Abstract
The consumption of nutrient-poor snack foods in Western diets is thought to be contributing to the increasing prevalence of obesity and diabetes. Soy offers unique potential to provide high quality protein, dietary fiber, and phytochemicals to snack foods to produce a more healthful nutritional profile. In this study, 27.3% of wheat flour was replaced with soy ingredients in a soft pretzel and evaluated for impact on satiety, glycemic index (GI), and insulinemic index (II). We first tested the soy pretzel for consumer acceptability by 51 untrained sensory panelists on a 9-point hedonic scale. Second, in a crossover trial, 20 healthy adults consumed soy and traditional pretzels (1000 kJ or 239 kcal each) after an overnight fast. They reported their levels of satiety on a 10 cm visual analog scale (VAS) for 2 h postprandially. Third, 12 healthy, non-diabetic subjects consumed soy or traditional pretzels (50 ± 2 g available carbohydrates) to determine the GI and II of both products. Blood glucose and insulin responses were monitored for 2 h after consumption and compared to a glucose reference. It was found that a consumer-acceptable soy soft pretzel had a lower mean (±SD) GI than its traditional counterpart: 39.1 (±20.4) for soy and 66.4 (±15.3) for wheat, (p = 0.002). However, soy addition did not statistically affect II (p = 0.15), or satiety (p = 0.91). In conclusion, a nutrient-dense soy pretzel formulation with 27.3% of wheat flour replaced by soy ingredients had attenuated postprandial glycemia without significantly affecting insulinemia or satiety in healthy adults.
Introduction
Snacks are defined as foods and beverages consumed at occasions other than meals.1 People in the United States are consuming up to 24% of their calories from snacks on average, a significant increase over the last few decades.1 The increase in food consumption frequency without compensatory energy reduction at each eating occasion may be contributing to the incidence of obesity and type 2 diabetes.2 Moreover, current snack food choices made by consumers tend to be high in fats and added sugars, contain highly processed carbohydrates that tend to increase blood glucose levels, and contain few essential nutrients or health-promoting phytochemicals.1 Habitual consumption of foods with these properties can additionally increase risk for nutrient deficiency or impact risk of chronic diseases of aging such as cardiovascular disease and type 2 diabetes.3 Therefore, snack foods offer an opportunity to include nutritious ingredients to potentially impact the quality of the typical American diet.
Soy incorporation into snack foods can provide nutritional benefits such as high quality protein, fiber, and various micro-nutrients and phytochemicals.4,5 It has been shown that soy products may enhance satiety6,7 which may reduce energy intake and risk of obesity.8 In addition, diets rich in soy protein may contribute to a lower risk of cardiovascular disease.4,9 Soy is the only non-animal food source that provides all 20 amino acids10 while remaining low in saturated fat and cholesterol. Soy flour may contain up to 17% dietary fiber including both soluble and insoluble fibers. These fibers may have a beneficial impact upon total and low density lipoprotein (LDL) cholesterol.9,11 Additionally, soy phytochemicals are proposed to potentially reduce the risk of several cancers.12,13
Grain-based snacks such as crackers, pretzels, and other bakery products are popular and offer a promising matrix for the delivery of soy. However, soy addition poses challenges for yeast-leavened bakery products.5,14–16 In bread, soy protein strongly binds water and dilutes the gluten matrix, decreasing loaf volume.17 However, baked snack foods such as pretzels (soft and hard), breadsticks, and crackers have a denser matrix which can accommodate increased soy and therefore provide a more promising consumer-acceptable delivery system.
The glycemic index (GI) of a food is defined as the area under the curve of glycemia vs. time (2 h) immediately following consumption of 50 g available carbohydrates (total carbohydrates minus fiber) from the test food, compared to 50 g pure glucose.18 The insulinemic index (II) is acquired and assessed with a protocol analogous to that of the GI and is used to compared postprandial insulinemia.19 Foods that are composed of sugars and refined grains generally possess high GI values (≥70 with pure glucose = 100) while foods with a lower amount of processed carbohydrates have lower GI values (≤55).21 Because habitual consumption of high GI foods has been associated with increased risk for type 2 diabetes,22 coronary heart disease,23 and increased appetite that may contribute to obesity,21 there is a desire to increase the availability of low GI snack foods.24 The effects of soy addition on satiety, GI, and II in baked products has not been reported.
The purpose of this investigation was to evaluate the acceptability of a soft pretzel formulated with 27.3% soy ingredients (dry weight) and determine the GI, II, and satiety of the soy-based soft pretzel compared to a conventional wheat soft pretzel. We hypothesized that incorporation of soy would lead to an increase in satiety and a decrease in GI and II in a soft pretzel.
Experimental
Sensory analysis, glycemic/insulinemic, and satiety studies were approved by the Institutional Review Board at The Ohio State University and performed at the Clinical Research Center at The Ohio State University Medical Center, Columbus, Ohio.
Pretzel production
Soy and wheat pretzels were produced using ingredients in Table 1. Wheat flour (350 g or 31.7 g/100 g), dough conditioner, wheat gluten, and 325 g water (37.5 g/100 g) were added to a KitchenAid mixer and stirred with a flat beater attachment at a low speed until moistened (about 30–60 s). To produce the sponge, the dough was proofed at 39 °C at 100% humidity for 2 h (CM2000 Holding/Proofing Combination Module; InterMetro Industries Corp., Wilkes-Barre, PA). The remaining ingredients except the shortening were added and stirred with the mixer. The flat beater attachment was used with the mixer on a low speed (“2”) until the ingredients were combined (about 1–2 min). The attachment was then replaced with a dough hook and the dough was stirred for approximately 6–8 min more. The shortening was added and the dough was blended until it sheeted (about 5 more min). The dough was rolled into an approximately 60 cm rope and formed into a soft pretzel shape. The pretzel was dipped into 1.0% sodium hydroxide solution (65 ± 5 °C) for 45–60 s and placed on a greased baking sheet (Pam 100% Canola cooking spray; ConAgra Foods, Omaha, NE). The pretzels were proofed for 30 min more then baked at 150 °C for 15 min (JA14 Jet-Air oven; Doyon, Linière, QC, Canada).
Table 1.
Ingredients used to produce wheat and soy soft pretzels
| Ingredient | Source | Soy pretzel (g/100 g) | Wheat pretzel (g/100 g) |
|---|---|---|---|
| Instant yeast | Lesaffre Yeast Corporation, Milwaukee WI | 0.30 | 0.30 |
| Bread flour | ConAgra Mills, Omaha NE | 40.97 | 56.40 |
| Vital wheat gluten | Bob’s Red Mill, Milwaukie OR | 0.90 | 0.90 |
| Dough conditioner | The Prepared Pantry, Rigby ID | 0.12 | 0.12 |
| Soy flour | ADM, Protein Specialties Division, Decatur IL | 11.53 | — |
| Benesoy soymilk powder | Davansoy, Inc., Carroll IA | 3.84 | — |
| Iodized salt | US Foodservice, Inc., Columbia MD | 0.72 | 0.72 |
| Pure granulated sugar | US Foodservice, Inc., Columbia MD | 2.26 | 2.26 |
| Vegetable shortening (Crisco®) | The J.M. Smucker Co, Orrville OH | 1.81 | 1.81 |
| Water | 37.53 ± 0.90 | 37.49 ± 0.90 |
To determine the energy density, the baked pretzel was dehydrated in a 60 °C cabinet (Curtin Matheson, Huston, TX) for 48 h and subjected to bomb calorimetry (Parr Adiabatic Calorimeter, Moline, IL). Benzoic acid was used to determine calorimeter efficiency. Amount of fat, carbohydrates, fiber, and protein were calculated based on certificate of analyses and nutrition facts labels.
Sensory analysis
Male and female participants between the ages of 18 and 40 yrs were recruited from the university campus to complete the sensory analysis. Samples were prepared by placing fresh pretzel pieces (less than 24 h old) into 2 oz plastic portion cup labeled with a random 3-digit number. The participants consumed samples of either a soy or wheat pretzel in random order (counterbalanced) in ambient lighting. The participants reported their level of acceptability on a 9-point hedonic scale with “1”, on the left, being “extremely dislike”, “5” being “neither like nor dislike”, and “9”, on the right, being “extremely like”.
Study 1 – glycemic and insulinemic indices
The GI and II protocols were based on those detailed in Brouns et al.25 Pretzel dough totalling 63.8 g (25.0 ± 1.0 g available carbohydrates25) was used to prepare pretzels for the GI and II studies. Participants consumed 50.0 ± 2.0 g available carbohydrates from the soft pretzels in the form of 2 soft pretzels. Table 2 shows the energy and macronutrient profiles of the dough. Baked soft pretzels were stored at −40 °C and thawed at room temperature the day before consumption.
Table 2.
Nutritional composition of the pretzels for studies 1 and 2
| Study 1 |
Study 2 |
|||
|---|---|---|---|---|
| Glycemic/ Insulinemic Index |
Satiety |
|||
| Soy | Wheat | Soy | Wheat | |
| Calories (kcal) | 332.1 | 270.8 | 239.0 | 239.0 |
| Fat (g) | 5.2 | 3.4 | 3.7 | 3.0 |
| Carbohydrates (g) | 54.7 | 51.4 | 39.4 | 45.4 |
| Fiber (g) | 4.7 | 1.4 | 3.4 | 1.2 |
| Protein (g) | 17.2 | 8.1 | 12.4 | 7.2 |
| Total soy isoflavones (µmol)a | 71.0 | — | 51.1 | — |
| Total weight consumed (g) | 72.9 | 62.0 | 52.4 | 54.5 |
Isoflavone content was obtained from a previous study with the same soy ingredients.20
Healthy non-smokers with a body mass index (BMI) less than 30 and without a history of diabetes, glucose intolerance, gastrointestinal disorders, or wheat or soy allergies were enrolled in the study. After an overnight fast, participants arrived at the Clinical Research Center (CRC). Their vital signs and weight were recorded and they rested for 30 min. During this time an intravenous catheter was inserted into the medial cubital vein in the left or right arm and, to assure adequate glycogen stores, a dietary record was assessed for intake of at least 150 g of carbohdyrates in each of the previous 3 days. At time t = 0, a baseline blood sample was drawn and, subsequently, they consumed either a glucose standard drink (Glucola, NERL Diagnostics LLC, East Providence, RI), white bread (Giant Eagle King Size enriched bread, Pittsburgh, PA), a soy pretzel, or a wheat pretzel, each containing 50 g available carbohydrates. The glucose drink was consumed three times- at the first session, the last session, and either session 3 or 4. The solid samples were all consumed once, the order determined by randomized block. Blood samples were drawn at t = 15, 30, 45, 60, 90, and 120 min. At least one week separated each of the 6 visits.
Blood samples were frozen the day of collection and analyzed in a single batch. A YSI 2300 State Plus Glucose and Lactate Analyzer with a sensitivity of 2.5 mg/dl was used to determine glycemia (YSI International, Yellow Springs, OH). Insulin concentrations were determined with an Immulite 1000 chemiluminescence method (Siemens Medical Solutions Diagnostics; Duluth, GA). This assay has a sensitivity of 2 µIU/mL, an intra-assay coefficient of 5.7%, and an inter-assay coefficient of 6.7%. Graphs of glycemia or insulinemia vs. time were generated and the area under the curve (AUC) was calculated for each by measuring the area above the baseline.25 The average of the AUCs for the three glucose standards was deemed a GI of 100; the same was performed for insulin. The GI and II were reported as the percent AUC as compared to the glucose standard. White bread served as a method validation.
Study 2 – satiety study
The satiety experiment employed a randomized, counter-balanced, cross-over design similar to that in Holt et al. except satiety values were compared between treatments instead of compared to a glucose treatment.8
Soy dough was weighed to 99.0 g and wheat dough to 98.5 g to obtain soft pretzels with 500 kJ (119.5 kcal) of energy (Table 2). Each participant consumed 1000 kJ per session in the form of 2 fresh pretzels (less than 48 h old).
Healthy adults age 18–45 yrs with no wheat or soy allergies were eligible. Pregnant women were excluded. Participants were randomly assigned to one of two groups; one group consumed the soy pretzel on day 1 while the other group consumed the wheat pretzel on day 1. After an overnight fast (10–12 h) and immediately before breakfast, the participants were instructed to report their state of hunger on a 10 cm visual analog scale (VAS) by placing a vertical line on the scale. The scale was flanked by “Extremely hungry” on the left and “Extremely full” on the right.26 The participant then consumed either the wheat or the soy pretzel, as instructed by the study designer. The participants were instructed to eat the pretzel as is, without any alterations such as heating or toasting or additions including salt or mustard. Participants reported satiety on congruent VASs at 15 min, 30 min, 1 h, 1.5 h, and 2 h after intake. During the 2 h period they refrained from eating and drinking with the exception of water with intakes recorded. The participants were allowed to eat or drink ad libitum for the rest of the day, although alcohol was discouraged. That night, the participants again fasted for 10–12 h and repeated the procedure the following morning for the other type of pretzel (soy or wheat). For analysis, the distance was measured between the left side of the scale and their vertical line. The data were then normalized by setting the baseline measurement at “0 mm” and the resulting values were plotted vs. time. Using the trapezoid rule, the AUC was calculated for the area above the baseline.25 Both the satiety declarations at each time point and the AUC of satiety vs. time were used in the statistical assessment.
Statistics
Differences between the soy and wheat soft pretzels were calculated for acceptability, GI, II, satiety values (AUCs), and water consumed during the satiety experiment using a paired, two-tailed, Student’s t-test using Microsoft® Office Excel® 2007 (Microsoft Corp., Redmond, WA). Individual time points of glycemic and insulinemic indices were analyzed using analysis of variance (ANOVA) with the model Y = Type + Participant + Time + Type × Time where Y = glycemia (mg/dl) or insulinemia (µIU/ml); Type = soy or wheat; Participant is 1–12; and Time = 0, 15, 30, 45, 60, 90, or 120 min. SAS statistical software was utilized (SAS 9.2 TS2M0, SAS Institute, Inc., Cary, NC). Individual satiety scores at each time point were evaluated using using Minitab statistical software (Minitab Inc., State College, PA) with the model Y = Time + Type + Particpant where Y = satiety; Participant = 1–20; and Type and Time are the same as above. Statistical significance was deemed at p < 0.05.
Results
No adverse effects were observed for any of the participants during the studies.
Sensory analysis
Fifty-one volunteers (19 male, 32 female) between the ages of 18 and 40 [mean (±SD) age = 26.6 (±6.3) yrs] completed sensory analysis. The mean (±SD) acceptability of the soy-based soft pretzel was 6.6 (±1.1) on a 9-point hedonic scale and 6.7 (±1.2) for the wheat pretzel (p = 0.59). These ratings fall between “slightly like” and “moderately like”.
Study 1 – glycemic and insulinemic indices
Thirteen participants were screened and 100% were eligible. One 29 year old male dropped out before the study began for personal reasons. Six recruits were male and 6 were female; 1 was East Asian and 11 were Caucasian. The age range was 19–33 years; mean (±SD) age was 23.8 (±4.5) years.
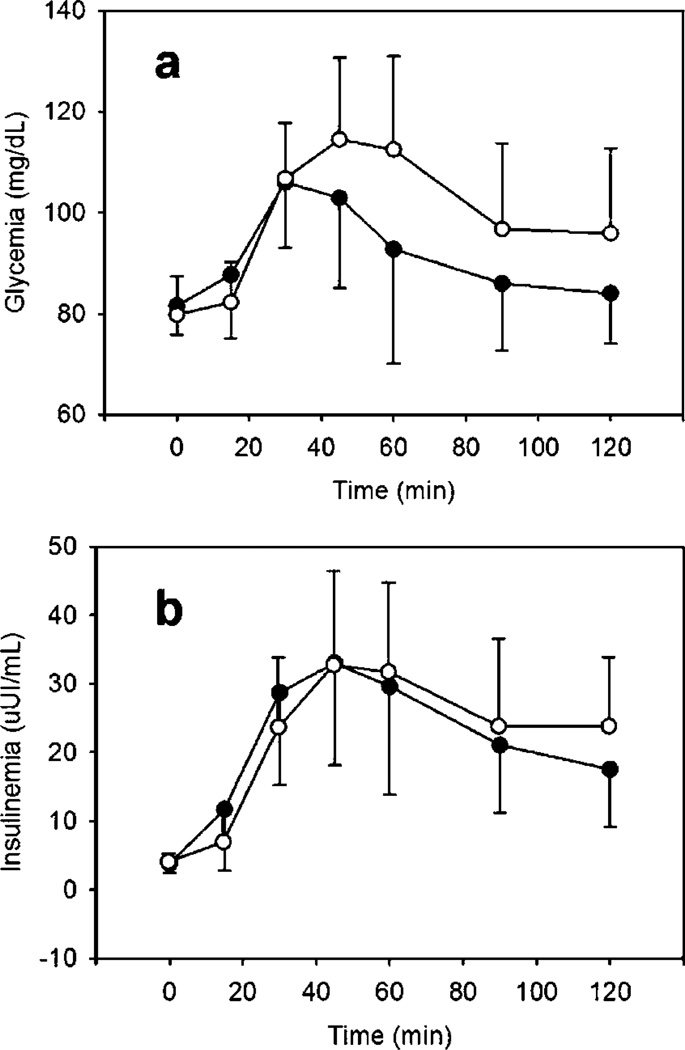
Blood glucose and insulin values at each of the assessment timepoints are presented in Fig. 1 and Table 3. The GI was calculated by measuring the AUC of the glycemic response compared to glucose. The mean (±SD) GI for the wheat pretzel was 66.4 (±15.3) vs. 39.1 (±20.4) for the soy pretzel (p = 0.002). The AUC for glycemia after soy pretzel consumption was lower than that post-wheat consumption for 10 out of 11 of the participants.
Fig. 1.
Postprandial glycemia (a) and insulinemia (b) vs. time averaged across 12 participants (● = soy, ○ = wheat). Error bars represent the standard deviation of participant distribution.
Table 3.
The numerical values for postprandial glycemia and insulinemia vs. time in Study 1, n = 12 participants. An asterisk (*) next to the soy glycemia value indicates statistical differences between soy and wheat values for a given time point. There were no significant differences between any of the insulinemia values for any given time point
| Glycemia (mg/dL) |
Insulinemia (µUI/mL) |
|||
|---|---|---|---|---|
| Soy | Wheat | Soy | Wheat | |
| Time (min) | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| 0 | 81.6 ± 5.8 | 79.8 ± 7.6 | 3.8 ± 1.4 | 4.3 ± 1.2 |
| 15 | 87.7 ± 12.6 | 82.3 ± 7.9 | 11.7 ± 8.9 | 7.2 ± 4.9 |
| 30 | 106.0 ± 13.0 | 106.8 ± 11.0 | 28.7 ± 13.5 | 23.9 ± 10.1 |
| 45 | 102.9 ± 17.8* | 114.6 ± 16.2 | 33.1 ± 15.0 | 32.9 ± 13.6 |
| 60 | 92.8 ± 22.6* | 112.5 ± 18.5 | 29.6 ± 15.8 | 31.9 ± 12.9 |
| 90 | 86.0 ± 13.4* | 96.7 ± 17.1 | 21.1 ± 9.8 | 24.0 ± 12.8 |
| 120 | 84.1 ± 10.0* | 95.8 ± 16.9 | 17.5 ± 8.4 | 24.0 ± 10.1 |
To confirm the reliability of this protocol, the GI for white bread was calculated. The calculated mean (±SD) GI for white bread was 60.4 ± 19.8, which is consistent with average GI (75 ± 2) reported by Atkinson et al.27
The AUC for insulinemia vs. time was calculated for the test foods and compared to that of the glucose standard. As a methods confirmation, white bread resulted in a mean (±SD) II of 62.8 (±18.9), consistent with reported value of 69 (±24) from Oku et al.28 Mean (±SD) II for the wheat pretzel was 79.0 (±22.6) vs. 75.0 (±19.6) for the soy pretzel (p = 0.44).
Study 2 – satiety
Twenty participants, 8 males and 12 females, age 20–43 years [mean (±SD) age = 25.3 (±6.4) years] were recruited for the satiety study. All screened applicants were eligible and 100% of participants completed the study.
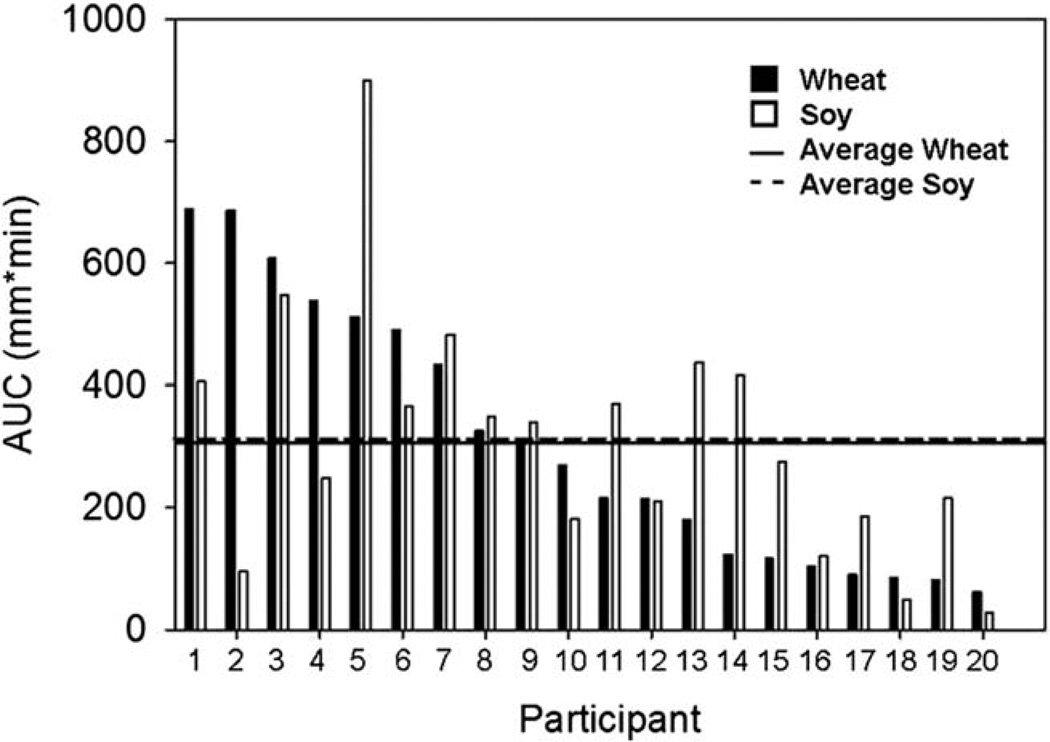
In order to assess the relative satiety levels for each of the pretzels, participants consumed 1000 kJ (239 kcal) of energy from pretzels for breakfast. The mean (±SD) satiety score (AUC) was 306.2 (±215.0) cm × min for wheat vs. 311.3 (±201.0) cm × min for the soy pretzel (p = 0.92). Moreover, the mean (±SD) amount of water consumed was similar for the 2 h after the consumption of both pretzels [342 (±273) mL for wheat vs. 336 (±319) mL for soy; p = 0.91].
Discussion
Current snack food formulations such as those for cookies, crackers, and hard pretzels tend to have high GIs, yet all but 3 of the 64 soy-based foods from the GI database are “low GI” (≤55).21,27 Despite this observation, there are no reported studies that examine the effect of soy substitution alone on the GI or II of bakery products or snack foods. Therefore, a soft pretzel enriched with soy was produced as a model bakery product or snack food.
In this study, consumer acceptability of the soy-based soft pretzel was compared to the traditional soft pretzel. Both pretzels were rated between “slightly like” and “moderately like” indicating that the soy pretzel is a consumer-acceptable substitute for the traditional soft pretzel. In contrast, Tsen reported that bread with more than 11% soy flour was unacceptable according to American bread quality standards, likely due to the denser, more moist crumb and darker color.14 However, Dhingra and Jood formulated an acceptable bread with 10% in an Indian population. 29 In accord with our results, Sabanis and Tzia utilized soy milk powder to incorporate up to 20% soy ingredients into bread while maintaining favorable sensory attributes in a Greek population.30 By incorporating approximately 1/3 of the soy ingredient as soy milk powder in this study, textural detriments were partly circumvented while maintaining high protein content. This is likely due to the higher ratio of soluble fiber : insoluble fiber in the soy milk powder compared to defatted soy flour.16 In addition, a chewy texture and darker brown color (imparted by the lye bath), while generally not acceptable in bread, is regarded as a positive quality in soft pretzels by consumers.
In the glycemic/insulinemic index study (Study 1), both pretzels were composed of 50 g available carbohydrates. But, because the soy pretzel formulation contained about 20% less starch per gram, each soy pretzel contained an additional 61.3 kcal, 1.8 g fat, 3.3 g fiber, and 9.1 g protein compared to the wheat pretzel (Table 2). Despite the elevation in these nutrients, the soy soft pretzel, composed of 27.3% soy ingredients, decreased the GI of a wheat-based soft pretzel. The standard deviations of our indices are higher than those that have generally been reported (Fig. 1), perhaps because we used venous blood instead of arterial or capillary blood in the interest of participant comfort;25 we had 100% compliance among those who began the study.
Reduced glycemia can arise from reduced rate of glucose introduction to the blood, increased rate of glucose uptake by tissues, or both.31 It is possible that at least the former mechanism is involved in the reduced glycemic response to the soy pretzel. The larger amount of total food in the stomach with the soy pretzel may have slowed the transit time from stomach to small intestine, reducing the rate of carbohydrate availability for absorption. The addition of protein has been shown to slow the rate of gastric emptying and, consequently, reduce postprandial glycemia in glucose/gelatin-based beverages.32 The insulin secretogenetic properties of protein may also have contributed to the lower postprandial glycemic response. Insulinemia has been shown to be higher after a meal with a whey protein pre-load, leading to attenuated post-prandial glycemia.33 Increased insulin sensitivity has been observed with longer soy interventions.34
Fiber can slow the rate of carbohydrate absorption in the small intestine, which can lead to reduced glycemic and/or insulinemic indices.35,36 Stachyose and raffinose are insoluble fibers that are present in defatted soy flour at about 1.4–4.1% and 0.1–1.2%, respectively.37 Both soy flour and soy milk powder contain about 2.5–3.0% soluble fiber but soy milk powder has considerably less insoluble fiber (14.2% vs. 21.3% for soy flour).16 Soluble fibers are more often associated with increasing the viscosity of the digesta, but stachyose and raffinose have also been shown specifically to slow the rate and extent of digestion.38 Goñi and Valentín-Gamazo similarly observed a reduction in GI when they supplemented spaghetti with 25% chickpea flour, attributing their observation partly to an increase in indigestable compounds (including non-starch polysaccharides) from the chickpea flour.39 This mechanism may in part contribute to the observed phenomena with soy addition to the soft pretzels.
Despite the attenuated glycemic response, the rate of insulin secretion did not significantly differ between ingestion of either pretzel. Although the carbohydrate concentration is diluted in the chyme, protein and lipid also stimulate glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) which stimulate the release of insulin. This finding is consistent with the observation that high protein foods, such as lentils, elicit insulin responses greater than that predicted from the glycemic response.19 Veldhorst et al. similarly showed that increasing soy protein concentration in a custard matrix increased insulinemia but not GI.7 However, Pereira et al. showed that habitual fibrous diets can increase insulin sensitivity, in effect attenuating blood glucose levels after a meal.40 In this study, because of the heterogeneity of the macronutrient composition in the pretzel matrix, we cannot correlate glycemia and insulinemia.41
In the satiety study (Study 2), soy and wheat pretzels containing 239 kilocalories (1000 kJ) were consumed. Despite the soy pretzel having 5.2 more grams of protein and 2.2 more grams of fiber than the wheat pretzel, there was no statistical difference in the feeling of satiety (Fig. 2). The large variation in satiety scores stems from the subjectivity of the assessment. To account for this, we used a cross-over design so that we could compare the satiety values directly from each individual. A pattern was not observed between satiety declarations after consumption of the wheat or soy pretzel. There were likely counteracting factors that led to this observation. Protein both stimulates the release of cholecystokinin (CCK), which inhibits gastric emptying.42 The increase in fiber can increase chyme viscosity, which leads to slowed gastric emptying and/or an increase in thirst which expands the stomach, leading to release of CCK, thus increasing satiety.6,43 However, the wheat pretzels were larger in appearance due to the facilitated formation of air cells from higher gluten concentrations, and may have subconsciously increased satiety.44 A study that includes a larger number of participants and can control for physical activity, food intake on days before the experiment, alcohol consumption, and sleep amount and quality may be able to detect subtle differences, if there are indeed any, between the satiety of these snack foods.
Fig. 2.
Satiety values for the participants as measured by AUC for satiety score vs. time relative to the baseline. Participants are ordered in descending order for wheat satiety values.
In order to control for the initial hunger level of the participants, the satiety study was designed so that the soy pretzel was consumed as the first meal of the day (breakfast) rather than as a snack. It has been estimated that university students, who comprised the majority of this participant pool, consume approximately 15–18% of their total energy intake from breakfast.45 Assuming a 2000 kcal diet, the 249 kcal soy pretzel might have been less food than their normal breakfast, resulting in insatiety for both pretzel varieties. Hence, future studies investigating satiety of a soy snack food in between meals or as a more appropriately sized breakfast may avoid this complication.
Due to the water-binding abilities of soy protein46 and the potential for the indigestible carbohydrates to increase chyme viscosity,43 we hypothesized that the soy pretzel would lead to a higher consumption of water that may contribute to increased satiety. However, the amount of water consumed was similar with the consumption of both pretzels.
Conclusion
The addition of 27.2% soy ingredients to a soft pretzel snack food can significantly decrease the GI without affecting consumer acceptability or satiety. These results show that it may be possible to supplement a variety of snack foods with soy at high enough quantities to achieve lower postprandial glycemia while maintaining favorable sensory characteristics.
References
- 1.Piernas C, Popkin BM. Health Affairs. 2010;29:398–404. doi: 10.1377/hlthaff.2009.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler DM, Glaeser EL, Shapiro JM. J. Econ. Perspect. 2003;17:93–118. [Google Scholar]
- 3.United States Department of Agriculture. Report of the dietary guidelines advisory committee on the dietary guidelines for Americans. 2010;(D5):11–15. [Google Scholar]
- 4.Xiao CW. J. Nutr. 2008;138:1244S–1249S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YC, Albrecht D, Bomser J, Schwartz SJ, Vodovotz YM. J. Agric. Food Chem. 2003;51:7611–7616. doi: 10.1021/jf034679c. [DOI] [PubMed] [Google Scholar]
- 6.Pupovac J, Anderson GH. J. Nutr. 2002;132:2775–2780. doi: 10.1093/jn/132.9.2775. [DOI] [PubMed] [Google Scholar]
- 7.Veldhorst MAB, Nieuwenhuizen AG, Westerterp KR, Engelen MPKJ, Brummer R-JM, Deutz NEP, Westerterp-Plentenga MS. Eur. J. Nutr. 2009;48:92–100. doi: 10.1007/s00394-008-0767-y. [DOI] [PubMed] [Google Scholar]
- 8.Holt SHA, Brand-Miller JC, Petocz P, Farmakalidis E. Eur. J. Clin. Nutr. 1995;49:675–690. [PubMed] [Google Scholar]
- 9.Erdman JW. Circulation. 2000;102:2555–2559. doi: 10.1161/01.cir.102.20.2555. [DOI] [PubMed] [Google Scholar]
- 10.Young VR. J. Am. Diet. Assoc. 1991;91:828–835. [PubMed] [Google Scholar]
- 11.Slavin J. J. Am. Diet. Assoc. 1991;91:816–819. [PubMed] [Google Scholar]
- 12.Birt DF, Hendrich S, Wang W. Pharmacol. Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 13.Wu AH, Yu MC, Tseng C-C, Pike MC. Br. J. Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsen CC. J. Am. Oil Chem. Soc. 1974;51:81–83. doi: 10.1007/BF00000018. [DOI] [PubMed] [Google Scholar]
- 15.Vittadini E, Vodovotz Y. J. Food Sci. 2003;68:2022–2027. [Google Scholar]
- 16.Nilufer D, Boyacioglu D, Vodovotz Y. J. Food Sci. 2008;73:C275–C281. doi: 10.1111/j.1750-3841.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 17.Ribotta PD, Arnulphi SA, León AE, Añón MC. J. Sci. Food Agric. 2005;85:1889–1896. [Google Scholar]
- 18.Jenkins DJA, Wolever TMS, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Am. J. Clin. Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 19.Holt SHA, Miller JCB, Petocz P. Am. J. Clin. Nutr. 1997;66:1264–1276. doi: 10.1093/ajcn/66.5.1264. [DOI] [PubMed] [Google Scholar]
- 20.Walsh KR, Zhang YC, Vodovotz Y, Schwartz SJ, Failla ML. J. Agric. Food Chem. 2003;51:4603–4609. doi: 10.1021/jf0342627. [DOI] [PubMed] [Google Scholar]
- 21.Brand-Miller JC, Holt SHA, Pawlak DB, McMillan J. Am. J. Clin. Nutr. 2002;76:281S–285S. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- 22.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willet WC. JAMA J. Am. Med. Assoc. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 23.Sieri S, Krogh V, Berrino F, Evangelista A, Agnoli C, Brighenti F, Pellegrini N, Palli D, Masala G, Sacerdote C, Veglia F, Tumino R, Frasca G, Grioni S, Pala V, Mattiello A, Chiodini P, Panico S. Arch. Intern. Med. 2010;170:640–647. doi: 10.1001/archinternmed.2010.15. [DOI] [PubMed] [Google Scholar]
- 24.Jahns L, Siega-Riz AM, Popkin BM. J. Pediatr. 2001;138:493–498. doi: 10.1067/mpd.2001.112162. [DOI] [PubMed] [Google Scholar]
- 25.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TMS. Nutr. Res. Rev. 2005;18:145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- 26.Flint A, Raben A, Blundell JE, Astrup A. Int. J. Obes. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson FS, Foster-Powell K, Brand-Miller JC. Am. J. Clin. Nutr. 2008;31:2218–2220. [Google Scholar]
- 28.Oku T, Nakamura M, Nakamura S. Int. J. Diabetes Mellit. 2010;2:88–94. [Google Scholar]
- 29.Dhingra S, Jood S. Int. J. Food Sci. Technol. 2004;39:213– 222. [Google Scholar]
- 30.Sabanis D, Tzia C. Food Bioprocess Technol. 2009;2:68–79. [Google Scholar]
- 31.Schenk S, Davidson CJ, Zderic TW, Byerley LO, Coyle EF. Am. J. Clin. Nutr. 2003;78:742–748. doi: 10.1093/ajcn/78.4.742. [DOI] [PubMed] [Google Scholar]
- 32.Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, Jones KL, Horowitz M, Rayner CK. Am. J. Clin. Nutr. 2007;86:1364–1368. doi: 10.1093/ajcn/86.5.1364. [DOI] [PubMed] [Google Scholar]
- 33.Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Cliton PM, Horowitz M, Rayner CK. Diabetes Care. 2009;32:1600–1602. doi: 10.2337/dc09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascencio C, Torres N, Isoard-Acosta F, Gómez-Pérez FJ, Hernández-Pando R, Tovar AR. J. Nutr. 2004;134:522–529. doi: 10.1093/jn/134.3.522. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins DJ, Wolever TMS, Leeds AR, Gassull MA, Haisman P, Dilawari J, Goff DV, Metz GL, Alberti KGMM. Br. Med. J. 1978;1:1392–1394. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papathanasopoulos A, Camilleri M. Gastroenterology. 2010;138:65–72. doi: 10.1053/j.gastro.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bainy E, Tosh S, Corredig M, Poysa V, Woodrow L. Carbohydr. Polym. 2008;72:664–672. [Google Scholar]
- 38.Choct M, McLeish J, Peisker M. Asian Austral. J. Anim. Sci. 2010;23:1386–1398. [Google Scholar]
- 39.Goñi I, Valentín-Gamazo C. Food Chem. 2003;81:511–515. [Google Scholar]
- 40.Pereira MA, Jacobs DR, Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Am. J. Clin. Nutr. 2002;75:848–855. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 41.Björck I, Liljeberg H, Östman E. Br. J. Nutr. 2000;83:149–155. doi: 10.1017/s0007114500001094. [DOI] [PubMed] [Google Scholar]
- 42.Debas HT, Farooq O, Grossman MI. Gastroenterology. 1975;68:1211–1217. [PubMed] [Google Scholar]
- 43.Eastwood MA. Annu. Rev. Nutr. 1992;12:19–35. doi: 10.1146/annurev.nu.12.070192.000315. [DOI] [PubMed] [Google Scholar]
- 44.Rolls BJ. Nutr. Rev. 1986:4493–4101. [Google Scholar]
- 45.Soriano J, Moltó JC, Maňnes J. Nutr. Res. 2000;20:1249–1258. [Google Scholar]
- 46.Kinsella JE. J. Am. Oil Chem. Soc. 1979;56:242–258. doi: 10.1007/BF02671544. [DOI] [PubMed] [Google Scholar]