
- 113.Katoh M, Egashira K, Mitsui T, Chishima S, Takeshita A, Narita H. Angiotensin-converting enzyme inhibitor prevents plasminogen activator inhibitor-1 expression in a rat model with cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis. J Mol Cell Cardiol. 2000;32:73–83. doi: 10.1006/jmcc.1999.1053. [DOI] [PubMed] [Google Scholar]
- 114.Kayanoki Y, Fujii J, Suzuki K, Kawata S, Matsuzawa Y, Taniguchi N. Suppression of antioxidative enzyme expression by transforming growth factor-beta 1 in rat hepatocytes. J Biol Chem. 1994;269:15488–15492. [PubMed] [Google Scholar]
- 115.Keeton MR, Curriden SA, van Zonneveld AJ, Loskutoff DJ. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J Biol Chem. 1991;266:23048–23052. [PubMed] [Google Scholar]
- 116.Kim KK, Flaherty KR, Long Q, Hattori N, Sisson TH, Colby TV, Travis WD, Martinez FJ, Murray S, Simon RH. A plasminogen activator inhibitor-1 promoter polymorphism and idiopathic interstitial pneumonia. Mol Med. 2003;9:52–56. [PMC free article] [PubMed] [Google Scholar]
- 117.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klinger KW, Winqvist R, Riccio A, Andreasen PA, Sartorio R, Nielsen LS, Stuart N, Stanislovitis P, Watkins P, Douglas R, et al. Plasminogen activator inhibitor type 1 gene is located at region q21.3-q22 of chromosome 7 and genetically linked with cystic fibrosis. Proc Natl Acad Sci USA. 1987;84:8548–8552. doi: 10.1073/pnas.84.23.8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kobayashi N, Nakano S, Mita S-i, Kobayashi T, Honda T, Tsubokou Y, Matsuoka H. Involvement of rho-kinase pathway for angiotensin ii-induced plasminogen activator inhibitor-1 gene expression and cardiovascular remodeling in hypertensive rats. J Pharmacol Exp Ther. 2002;301:459–466. doi: 10.1124/jpet.301.2.459. [DOI] [PubMed] [Google Scholar]
- 120.Konecny R, Leonard S, Shi X, Robinson V, Castranova V. Reactivity of free radicals on hydroxylated quartz surface and its implications for pathogenicity: experimental and quantum mechanical study. J Environ Pathol Toxicol Oncol. 2001;20(suppl 1):119–132. [PubMed] [Google Scholar]
- 121.Kotani I, Sato A, Hayakawa H, Urano T, Takada Y, Takada A. Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res. 1995;77:493–504. doi: 10.1016/0049-3848(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 122.Krag S, Danielsen CC, Carmeliet P, Nyengaard J, Wogensen L. Plasminogen activator inhibitor-1 gene deficiency attenuates TGF-beta1-induced kidney disease. Kidney Int. 2005;68:2651–2666. doi: 10.1111/j.1523-1755.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 123.Kruithof EK. Plasminogen activator inhibitors: a review. Enzyme. 1988;40:113–121. doi: 10.1159/000469153. [DOI] [PubMed] [Google Scholar]
- 124.Kurata A, Nishizawa H, Kihara S, Maeda N, Sonoda M, Okada T, Ohashi K, Hibuse T, Fujita K, Yasui A, Hiuge A, Kumada M, Kuriyama H, Shimomura I, Funahashi T. Blockade of angiotensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. 2006;70:1717–1724. doi: 10.1038/sj.ki.5001810. [DOI] [PubMed] [Google Scholar]
- 125.Kutz SM, Hordines J, McKeown-Longo PJ, Higgins PJ. TGF-beta1-induced PAI-1 gene expression requires MEK activity and cell-to-substrate adhesion. J Cell Sci. 2001;114:3905–3914. doi: 10.1242/jcs.114.21.3905. [DOI] [PubMed] [Google Scholar]
- 126.Kuusniemi A-M, Lapatto R, Holmberg C, Karikoski R, Rapola J, Jalanko H. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotypic change. Kidney Int. 2005;68:121–132. doi: 10.1111/j.1523-1755.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 127.Lakari E, Paakko P, Pietarinen-Runtti P, Kinnula VL. Manganese superoxide dismutase and catalase are coordinately expressed in the alveolar region in chronic interstitial pneumonias and granulomatous diseases of the lung. Am J Respir Crit Care Med. 2000;161:615–621. doi: 10.1164/ajrccm.161.2.9904091. [DOI] [PubMed] [Google Scholar]
- 128.Lardot C, Heusterpreute M, Mertens P, Philippe M, Lison D. Expression of plasminogen activator inhibitors type-1 and type-2 in the mouse lung after administration of crystalline silica. Eur Respir J. 1998;11:912–921. doi: 10.1183/09031936.98.11040912. [DOI] [PubMed] [Google Scholar]
- 129.Laurent GJ. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol. 1987;252:C1–C9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- 130.Lavoie J-C, Rouleau T, Gagnon C, Chessex P. Photoprotection prevents TPN-induced lung procollagen mRNA in newborn guinea pigs. Free Radic Biol Med. 2002;33:512–520. doi: 10.1016/s0891-5849(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 131.Lawrence DA, Loskutoff DJ. Inactivation of plasminogen activator inhibitor by oxidants. Biochemistry. 1986;25:6351–6355. doi: 10.1021/bi00369a001. [DOI] [PubMed] [Google Scholar]
- 132.Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005;67:1762–1771. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 133.Levin EG, Santell L. Conversion of the active to latent plasminogen activator inhibitor from human endothelial cells. Blood. 1987;70:1090–1098. [PubMed] [Google Scholar]
- 134.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86:324–333. [PubMed] [Google Scholar]
- 135.Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost. 2005;3:35–45. doi: 10.1111/j.1538-7836.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 136.Lindahl TL, Sigurdardottir O, Wiman B. Stability of plasminogen activator inhibitor 1 (PAI-1) Thromb Haemost. 1989;62:748–751. [PubMed] [Google Scholar]
- 137.Liu R, Ahmed KM, Nantajit D, Rosenthal FS, Hai CX, Li JJ. Therapeutic effects of alpha-lipoic acid on bleomycin-induced pulmonary fibrosis in rats. Int J Mol Med. 2007;19:865–873. [PubMed] [Google Scholar]
- 138.Liu R-M, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-{beta}-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2004;286:L121–L128. doi: 10.1152/ajplung.00231.2003. [DOI] [PubMed] [Google Scholar]
- 139.Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 140.Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106:1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Loskutoff DJ, Samad F. The adipocyte and hemostatic balance in obesity: studies of PAI-1. Arterioscler Thromb Vasc Biol. 1998;18:1–6. doi: 10.1161/01.atv.18.1.1. [DOI] [PubMed] [Google Scholar]
- 142.Loskutoff DJ, Sawdey M, Keeton M, Schneiderman J. Regulation of PAI-1 gene expression in vivo. Thromb Haemost. 1993;70:135–137. [PubMed] [Google Scholar]
- 143.Lyon CJ, Hsueh WA. Effect of plasminogen activator inhibitor-1 in diabetes mellitus and cardiovascular disease. Am J Med. 2003;115(suppl 8A):62S–68S. doi: 10.1016/j.amjmed.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 144.Ma JY, Barger MW, Hubbs AF, Castranova V, Weber SL, Ma JK. Use of tetrandrine to differentiate between mechanisms involved in silica-versus bleomycin-induced fibrosis. J Toxicol Environ Health A. 1999;57:247–266. doi: 10.1080/009841099157692. [DOI] [PubMed] [Google Scholar]
- 145.Ma L-J, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. transforming growth factor-{beta}-dependent and -independent pathways of induction of tubulointerstitial fibrosis in {beta}6−/− Mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Macfelda K, Weiss TW, Kaun C, Breuss JM, Zorn G, Obern-dorfer U, Voegele-Kadletz M, Huber-Beckmann R, Ullrich R, Binder BR, Losert UM, Maurer G, Pacher R, Huber K, Wojta J. Plasminogen activator inhibitor 1 expression is regulated by the inflammatory mediators interleukin-1alpha, tumor necrosis factor-alpha, transforming growth factor-beta and oncostatin M in human cardiac myocytes. J Mol Cell Cardiol. 2002;34:1681–1691. doi: 10.1006/jmcc.2002.2117. [DOI] [PubMed] [Google Scholar]
- 147.Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, Arguiri E, Shuvaev VV, Sun J, Cengel K, Solomides CC, Christofidou-Solomidou M. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81:196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mackay AR, Corbitt RH, Hartzler JL, Thorgeirsson UP. Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res. 1990;50:5997–6001. [PubMed] [Google Scholar]
- 149.MacNee W, Rahman I. Oxidants/antioxidants in idiopathic pulmonary fibrosis. Thorax. 1995;50(suppl 1):S53–S58. doi: 10.1136/thx.50.suppl_1.s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maier K, Leuschel L, Costabel U. Increased levels of oxidized methionine residues in bronchoalveolar lavage fluid proteins from patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143:271–274. doi: 10.1164/ajrccm/143.2.271. [DOI] [PubMed] [Google Scholar]
- 151.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Margaglione M, Cappucci G, d’Addedda M, Colaizzo D, Giuliani N, Vecchione G, Mascolo G, Grandone E, Di Minno G. PAI-1 plasma levels in a general population without clinical evidence of atherosclerosis: relation to environmental and genetic determinants. Arterioscler Thromb Vasc Biol. 1998;18:562–567. doi: 10.1161/01.atv.18.4.562. [DOI] [PubMed] [Google Scholar]
- 153.Mata M, Ruiz A, Cerda M, Martinez-Losa M, Cortijo J, Santangelo F, Serrano-Mollar A, Llombart-Bosch A, Morcillo EJ. Oral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in rats. Eur Respir J. 2003;22:900–905. doi: 10.1183/09031936.03.00018003. [DOI] [PubMed] [Google Scholar]
- 154.Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int. 2005;67:2221–2238. doi: 10.1111/j.1523-1755.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 155.Matsuoka H, Sisson TH, Nishiuma T, Simon RH. Plasminogen-mediated activation and release of hepatocyte growth factor from extracellular matrix. Am J Respir Cell Mol Biol. 2006;35:705–713. doi: 10.1165/rcmb.2006-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Matsusaka T, Hymes J, Ichikawa I. Angiotensin in progressive renal diseases: theory and practice. J Am Soc Nephrol. 1996;7:2025–2043. doi: 10.1681/ASN.V7102025. [DOI] [PubMed] [Google Scholar]
- 157.Meyer A, Buhl R, Kampf S, Magnussen H. Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals. Am J Respir Crit Care Med. 1995;152:1055–1060. doi: 10.1164/ajrccm.152.3.7663783. [DOI] [PubMed] [Google Scholar]
- 158.Meyer A, Buhl R, Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J. 1994;7:431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- 159.Miles LA, Plow EF. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985;260:4303–4311. [PubMed] [Google Scholar]
- 160.Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 161.Monea S, Lehti K, Keski-Oja J, Mignatti P. Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol. 2002;192:160–170. doi: 10.1002/jcp.10126. [DOI] [PubMed] [Google Scholar]
- 162.Montaldo C, Cannas E, Ledda M, Rosetti L, Congiu L, Atzori L. Bronchoalveolar glutathione and nitrite/nitrate in idiopathic pulmonary fibrosis and sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:54–58. [PubMed] [Google Scholar]
- 163.Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med. 1998;158:1524–1527. doi: 10.1164/ajrccm.158.5.9803102. [DOI] [PubMed] [Google Scholar]
- 164.Motojima M, Ando T, Yoshioka T. Sp1-like activity mediates angiotensin-II-induced plasminogen-activator inhibitor type-1 (PAI-1) gene expression in mesangial cells. Biochem J. 2000;349:435–441. doi: 10.1042/0264-6021:3490435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mottonen J, Strand A, Symersky J, Sweet RM, Danley DE, Geoghegan KF, Gerard RD, Goldsmith EJ. Structural basis of latency in plasminogen activator inhibitor-1. Nature. 1992;355:270–273. doi: 10.1038/355270a0. [DOI] [PubMed] [Google Scholar]
- 166.Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 167.Nakstad B, Lyberg T, Skjonsberg OH, Boye NP. Local activation of the coagulation and fibrinolysis systems in lung disease. Thromb Res. 1990;57:827–838. doi: 10.1016/0049-3848(90)90150-b. [DOI] [PubMed] [Google Scholar]
- 168.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 169.Nath KA, Grande J, Croatt A, Haugen J, Kim Y, Rosenberg ME. Redox regulation of renal DNA synthesis, transforming growth factor- beta1 and collagen gene expression. Kidney Int. 1998;53:367–381. doi: 10.1046/j.1523-1755.1998.00778.x. [DOI] [PubMed] [Google Scholar]
- 170.Nieto N, Cederbaum AI. S-Adenosylmethionine blocks collagen I production by preventing transforming growth factor-{beta} induction of the COL1A2 promoter. J Biol Chem. 2005;280:30963–30974. doi: 10.1074/jbc.M503569200. [DOI] [PubMed] [Google Scholar]
- 171.Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Glie-mann J, Blasi F. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J. 1997;16:2610–2620. doi: 10.1093/emboj/16.10.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002;294:1155–1160. doi: 10.1016/S0006-291X(02)00577-6. [DOI] [PubMed] [Google Scholar]
- 173.Oikawa T, Freeman M, Lo W, Vaughan DE, Fogo A. Modulation of plasminogen activator inhibitor-1 in vivo: a new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int. 1997;51:164–172. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- 174.Okada H, Woodcock-Mitchell J, Mitchell J, Sakamoto T, Marutsuka K, Sobel BE, Fujii S. Induction of plasminogen activator inhibitor type 1 and type 1 collagen expression in rat cardiac microvascular endothelial cells by interleukin-1 and its dependence on oxygen-centered free radicals. Circulation. 1998;97:2175–2182. doi: 10.1161/01.cir.97.21.2175. [DOI] [PubMed] [Google Scholar]
- 175.Okada Y, Gonoji Y, Naka K, Tomita K, Nakanishi I, Iwata K, Yamashita K, Hayakawa T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells: purification and activation of the precursor and enzymic properties. J Biol Chem. 1992;267:21712–21719. [PubMed] [Google Scholar]
- 176.Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 177.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxy-nitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 178.Olman MA, Mackman N, Gladson CL, Moser KM, Loskutoff DJ. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin Invest. 1995;96:1621–1630. doi: 10.1172/JCI118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pan LH, Ohtani H, Yamauchi K, Nagura H. Co-expression of TNF alpha and IL-1 beta in human acute pulmonary fibrotic diseases: an immunohistochemical analysis. Pathol Int. 1996;46:91–99. doi: 10.1111/j.1440-1827.1996.tb03584.x. [DOI] [PubMed] [Google Scholar]
- 180.Pandey M, Loskutoff DJ, Samad F. Molecular mechanisms of tumor necrosis factor-alpha-mediated plasminogen activator inhibitor-1 expression in adipocytes. FASEB J. 2005:4–3459fje. doi: 10.1096/fj.04-3459fje. [DOI] [PubMed] [Google Scholar]
- 181.Paola Rottoli BM, Riccardo Cianti, Elena Bargagli, Cecilia Vagaggini, Nikolaos Nikiforakis, Vitaliano Pallini, Luca Bini. Carbonylated proteins in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics. 2005;5:2612–2618. doi: 10.1002/pmic.200401206. [DOI] [PubMed] [Google Scholar]
- 182.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 183.Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol. 2004;35:1000–1007. doi: 10.1016/j.humpath.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 184.Perlemuter G, Letteron P, Carnot F, Zavala F, Pessayre D, Nalpas B, Brechot C. Alcohol and hepatitis C virus core protein additively increase lipid peroxidation and synergistically trigger hepatic cytokine expression in a transgenic mouse model. J Hepatol. 2003;39:1020–1027. doi: 10.1016/s0168-8278(03)00414-8. [DOI] [PubMed] [Google Scholar]
- 185.Pilger A, Germadnik D, Schaffer A, Theiler A, Pils P, Sluka F, Winker N, Rudiger HW. 8-Hydroxydeoxyguanosine in leukocyte DNA and urine of quartz-exposed workers and patients with silicosis. Int Arch Occup Environ Health. 2000;73:305–310. doi: 10.1007/s004200000117. [DOI] [PubMed] [Google Scholar]
- 186.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 187.Portal BC, Richard M-J, Faure HS, Hadjian AJ, Favier AE. Altered antioxidant status and increased lipid peroxidation in children with cystic fibrosis. Am J Clin Nutr. 1995;61:843–847. doi: 10.1093/ajcn/61.4.843. [DOI] [PubMed] [Google Scholar]
- 188.Porter DW, Hubbs AF, Mercer R, Robinson VA, Ramsey D, McLaurin J, Khan A, Battelli L, Brumbaugh K, Teass A, Castranova V. Progression of lung inflammation and damage in rats after cessation of silica inhalation. Toxicol Sci. 2004;79:370–380. doi: 10.1093/toxsci/kfh110. [DOI] [PubMed] [Google Scholar]
- 189.Porter DW, Millecchia LL, Willard P, Robinson VA, Ramsey D, McLaurin J, Khan A, Brumbaugh K, Beighley CM, Teass A, Castranova V. Nitric oxide and reactive oxygen species production causes progressive damage in rats after cessation of silica inhalation. Toxicol Sci. 2006;90:188–197. doi: 10.1093/toxsci/kfj075. [DOI] [PubMed] [Google Scholar]
- 190.Prendergast GC, Diamond LE, Dahl D, Cole MD. The c-myc-regulated gene mrl encodes plasminogen activator inhibitor 1. Mol Cell Biol. 1990;10:1265–1269. doi: 10.1128/mcb.10.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, Siafakas NM, Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest. 2006;36:362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 192.Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43:872–878. doi: 10.1002/hep.21107. [DOI] [PubMed] [Google Scholar]
- 193.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 194.Rahman I, Skwarska E, Henry M, Davis M, O’Connor CM, FitzGerald MX, Greening A, MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic Biol Med. 1999;27:60–68. doi: 10.1016/s0891-5849(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 195.Reid DW, Misso N, Aggarwal S, Thompson PJ, Walters EH. Oxidative stress and lipid-derived inflammatory mediators during acute exacerbations of cystic fibrosis. Respirology. 2007;12:63–69. doi: 10.1111/j.1440-1843.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 196.Reilly CF, Hutzelmann JE. Plasminogen activator inhibitor-1 binds to fibrin and inhibits tissue-type plasminogen activator-mediated fibrin dissolution. J Biol Chem. 1992;267:17128–17135. [PubMed] [Google Scholar]
- 197.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 198.Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulmon Med. 2005;11:169–173. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- 199.Rosias PPR, Hartog GJMD, Robroeks CMHHT, Bast A, Donckerwolcke RAMG, Heynens JWCM, Suykerbuyk J, Hendriks HJE, Joumlbsis Q, Dompeling E. Free radicals in exhaled breath condensate in cystic fibrosis and healthy subjects. Free Radic Res. 2006;40:901–909. doi: 10.1080/10715760500522648. [DOI] [PubMed] [Google Scholar]
- 200.Roum JH, Borok Z, McElvaney NG, Grimes GJ, Bokser AD, Buhl R, Crystal RG. Glutathione aerosol suppresses lung epithelial surface inflammatory cell-derived oxidants in cystic fibrosis. J Appl Physiol. 1999;87:438–443. doi: 10.1152/jappl.1999.87.1.438. [DOI] [PubMed] [Google Scholar]
- 201.Roum JH, Buhl R, McElvany NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol Respir Environ Exerc Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 202.Rozy A, Czerniawska J, Stepniewska A, Wozbinska B, Goljan A, Puscinska E, Gorecka D, Chorostowska-Wynimko J. Inflammatory markers in the exhaled breath condensate of patients with pulmonary sarcoidosis. J Physiol Pharmacol. 2006;57(suppl 4):335–340. [PubMed] [Google Scholar]
- 203.Sakamoto T, Woodcock-Mitchell J, Marutsuka K, Mitchell JJ, Sobel BE, Fujii S. TNF-alpha and insulin, alone and synergistically, induce plasminogen activator inhibitor-1 expression in adipocytes. Am J Physiol. 1999;276:C1391–C1397. doi: 10.1152/ajpcell.1999.276.6.C1391. [DOI] [PubMed] [Google Scholar]
- 204.Samarakoon R, Higgins CE, Higgins SP, Kutz SM, Higgins PJ. Plasminogen activator inhibitor type-1 gene expression and induced migration in TGF-beta1-stimulated smooth muscle cells is pp60(c-src)/MEK-dependent. J Cell Physiol. 2005;204:236–246. doi: 10.1002/jcp.20279. [DOI] [PubMed] [Google Scholar]
- 205.Sanchez A, Alvarez AM, Benito M, Fabregat I. Cycloheximide prevents apoptosis, reactive oxygen species production, and glutathione depletion induced by transforming growth factor beta in fetal rat hepatocytes in primary culture. Hepatology. 1997;26:935–943. doi: 10.1002/hep.510260420. [DOI] [PubMed] [Google Scholar]
- 206.Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- 207.Sato M, Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Ohyama Y, Suga T, Maeno T, Aoki Y, Tamura J, Sakamoto H, Nagai R, Kurabayashi M. c-Src and hydrogen peroxide mediate transforming growth factor-{beta}1-induced smooth muscle cell-gene expression in 10T1/2 cells. Arterioscler Thromb Vasc Biol. 2005;25:341–347. doi: 10.1161/01.ATV.0000152608.29351.8f. [DOI] [PubMed] [Google Scholar]
- 208.Schultze-Mosgau S, Kopp J, Thorwarth M, Rodel F, Melnychenko I, Grabenbauer GG, Amann K, Wehrhan F. Plasminogen activator inhibitor-I-related regulation of procollagen I ([alpha]1 and [alpha]2) by antitransforming growth factor-[beta]1 treatment during radiation-impaired wound healing. Int J Radiat Oncol Biol Phys. 2006;64:280–288. doi: 10.1016/j.ijrobp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 209.Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, Bohm M. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 210.Seite S, Colige A, Deroanne C, Lambert C, Piquemal-Vivenot P, Montastier C, Fourtanier A, Lapiere C, Nusgens B. Changes in matrix gene and protein expressions after single or repeated exposure to one minimal erythemal dose of solar-simulated radiation in human skin in vivo. Photochem Photobiol. 2004;79:265–271. doi: 10.1562/yg-03-09.1. [DOI] [PubMed] [Google Scholar]
- 211.Shihab FS, Bennett WM, Tanner AM, Andoh TF. Angiotensin II blockade decreases TGF-beta1 and matrix proteins in cyclosporin nephropathy. Kidney Int. 1997;52:660–673. doi: 10.1038/ki.1997.380. [DOI] [PubMed] [Google Scholar]
- 212.Siegmund SV, Dooley S, Brenner DA. Molecular mechanisms of alcohol-induced hepatic fibrosis. Dig Dis. 2005;23:264–274. doi: 10.1159/000090174. [DOI] [PubMed] [Google Scholar]
- 213.Sisson TH, Hanson KE, Subbotina N, Patwardhan A, Hattori N, Simon RH. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1023–L1032. doi: 10.1152/ajplung.00049.2002. [DOI] [PubMed] [Google Scholar]
- 214.Sisson TH, Hattori N, Xu Y, Simon RH. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum Gene Titer. 1999;10:2315–2323. doi: 10.1089/10430349950016960. [DOI] [PubMed] [Google Scholar]
- 215.Sogut S, Ozyurt H, Armutcu F, Kart L, Iraz M, Akyol O, Ozen S, Kaplan S, Temel I, Yildirim Z. Erdosteine prevents bleomycin-induced pulmonary fibrosis in rats. Eur J Pharmacol. 2004;494:213–220. doi: 10.1016/j.ejphar.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 216.Srivastava KD, Rom WN, Jagirdar J, Yie TA, Gordon T, Tchou-Wong KM. Crucial role of interleukin-1beta and nitric oxide synthase in silica-induced inflammation and apoptosis in mice. Am J Respir Crit Care Med. 2002;165:527–533. doi: 10.1164/ajrccm.165.4.2106009. [DOI] [PubMed] [Google Scholar]
- 217.Starosta V, Rietschel E, Paul K, Baumann U, Griese M. Oxidative changes of bronchoalveolar proteins in cystic fibrosis. Chest. 2006;129:431–437. doi: 10.1378/chest.129.2.431. [DOI] [PubMed] [Google Scholar]
- 218.Strandberg L, Lawrence D, Johansson L, Ny T. The oxidative inactivation of plasminogen activator inhibitor type 1 results from a conformational change in the molecule and does not require the involvement of the P1′ methionine. J Biol Chem. 1991;266:13852–13858. [PubMed] [Google Scholar]
- 219.Strausz J, Muller-Quernheim J, Steppling H, Borkowski A, Ferlinz R. [Oxygen radical production of alveolar inflammatory cells in sarcoidosis and idiopathic lung fibrosis] Pneumologie. 1989;43:440–445. [PubMed] [Google Scholar]
- 220.Strausz J, Muller-Quernheim J, Steppling H, Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- 221.Sun Y, Zhang J, Lu L, Bedigian MP, Robinson AD, Weber KT. Tissue angiotensin II in the regulation of inflammatory and fibrogenic components of repair in the rat heart. J Lab Clin Med. 2004;143:41–51. doi: 10.1016/j.lab.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 222.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol. 2000;157:177–187. doi: 10.1016/S0002-9440(10)64529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Swiatkowska M, Szemraj J, Al-Nedawi KN, Pawlowska Z. Reactive oxygen species upregulate expression of PAI-1 in endothelial cells. Cell Mol Biol Lett. 2002;7:1065–1071. [PubMed] [Google Scholar]
- 224.Swiatkowska M, Szemraj J, Cierniewski CS. Induction of PAI-1 expression by tumor necrosis factor B in endothelial cells is mediated by its responsive element located in the 4G/5G site. FEBS J. 2005;272:5821–5831. doi: 10.1111/j.1742-4658.2005.04979.x. [DOI] [PubMed] [Google Scholar]
- 225.Takeshita K, Yamamoto K, Ito M, Kondo T, Matsushita T, Hirai M, Kojima T, Nishimura M, Nabeshima Y, Loskutoff DJ, Saito H, Murohara T. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin Thromb Hemost. 2002;28:545–554. doi: 10.1055/s-2002-36699. [DOI] [PubMed] [Google Scholar]
- 226.Takeshita Y, Takamura T, Hamaguchi E, Shimizu A, Ota T, Sakurai M, Kaneko S. Tumor necrosis factor-[alpha]-induced production of plasminogen activator inhibitor 1 and its regulation by pioglitazone and cerivastatin in a nonmalignant human hepatocyte cell line. Metabolism. 2006;55:1464–1472. doi: 10.1016/j.metabol.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 227.Thannickal VJ, Day RM, Klinz SG, Bastien MC, Larios JM, Fanburg BL. Ras-dependent and -independent regulation of reactive oxygen species by mitogenic growth factors and TGF-beta1. FASEB J. 2000;14:1741–1748. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- 228.Tiitto L, Kaarteenaho-Wiik R, Sormunen R, Holmgren A, Paakko P, Soini Y, Kinnula VL. Expression of the thioredoxin system in interstitial lung disease. J Pathol. 2003;201:363–370. doi: 10.1002/path.1435. [DOI] [PubMed] [Google Scholar]
- 229.Tiitto LH, Peltoniemi MJ, Kaarteenaho-Wiik RL, Soini YM, Paakko PK, Sormunen RT, Kinnula VL. Cell-specific regulation of gamma-glutamylcysteine synthetase in human interstitial lung diseases. Hum Pathol. 2004;35:832–839. doi: 10.1016/j.humpath.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 230.Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, Sakamoto H, Iwasaki H. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005;10:449–455. doi: 10.1111/j.1440-1843.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 231.Tuan TL, Wu H, Huang EY, Chong SS, Laug W, Messadi D, Kelly P, Le A. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol. 2003;162:1579–1589. doi: 10.1016/S0002-9440(10)64292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Uria JA, Jimenez MG, Balbin M, Freije JM, Lopez-Otin C. Differential effects of transforming growth factor-beta on the expression of collagenase-1 and collagenase-3 in human fibroblasts. J Biol Chem. 1998;273:9769–9777. doi: 10.1074/jbc.273.16.9769. [DOI] [PubMed] [Google Scholar]
- 233.Vallyathan V, Mega JF, Shi X, Dalal NS. Enhanced generation of free radicals from phagocytes induced by mineral dusts. Am J Respir Cell Mol Biol. 1992;6:404–413. doi: 10.1165/ajrcmb/6.4.404. [DOI] [PubMed] [Google Scholar]
- 234.Vayalil P, Iles K, Choi J, Yi A-K, Postlethwait E, Liu R-M. Glutathione suppresses TGF-beta-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00128.2007. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vayalil PK, Olman M, Murphy-Ullrich JE, Postlethwait EM, Liu RM. Glutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogen. Am J Physiol Lung Cell Mol Physiol. 2005;289:L937–L945. doi: 10.1152/ajplung.00150.2005. [DOI] [PubMed] [Google Scholar]
- 236.Venkatesan N, Punithavathi D, Babu M. Protection from acute and chronic lung diseases by curcumin. Adv Exp Med Biol. 2007;595:379–405. doi: 10.1007/978-0-387-46401-5_17. [DOI] [PubMed] [Google Scholar]
- 237.Vulin AI, Stanley FM. Oxidative stress activates the plasminogen activator inhibitor type 1 (PAI-1) promoter through an AP-1 response element and cooperates with insulin for additive effects on PAI-1 transcription. J Biol Chem. 2004;279:25172–25178. doi: 10.1074/jbc.M403184200. [DOI] [PubMed] [Google Scholar]
- 238.Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, Vaughan DE, Brown NJ. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- 239.Welsh MJ, Tsui LC, Boat TF, Beaudet AL. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited diseases. New York: McGraw-Hill; 1995. pp. 3799–3876. [Google Scholar]
- 240.Wong AP, Cortez SL, Baricos WH. Role of plasmin and gelatinase in extracellular matrix degradation by cultured rat mesangial cells. Am J Physiol. 1992;263:F1112–F1118. doi: 10.1152/ajprenal.1992.263.6.F1112. [DOI] [PubMed] [Google Scholar]
- 241.Woodward RN, Finn AV, Dichek DA. Identification of intracellular pathways through which TGF-[beta]1 upregulates PAI-1 expression in endothelial cells. Atherosclerosis. 2006;186:92–100. doi: 10.1016/j.atherosclerosis.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 242.Xiao W, Hsu Y-P, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest. 2005;128:2316–2326. doi: 10.1378/chest.128.4.2316. [DOI] [PubMed] [Google Scholar]
- 243.Yadav D, Hertan HI, Schweitzer P, Norkus EP, Pitchumoni CS. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97:2634–2639. doi: 10.1111/j.1572-0241.2002.06041.x. [DOI] [PubMed] [Google Scholar]
- 244.Yang C, Patel K, Harding P, Sorokin A, Glass IIWF. Regulation of TGF-[beta]1/MAPK-mediated PAI-1 gene expression by the actin cytoskeleton in human mesangial cells. Exp Cell Res. 2007;313:1240–1250. doi: 10.1016/j.yexcr.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yildirim Z, Kotuk M, Erdogan H, Iraz M, Yagmurca M, Kuku I, Fadillioglu E. Preventive effect of melatonin on bleomycin-induced lung fibrosis in rats. J Pineal Res. 2006;40:27–33. doi: 10.1111/j.1600-079X.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 246.Yildirim Z, Kotuk M, Iraz M, Kuku I, Ulu R, Armutcu F, Ozen S. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: erdosteine and N-acetylcysteine. Pulmon Pharmacol Ther. 2005;18:367–373. doi: 10.1016/j.pupt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 247.Yingling JM, Datto MB, Wong C, Frederick JP, Liberati NT, Wang XF. Tumor suppressor Smad4 is a transforming growth factor beta-inducible DNA binding protein. Mol Cell Biol. 1997;17:7019–7028. doi: 10.1128/mcb.17.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yoshimoto T, Fukai N, Sato R, Sugiyama T, Ozawa N, Shichiri M, Hirata Y. Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology. 2004;145:3331–3337. doi: 10.1210/en.2003-1583. [DOI] [PubMed] [Google Scholar]
- 249.Yoshimoto T, Gochou N, Fukai N, Sugiyama T, Shichiri M, Hirata Y. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertens Res. 2005;28:165–172. doi: 10.1291/hypres.28.165. [DOI] [PubMed] [Google Scholar]
- 250.Yucesoy B, Vallyathan V, Landsittel DP, Sharp DS, Weston A, Burleson GR, Simeonova P, McKinstry M, Luster MI. Association of tumor necrosis factor-[alpha] and interleukin-1 gene polymorphisms with silicosis. Toxicol Appl Pharmacol. 2001;172:75–82. doi: 10.1006/taap.2001.9124. [DOI] [PubMed] [Google Scholar]
- 251.Zhang L, Seiffert D, Fowler BJ, Jenkins GR, Thinnes TC, Loskutoff DJ, Parmer RJ, Miles LA. Plasminogen has a broad extrahepatic distribution. Thromb Haemost. 2002;87:493–501. [PubMed] [Google Scholar]
- 252.Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J Immunol. 1993;150:4188–4196. [PubMed] [Google Scholar]
- 253.Zhang Y, Ma J. Changes of coagulation and fibrinolysis system in bronchoalveolar lavage fluid in lung fibrosis. Beijing Da Xue Xue Bao. 2005;37:516–519. [PubMed] [Google Scholar]
- 254.Zhao W, O’Malley Y, Robbins ME. Irradiation of rat mesangial cells alters the expression of gene products associated with the development of renal fibrosis. Radiat Res. 1999;152:160–169. [PubMed] [Google Scholar]
- 255.Zhao W, Spitz DR, Oberley LW, Robbins ME. Redox modulation of the pro-fibrogenic mediator plasminogen activator inhibitor-1 following ionizing radiation. Cancer Res. 2001;61:5537–5543. [PubMed] [Google Scholar]