Abstract
Transforming growth factor (TGF)-β upregulates plasminogen activator inhibitor type 1 (PAI-1) in a variety of cell types, and PAI-1 is considered to be an essential factor for the development of fibrosis. Our previous studies demonstrated that TGF-β decreased intracellular glutathione (GSH) content in murine embryonic fibroblasts (NIH/3T3 cells), whereas treatment of the cells with GSH, which restored intracellular GSH concentration, inhibited TGF-β-induced collagen accumulation by blocking PAI-1 expression and enhancing collagen degradation. In the present study, we demonstrate that GSH blocks TGF-β-induced PAI-1 promoter activity in NIH/3T3 cells, which is associated with an inhibition of TGF-β-induced JNK and p38 phosphorylation. Interestingly, although exogenous GSH does not affect phosphorylation and/or nuclear translocation of Smad2/3 and Smad4, it completely eliminates TGF-β-induced binding of transcription factors to not only AP-1 and SP-1 but also Smad cis elements in the PAI-1 promoter. Decoy oligonucleotides (ODN) studies further demonstrate that AP-1, SP-1, and Smad ODNs abrogate the inhibitory effect of GSH on TGF-β-induced PAI-1 promoter activity and inhibit TGF-β-induced expression of endogenous PAI-1. Furthermore, we show that GSH reduces TGF-β-stimulated reactive oxygen species (ROS) signal. Blocking ROS production with diphenyleneiodonium or scavenging ROS with a superoxide dismutase and catalase mimetic MnTBaP dramatically reduces TGF-β-induced p38 and JNK phosphorylation as well as PAI-1 gene expression. In composite, these findings suggest that GSH inhibits TGF-β-stimulated PAI-1 expression in fibroblasts by blocking the JNK/p38 pathway, probably by reducing ROS, which leads to an inhibition of the binding of transcription factors to the AP-1, SP-1, and Smad cis elements in the PAI-1 promoter.
Keywords: transforming growth factor-β, plasminogen activator inhibitor-1, mitogen-activated protein kinase
Coordinated control of the production and turnover of extracellular matrix (ECM) components is crucial for normal tissue homeostasis. Fibrotic disease occurs when normal control of this process is compromised and excess fibrous material accumulates in the tissues (27). Fibrogenesis occurs in most tissues, suggesting that a common pathway(s) may mediate this response (27). Although little is known about specific mechanisms, altered expression of transforming growth factor (TGF)-β has been strongly correlated with fibrosis (5, 6, 28, 36). TGF-β induces fibrosis by promoting net matrix protein accumulation through increasing ECM production and inhibiting ECM degradation. Type I plasminogen activator inhibitor (PAI-1), a physiological inhibitor of plasminogen activators, inhibits protease-dependent fibrinolytic activity and subsequent ECM degradation. PAI-1 expression is consistently and dramatically upregulated in a variety of fibrotic diseases as well as in experimental animal models such as carbon tetrachloride-induced liver fibrosis (48) and bleomycin-induced pulmonary fibrosis (30). It has also been reported that bleomycin induces a more severe lung fibrosis in transgenic mice overexpressing PAI-1 but comparatively less severe fibrosis in PAI-1-deficient mice (12), suggesting an important role of PAI-1 in the development of fibrosis.
Our previous studies demonstrated that TGF-β decreased intracellular concentration of glutathione (GSH), the most abundant intracellular free thiol and an important antioxidant, and stimulated collagen accumulation in murine embryonic fibroblasts (NIH/3T3 cells). Restoration of intracellular GSH levels with N-acetyl cysteine (NAC), glutathione ester, or GSH inhibited TGF-β-induced collagen accumulation (26). Furthermore, we showed that GSH inhibited TGF-β-induced collagen accumulation by blocking TGF-β-induced PAI-1 expression and thus stimulating collagen degradation (42). Because of the importance of PAI-1 as a major downstream effecter of TGF-β-induced fibrosis, defining the molecular mechanism whereby GSH inhibits TGF-β-initiated PAI-1 expression may lead to the identification of new therapeutic targets for the fibrotic diseases associated with an elevated PAI-1 expression.
The Smad pathway mediates the induction of many TGF-β responsive genes including PAI-1. TGF-β signaling through the Smad pathway has been well described in the past. Following the binding of TGF-β to the type II receptor, the type I receptor is phosphorylated, which further phosphorylates Smad2 or Smad3. Phosphorylated Smad2 and Smad3 form a heteromeric complex with Smad4, which translocates to the nucleus where the complex can directly or indirectly regulate gene transcription by interacting with other transcription factors. Increasing evidence indicates that, in addition to the Smad pathway, other pathways, including mitogen-activated protein kinase (MAPK) pathways, are also important in TGF-β signaling. Importantly, several recent studies have demonstrated that MAPKs are involved in TGF-β-induced PAI-1 expression in renal epithelial cells (24), smooth muscle cells (33), endothelial cells (44), and mesangial cells (15). Whether GSH inhibits TGF-β-induced PAI-1 gene expression by blocking Smad, MAPK, and/or other signaling pathways remains to be determined.
In the present study, we show that GSH reduces TGF-β-stimulated reactive oxygen species (ROS) signal in NIH/3T3 cells and blocks TGF-β-induced JNK and p38 phosphorylation, associated with an inhibition of TGF-β-induced PAI-1 promoter activity. Most interestingly, we show that although GSH has no effect on the phosphorylation and nuclear translocation of Smad proteins, it suppresses the binding of the transcription factors to activator protein (AP)-1, specificity protein (SP)-1, and Smad cis elements in the PAI-1 promoter. Furthermore, we show that AP-1, SP-1, and Smad decoy oligonucleotides (ODN) abrogate the inhibitory effect of GSH on TGF-β-induced PAI-1 promoter activity and inhibit TGF-β-induced expression of endogenous PAI-1 gene. These results suggest that GSH inhibits TGF-β-induced PAI-1 gene expression in part by blocking JNK and p38 pathways, probably by reducing ROS, and the subsequent binding of the transcription factors to AP-1, SP-1, and Smad cis elements in the PAI-1 promoter.
MATERIALS AND METHODS
Cell culture and treatment
Mouse embryonic fibroblast NIH/3T3 cells were originally obtained from American Type Culture Collection (Manassas, VA). Mv1Lu cells (kindly provided by Dr. Daniel Rifkin, New York University) are mink lung epithelial cells stably transfected with a luciferase reporter gene driven by 800 bp of human PAI-1 promoter (1). Cells were routinely cultured in DMEM (Cellgro, Herndon, VA) containing 4 mM L-glutamine, 1.5 g/l sodium bicarbonate, and 4.5 g/l glucose supplemented with 10% (vol/vol) FBS (Hyclone, Logan, UT), 100 U/ml penicillin, and 100 µg/ml streptomycin (GIBCO BRL) in a humidified atmosphere with 5% CO2 at 37°C.
Plasmids
The deletion constructs of PAI-1 were kindly provided by Dr. David Luskotoff (The Scripps Research Institute, La Jolla, CA) (41). All the fragments generated had an identical 3′ end EcoRI site at position +71 and with different 5′ ends. p800luc was generated from the HindIII site at position −800, p549luc from the SphI site at position −549, and p187luc from the RsaI site at position −189 of human PAI-1 promoter and subcloned into vector p19LUC. pRL-TK-luciferase expressing Renilla luciferase was from Promega (Madison, Wisconsin). The dominant negative (DN) JNK and DN p38 constructs as well as the empty vectors were generated as described previously by Yeo et al. (45).
Transient transfection and reporter gene assays
NIH/3T3 cells were plated into 24-well culture plates (1 × 105 cells/well) and cotransfected with p800Luc (luciferase) reporter constructs (0.4 µg/ well) and pRL-TK-luciferase (Renilla luciferase, used to normalize the transfection efficiency; 2.5 ng/well) using Lipofectamine reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. In experiments with DN, cells were transfected with an equal amount (1 µg) of control empty vector (pIRES2-EGFP for p38; pCDNA3 for JNK), DN-p38, or DN-JNK1 plus pRL-TK-luciferase with (for promoter activity analysis) or without (for determination of endogenous PAI-1 expression) reporter construct p800Luc (1 µg). After transfection, the cells were carefully washed three times with culture media and treated with GSH or TGF-β (1 ng/ml) with or without GSH (5 mM) for various periods of time as indicated in the figure legends. Luciferase activity (firefly luciferase and Renilla luciferase) in the cell lysates were evaluated using the Dual Luciferase Reporter Assay System (Promega) and normalized based on transfection controls in each experiment. The expression of endogenous PAI-1 was determined by measuring PAI-1 antigen in the medium by ELISA.
To inhibit the binding of transcription factor to specific cis elements in the PAI-1 promoter, double-stranded decoy ODN for AP-1 (5′-GGAACATGAGTTCATCTATTTC-3′, P-Box), SP1-1 (5′-GT-GGGTGGGGCTGGAACAT-3′), and Smad (5′-GGGAGAGACAGACACAGGCAG-3′) were synthesized commercially (Invitrogen) based on the published human PAI-1 promoter sequence (7, 8, 10, 21, 22, 41). Complementary strands were annealed by heating equimolar mixtures of positive and negative strands in 10 mM Tris·HCl, pH 8.0, 200 mM NaCl, and 1 mM EDTA at 95°C for 5 min, followed by cooling to room temperature over a period of 1 h. Transfections were performed in 24 wells as described above. Each decoy was used at a final concentration of 312 nM in transfection studies.
Preparation of cell lysates and isolation of nuclear fractions
After the incubation period, the cells were washed twice with PBS and lysed with 200 µl of ice-cold lysis buffer for 30 min on ice. The lysates were collected by scraping cells and passing them through a 22 G needle several times to shear chromosomal DNA and to reduce the viscosity of the lysates. Unbroken cell debris were removed by centrifugation at 15,000 g for 30 min. The supernatants were collected, and protein content was measured using the BCA protein assay reagent kit (Pierce, Rockford, IL).
The nuclear fractions were isolated as described previously (11). Briefly, cells were washed twice with ice-cold PBS, scraped off into cold 0.75 ml of PBS, and packed by brief centrifugation (14,000 g for 1 min at 4°C). The pellets were resuspended in 400 µl of hypotonic buffer (10 mM HEPES, pH 7.9, 0.5% Igepal, 2 mM MgCl2, 10 mM KCl, 0.1 mM EDTA, 1.0 mM DTT, 0.5 mM PMSF, 1.0 µg/ml leupeptin, 1.0 µg/ml aprotinin) in a microfuge tube and incubated for 10 min on ice. Cell suspensions were packed again at 14,000 g for 1 min at 4°C, and the pellets incubated on ice for 30 min with 100 µl of high-salt buffer (50 mM HEPES, pH 7.9, 300 mM NaCl, 50 mM KCl, 0.1 mM EDTA, 1.0 mM DTT, 0.5 mM PMSF). After centrifugation at 14,000 g for 5 min, the supernatants were collected, and protein content was measured using BCA protein assay reagent kit and stored immediately at − 80°C until use.
Western blot analysis
Cell lysates or nuclear fractions equivalent to 20–50 µg of protein were resolved on a SDS-PAGE gel. The separated proteins were electrophoretically transferred onto a PVDF membrane and blocked with 5% nonfat dry milk. The membranes were probed overnight with specific antibodies to phospho-ERK (1:500), ERK (1:1,000), phospho-p38 (1:500), p38 (1:1,000), phos-pho-JNK (1:500), JNK (1:1,000), Smad4 (1:500), Smad2/3 (1:500), phospho-Smad2/3 (1:1,000), or TATA binding protein, a loading control for nuclear protein, and then with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h after washing. The protein bands were visualized by chemiluminescence using the ECL detection system (Amersham, Piscataway, NY). The bands were analyzed semiquantitatively using QuantityOne imaging software (Bio-Rad, Hercules, CA). All the antibodies except phospho-Smad2/3 (Cell Signaling Technology, Beverly, CA) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Isolation of RNA and northern blot analysis
Total cellular RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. RNA (20 µg) was separated on 1.2% agarose gels containing 5% (vol/vol) formaldehyde. The RNA was transferred overnight to nylon membranes and UV-crosslinked to the membranes. The blots were hybridized using a standard protocol as we have described previously (26) with cDNA probes labeled with [32P] by random priming (Invitrogen). The blots were probed consecutively with a human PAI-1 and β-actin cDNAs. After hybridization, the membranes were washed with 2× SSC/0.1% SDS solution and then 0.1× SSC/0.1% SDS at 65°C. Membranes were scanned, and radioactivity was quantitated using an InstantImager (Packard).
Total PAI-1 ELISA
A total PAI-1 ELISA assay was developed in our laboratory based on a previously described method with modifications (3). Plates were coated with 100 µl of capture antibody (10 µg/ml; cat. no. H34G6; Molecular Innovations, Southfield, MI) diluted in 25 mM carbonate buffer (pH 9.4) and incubated overnight at 4°C. The wells were then blocked with 250 µl of blocking buffer (TBS containing 3% BSA Fraction V; Fisher Scientific, Pittsburgh, PA) by incubating at 37°C for 30 min and then washed three times with 300 µl of wash buffer (TBS containing 0.05% Tween 20). PAI-1 standards (cat. no. MPAI-I91L, Molecular Innovations) ranging from 0 to 5,000 pg/ml were used in the assay. Samples and standards (100 µl), diluted in the blocking buffer, were added to each well and incubated at 25°C for 30 min with vigorous shaking (500 rpm). The wells were washed and then incubated with 100 µl of 1:5,000 diluted rabbit polyclonal anti-mouse PAI-1 antibody (cat. no. ASMPAI-GF, Molecular Innovations) at 25°C for 30 min. After washing, 100 µl of HRP-conjugated anti-rabbit antibody (1:10,000, Santa Cruz Biotechnology) was added to each well, and the plate was incubated for another 30 min at room temperature. One-hundred microliters of TMB ready-to-use substrate (Sigma, St. Louis, MO) was added to each well for color development. The enzyme reaction was stopped after 5 min with 50 µl of 1 N H2SO4, and the absorbance was measured at 450 nm. The results were calculated based on the standard curve run under the same conditions.
Measurement of intracellular ROS
After treatment, the cells were washed with PBS and then incubated with 10 µM 2′,7′-dichlorodi-hydrofluorescein diacetate (DCFDA) in the dark with rotation for 30 min. After further washing, fluorescence was measured, and multiple images were captured using a Nikon TE2000E2 microscope. The intensity of fluorescent staining (green) due to oxidation of DCFDA was quantified using the imaging software. The average fluorescence intensity from four independent samples for each treatment is presented.
EMSA
Nuclear protein extracts were prepared for EMSA as described earlier (11). The protein content of the nuclear extract was determined using the BCA kit (Pierce). The ODNs for AP-1 (P-Box), SP1-1, and Smad (the same as used in transfection experiments) were end-labeled with [γ-32P]ATP using T4 polynucleotide kinase as recommended by the manufacturer (Promega). Nuclear proteins (2 µg) from control and treated cells were incubated with 32P-labeled ODN probe under binding conditions for 20 min at room temperature in a final volume of 20 µl. Ten times non-radiolabeled (cold) ODNs were used for competition studies to reveal the specificity of the binding. After binding, protein-DNA complexes were electrophoresed on a native 4.0% polyacrylamide gel using 0.5× TBE buffer. The gel was then dried and scanned, and signals were quantified using InstantImager (Packard).
Statistical analysis
Data were presented as means ± SE and were evaluated by one-way ANOVA. Statistical significance was determined post hoc by Tukey’s test wherein P < 0.05 was considered significant.
RESULTS
GSH blocks TGF-β-induced PAI-1 promoter activity
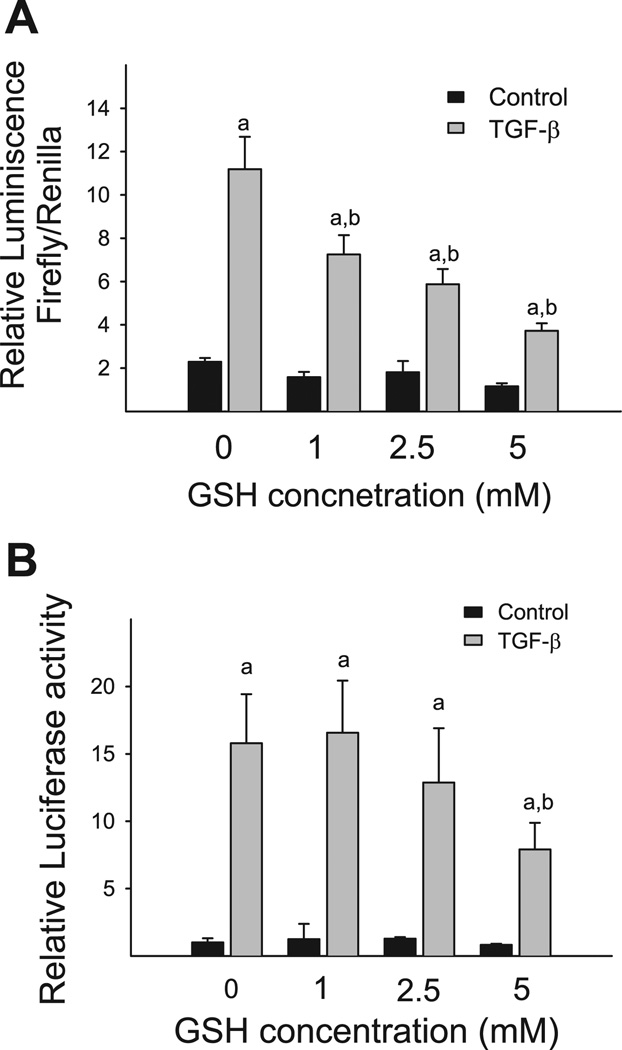
In the previous studies, we have demonstrated that GSH selectively blocked TGF-β-induced PAI-1 expression at both mRNA and protein levels in NIH/3T3 cells without affecting the autocrine function of TGF-β or TGF-β-induced collagen mRNA expression (42). To evaluate whether the inhibition of PAI-1 expression by GSH occurred at the transcriptional level, we studied the effect of GSH on TGF-β-induced PAI-1 promoter activity using p800luc reporter construct that contains 800 bp of the proximal promoter sequences of human PAI-1 gene (Fig. 1). As shown in Fig. 2A, 1 ng/ml TGF-β increases the luciferase activity by 7.5-fold compared with the unstimulated cells after normalized by transfection control. Importantly, treatment of the cells with exogenous GSH causes a concentration-dependent inhibition of PAI-1 promoter activity induced by TGF-β (Fig. 2A). To confirm this finding, Mv1Lu cells, a mink lung epithelial cell line stably transfected with luciferase reporter gene driven by the same 800 bp of human PAI-1 promoter sequence, were treated with 1 ng/ml TGF-β in the presence or absence of GSH. The luciferase activity in the cell lysate was measured 24 h after treatment. The results show that 5 mM GSH also dramatically reduces TGF-β-induced luciferase activity in Mv1Lu cells, although 1 or 2.5 mM GSH have no significant effect (Fig. 2B). GSH under the concentrations used does not cause obvious cytotoxicity based on the cell number and morphology study (data not shown). Collectively, these findings indicate that exogenous GSH inhibits TGF-β-induced PAI-1 expression by blocking transcription of PAI-1 gene.
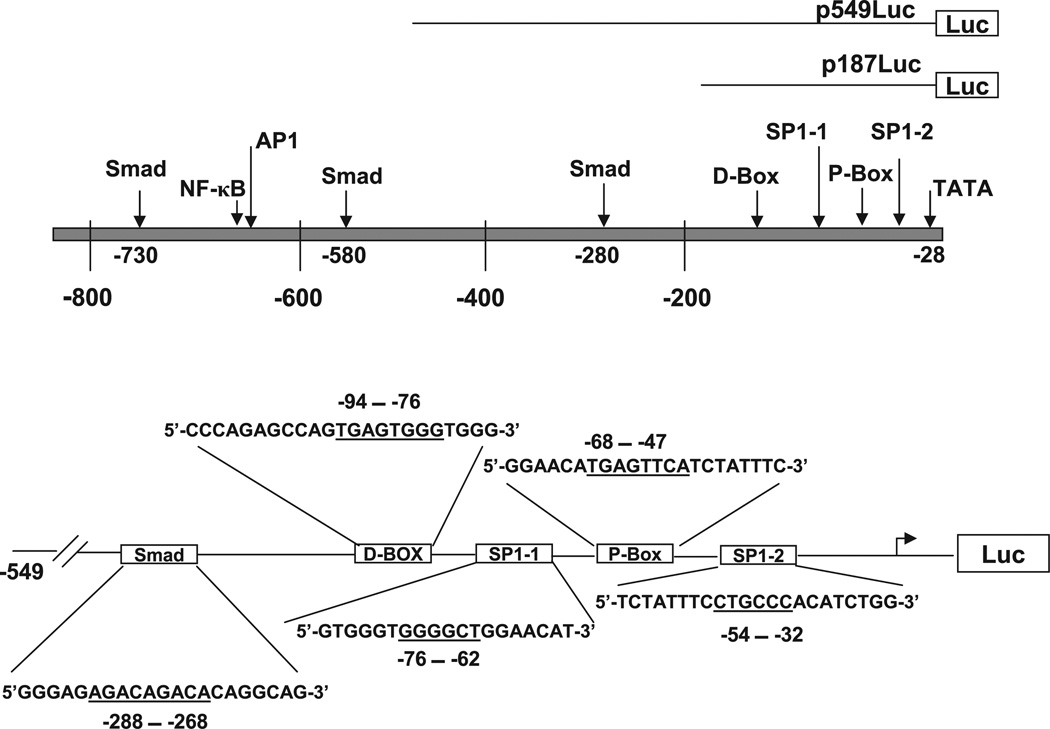
Fig. 1.
Schematic representation of the promoter region of human plasminogen activator inhibitor type 1 (PAI-1) gene. The top represents the promoter region up to − 800 bp. The arrows indicate the transcription factor binding sites in the promoter region, based on the published sequences (7, 8, 10, 21, 22, 32). D-Box and P-Box contain AP-1-like cis elements. The bottom illustrates major transcription factor binding sites in p549Luc construct. The boxes represent the locations of the transcription factor binding sites, and the sequences are the decoy oligonucleotides (ODNs) that are used in the study. The underlined sequences represent the binding sites of transcription factors (22).
Fig. 2.
Glutathione (GSH) inhibits transforming growth factor (TGF)-β-induced PAI-1 expression at the transcriptional level. A: NIH/3T3 cells were transiently cotransfected with p800luc reporter gene construct or p19luc control construct and pRL-TK-luciferase (transfection control) and then stimulated with TGF-β (1 ng/ml) for 24 h in the presence or absence of GSH (1–5 mM). B: Mv1Lu cells were treated with 1 ng/ml TGF-β with or without GSH (1–5 mM). The luciferase activity was measured 24 h after TGF-β treatment and normalized with transfection control as described in materials and methods. a, Significantly different from the corresponding TGF-β untreated control; b, significantly different from TGF-β-alone group (P < 0.05, n = 3).
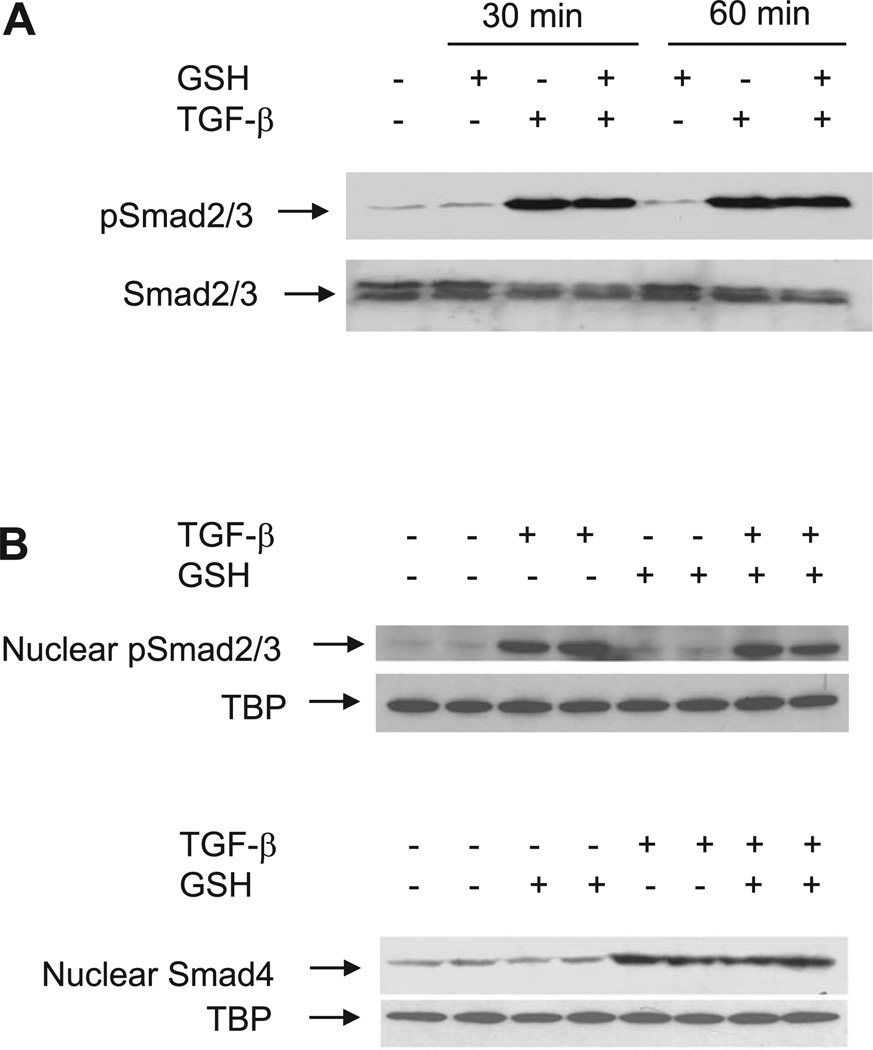
GSH has no effect on TGF-β-induced Smad2/3 phosphorylation or Smad2/3/4 nuclear translocation
Smads are the major signaling molecules that mediate TGF-β’s cellular responses including PAI-1 gene expression. Therefore, we explored the effect of GSH on Smad signaling components in TGF-β-stimulated NIH/3T3 cells. The results show that within 10 min of TGF-β treatment, there is a significant increase in the phosphorylation of Smad2/3 and the nuclear translocation of phosphorylated Smad2/3 and Smad4 (data not shown), which persists until the end of the experiment (60 min) (Fig. 3). Importantly, GSH treatment has no significant effect on the TGF-β-induced Smad2/3 phosphorylation or nuclear translocation of phosphorylated Smad2/3 or Smad4 (Fig. 3A and Fig. 3B).
Fig. 3.
GSH has no effect on TGF-β-induced Smad phosphorylation and/or nuclear translocation. A: effect of GSH on TGF-β-induced phosphorylation of Smad2/3. B: effect of GSH on TGF-β-induced nuclear translocation of pSmad2/3 and Smad4. NIH/3T3 cells were treated with TGF-β (1 ng/ml) in the presence or absence of GSH (5 mM). The cells were collected at different time points as indicated. Cell lysates and nuclear extracts were prepared, and equal amounts of cell lysates or nuclear extract (50 µg/lane) were subjected to SDS-PAGE. Western blotting was performed using specific antibodies as described in materials and methods. TATA binding protein (TBP) was used as a loading control for nuclear proteins.
JNK and p38 MAPKs mediate TGF-β-induced PAI-1 expression, whereas GSH blocks TGF-β-induced JNK and p38 MAPK phosphorylation
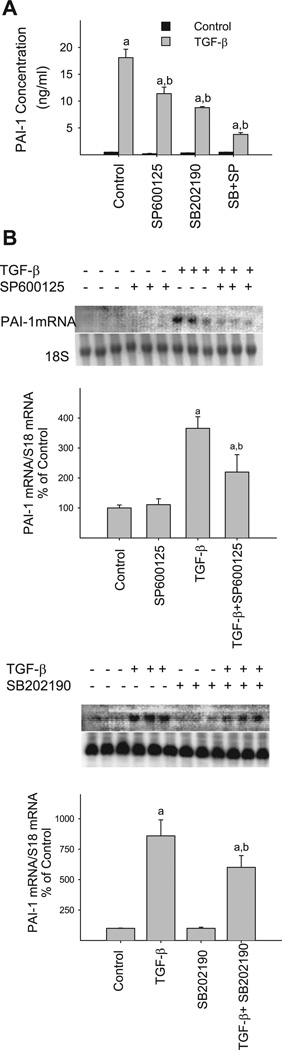
In addition to Smad pathway, three members of the MAPK family, ERK, JNK, and p38, are also implicated in TGF-β-induced gene expression. To know whether MAP kinases are involved in TGF-β-stimulated PAI-1 gene expression in our cell model, we examined the effects of pharmacological inhibitors for p38 (SB-202190) and JNK (SP-600125) on TGF-β-induced PAI-1 protein and mRNA expression in NIH/3T3 cells (TGF-β has no effect on ERK phosphorylation, see below). The results show that incubation of the cells with either p38 or JNK inhibitor significantly reduces TGF-β-stimulated PAI-1 protein (Fig. 4A) and mRNA (Fig. 4B) expression. Neither JNK nor p38 inhibitor at the concentration used caused obvious cytotoxicity based on the cell morphology and number analyses (data not shown). These data indicate that both p38 and JNK pathways play a significant role in TGF-β-induced PAI-1 expression in our cell model.
Fig. 4.
Pharmacological inhibitors of JNK and p38 MAPKs block TGF-β-induced PAI-1 expression. 70–80% Confluent, serum-starved NIH/3T3 cells were treated with 1 ng/ml TGF-β in the presence or absence of p38 inhibitor SB-202190 (3 µM) or JNK inhibitor SP-600125 (10 µM) for 24 h. The medium was collected for PAI-1 protein analysis by ELISA (A) while the cells were collected for PAI-1 mRNA measurement by Northern analysis (B) as described in materials and methods. a, Significantly different from the corresponding TGF-β untreated control; b, significantly different from TGF-β-alone group (P < 0.05, n = 3–4).
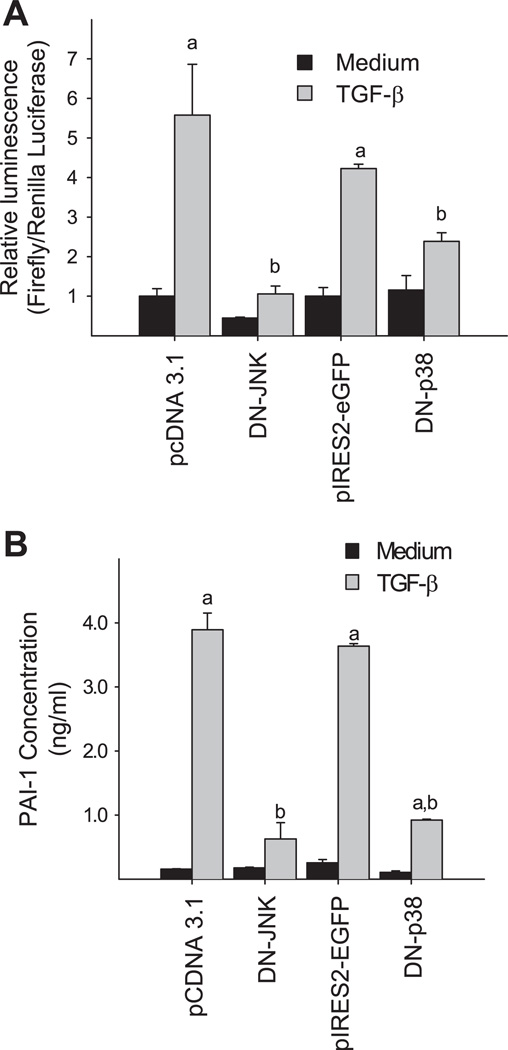
To further validate the role of MAPKs in the induction of PAI-1 by TGF-β, we tested whether DN-p38 and/or DN-JNK could block TGF-β-induced PAI-1 promoter activity or endogenous PAI-1 expression. NIH/3T3 cells were cotransfected with p800luc and the expression vectors encoding DN-p38 or DN-JNK or control vectors and then treated with TGF-β. The luciferase activity was measured in the cell lysates while PAI-1 protein concentration was determined in the medium 24 h after TGF-β treatment. The data show that overexpressing DN-p38 or DN-JNK markedly inhibits TGF-β-induced PAI-1 promoter activity (Fig. 5A) and blocks the endogenous PAI-1 expression induced by TGF-β (Fig. 5B). Together, our data indicate that p38 and JNK signaling pathways are important in the induction of PAI-1 expression by TGF-β in NIH/3T3 cells.
Fig. 5.
Dominant negative (DN) mutants of p38 or JNK inhibit TGF-β-induced PAI-1 expression. A: DN-p38 and DN-JNK block TGF-β-induced p800luc promoter activity. NIH/3T3 cells were cotransfected with the reporter gene and DN constructs or corresponding vector (pCDNA3.1 for DN-JNK and pIRES2-EGFP for DN-p38) as indicated and then treated with TGF-β (1 ng/ml). The luciferase activity was measured in the cell lysates 24 h after treatment. Renilla luciferase was used to normalize the transfection efficiency. B: DN-p38 and DN-JNK block TGF-β-induced endogenous PAI-1 expression. The experimental conditions were the same as those described for A. The PAI-1 was detected in the media by ELISA as described in materials and methods. a, Significantly different from the corresponding TGF-β untreated control; b, significantly different from the corresponding vector-transfected and TGF-β-treated group (P < 0.05, n = 4–6).
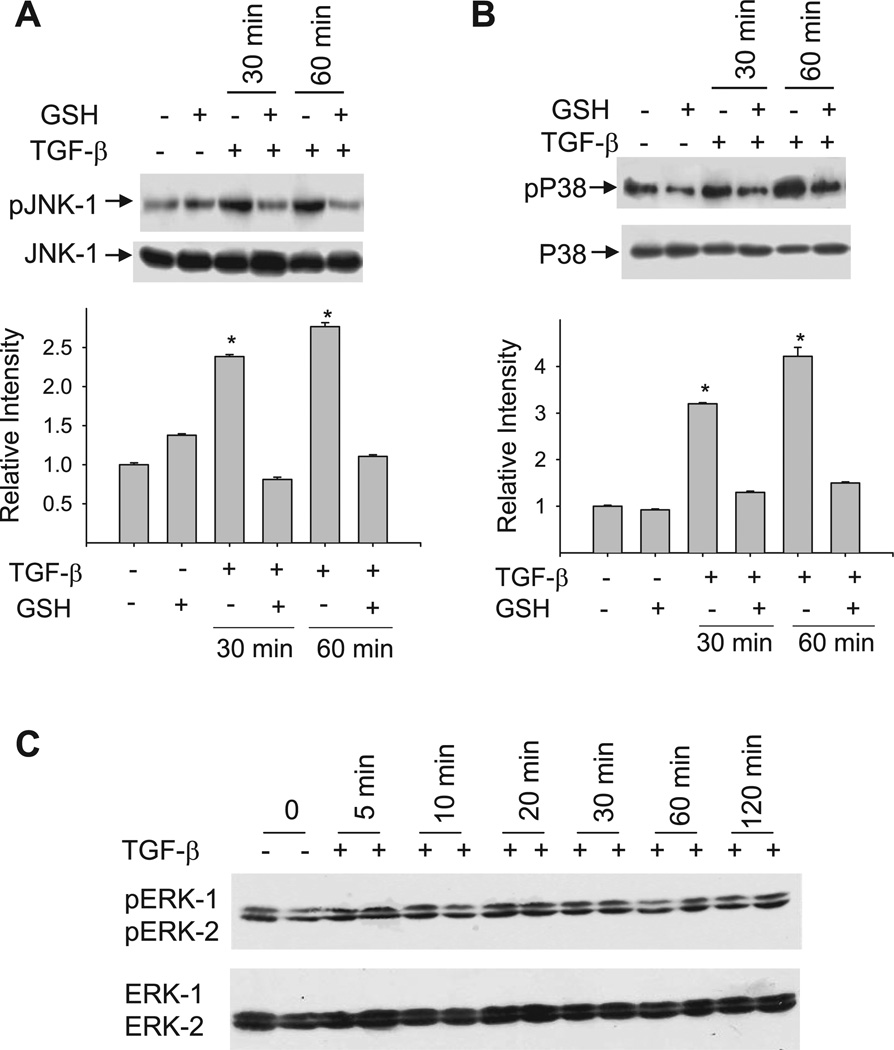
To determine whether GSH inhibits TGF-β-induced PAI-1 expression by blocking MAPK pathways, effects of GSH on the phosphorylation of JNK, p38, and ERK MAPKs in TGF-β-treated NIH/3T3 cells were examined by Western blot using phospho- and non-phosphorylated p38, ERK, and JNK antibodies. As shown in Fig. 6, TGF-β increases the phosphorylation of both JNK (Fig. 6A) and p38 MAPK (Fig. 6B) but has no effect on ERK phosphorylation up to 120 min (Fig. 6C). Importantly, treatment of NIH/3T3 cells with GSH completely blocks TGF-β-stimulated phosphorylation of p38 and JNK MAPKs (Fig. 6A and B).
Fig. 6.
GSH blocks TGF-β-induced phosphorylation of JNK and p38 MAPKs. Serum-starved cells were incubated with TGF-β in the presence or absence of GSH (5 mM). Cell lysates were prepared at different time points as indicated. Equal amounts of protein (50 µg/lane) were fractionated on SDS-PAGE gel and probed with specific antibodies for the phosphorylated or unphosphorylated JNK (A), p38 (B), or ERK (C). *Significantly different from untreated control (P < 0.05, n = 3).
ROS mediate TGF-β activation of JNK/p38 pathway and induction of PAI-1, whereas GSH attenuates ROS signal in TGF-β treated fibroblasts
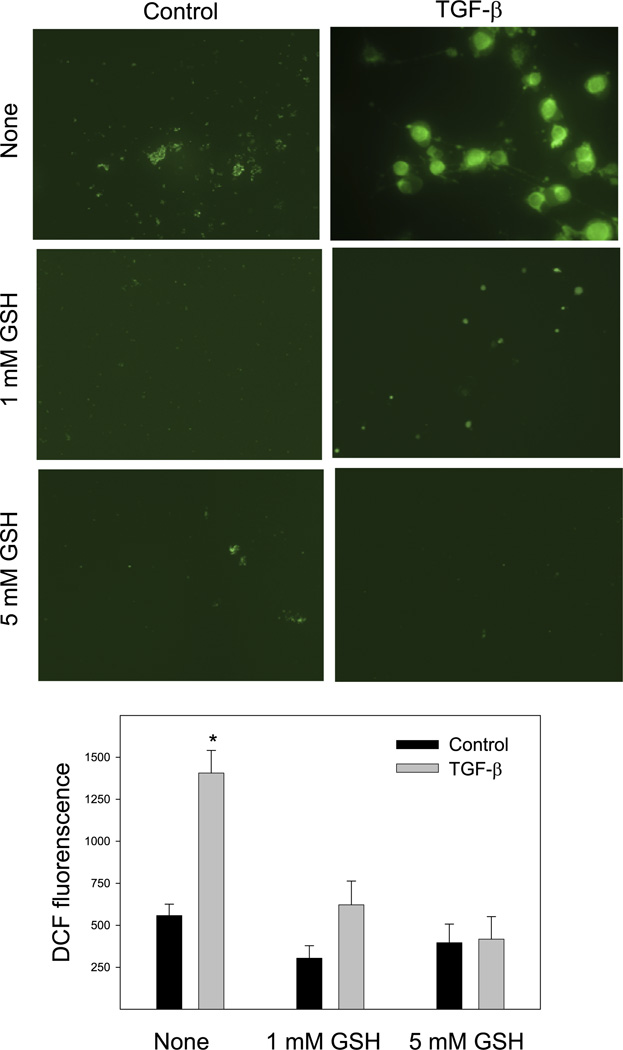
TGF-β has been shown to increase ROS production through NAD(P)H oxidases, and ROS mediate the induction of many TGF-β-responsive genes. Our previous studies also showed that TGF-β increased superoxide production in NIH/3T3 cells (26). To further elucidate whether GSH blocks MAPK activation and PAI-1 induction in TGF-β-treated fibroblasts by reducing ROS signal, the effect of exogenous GSH on TGF-β-stimulated ROS production was studied using the redox-sensitive fluorescence dye DCFDA. The results show that treatment of NIH/3T3 cells with 1 ng/ml TGF-β for 1 h leads to a significant increase in the fluorescence intensity in the cells, suggesting an increased ROS level. Both 1 and 5 mM GSH almost completely block TGF-β-induced fluorescence signal, although GSH by itself has no significant effect on the basal level of ROS (Fig. 7).
Fig. 7.
GSH attenuates TGF-β-induced reactive oxygen species signals in fibroblasts. NIH/3T3 cells were treated with 1 ng/ml TGF-β in the presence or absence of 1 or 5 mM GSH in a serum-free medium for 1 h. After being washed, the cells were incubated with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) in the dark with rotation for 30 min and then washed with warm PBS twice. Fluorescence was measured, and multiple images were captured using a Nikon TE2000E2 microscope. The intensity of fluorescent staining (green) due to oxidation of DCFDA was quantified using the imaging software. A representative image from each treatment group is presented in the top panel, whereas the average fluorescence intensity from 4 independent samples for each treatment is presented in the bottom panel. *Significantly different from untreated control (n = 4, P < 0.05).
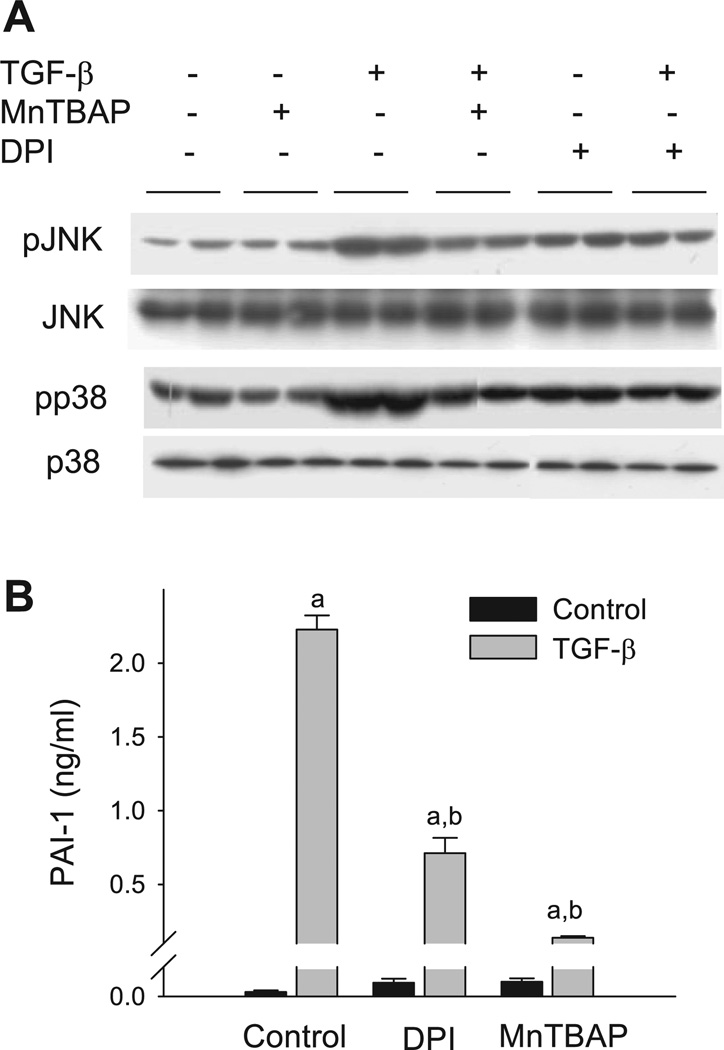
To determine whether ROS are involved in the activation of p38 and JNK pathways and subsequent induction of PAI-1 by TGF-β, we examined the effect of diphenyleneiodonium (DPI), which inhibits NAD(P)H oxidase and other flanoproteins, or MnTBaP, a mimetic of catalase and superoxide dismutase, on TGF-β-induced PAI-1 protein expression by ELISA and the phosphorylation of p38 and JNK by Western blotting. The results show that MnTBaP alone has no significant effect on the basal level of p38 or JNK phosphorylation, although DPI slightly increases the phosphorylation. Importantly, phosphorylation of p38 and JNK induced by TGF-β is substantially inhibited by DPI or MnTBaP (Fig. 8A). In parallel with the effect on JNK and p38 phosphorylation, DPI and MnTBaP also significantly reduce TGF-β-stimulated PAI-1 protein expression (Fig. 8B). Neither DPI nor MnTBaP at the concentration used causes obvious toxicity to the cells based on the cell morphology and cell number analysis (data not shown). Together, the data suggest that ROS are involved in TGF-β-induced p38 and JNK activation and PAI-1 expression and that GSH blocks TGF-β-induced MAPK activation and PAI-1 expression probably by reducing cellular ROS levels.
Fig. 8.
Diphenyleneiodonium (DPI) and MnTBaP inhibit TGF-β-induced MAPK phosphorylation and PAI-1 expression. A: DPI and MnTBaP inhibit TGF-β-induced p38 and JNK phosphorylation. 70–80% Confluent NIH/3T3 cells were treated with TGF-β (1.0 ng/ml) in the presence or absence of DPI (2 µM) or MnTBaP (50 µM) for 30 min. The cell lysates were prepared and analyzed by Western blot using specific antibodies for phosphorylated or unphosphorylated p38 and JNK. B: DPI and MnTBaP inhibit TGF-β-induced PAI-1 expression. The cells were pretreated with DPI (2 µM) for 2 h and then with TGF-β (1.0 ng/ml) in the absence of DPI for 24 h or the cells were treated with TGF-β (1.0 ng/ml) in the presence or absence of MnTBaP (50 µM) for 24 h. The media were collected to analyze the PAI-1 protein by ELISA. a, Significantly different from the corresponding TGF-β untreated control; b, significantly different from TGF-β-alone treated group (P < 0.05, n = 4).
AP-1, SP-1, and Smad decoy ODN abrogate the inhibitory effect of GSH on TGF-β-induced PAI-1 promoter activity and inhibit the expression of endogenous PAI-1 gene induced by TGF-β
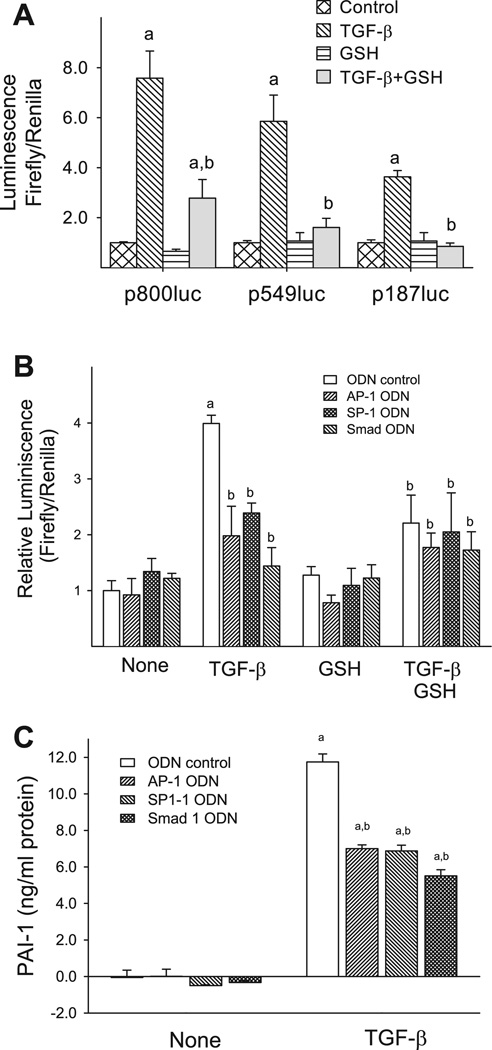
Both human and mouse PAI-1 promoter contains multiple transcription factor binding sites such as Smad binding elements, AP-1, SP-1, and NF-κB binding sites. These sites have been shown to be responsive to different stimuli. To identify the cis acting elements in the PAI-1 gene promoter that mediate the inhibitory effects of GSH, transient transfection was performed with p800Luc, a luciferase reporter construct containing the major cis regulatory elements from the promoter region of human PAI-1 gene (41), and two deletion constructs, p549luc and p187luc (see Fig. 1, top, for the detailed structures of these constructs). The luciferase activity was then analyzed in the cells treated with TGF-β in the presence or absence of GSH. As shown in Fig. 9A, TGF-β treatment increases the luciferase activity in the cells transfected with p800Luc as well as the deletion constructs. The degree of the induction is proportional to the length of the promoter region present in the constructs. Importantly, treatment of cells with GSH significantly reduces TGF-β-induced luciferase activity in all three constructs used. It is interesting to note that GSH almost completely inhibits TGF-β-induced luciferase activity in p549Luc- or p187Luc-transfected cells but only partially in p800Luc-transfected cells. These data suggest that GSH inhibits TGF-β-induced PAI-1 expression by interfering with cis element(s) that are presented in these two short constructs.
Fig. 9.
AP-1, SP-1, or Smad decoy ODN abrogates GSH inhibitory effect on TGF-β-induced PAI-1 promoter activity and inhibits TGF-β-induced PAI-1 expression. A: NIH/3T3 cells were cotransfected with luciferase reporter constructs driven by 187 bp, 549 bp, or 800 bp of human PAI-1 promoter region, as shown in Fig. 1, and pRL-TK Renilla luciferase reporter construct (transfection control). After transfection, the cells were treated with TGF-β in the presence or absence of GSH (5 mM) for 24 h. The luciferase activities (firefly luciferase and Renilla luciferase) in the cell lysates were determined using the Dual Luciferase Reporter Assay System (Promega), and the results were normalized based on transfection controls in each experiment. a, Significantly different from the corresponding (transfected with the same reporter construct) untreated control; b, significantly different from the corresponding TGF-β-alone treated cells (P < 0.05, n = 5–9). B: NIH/3T3 cells were transfected with p800luc reporter construct and pRL-TK-luciferase construct (transfection control) as well as specific ODN for AP-1, SP-1, or Smad (final concentration 315 nM) as indicated and then stimulated with TGF-β (1 ng/ml) for 24 h in the presence or absence of 5 mM GSH. The luciferase activities in cell lysates were measured as described above. a, Significantly different from the untreated ODN control group (bar 1); b, significantly different from TGF-β-treated ODN control group (bar 5) (P < 0.05, n = 5–9). C: NIH/3T3 cells were transfected with AP-1, SP-1, or Smad ODN at a final concentration of 315 nM and then treated with 1 ng/ml TGF-β in a serum-free medium for 24 h. The conditioned media were collected, and PAI-1 protein was determined by ELISA. a, Significantly different from the untreated control group; b, significantly different from TGF-β-alone treated group (P < 0.05, n = 4).
To further identify the cis element(s) in PAI-1 promoter that mediates GSH effect, we examined the effects of decoy ODNs corresponding to AP-1-like (P-Box), SP1-1, and Smad elements present in these two short constructs (see materials and methods as well as Fig. 1 for detailed information of the sequences) on TGF-β-induced PAI-1 promoter activity in the presence or absence of GSH. NIH/3T3 cells were cotransfected with p800Luc plasmid and SP1–1, AP-1-like (P-Box), or Smad ODN and then treated with TGF-β with or without GSH. The results show that all three ODNs significantly inhibit TGF-β-induced luciferase activity (Fig. 9B). Importantly, the degree of inhibition of TGF-β-induced p800Luc activity by SP-1 ODN, AP-1 ODN, or Smad ODN alone is comparable to that caused by the ODN plus GSH. In other words, addition of GSH does not further inhibit TGF-β-induced p800Luc activity in the presence of the ODNs. The results suggest that GSH inhibits TGF-β-induced PAI-1 promoter activity by blocking the binding of transcription factors to SP-1-like, AP-1-like, and Smad cis elements in the PAI-1 promoter even though GSH has no effect on TGF-β-induced phosphorylation and/or nuclear translocation of Smad2/3/4.
The luciferase reporter constructs used in this study are driven by human PAI-1 promoter. To confirm that TGF-β induces PAI-1 expression in NIH/3T3 cells through increasing the binding of the transcription factors to AP-1, SP-1, and Smad cis elements, effects of AP-1, SP-1, and Smad ODNs on TGF-β-induced expression of endogenous PAI-1 were studied. The results show that treatment of NIH/3T3 cells with 1 ng/ml TGF-β for 24 h increases PAI-1 protein concentration in the medium 12-fold, whereas AP-1, SP-1, and Smad ODNs significantly reduce TGF-β-induced PAI-1 expression by 40–50% (Fig. 9C). These data suggest that TGF-β induces the binding of the transcription factors to these cis elements in PAI-1 promoter, which mediate the induction of PAI-1 gene in NIH/3T3 cells.
GSH blocks TGF-β-induced binding of transcription factors to SP-1, AP-1, and Smad elements in PAI-1 promoter
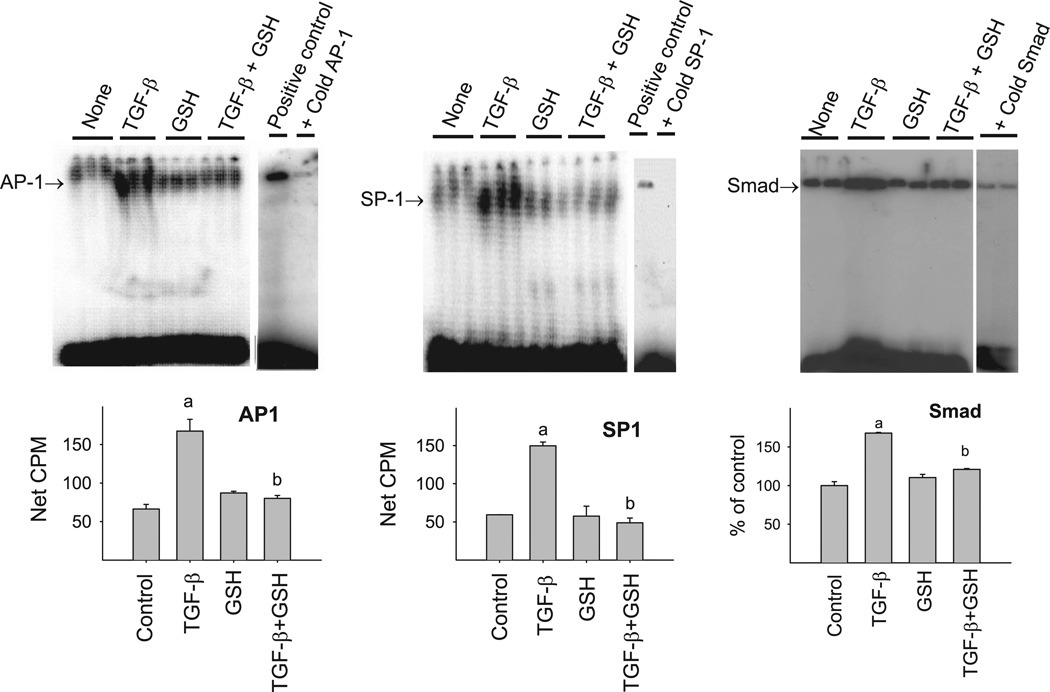
To further confirm the involvement of AP-1, SP-1, and Smad sites in the inhibitory effect of GSH on TGF-β-induced PAI-1 expression, EMSA was performed using the same ODNs that were used for the decoy experiments. As shown in Fig. 10, treatment of NIH/3T3 cells with TGF-β enhances the binding of transcription factors to AP-1 (left), SP-1 (middle), and Smad (right) ODNs, which was blocked by the corresponding cold ODNs, indicating the specificity of the binding. Importantly, GSH completely blocks the TGF-β-induced binding of transcription factors to these cis elements. These data further suggest that GSH inhibits TGF-β-induced PAI-1 promoter activity by blocking the binding of transcription factors to AP-1, SP-1, and Smad sites in the PAI-1 promoter region and therefore the transcription of the gene.
Fig. 10.
GSH inhibits TGF-β-induced binding of transcription factors to AP-1, SP-1, and Smad cis elements in PAI-1 promoter. The ODNs used for PAI-1 promoter decoy studies were end-labeled with [γ-32P]ATP and incubated with nuclear extracts from NIH/3T3 cells treated with TGF-β with or without GSH for 30 min. The protein DNA complexes were then electrophoresed on native PAGE as described in materials and methods. Tenfold non-radiolabeled (cold) ODNs were used to confirm the specificity of the binding. a, Significantly different from the untreated control; b, significantly different from TGF-β-treated group (P < 0.05, n = 3–4).
DISCUSSION
PAI-1 is of particular interest because its expression is elevated in variety of fibrotic disorders. TGF-β, a predominant fibrotic cytokine, is a major inducer of PAI-1. Therefore, defining the signaling pathways that mediate GSH inhibition of TGF-β-induced PAI-1 expression reported in our previous study could further reveal the molecular mechanism underlying GSH’s antifibrotic effects. The major new finding from the present study is that GSH inhibits TGF-β-induced PAI-1 expression by blocking JNK and p38 MAPK phosphorylation, probably by reducing ROS, which leads to an inhibition of the binding of transcription factors to AP-1, SP-1, and Smad cis elements in PAI-1 promoter and thereby PAI-1 gene transcription. This conclusion is supported by the following results: 1) pharmacological inhibitors and DN mutants of p38 and JNK block TGF-β-induced PAI-1 expression, suggesting that JNK and p38 pathways are involved in the TGF-β-induced PAI-1 expression in our cell model; 2) GSH inhibits TGF-β-stimu-lated phosphorylation of p38 and JNK; 3) GSH attenuates TGF-β-induced ROS signal, whereas DPI and MnTBaP, which reduce ROS by different mechanisms, block TGF-β-induced p38/JNK phosphorylation and PAI-1 expression; 4) GSH inhibits TGF-β-induced binding of transcription factors to AP-1, SP-1, and Smad cis elements in the PAI-1 promoter, although it has no effect on the phosphorylation and/or nuclear translocation of Smad2/3 and Smad4; 5) AP-1, SP-1, and Smad decoy ODN inhibit TGF-β-induced endogenous PAI-1 gene expression and abrogate GSH inhibitory effects on TGF-β-induced PAI-1 promoter activity.
The Smad signaling pathway is critical for the induction of many TGF-β-responsive genes including PAI-1. Upon the binding of TGF-β to the membrane receptor, TGF-β signaling is transduced to the nucleus by a series of events involving phosphorylation of Smad2/3 and nuclear translocation of Smad2/3 and Smad4 complex. Interestingly, we show, in the present study, that GSH has no effect on the phosphorylation of Smad2/3 or nuclear translocation of Smad2/3 and Smad4; however, it completely blocks the binding of transcription factors to the sequences containing Smad binding elements in human PAI-1 promoter. Decoy ODN studies further show that Smad ODN abrogates the inhibitory effect of GSH on TGF-β-induced PAI-1 promoter activity and inhibits TGF-β induction of endogenous PAI-1 gene expression. These data suggest that GSH inhibits TGF-β/Smad signal transduction by interfering with Smad DNA binding rather than the phosphorylation or nuclear translocation of Smad proteins. It should be mentioned that, although similar, the Smad binding site identified in the mouse PAI-1 promoter is only 44% identical to that identified in human PAI-1 promoter. Therefore, the conclusion is not exclusive, and the possibility of the mouse Smad binding site being regulated differently from the human sequence cannot be ruled out.
The MAPKs are a major signaling system used by eukaryotic cells to transduce extracellular signals to intracellular responses (35, 43). Studies have shown that MAPK pathways are also involved in the regulation of TGF-β-induced PAI-1 expression in various cell types with different MAPK pathways functioning in different cells (15, 33, 44). In mesangial cells, both JNK and ERK phosphorylation are essential for the induction of PAI-1 by TGF-β (15), whereas in smooth muscle cells, ERK phosphorylation plays a major role for TGF-β-induced PAI-1 expression (33). On the other hand, MEK and JNK have been shown to be important in mediating TGF-β-induced PAI-1 expression in endothelial cells (44). In this study, we show that TGF-β stimulates the phosphorylation of JNK and p38 but not ERK in NIH/3T3 cells (Fig. 6). Using specific inhibitors and DN, we further demonstrate that the phosphorylation of JNK and p38 MAPKs is required for PAI-1 induction by TGF-β. Importantly, we have shown that GSH almost completely blocks TGF-β-stimulated phosphorylation of JNK and p38 in the same concentration that significantly inhibited TGF-β-induced PAI-1 promoter activity (Fig. 2) and PAI-1 mRNA/protein expression (26). These data suggest that inhibition of JNK/p38 pathway may underlie the suppression of TGF-β-induced PAI-1 gene expression by GSH.
Both human and mouse PAI-1 promoters near the TATA box contain AP-1-like and SP-1-like binding sites (7, 31). It has been reported that the c-Jun homodimers and c-Jun/c-Fos heterodimers, MAPK-activated transcription factors, bind to these elements to mediate TGF-β responses (21). In this study, we show that GSH blocks TGF-β-induced binding of transcription factors to AP-1 and SP-1 cis elements in human PAI-1 promoter, whereas AP-1 and SP-1 ODNs abrogate GSH inhibitory effect on TGF-β-induced PAI-1 promoter activity and reduce TGF-β-induced expression of endogenous PAI-1 gene. Together, these data further suggest that inhibition of JNK/p38 pathway signaling, which leads to an inhibition of the binding of transcription factors to the AP-1 and SP-1 cis elements in PAI-1 promoter, may underlie the suppression of TGF-β-induced PAI-1 gene transcription by GSH.
MAPKs interact with Smad signaling in different ways. In addition to affecting phosphorylation and nuclear translocation of Smad proteins (20, 23, 29), MAPKs also interact with Smad proteins and therefore modulate their DNA binding activity (17, 18, 34, 46). It has been reported that MAPK-activated transcription factors such as c-Jun and c-Fos interact with Smad complexes and facilitate their DNA binding (18). It has also been reported that the transcriptional activation of PAI-1 gene by Smad is mediated through the AP-1 binding sites in the PAI-1 promoter (46). ATF-2 is a downstream substrate of both JNK and p38 pathways. It has been shown that ATF-2 participates in transcription complexes in association with Smad proteins (34). Furthermore, it has been shown that TGF-β upregulates PAI-1 gene expression by activating SP-1-dependent transcription through the induction of Smad/SP-1 complex formation (17). Using decoy ODNs and EMSA techniques, we demonstrate in this study that GSH blocks TGF-β-induced binding of transcription factors to not only AP-1 and SP-1 but also Smad cis elements in PAI-1 promoter although it has no effect on the phosphorylation and nuclear translocation of Smad2/3/4. Based on these results and the results from other laboratories, it is speculated that AP-1 and SP-1 may be involved in the binding of Smad to its cis elements in PAI-1 promoter and that GSH blocks TGF-β-Smad signal transduction by inhibiting the binding of these cofactors to Smad proteins and thereby the binding of Smad proteins to DNA. Nevertheless, additional studies will be needed to confirm our hypothesis.
GSH, a tripeptide, is the most abundant intracellular free thiol and plays a critical role in regulating a variety of cellular functions including detoxification of xenobiotics, synthesis of DNA and other endogenous compounds, and regulation of gene expression and the cell cycle. However, the most important and well-known function of GSH is antioxidant. GSH can directly scavenge ROS or reduce H2O2 and lipid peroxides through glutathione peroxidase and/or glutathione S-trans-ferase-catalyzed reactions. GSH can also reduce oxidized protein cysteine residues through glutaredoxin-mediated reactions. The molecular mechanism whereby GSH modulates TGF-β signaling is not entirely understood at the moment. As TGF-β increases ROS production and ROS mediate the induction of PAI-1 by different stimuli (13, 19, 25, 37, 49), one potential mechanism whereby GSH inhibits TGF-β-induced PAI-1 expression would be reducing ROS. Indeed, we have shown in this study that GSH decreases DCFDA fluorescence (ROS) signal in TGF-β-treated cells (Fig. 7), whereas DPI and MnTBaP, which reduce ROS by differentmechanisms, inhibit TGF-β induction of PAI-1 expression. These data suggest that ROS are involved in the induction of PAI-1 by TGF-β in our cell model and that GSH blocks TGF-β-induced PAI-1 expression probably by reducing ROS.
It has been reported that TGF-β increases ROS production by activating the membrane bond enzyme NADPH oxidase(s) (38, 39) and by impairing mitochondrial complex IV (47). Whether TGF-β stimulates ROS production in NIH/3T3 cells by activating NADPH oxidase or impairing mitochondrial electron transport is unknown. Although our data show that GSH reduces intracellular ROS signals in the TGF-β-treated cells (Fig. 7), whether GSH does so by reducing extracellular ROS or intracellular ROS is unclear as H2O2 can diffuse across the membrane in either direction. A decrease in the intracellular ROS could result from scavenging of extracellular ROS, which leads to a decreased diffusion of extracellular ROS into the cells or an increased removal of intracellular ROS (speeding up the outward diffusion), and/or a direct reduction of intracellular ROS. Extensive studies will be needed to fully address the question of whether GSH blocks TGF-β’s signaling by scavenging extracellular ROS and/or by restoring intracellular GSH level (26) and thereby reducing intracellular ROS/restoring the functions of GSH-dependent proteins/enzymes, which are beyond the scope of the current study. It should be mentioned that although DPI and MnTBaP inhibit TGF-β-induced PAI-1 expression as GSH does, whether other antioxidant compounds, such as ascorbic acid and vitamin E, would produce the same effects is unknown. It should also be emphasized that although DPI, MnTBaP, and GSH share the capacity to reduce ROS, the mechanisms of their actions are different. DPI inhibits ROS production, whereas MnTBaP scavenges ROS. GSH, on the other hand, can scavenge ROS and supply substrate for glutathione peroxidase and other GSH-dependent reactions. In addition to reducing ROS, GSH can also reduce oxidized protein cysteine residues through glutaredoxin-mediated reactions. Therefore, the biological effects of these three compounds could be dramatically different in different cell systems and/or in vivo.
Currently, therapeutic options for pulmonary fibrosis are very limited. GSH and its precursor NAC have been used clinically for the treatment of lung fibrotic diseases. It has been reported that aerosol administration of GSH or NAC restored GSH concentration in lung lining fluid and slowed the deterioration of lung functions in idiopathic pulmonary fibrosis (IPF) and cystic fibrosis patients (2, 4, 9, 14, 16, 40), indicating a potential therapeutic value of GSH/NAC in the treatment of lung fibrotic diseases. However, the mechanism underlying the therapeutic/antifibrotic effects of GSH/NAC is not completely defined. Our previous studies have shown that GSH blocks TGF-β-induced collagen accumulation in fibroblast cells by blocking TGF-β-induced PAI-1 expression and thus stimulating collagen degradation (26, 42). The present study further shows that GSH inhibits TGF-β-induced PAI-1 expression by blocking PAI-1 gene transcription through inhibition of JNK and p38 MAPK phosphorylation and the binding of transcription factors to AP-1, SP-1, and Smad cis elements in the promoter of PAI-1 gene. It should be mentioned that the GSH concentrations used in this study (1–5 mM) are not physiological extracellularly, although they are intracellularly. The effects observed may be unique to the pharmacological concentrations used. Nevertheless, our data provide strong evidence that GSH and NAC treatment may be of benefit in the treatment of lung fibrosis.
In conclusion, our data suggest that GSH blocks TGF-β-induced PAI-1 expression in part by suppressing p38 and JNK signaling and therefore the binding of transcription factors to AP-1, SP-1, and Smad elements in PAI-1 promoter, leading to a suppression of PAI-1 gene transcription. This study reveals a molecular mechanism by which GSH inhibits PAI-1 gene expression and may help explain how GSH exerts its antifi-brogenic effects in fibrotic diseases such as IPF.
ACKNOWLEDGMENTS
The authors thank Dr. Joanne Murphy-Ullrich and Dr. James Hagood for insightful suggestions on the project and critical comments on the manuscript.
GRANTS
This work was supported by National Institutes of Health Grant ES-011831 and a grant from the American Lung Association to R.-M. Liu; National Institutes of Health Grant HL-54696 to E. M. Postlethwait; and a grant from American Heart Association Beginning Grant in Aid and a grant from the Francis Families Foundation to K. E. Iles.
REFERENCES
- 1.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 2.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxi-dative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156:1897–1901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 3.Berk RS, Katar M, Dong Z, Day DE. Plasminogen activators and inhibitors in the corneas of mice infected with Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 2001;42:1561–1567. [PubMed] [Google Scholar]
- 4.Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest. 2005;127:308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- 5.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 7.Chen YQ, Su M, Walia RR, Hao Q, Covington JW, Vaughan DE. Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem. 1998;273:8225–8231. doi: 10.1074/jbc.273.14.8225. [DOI] [PubMed] [Google Scholar]
- 8.Dawson SJ, Wiman B, Hamsten A, Green F, Humphries S, Henney AM. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem. 1993;268:10739–10745. [PubMed] [Google Scholar]
- 9.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 10.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eitzman DT, Krauss JC, Shen T, Ginsburg Cui J. Lack of plasminogen activator inhibitor-1 effect in a transgenic mouse model of metastatic melanoma. Blood. 1996;87:4718–4722. [PubMed] [Google Scholar]
- 13.Ferroni P, Guagnano MT, Manigrasso MR, Ciabattoni G, Davi G. Increased plasminogen activator inhibitor-1 levels in android obesity: correlation with oxidative stress. J Thromb Haemostasis. 2005;3:1086–1087. doi: 10.1111/j.1538-7836.2005.01395.x. [DOI] [PubMed] [Google Scholar]
- 14.Griese M, Ramakers J, Krasselt A, Starosta V, Van Koningsbruggen S, Fischer R, Ratjen F, Mullinger B, Huber RM, Maier K, Rietschel E, Scheuch G. Improvement of alveolar glutathione and lung function but not oxidative state in cystic fibrosis. Am J Respir Crit Care Med. 2004;169:822–828. doi: 10.1164/rccm.200308-1104OC. [DOI] [PubMed] [Google Scholar]
- 15.Guo B, Inoki K, Isono M, Mori H, Kanasaki K, Sugimoto T, Akiba S, Sato T, Yang B, Kikkawa R, Kashiwagi A, Haneda M, Koya D. MAPK/AP-1-dependent regulation of PAI-1 gene expression by TGF-β in rat mesangial cells. Kidney Int. 2005;68:972–984. doi: 10.1111/j.1523-1755.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 16.Hartl D, Starosta V, Maier K, Beck-Speier I, Rebhan C, Becker BF, Latzin P, Fischer R, Ratjen F, Huber RM, Rietschel E, Krauss-Etschmann S, Griese M. Inhaled glutathione decreases PGE2 and increases lymphocytes in cystic fibrosis lungs. Free Radic Biol Med. 2005;39:463–472. doi: 10.1016/j.freeradbiomed.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Hua X, Liu X, Ansari DO, Lodish HF. Synergistic cooperation of TFE3 and smad proteins in TGF-beta-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev. 1998;12:3084–3095. doi: 10.1101/gad.12.19.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Seo JY, Ha H, Lee EA, Kim YS, Han DC, Uh ST, Park CS, Lee HB. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem Biophys Res Commun. 2003;309:961–966. doi: 10.1016/j.bbrc.2003.08.102. [DOI] [PubMed] [Google Scholar]
- 20.Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem. 2005;280:1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- 21.Keeton MR, Curriden SA, van Zonneveld AJ, Loskutoff DJ. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J Biol Chem. 1991;266:23048–23052. [PubMed] [Google Scholar]
- 22.Knudsen H, Olesen T, Riccio A, Ungaro P, Christensen L, Andreasen PA. A common response element mediates differential effects of phorbol esters and forskolin on type-1 plasminogen activator inhibitor gene expression in human breast carcinoma cells. Eur J Biochem. 1994;220:63–74. doi: 10.1111/j.1432-1033.1994.tb18599.x. [DOI] [PubMed] [Google Scholar]
- 23.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutz SM, Hordines J, McKeown-Longo PJ, Higgins PJ. TGF-beta1-induced PAI-1 gene expression requires MEK activity and cell-to-substrate adhesion. J Cell Sci. 2001;114:3905–3914. doi: 10.1242/jcs.114.21.3905. [DOI] [PubMed] [Google Scholar]
- 25.Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 upregulation in mesangial cells and in diabetic kidney. Kidney Int. 2005;67:1762–1771. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu RM, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-β-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2004;286:L121–L128. doi: 10.1152/ajplung.00231.2003. [DOI] [PubMed] [Google Scholar]
- 27.Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106:1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori S, Matsuzaki K, Yoshida K, Furukawa F, Tahashi Y, Yamagata H, Sekimoto G, Seki T, Matsui H, Nishizawa M, Fujisawa J, Okazaki K. TGF-beta and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene. 2004;23:7416–7429. doi: 10.1038/sj.onc.1207981. [DOI] [PubMed] [Google Scholar]
- 30.Olman MA, Mackman N, Gladson CL, Moser KM, Loskutoff DJ. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin Invest. 1995;96:1621–1630. doi: 10.1172/JCI118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prendergast GC, Diamond LE, Dahl D, Cole MD. The c-myc-regulated gene mrl encodes plasminogen activator inhibitor 1. Mol Cell Biol. 1990;10:1265–1269. doi: 10.1128/mcb.10.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rerolle JP, Hertig A, Nguyen G, Sraer JD, Rondeau EP. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000;58:1841–1850. doi: 10.1111/j.1523-1755.2000.00355.x. [DOI] [PubMed] [Google Scholar]
- 33.Samarakoon R, Higgins CE, Higgins SP, Kutz SM, Higgins PJ. Plasminogen activator inhibitor type-1 gene expression and induced migration in TGF-beta1-stimulated smooth muscle cells is pp60(c-src)/ MEK-dependent. J Cell Physiol. 2005;204:236–246. doi: 10.1002/jcp.20279. [DOI] [PubMed] [Google Scholar]
- 34.Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- 35.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 36.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 37.Swiatkowska M, Szemraj J, Al-Nedawi KN, Pawlowska Z. Reactive oxygen species upregulate expression of PAI-1 in endothelial cells. Cell Mol Biol Lett. 2002;7:1065–1071. [PubMed] [Google Scholar]
- 38.Thannickal VJ, Day RM, Klinz SG, Bastien MC, Larios JM, Fanburg BL. Ras-dependent and -independent regulation of reactive oxygen species by mitogenic growth factors and TGF-beta1. FASEB J. 2000;14:1741–1748. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- 39.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 40.Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, Sakamoto H, Iwasaki H. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005;10:449–455. doi: 10.1111/j.1440-1843.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 41.van Zonneveld AJ, Curriden SA, Loskutoff DJ. Type 1 plasminogen activator inhibitor gene: functional analysis and glucocorticoid regulation of its promoter. Proc Natl Acad Sci USA. 1988;85:5525–5529. doi: 10.1073/pnas.85.15.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vayalil PK, Olman M, Murphy-Ullrich JE, Postlethwait EM, Liu RM. Glutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogen. Am J Physiol Lung Cell Mol Physiol. 2005;289:L937–L945. doi: 10.1152/ajplung.00150.2005. [DOI] [PubMed] [Google Scholar]
- 43.Vojtek AB, Cooper JA. Rho family members: activators of MAP kinase cascades. Cell. 1995;82:527–529. doi: 10.1016/0092-8674(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 44.Woodward RN, Finn AV, Dichek DA. Identification of intracellular pathways through which TGF-β1 upregulates PAI-1 expression in endothelial cells. Atherosclerosis. 2006;186:92–100. doi: 10.1016/j.atherosclerosis.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Yeo SJ, Gravis D, Yoon JG, Yi AK. Myeloid differentiation factor 88-dependent transcriptional regulation of cyclooxygenase-2 expression by CpG DNA: role of NF-kappaB and p38. J Biol Chem. 2003;278:22563–22573. doi: 10.1074/jbc.M302076200. [DOI] [PubMed] [Google Scholar]
- 46.Yingling JM, Datto MB, Wong C, Frederick JP, Liberati NT, Wang XF. Tumor suppressor Smad4 is a transforming growth factor beta-inducible DNA binding protein. Mol Cell Biol. 1997;17:7019–7028. doi: 10.1128/mcb.17.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene. 2005;24:1895–1903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Shen HM, Zhang QF, Ong CN. Critical role of GSH in silica-induced oxidative stress, cytotoxicity, and genotoxicity in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 1999;277:L743–L748. doi: 10.1152/ajplung.1999.277.4.L743. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W, Spitz DR, Oberley LW, Robbins ME. Redox modulation of the pro-fibrogenic mediator plasminogen activator inhibitor-1 following ionizing radiation. Cancer Res. 2001;61:5537–5543. [PubMed] [Google Scholar]