Abstract
Light to moderate alcohol consumption and leisure time physical activity (LTPA) are independently associated with lower levels of high sensitivity C-reactive protein (CRP), a predictor of cardiometabolic risk. In contrast, depression, ranging from low mood disturbance to major depressive disorder, has been associated with elevated CRP. To test the hypothesis that depression attenuates the anti-inflammatory effects of LTPA and alcohol consumption, the current study tested the moderating effect of severity of depressive symptomatology on the relation of alcohol consumption and LTPA to CRP in 222 healthy adult men and women (18–65 years of age). Given the known effects of gender on inflammation, we also examined the effects of gender on the tested interactions. Depression was assessed using the Beck Depression Inventory. Frequency of alcohol consumption, hours of LTPA per week and other coronary risk/protective factors were assessed via self-report and structured interview. Fasting blood samples were used to measure CRP and lipids. As predicted, the interaction between LTPA and depressive symptomatology was significant (F = 5.29, p < .03) such that lower CRP was associated with the combination of decreased depressive symptomatology and increased LTPA. Among those with increased depressive symptoms, increased LTPA was not associated with higher CRP. Similarly, depression interacted with alcohol consumption in predicting CRP in men but not women (F = 5.03, p < .008) such that for men light to moderate alcohol consumption was associated with lower CRP but only among those with decreased depressive symptoms. Light to moderate alcohol consumption was not associated with lower CRP in those with increased depressive symptom severity. The pattern of the interactions between anti-inflammatory activities such as light to moderate alcohol consumption and LTPA and psychological distress as indexed by severity of depressive symptomatology suggests an important new avenue for future research.
Keywords: depression, leisure time physical activity, alcohol consumption, C-reactive protein, Gender differences
Introduction
Lifestyle factors such as leisure time physical activity (LTPA) and light-to-moderate alcohol consumption are associated with lower risk of cardiovascular disease (CVD) and type 2 diabetes (T2D) (Blair et al., 1989; Brien, Ronksley, Turner, Mukamal, & Ghali, 2011; Dunkley et al., 2012; Fagard, 1993; Golbidi & Laher, 2012; Joosten et al., 2010; Kelley, Kelley, Roberts, & Haskell, 2012a, 2012b; Lee & Skerrett, 2001; Oguma & Shinoda-Tagawa, 2004; Okada et al., 2010; Paffenbarger, Hyde, Wing, & Hsieh, 1986; Pietraszek, Gregersen, & Hermansen, 2010; Ronksley, Brien, Turner, Mukamal, & Ghali, 2011; Umpierre et al., 2011). It is believed that the benefits of LTPA and moderate alcohol consumption on reducing risk reflect improvements in cardiometabolic risk factors. Thus, LTPA and moderate alcohol consumption have been associated with lower blood pressure (Fagard, 1993), improved lipid and lipoprotein profile (Brien et al., 2011; Kelley et al., 2012a), reduced coagulation and platelet aggregation (Brien et al., 2011) and improved insulin sensitivity (Pietraszek et al., 2010).
Researchers have shown that LTPA and alcohol consumption also reduce inflammation. In cross-sectional, longitudinal and experimental studies, LTPA (Kasapis & Thompson, 2005; Panagiotakos, Pitsavos, Chrysohoou, Kavouras, & Stefanadis, 2005) and moderate alcohol consumption (i.e., 1–2 drinks/day) are associated with lower levels of high-sensitivity C-reactive protein (hsCRP) (Kelley & Kelley, 2006; Michigan, Johnson, & Master, 2011; Raum, Gebhardt, Buchner, Schiltenwolf, & Brenner, 2007), an acute-phase protein that predicts future risk of CVD (Ridker, 2001; Ridker, Buring, Shih, Matias, & Hennekens, 1998; Ridker, Hennekens, Buring, & Rifai, 2000; Zairis et al., 2004) and T2D (Han et al., 2002; Thorand et al., 2007). While CRP is a non-specific marker of inflammation (Saito et al., 2003), evidence suggests that it plays an active role in the pathogenesis of atherosclerotic plaque formation (Du Clos, 2000; Ishikawa et al., 2004) through its facilitation of low density lipoprotein (LDL) cholesterol absorption (Zwaka, Hombach, & Torzewski, 2001), recruitment of monocytes to arterial wall (Pasceri, Willerson, & Yeh, 2000) and reduction of vasoreactivity (Silva & Pais de Lacerda, 2012). Such effects have led some to suggest that CRP is not only a surrogate biomarker for disease risk but also a mediator in disease pathophysiology (Verma & Yeh, 2003).
In contrast to the beneficial effects of LTPA and moderate alcohol consumption, depression and its defining symptoms are prospectively associated with an increased risk of both CVD (Khan, Kulaksizoglu, & Cilingiroglu, 2010; Lippi, Montagnana, Favaloro, & Franchini, 2009; Nemeroff & Goldschmidt-Clermont, 2012) and T2D (Brown, Majumdar, Newman, & Johnson, 2005; Carnethon et al., 2007). Evidence suggests that depression increases the risk of CVD by 50% to 100%, and also worsens the outcome for patients who experience a cardiac event (Lippi et al., 2009). Similarly, depression increases the risk of T2D by 60% (Mezuk, Eaton, Albrecht, & Golden, 2008). While underlying mechanisms have not been well elucidated, a number of studies have reported that depression, ranging from low mood to major depressive disorder, is associated with higher levels of inflammatory biomarkers including hsCRP (Davidson et al., 2009; Elovainio et al., 2009; Ferketich, Ferguson, & Binkley, 2005; Lindqvist et al., 2009; Musselman et al., 2001; Pikhart et al., 2009; Suarez, 2004; Suarez & Boyle, 2005).
The link between depression and disease outcomes has been extensively explored with a number of studies examining putative physiological mechanisms linking depression and disease endpoints (Rozanski, Blumenthal, & Kaplan, 1999). For the most part, the primary approach employed by studies that examine putative mechanisms emphasizes the direct effect of depression on plausible mechanisms and subsequent clinical outcomes. While this approach has yielded a wealth of evidence for the role of depression in onset and progression of various clinical conditions (Herbert & Cohen, 1993; Howren, Lamkin, & Suls, 2009), it is reasonable to speculate that the impact of depression may also include inhibition of beneficial effects associated with risk-reducing activities. In other words, depression may act as a rate-limiting factor that inhibits or attenuates the cardioprotective effects of activities known to lower the risk of CVD and T2D such as LTPA and moderate alcohol consumption. Such an approach may reveal that depression, and more broadly psychological distress, not only has direct effects on putative mechanisms but also indirect effects that are characterized by diminished improvements in traditional and emerging risk factors following health promoting activities.
Recently, two studies have assessed the moderating effects of indicators of psychological distress on the relation of physical activity to immune responses to influenza vaccination. For example, Segerstrom et al. (2012) showed that higher levels of psychological distress, a latent construct that incorporated items from the Geriatric Depression Scale, attenuated antibody responses to vaccinations in persons who were physically active. In contrast, a more robust antibody response to vaccination was observed in subjects who were physically activity and reported low levels of psychological distress (Segerstrom, Hardy, Evans, & Greenberg, 2012). Similarly, Emeny et al. also examined the interaction between psychological distress as indexed by level of job strain and physical activity on CRP (Emeny et al., 2012). Emeny et al. showed that the beneficial effects of physical activity on reducing CRP were absent in people reporting high job strain but present in people with low job strain (Emeny et al., 2012). While preliminary, these findings support the general hypothesis that markers of psychological distress may act to inhibit or diminish the anti-inflammatory or immunological responses to health promoting behaviors. In contrast, the positive consequences of health promoting behaviors would be more likely to be observed among those with lower psychological distress. In testing this general hypothesis, the present study examined the moderating effect of psychological distress, as indexed by severity of depressive symptoms, on the relation of LTPA and alcohol consumption to hsCRP.
To fully explore the hypothesized interactions, we also examined the role of gender. Previous reports have shown that gender moderates the health-related effects of ethanol (Albert, Glynn, & Ridker, 2003; Greenfield, Rehm, & Rogers, 2002; Oliveira, Rodríguez-Artalejo, & Lopes, 2010). It is also acknowledged that the prevalence of depression differs between men and women. Combined, these observations raise the possibility that gender may moderate the joint effect of depression and alcohol consumption on CRP. To test this possibility, we examined the three-way interaction between gender, alcohol use and depression as well as the three-way interaction between gender, LTPA and depression.
Methods
Participants
Participants were 222 nonsmoking healthy men and women between the ages of 18 and 65 years. Subjects were enrolled in studies examining the relation of inflammatory biomarkers (e.g., C-reactive protein) to psychosocial risk factors of cardiovascular disease (Suarez, 2004). Subjects were recruited from the general community via advertisements placed in newspapers, online websites and fliers distributed throughout the community. Interested individuals were screened for entry criteria using a self-report health questionnaire and interview. Inclusion criteria included the following: no history or current diagnosis of psychiatric conditions; no current or previous use of anti-depressant medications; and no chronic medical conditions related to inflammation, such as asthma, allergies, arthritis, diabetes, all forms of cancer and cardiovascular diseases including hypertension. Women who used oral contraceptives or hormone replacement therapy were excluded.
Assessments
Depression
The 21-item Beck Depression Inventory (BDI) was used to assess symptoms of depression for the two weeks prior to administration (Beck, Steer, & Garbin, 1988). Subjects were instructed to respond to each item (e.g., “I often feel sad.”) using a four-point scale ranging from 0 (symptom not present) to 3 (symptom very intense) with total score ranging from 0 to 63. The depressive symptoms characterized were negative mood, sadness, pessimism, decreased functioning (e.g., indecisiveness), work inhibition, and somatic problems (e.g., insomnia and fatigue) (Beck et al., 1988). The BDI was administered on the day that blood samples were collected. While the BDI is not designed to yield a clinical diagnosis, scores are significantly correlated with a diagnosis of major depressive disorder (Beck et al., 1988). A previous study using the BDI to screen for depression in a general population reported that, of 1240 subjects between the ages of 18 and 64, 4.16% scored 13 and above on the BDI, a cut-off point with 100% sensitivity, 99% specificity and 98% overall diagnostic value for depressive disorder (Manrique, & Dowrick, 2000). In our sample, 4.5% scored 13 and above, with none of these subjects having been previously diagnosed with major depressive disorder. Due to the skewness of the distribution, BDI was log-transformed and used as a continuous variable in all analysis.
Alcohol consumption
Alcohol consumption was classified according to the scheme used by Albert et al. (Albert et al., 2003): never/former (< 1 drink in past 12-months), infrequently (1–3 drinks/month), occasionally (1–7 drinks/week), and regularly (2 or more drinks/day). Due to the low number of subjects in the “regular” category (men: 9.9%; women: 6.6%), we combined the categories of “occasionally” and “regularly.” Combining these two categories resulted in a subgroup where women reported drinking an average of ½ drink/day (median = 0.43 drinks/day, IQ range: 0.29–1.14) and men drinking slightly more than 1 drink/day (median = 1 drink/day, IQ range: 0.57–1.86). Thus, the result of combining these two categories yielded a classification that is consistent with gender-specific recommendations for the number of drinks/day associated with the greatest cardiovascular benefits (Movva & Figueredo, in press; Pai et al., 2006).
Leisure Time Physical Activity
Physical activity was assessed as a continuous variable. On the day of their laboratory visit subjects were asked to report the number of hours of LTPA in the week prior to their study visit. We did not question subjects on the type of physical activity or intensity thus metabolic equivalent task (MET) could not be calculated. Due to the skewness of the distribution, all analyses used the log-transformed hour of LTPA.
Biochemical Measures
Fasting venous blood samples were collected after an overnight fast, between the hours of 0830 and 0930 while subjects were seated in a reclined position. To minimize menstrual cycle effects, samples from pre-menopausal women were collected during the follicular phase. Samples were frozen at −80°C until assays were performed. High sensitivity CRP was measured using an ultrasensitive, enzyme-linked, immunometric latex-enhanced assay (Diagnostic Products Corporation, Los Angeles, CA) using purified protein and polyclonal anti-CRP antibodies from Diagnostic Products Corporation (Los Angeles, CA). This system has a low detection threshold of < 0.10 mg/L with coefficients of variation ranging from 6.6% to 9.3%. Total cholesterol (TC), high density lipoprotein (HDL) cholesterol and triglycerides (trig) were determined by the Duke University Clinical Laboratories.
Data collection
Subjects were instructed not use prescription medications or over-the-counter preparations, including low-dose aspirin, for two weeks prior to the study visit. On the day of the study visit, staff interviewed participants to determine their participation in LTPA in the week prior to their study visit and to verify that they were free of acute infections and recent injuries and had not undergone medical/dental procedures two weeks prior. Each subject’s temperature was also taken orally. Informed consent was obtained prior to the study. Subjects were compensated for their participation. The Institutional Review Board of Duke University approved this protocol.
Statistical Analyses
Descriptive statistics were performed using ANOVAs to compare means for continuous variables and Chi-squared tests for categorical variables such as alcohol use and gender. Tests of the hypotheses relating to the factors determining CRP level were conducted using multiple linear regression using SAS version 9.3 statistical software (SAS Institute Inc., Cary, North Carolina). Guided by previous research, covariates were selected a priori and included age, gender, body mass index (BMI), and race. Using the same covariates, we developed separate models for alcohol consumption and LTPA. Analysis employed a two-step approach. The first step, referred to as the full model, included all covariates, the main effects of log-transformed BDI, gender and behavior (i.e., alcohol use group or log-transformed LTPA), the three 2-way interactions between BDI, sex and behavior and the 3-way interaction between log-transformed BDI, protective behavior and gender predicting log-transformed hsCRP. If the 3-way interaction was non-significant, the 3-way interaction was eliminated and the model was revised to only include the 2-way interaction between depression score and lifestyle factor.
Probing of significant interactions was accomplished by performing simple slope analysis that included main effects and interactions at both levels as presented in the figures. Analysis of trimmed models centering the appropriate variables was used to test simple main effects (Aiken & West, 1991). For the analysis using trimmed models, the interactions retained their significance. For simple slope analysis, we followed statistical methods suggested by Aitken and West (Aiken & West, 1991) and Preacher, Curran and Bauer (Preacher, Curran, & Bauer, 2006) and implemented R scripts available freely from Dr. K. J. Preacher’s web site www.quantpsy.org. Aitken and West (1991) originally suggested probing interactions (albeit somewhat arbitrarily) at −/+1 SD above and below the mean of the moderator. We probed the interactions at −/+.5 SD of the mean of log BDI and converted results back to original BDI scale because even after log transformation, probing at −/+ 1SD of the log BDI put the original BDI values at very low and high extremes of the original BDI scale (because of the skewed BDI scale). The simple slope analysis allowed us to address two questions: is the slope of the simple regression line significantly different than zero, and do the slopes of a pair of simple regression lines differ from one another as a function of the moderator, in this case, BDI.
Results
Data were collected on 222 subjects. One subject was excluded from the alcohol analysis due to missing BDI and 11 subjects were excluded from the LTPA analysis due to missing LTPA values. Table 1 shows the participant characteristics stratified by gender.
Table 1.
Participant characteristics and test of gender differences
| Total Sample | Men | Women | Gender effect | |
|---|---|---|---|---|
| Age, years | 28.9 (10) | 27.7 (9.5) | 30.2 (10.3) | 0.06 |
| BMI, kg/m2 | 25.1 (4.7) | 25.3 (4.5) | 25.0 (4.8) | ns |
| Total cholesterol (mg/dL) | 168 (36) | 167.3 (36) | 169.6 (37) | ns |
| Race/Ethnicity (%) | ns | |||
| White | 54 | 55 | 53 | |
| Black | 27 | 22 | 33 | |
| Asian | 15 | 19 | 12 | |
| Hispanic | 1 | 1 | 0 | |
| Mixed/Other | 3 | 3 | 2 | |
| Education level (%) | ns | |||
| Less than High School | <1 | <1 | 0 | |
| High School Graduate | 5 | 5 | 5 | |
| Some College | 31 | 31 | 30 | |
| College Graduate | 44 | 29 | 49 | |
| Post-College | 20 | 24 | 16 | |
| Alcohol consumption frequency (%) | ns | |||
| Never/Former | 27 | 26 | 29 | |
| Infrequent | 31 | 29 | 33 | |
| Occasional/Regular | 42 | 45 | 38 | |
| Leisure time Physical Activity (LTPA) (hr/wk)* | 3.5 (1.0–6.0) | 4.0 (1.75–6.0) | 3.0 (0.5–5.5) | < 0.05 |
| Beck Depression Score* | 3.0 (1.0 – 6.0) | 3.0 (1.0 – 5.0) | 3.0 (1.0–7.0) | < 0.02 |
| hsCRP (mg/L)* | 0.72 (0.16–5.13) | 0.71 (0.18–4.96) | 0.73 (0.15–5.50) | ns |
Continuous parametric results are given as Mean (SD); categorical results as percentage; and continuous non-parametric results as median (IQR); ns = not significant
The relation of the covariates to CRP was as expected. Log CRP was significantly associated with age (r = .23, p < .001) and BMI (r = .43, p < .0001). There was a trend for log CRP to differ among races (F(4, 221) = 1.78, p = .13), with post-hoc comparison among groups suggesting that relative to whites, African Americans (AA) have significantly higher CRP (p = .01), a finding that is consistent with previous reports of higher CRP in AA than whites (Fox, Merali, & Harrison, 2006). CRP levels were similar in men and women (F(1, 220) = 0.00, ns).
We also examined the relation of the independent variable to the selected covariates. BDI did not differ based on the selected covariates of race (F(4, 220)=0.91, ns), age (r= 0.09, ns) and BMI (r = 0.0, ns). Women had higher BDI scores than men (F(1,223)=6.14, p=0.014), consistent with previous findings . BDI scores, however, were not associated with either LTPA (r = −0.06, ns) or alcohol consumption (F(2,221)=0.53, ns), nor were these relationships moderated by gender (for LTPA X gender (F = 1.88, ns); for alcohol group X gender (F (2, 216) = 0.62, ns), confirming that collinearity did not influence the results of our hypothesis-testing.
There was a trend toward a significant main effect of alcohol use (F(2, 219) = 2.99, p=0.052) with subjects who reported occasional/regular alcohol consumption showing significantly lower CRP relative to nondrinkers (p = .016) and infrequent drinkers having lower CRP than nondrinkers, but this latter comparison was not significant (p = .087). CRP did not differ between infrequent and occasional regular users. Although not significant, lower CRP values were associated with increasing hours of LTPA (β = −0.098, SE =.09, p = .10), consistent with the notion that the effects of hours of LTPA on CRP may be moderated by other factors such as severity of depressive symptoms.
Alcohol Consumption
Our sample was generally healthy with respect to alcohol consumption. No participants met criteria for “heavy” drinking, defined as more than 5 drinks per day (35 drinks per week) by Alatalo et al (Alatalo et al., 2009). In our sample, the median number of drinks per week was 4 (approximately ½ drink per day), with an interquartile range of 1–8 drinks per week. Five subjects drank more than 20 drinks per week and the highest consumption level occurred in 1 subject who consumed 28 drinks per week. As previously noted, alcohol consumption was not associated with BDI score. In fact, the subject who consumed the highest number of drinks per week (28 drinks per week) had a BDI score of 0. Regression analyses were performed by both including and excluding this subject, with similar results. Therefore, results described herein include all subjects.
In testing our hypothesis, we first examined whether gender moderated the relation of alcohol consumption and severity of depressive symptoms in predicting CRP levels (See table 2). Results revealed a significant 3-way interaction between BDI score, alcohol use and gender (F(2, 204) = 4.89, p < 0.008, R2 = 0.035) leading us to conduct separate regression analysis for men and women. Results of these post hoc analyses indicated that the 2-way interaction between BDI score and alcohol consumption significantly predicted CRP levels in men (F(2, 109) = 3.79, p < .03, R2 = 0.047) but not in women (F(2, 90) = 1.92, p = .098, R2 = 0.036).
Table 2a.
Regression model included alcohol groups (abstainers, infrequent, occasional/regular), log-transformed BDI scores, age, sex body mass index and race as predictors. Significance testing used Type III test statistics.
| Source | DF | F-Value | p-value |
|---|---|---|---|
| BDI | 1 | 0.62 | 0.4319 |
| ALCOHOL | 2 | 0.35 | 0.7043 |
| BDI ALCOHOL | 2 | 0.35 | 0.7057 |
| SEX | 1 | 0.47 | 0.4959 |
| BDI× SEX | 1 | 0.78 | 0.3794 |
| BDI× SEX×ALCOHOL | 2 | 5.03 | 0.0074 |
| AGE | 1 | 8.72 | 0.0035 |
| BMI | 1 | 34.87 | <0.0001 |
| RACE | 4 | 1.11 | 0.3518 |
| R-square | 0.280582 | ||
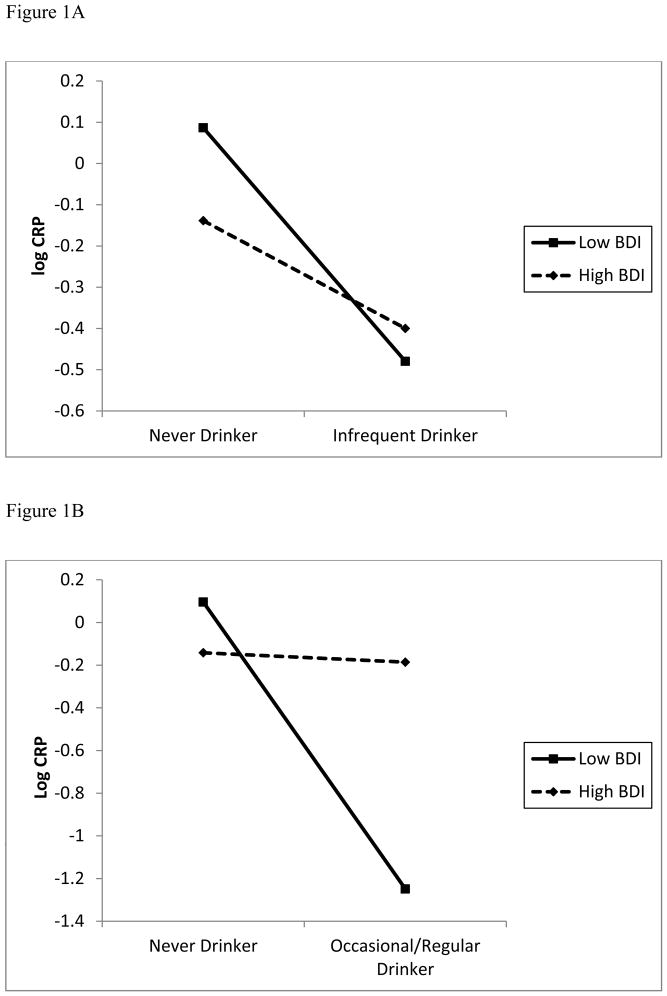
To explore the moderating effect of BDI on the association between alcohol consumption and CRP in men, we performed simple slope analyses (Aiken & West, 1991; Preacher et al., 2006). Note that Z-statistics here are the normal approximation to t-statistics due to large degrees of freedom. Figure 1A graphs the relation of alcohol consumption to CRP as a function of high and low BDI scores using 0.5 SD above and below the mean of BDI (low BDI ≈ 2 and high BDI ≈ 7). For comparing never drinkers against occasional/regular drinkers, simple slopes were significant at lower BDI (β = −0.7544, SE =0.258, z = −2.9244, p < 0.004), such that occasional/regular alcohol users with low BDI had lower CRP. For high BDI, the simple main effect of alcohol consumption was not significant (β = −0.1665, SE =0.226, z = −0.7362, p = 0.46).
Figure 1.
Figure 1A and B: For men, slopes at high (+ 0.5 SD) and low (−0.5 SD) levels of severity of depressive symptomatology for comparisons between infrequent drinkers and never drinkers (Fig 1A) and at high (+ 0.5 SD) and low (− 0.5 SD) severity of depressive symptomatology for comparisons between never drinkers and occasional/regular drinkers (Fig 1B) and
To get a complete picture of the moderation/non-moderation nature of BDI on different alcohol use behaviors, we also performed simple slope analyses comparing the infrequent alcohol users to those who abstained. As illustrated in Figure 1B, at lower BDI (β = −0.3584, SE = 0.2837, z = −1.2636, p = 0.21, and at the higher BDI, (β = −0.1736, SE = 0.2471, z = −0.7023, p=0.47) (see Figure 1B).
Leisure Time Physical Activity
Our sample group was also healthy in terms of hours of LTPA in the week prior to their laboratory visit. In response to the question, “how many hours per week of leisure physical activity did you participate in during the previous week?’. In this sample, the median was 3.5 hours of LTPA per week (IQ: 1–6 hours/week). There was a trend for men to report more hours/week of LTPA (t(211) = 1.92, p = 0.057, men: mean = 4; IQ 1.75 - 6.0 hours/week; women: median = 3; IQ 0.5–5.5 hours/week). Analysis excluded all subjects whose hour/week LTPA > 3 SD from the mean.
With regard to CRP level, the 3-way interaction between gender, BDI score, and log-transformed hours of LTPA did not significantly predict log-transformed hsCRP (F(1, 198) = 0.02, R2 = .008). Therefore, data from men and women were combined and we examined the effect of the 2-way interaction between BDI score and log-transformed hours of LTPA (F(1, 199) = 5.29, p < .03, R2 = 0.020) (see Table 3).
Table 2b.
Parameter estimates from the final regression model.
| Parameter | Estimate | SE | t-value (df=207) | p-value |
|---|---|---|---|---|
| Intercept | −3.347 | 0.629 | −5.32 | <.0001 |
| BDI | −0.040 | 0.178 | −0.22 | 0.8245 |
| ALCOHOL (0 vs 2) | −0.622 | 0.421 | −1.48 | 0.1404 |
| ALCOHOL (1 vs 2) | 0.278 | 0.432 | 0.64 | 0.5212 |
| BDI×ALCOHOL (0 vs 2) | 0.538 | 0.264 | 2.04 | 0.0424 |
| BDI×ALCOHOL (1 vs 2) | −0.035 | 0.255 | −0.14 | 0.8899 |
| SEX (M vs F) | −0.328 | 0.366 | −0.90 | 0.3715 |
| BDI×SEX (M vs F) | 0.326 | 0.237 | 1.38 | 0.1705 |
| SEX×ALCOHOL (M&0 vs M&2) | 1.587 | 0.561 | 2.83 | 0.0052 |
| SEX×ALCOHOL (M&1 vs M&2) | −0.114 | 0.567 | −0.20 | 0.8409 |
| BDI ×SEX×ALCOHOL (M&0 vs M&2) | −1.155 | 0.374 | −3.09 | 0.0023 |
| BDI×SEX ×ALCOHOL (M&0 vs M&2) | −0.227 | 0.360 | −0.63 | 0.5286 |
| AGE | 0.022 | 0.007 | 2.95 | 0.0035 |
| BMI | 0.096 | 0.016 | 5.91 | <.0001 |
| RACE (White vs Other) | −0.092 | 0.425 | −0.22 | 0.8298 |
| RACE (Black vs Other) | −0.026 | 0.450 | −0.06 | 0.9539 |
| RACE (Asian vs Other) | 0.319 | 0.447 | 0.71 | 0.4763 |
| RACE (Hispanic vs Other) | −0.333 | 1.072 | −0.31 | 0.7566 |
Alcohol groups: 0 = abstainers; 1 = infrequent; 2 = occasional/regular, M = males, F = females, BDI = Beck Depression Inventory (log-transformed).
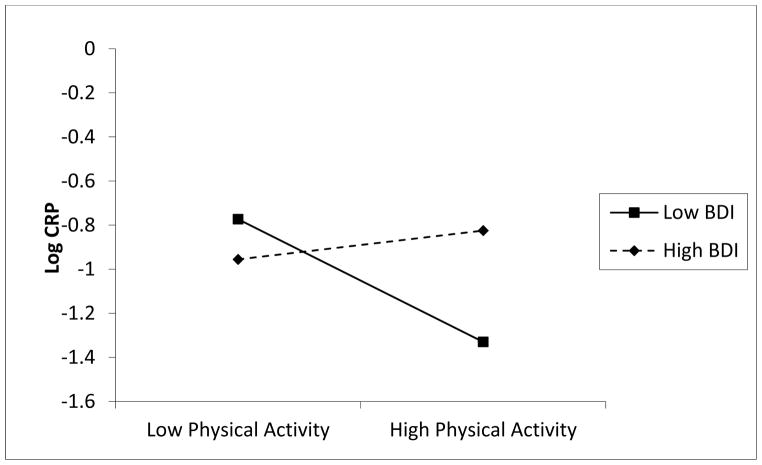
Figure 2 illustrates the result of the simple slope by graphing log-CRP at 0.5 SD above and below BDI (low BDI ≈ 2 and high BDI ≈ 7). In these analyses, we observed a significant main effect of hours of LTPA per week (β = −0.4707, SE =0.2125, z = −2.2152, p < .03) when BDI was lower than average, but not when BDI was higher than average (β = −0.0956, SE = 0.1764, z = −0.5419, p = 0.59). Thus, when subjects had decreased symptom severity, increased LTPA was associated with lower CRP. For those with increased depressive symptom severity, we failed to observe a simple main effect for LTPA on CRP.
Figure 2.
Slopes of leisure time physical activity (LTPA) at high (+ 0.5 SD) and low (− 0.5 SD) severity of depressive symptomatology.
Effects of Depression, Alcohol Consumption and Leisure Time Physical Activity on Fasting Lipids
It was important to determine if the moderating effects of BDI were also observed for the relation of alcohol consumption and LTPA to fasting lipid levels. Both alcohol consumption and LTPA are associated with lower total cholesterol (TC) and triglyceride (TRIG) levels and higher high-density lipoprotein (HDL) cholesterol (Panagiotakos et al., 2003; Rimm, Williams, Fosher, Criqui, & Stampfer, 1999), We therefore examined the interactions found to be significant in predicting hsCRP to determine if they also predicted TC, HDL cholesterol and TRIG. Tests of the alcohol group by BDI score by gender interaction did not predict fasting TC (F(2, 203) = 0.51, ns), HDL (F(2,203) =2.00, ns) or TRIG (F(2, 203) = 0.92, ns). Similarly, the LTPA by BDI score interaction failed to predict TC (F(1,200) = 1.05, ns), HDL (F(1, 200) = 0.05, ns) and TRIG (F(1, 200) = 0.94, ns). Thus, it appears that the moderating effects of depression on the relation of physical activity and alcohol consumption do not generalize to fasting lipids but are specific to CRP.
Discussion
The present study examined whether current untreated depression moderated the well-recognized anti-inflammatory effects of lifestyle factors (moderate alcohol consumption and LTPA) and whether these effects differed in men and women. Imposing methodological constraints on current health status (no acute or chronic medical conditions), use of over-the-counter medications, smoking status (non-smokers only), steroid use (both oral contraceptives and hormone replacement therapy) and menstrual cycle phase (follicular phase) for premenopausal women, we observed that the best predictor of hsCRP was the interaction between health-related behaviors and depressive symptomatology. For men, moderate alcohol consumption (≈ 1 drink/day) was associated with lower CRP but only for those with decreased severity of depressive symptomatology. This was not the case for men with increased depressive symptoms, where the beneficial effects of moderate consumption of alcohol were abolished. We also observed that severity of depressive symptoms did not moderate the level of CRP in infrequent drinkers relative to non-drinkers. Depressive symptomatology also moderated the positive anti-inflammatory effects of hours of LTPA per week. In this case, individuals with increased hours of LTPA per week showed lower CRP but only if they had decreased levels of depressive symptomatology. For those with increased severity of depressive symptoms, LTPA was not associated with CRP. Using two disparate lifestyle factors that are independently associated with lower CRP, the pattern of findings suggest that the effects of depression go beyond “main effects” characterized by dysregulation of inflammatory system to include moderation effects that take the form of attenuation of the anti-inflammatory effects of lifestyle factors.
In contrast to our findings predicting CRP, the same interactions failed to predict lipid concentrations. Previous studies have shown that both LTPA and moderate alcohol consumption are associated with lower TC and higher HDL (Panagiotakos et al., 2005; Panagiotakos et al., 2003). With the exception of the current study, we know of no other study that has examined whether depression moderates the effect of lifestyle factors on lipid levels. In our laboratory we have observed that depression is associated with lower TC (Suarez, 1999), a relation that is independent of BMI, age and other relevant factors. Similar findings have been reported by others, who have shown lower HDL among those with MDD (Ancelin et al., 2010; Maes et al., 1997; Sutin et al., 2010). While depression may not moderate the relation of lifestyle factors to lipids, it remains to be determined whether depression has similar moderating effects on the relation of lifestyle factors to other cardiometabolic indicators such as glucose metabolism and insulin resistance.
The observed patterns are not the first to suggest that indicators of psychological distress modify or inhibit immune-related responses to health promoting activities and physical traits. Segerstrom et al. (Segerstrom et al., 2012) assessed antibody responses to influenza vaccination as a function of physical activity and psychological distress, a latent construct that included aspects of depression. In that study, greater physically activity (equivalent to 1 hour of walking daily) was associated with a robust antibody response but only among those adults with low levels of psychological distress. In contrast, among adults who were at high levels of psychological distress, physical activity was not associated with a robust antibody response (Segerstrom et al., 2012). Similarly, Emeny et al. showed that physical activity was significantly associated with lower CRP but only in subjects with low job strain (Emeny et al., 2012). For subjects reporting high job strain, physical activity failed to have a beneficial effect on CRP (Emeny et al., 2012). As with the latent construct of psychological distress used by Segerstrom et al, high job strain is associated with greater depressive symptomatology (Ahola & Hakanen, 2007; Ahola et al., 2006). Thus, the current findings provide additional evidence in support of the hypothesis that the presence of depression and depression-related distress inhibits the beneficial health effects of physical activity on a measure of inflammation.
Depression also attenuates the effects of moderate alcohol consumption on CRP levels, in men but not women. In depressed men, alcohol drinking was associated with elevated CRP. At this time, we can only speculate about the reasons for the observed gender-specific differences for the joint effect of depression and alcohol use on CRP. It is important to note that gender-differences for the joint effect of alcohol use and depression on health outcomes have been reported previously. Using the 1984 US National Alcohol Survey, Greenfield et al. (Greenfield et al., 2002) showed that, for depressed women, the relative risk of all-cause mortality was highest for former drinkers with heavy occasions. For depressed men, however, highest risk occurred in those who currently drank more than 6 drinks per day. In the absence of depression, the relative risk of mortality was significantly lower for these two alcohol consumption groups. In light of our findings and those of Greenfield et al. and others (Albert et al., 2003; Oliveira et al., 2010), the complex interaction between gender, ethanol consumption and depression should be carefully considered in future clinical studies.
The findings of the current study are consistent with previous findings that measures of depressed affect, such as the Center for Epidemiological Studies Depression scale, are not associated with frequency of alcohol consumption (Graham, Massak, Demers, & Rehm, 2007; Graham & Schmidt, 1999). Similarly, we did not find an association between LTPA and severity of depressive symptoms. The cross-sectional nature of our design, however, does not allow for testing causality but previous studies have shown that LTPA does not have a causal effect on depressive symptoms (De Moor Mm, 2008). While our findings are consistent with previous observations, we are limited by the types of measures used, not only for depression where we measured symptom severity, but also for alcohol consumption and LTPA, where the emphasis was on frequency and total hours per week, respectively. Thus, it remains to be determined whether clinical depression has similar moderating effects on the relation of LTPA and alcohol consumption to CRP and other measures of inflammation.
The current study has a number of limitations. First, we used single items to assess amount of physical activity or alcohol consumption. More detailed assessments are likely to provide additional information not captured by a single item (e.g., Graham et al., 2007). This is also the case for physical activity where we did not collect type of activity and intensity, aspects that would have allowed us to calculate MET. Second, the cross-sectional nature of the study does not allow us to address directionality. Most (though not all) intervention studies published to date have demonstrated that exercise improves symptoms of depression in cardiac patients (Blumenthal, 2011). Depression, however, has not been shown to impact the likelihood of exercising (Ku, Fox, Chen, & Chou, 2012) nor is regular physical activity predictive of changes in depressive symptoms (De Moor, Beem, Stubbe, Boomsma, & De Geus, 2006). With our cross-sectional design, we have no way of knowing whether reduction in CRP (and implied risk of CVD and T2D) would be greater or less in depressed relative to non-depressed patients given an exercise intervention. Interestingly, preliminary evidence suggests that for cardiac patients who are depressed, 16-weeks of exercise improved severity of depressive symptoms but did not significantly lower CRP (Blumenthal et al., 2012). What is not known is whether a similar exercise program would reduce CRP in non-depressed patients.
In summary, the current data suggest a novel role for the deleterious effects of untreated depression. Depression has been linked to poor health outcomes via direct effects on mechanisms implicated in heart disease and T2D such as inflammation. In the current study, we showed that current depression inhibits the anti-inflammatory effect of two disparate activities that are associated with lower hsCRP and reduced risk of future CHD and T2D. The inhibiting effects of depression were not observed for other health benefits of physical activity and light-to-moderate alcohol consumption such as lower triglyceride and lipid levels. In both depressed and non-depressed subjects, physical activity and light-to-moderate alcohol consumption were associated with favorable lipid profiles (e.g., low LDL, high HDL).
The current data, combined with emerging evidence, have implications for the planning of preventive intervention strategies and education programs aimed at reducing the risk of heart disease and diabetes. The American Heart Association recommends screening of depression in patients with coronary heart disease (Kasim-Karakas, 2000), but depression and depressive symptoms are often unrecognized or untreated in primary clinical practice (Coyne, Schwenk, & Fechner-Bates, 1995). Primary care providers have the opportunity to have substantial preventive impact, especially if they are able to recognize symptoms arising de novo, when patients frequently attribute changes in mood and behaviors to everyday stressors (Ylonen et al., 2003). Early intervention is particularly important since any episode of depression, if untreated, may last months to years (Rost et al., 1998). Aside from depressed mood, reduced energy level, difficulty with concentration, loss of interest in previously pleasurable activities and sleep dysregulation, the current findings suggest that the effects of depression extend beyond these psychiatric symptoms and include attenuation of positive health consequences of lifestyle factors. These findings argue for medical providers to combine management of depression alongside reduction of other forms of cardiovascular risk, instead of the more traditional approach of managing conditions separately.
Table 3a.
Regression model included log-transformed leisure time physical activity (hours/week), log-transformed Beck Depression Inventory (BDI) scores, age, sex body mass index and race as predictors. Significance testing used Type III test statistics.
| Source | DF | F-Value | p-value |
|---|---|---|---|
| BDI | 1 | 1.86 | 0.1741 |
| LTPA | 1 | 5.43 | 0.0208 |
| BDI LTPA | 1 | 5.08 | 0.0252 |
| SEX | 1 | 0.01 | 0.9412 |
| AGE | 1 | 8.43 | 0.0001 |
| BMI | 1 | 37.35 | <.0001 |
| RACE | 4 | 1.60 | 0.1747 |
| R-square | 0.252226 | ||
Table 3b.
Parameter estimates from the final model.
| Parameter | Estimate | SE | t-value (df=200) | p-value |
|---|---|---|---|---|
| Intercept | −3.478 | 0.642 | −5.42 | <.0001 |
| BDI | −0.198 | 0.145 | −1.36 | 0.1741 |
| LTPA | −0.331 | 0.142 | −2.33 | 0.0208 |
| BDI×LTPA | 0.209 | 0.093 | 2.25 | 0.0252 |
| SEX (M vs F) | 0.011 | 0.139 | 0.07 | 0.9412 |
| AGE | 0.021 | 0.007 | 2.90 | 0.0041 |
| BMI | 0.090 | 0.016 | 5.60 | <.0001 |
| RACE (White vs Other) | 0.545 | 0.496 | 1.14 | 0.2562 |
| RACE (Black vs Other) | 0.632 | 0.511 | 1.14 | 0.2175 |
| RACE (Asian vs Other) | 0.983 | 0.515 | 1.91 | 0.0579 |
| RACE (Hispanic vs Other) | 0.390 | 1.100 | 0.36 | 0.7212 |
LTPA = leisure time physical activity (hours/week); BDI = Beck Depression Inventory score; M = male, F = female.
Highlight.
Depression attenuates the anti-inflammatory effects of leisure time physical activity and light-to-moderate alcohol consumption on the cardiometabolic r risk marker, C-reactive protein.
Acknowledgments
This work was supported by a grant from National Heart, Lung and Blood Institute (NHLBI) HL67459 to ECS. The NHLBI did not contribute to study design.
This work was supported by a grant from the National Institutes of Health (HL67459) to ECS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edward C. Suarez, Department of Psychiatry, Duke University Medical Center, Durham, NC
Nicole L. Schramm-Sapyta, Department of Psychiatry, Duke University Medical Center, Durham, NC
Tracey Vann Hawkins, Department of Psychiatry, Duke University Medical Center, Durham, NC.
Alaattin Erkanli, Department of Biostatistics and Bioinformatics, Duke University School of Medicine, Durham, NC.
References
- 1.Ahola K, Hakanen J. Job strain, burnout, and depressive symptoms: A prospective study among dentists. Journal of Affective Disorders. 2007;104(13):103–110. doi: 10.1016/j.jad.2007.03.004. doi: http://dx.doi.org/10.1016/j.jad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ahola K, Honkonen T, Kivimäki M, Virtanen M, Isometsä E, Aromaa A, Lönnqvist J. Contribution of Burnout to the Association Between Job Strain and Depression: the Health 2000 Study. Journal of Occupational and Environmental Medicine. 2006;48(10):1023–1030. doi: 10.1097/01.jom.0000237437.84513.92. 1010.1097/1001.jom.0000237437.0000284513.0000237492. [DOI] [PubMed] [Google Scholar]
- 3.Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 4.Alatalo P, Koivisto H, Puukka K, Hietala J, Anttila P, Bloigu R, Niemela O. Biomarkers of liver status in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol. 2009;44(2):199–203. doi: 10.1093/alcalc/agn099. [DOI] [PubMed] [Google Scholar]
- 5.Albert MA, Glynn RJ, Ridker PM. Alcohol Consumption and Plasma Concentration of C-Reactive Protein. Circulation. 2003;107(3):443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 6.Ancelin M-L, Carrière I, Boulenger J-P, Malafosse A, Stewart R, Cristol J-P, Dupuy A-M. Gender and Genotype Modulation of the Association Between Lipid Levels and Depressive Symptomatology in Community-Dwelling Elderly (The ESPRIT Study) Biological Psychiatry. 2010;68(2):125–132. doi: 10.1016/j.biopsych.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 8.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal JA. New frontiers in cardiovascular behavioral medicine: comparative effectiveness of exercise and medication in treating depression. Cleveland Clinic Journal of Medicine. 2011;78(Suppl 1):S35–43. doi: 10.3949/ccjm.78.s1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, Hinderliter AL. Exercise and Pharmacological Treatment of Depressive Symptoms in Patients With Coronary Heart DiseaseResults From the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) Study. Journal of the American College of Cardiology. 2012;60(12):1053–1063. doi: 10.1016/j.jacc.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown LC, Majumdar SR, Newman SC, Johnson JA. History of depression increases risk of Type 2 Diabetes in younger adults. Diabetes Care. 2005;28(5):1063–1067. doi: 10.2337/diacare.28.5.1063. [DOI] [PubMed] [Google Scholar]
- 13.Carnethon MR, Biggs ML, Barzilay JI, Smith NL, Vaccarino V, Bertoni AG, Siscovick DS. Longitudinal association between depressive symptoms and incident Type 2 Diabetes Mellitus in older adults: The Cardiovascular Health Study. Arch Intern Med. 2007;167:802–807. doi: 10.1001/archinte.167.8.802. [DOI] [PubMed] [Google Scholar]
- 14.Coyne JC, Schwenk TL, Fechner-Bates S. Nondetection of depression by primary care physicians reconsidered. General Hospital Psychiatry. 1995;17(1):3–12. doi: 10.1016/0163-8343(94)00056-j. [DOI] [PubMed] [Google Scholar]
- 15.Davidson KW, Schwartz JE, Kirkland SA, Mostofsky E, Fink D, Guernsey D, Shimbo D. Relation of Inflammation to Depression and Incident Coronary Heart Disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study) The American Journal of Cardiology. 2009;103(6):755–761. doi: 10.1016/j.amjcard.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Moor MHM, Beem AL, Stubbe JH, Boomsma DI, De Geus EJC. Regular exercise, anxiety, depression and personality: A population-based study. Preventive Medicine. 2006;42(4):273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.DeMoor Mm, BDISJHWGdGEC Testing causality in the association between regular exercise and symptoms of anxiety and depression. Archives of General Psychiatry. 2008;65(8):897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- 18.Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 19.Dunkley AJ, Charles K, Gray LJ, Camosso-Stefinovic J, Davies MJ, Khunti K. Effectiveness of interventions for reducing diabetes and cardiovascular disease risk in people with metabolic syndrome: systematic review and mixed treatment comparison meta-analysis. Diabetes Obes Metab. 2012;14(7):616–625. doi: 10.1111/j.1463-1326.2012.01571.x. [DOI] [PubMed] [Google Scholar]
- 20.Elovainio M, Aalto AM, Kivimaki M, Pirkola S, Sundvall J, Lonnqvist J, Reunanen A. Depression and C-reactive protein: population-based Health 2000 Study. Psychosomatic Medicine. 2009;71(4):423–430. doi: 10.1097/PSY.0b013e31819e333a. [DOI] [PubMed] [Google Scholar]
- 21.Emeny R, Lacruz ME, Baumert J, Zierer A, von Eisenhart Rothe A, Autenrieth C, Ladwig KH. Job strain associated CRP is mediated by leisure time physical activity: Results from the MONICA/KORA study. Brain, Behavior, and Immunity. 2012;26(7):1077–1084. doi: 10.1016/j.bbi.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Fagard RH. Physical fitness and blood pressure. Journal of Hypertension Supplement. 1993;11(5):S47–52. [PubMed] [Google Scholar]
- 23.Ferketich AK, Ferguson JP, Binkley PF. Depressive symptoms and inflammation among heart failure patients. American Heart Journal. 2005;150(1):132–136. doi: 10.1016/j.ahj.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behavioural Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Golbidi S, Laher I. Exercise and the cardiovascular system. Cardiol Res Pract. 2012;2012:210852. doi: 10.1155/2012/210852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham K, Massak A, Demers A, Rehm J. Does the association between alcohol consumption and depression depend on how they are measured? Alcoholism, Clinical and Experimental Research. 2007;31(1):78–88. doi: 10.1111/j.1530-0277.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- 27.Graham K, Schmidt G. Alcohol use and psychological well-being among older adults. J Stud Alcohol. 1999;60:345–351. doi: 10.15288/jsa.1999.60.345. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield TK, Rehm J, Rogers JD. Effects of depression and social integration on the relationship between alcohol consumption and all-cause mortality. Addiction. 2002;97(1):29–38. doi: 10.1046/j.1360-0443.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 29.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean MEJ, Haffner SM. Prospective Study of C-Reactive Protein in Relation to the Development of Diabetes and Metabolic Syndrome in the Mexico City Diabetes Study 10.2337/diacare.25.11.2016. Diabetes Care. 2002;25(11):2016–2021. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 30.Herbert TB, Cohen S. Depression and immunity: A meta-analytic review. Psychological Bulletin. 1993;113(3):472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 31.Howren MB, Lamkin DM, Suls J. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosomatic Medicine. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa T, Imamura T, Hatakeyama K, Date H, Nagoshi T, Kawamoto R, Eto T. Possible contribution of C-reactive protein within coronary plaque to increasing its own plasma levels across coronary circulation. The American Journal of Cardiology. 2004;93(5):611–614. doi: 10.1016/j.amjcard.2003.11.030. doi: http://dx.doi.org/10.1016/j.amjcard.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Joosten MM, Grobbee DE, van der AD, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. American Journal of Clinical Nutrition. 2010;91(6):1777–1783. doi: 10.3945/ajcn.2010.29170. [DOI] [PubMed] [Google Scholar]
- 34.Kasapis C, Thompson PD. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory MarkersA Systematic Review. Journal of the American College of Cardiology. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 35.Kasim-Karakas SE. Ethnic differences in the insulin resistance syndrome. American Journal of Clinical Nutrition. 2000;71(3):670–671. doi: 10.1093/ajcn/71.3.670. [DOI] [PubMed] [Google Scholar]
- 36.Kelley GA, Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism: Clinical and Experimental. 2006;55(11):1500–1507. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Kelley GA, Kelley KS, Roberts S, Haskell W. Combined effects of aerobic exercise and diet on lipids and lipoproteins in overweight and obese adults: a meta-analysis. J Obes. 2012a;2012:985902. doi: 10.1155/2012/985902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley GA, Kelley KS, Roberts S, Haskell W. Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Clinical Nutrition. 2012b;31(2):156–167. doi: 10.1016/j.clnu.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan FM, Kulaksizoglu B, Cilingiroglu M. Depression and coronary heart disease. Curr Atheroscler Rep. 2010;12(2):105–109. doi: 10.1007/s11883-010-0096-5. [DOI] [PubMed] [Google Scholar]
- 40.Ku P-W, Fox KR, Chen L-J, Chou P. Physical Activity and Depressive Symptoms in Older Adults: 11-Year Follow-Up. American Journal of Preventive Medicine. 2012;42(4):355–362. doi: 10.1016/j.amepre.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. Journal of Affective Disorders. 2000;57(1–3):261–265. doi: 10.1016/s0165-0327(99)00088-9. doi: http://dx.doi.org/10.1016/S0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- 42.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(6 Suppl):S459–471. doi: 10.1097/00005768-200106001-00016. discussion S493–454. [DOI] [PubMed] [Google Scholar]
- 43.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 44.Lippi G, Montagnana M, Favaloro EJ, Franchini M. Mental depression and cardiovascular disease: a multifaceted, bidirectional association. Seminars in Thrombosis and Hemostasis. 2009;35(3):325–336. doi: 10.1055/s-0029-1222611. [DOI] [PubMed] [Google Scholar]
- 45.Maes M, Smith R, Christophe A, Vandoolaeghe E, Gastel AV, Neels H, Meltzer HY. Lower serum high-density lipoprotein cholesterol (HDL-C) in major depression and in depressed men with serious suicidal attempts: relationship with immune-inflammatory markers. Acta Psychiatrica Scandinavica. 1997;95(3):212–221. doi: 10.1111/j.1600-0447.1997.tb09622.x. [DOI] [PubMed] [Google Scholar]
- 46.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michigan A, Johnson TV, Master VA. Review of the Relationship between C-Reactive Protein and Exercise. Mol Diagn Ther. 2011 doi: 10.2165/11593400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Movva R, Figueredo VM. Alcohol and the heart: To abstain or not to abstain? International Journal of Cardiology. doi: 10.1016/j.ijcard.2012.01.030. (in press) [DOI] [Google Scholar]
- 49.Musselman D, Miller A, Porter M, Manatunga A, Gao F, Penna S, Nemeroff C. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am J Psychiatry. 2001;158(8):1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 50.Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak-the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012 doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- 51.Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med. 2004;26(5):407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, Miyamoto Y. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb. 2010;17(8):828–833. doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira A, Rodríguez-Artalejo F, Lopes C. Alcohol Intake and Systemic Markers of Inflammation—Shape of the Association According to Sex and Body Mass Index. Alcohol and Alcoholism. 2010;45(2):119–125. doi: 10.1093/alcalc/agp092. [DOI] [PubMed] [Google Scholar]
- 54.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 55.Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186(1):113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 56.Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Preventive Medicine. 2005;40(4):432–437. doi: 10.1016/j.ypmed.2004.07.010. doi: http://dx.doi.org/10.1016/j.ypmed.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Zeimbekis A, Papaioannou I, Stefanadis C. Effect of leisure time physical activity on blood lipid levels: the ATTICA study. Coronary Artery Disease. 2003;14(8):533–539. doi: 10.1097/00019501-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Pasceri V, Willerson JT, Yeh ETH. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;89:763–771. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 59.Pietraszek A, Gregersen S, Hermansen K. Alcohol and type 2 diabetes. A review. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Pikhart H, Hubacek JA, Kubinova R, Nicholson A, Peasey A, Capkova N, Bobak M. Depressive symptoms and levels of C-reactive protein: a population-based study. Social Psychiatry and Psychiatric Epidemiology. 2009;44(3):217–222. doi: 10.1007/s00127-008-0422-1. [DOI] [PubMed] [Google Scholar]
- 61.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling and latent curve analysis. J Educ Behavioral Statistics. 2006;2006:437–448. [Google Scholar]
- 62.Raum E, Gebhardt K, Buchner M, Schiltenwolf M, Brenner H. Long-term and short-term alcohol consumption and levels of C-reactive protein. Int J Cardiol. 2007;121(2):224–226. doi: 10.1016/j.ijcard.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 63.Ridker PM. High-Sensitivity C-Reactive Protein : Potential Adjunct for Global Risk Assessment in the Primary Prevention of Cardiovascular Disease. Circulation. 2001;103(13):1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 64.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women.[see comment] Circulation. 1998;98(8):731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 65.Ridker PM, Hennekens CH, Buring J, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 66.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319(7224):1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rost K, Zhang M, Fortney J, Smith J, Coyne J, Richard Smith G., Jr Persistently poor outcomes of undetected major depression in primary care. General Hospital Psychiatry. 1998;20(1):12–20. doi: 10.1016/s0163-8343(97)00095-9. [DOI] [PubMed] [Google Scholar]
- 69.Rozanski A, Blumenthal JA, Kaplan JR. Impact of psychological factors on the pathogenesis of cardiovascular disease and implication for therapy. Circulation. 1999;99:2129–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 70.Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 71.Segerstrom SC, Hardy JK, Evans DR, Greenberg RN. Vulnerability, distress, and immune response to vaccination in older adults. Brain, Behavior, and Immunity. 2012;26(5):747–753. doi: 10.1016/j.bbi.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silva D, Pais de Lacerda A. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev Port Cardiol. 2012;31(11):733–745. doi: 10.1016/j.repc.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Suarez EC. Relations of Trait Depression and Anxiety to Low Lipid and Lipoprotein Concentrations in Healthy Young Adult Women. Psychosomatic Medicine. 1999;61(3):273–279. doi: 10.1097/00006842-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Suarez EC. C-reactive protein is associated with psychologic risk factors of cardiovascular disease in apparently healthy adults. Psychosomatic Medicine. 2004;66:684–691. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- 75.Suarez EC, Boyle S. The relationship between insulin resistance and psychological risk factors of cardiovascular disease: Is inflammation the link?. Annual Meeting of the American Psychosomatic Society; Vancouver, Canada. 2005. [Google Scholar]
- 76.Sutin AR, Terracciano A, Deiana B, Uda M, Schlessinger D, Lakatta EG, Costa PT., Jr Cholesterol, triglycerides, and the Five-Factor Model of personality. Biological Psychology. 2010;84(2):186–191. doi: 10.1016/j.biopsycho.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thorand B, Baumert J, Kolb H, Meisinger C, Chambless L, Koenig W, Herder C. Sex differences in the prediction of Type 2 Diabetes by inflammatory markers: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Diabetes Care. 2007;30(4):854–860. doi: 10.2337/dc06-1693. [DOI] [PubMed] [Google Scholar]
- 78.Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, Schaan BD. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305(17):1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 79.Verma S, Yeh ETH. C-reactive protein and atherothrombosis—Beyond a biomarker: an actual partaker of lesion formation. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2003;285(5):R1253–R1256. doi: 10.1152/ajpregu.00170.2003. [DOI] [PubMed] [Google Scholar]
- 80.Ylonen K, Alfthan G, Groop L, Saloranta C, Aro A, Virtanen SM. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: the Botnia Dietary Study. American Journal of Clinical Nutrition. 2003;77(6):1434–1441. doi: 10.1093/ajcn/77.6.1434. [DOI] [PubMed] [Google Scholar]
- 81.Zairis MN, Ambrose JA, Lyras AG, Thoma MA, Psarogianni PK, Psaltiras PG, Foussas SG. C Reactive protein, moderate alcohol consumption, and long term prognosis after successful coronary stenting: four year results from the GENERATION study. Heart. 2004;90(4):419–424. doi: 10.1136/hrt.2003.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: Implications for atherosclerosis. Circulation. 2001;103(9):1194–1197. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
Additional References
- 83.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 84.Preacher KJ, Curran PJ, Bauer DJ. Computational Tools for Probing Interactions in Multiple Linear Regression, Multilevel Modeling, and Latent Curve Analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- 85.Johnson PO, Neyman J. Tests of certain linear hypotheses and their applications to some educational problems. Statistical Research Memoirs. 1936;1:57–93. [Google Scholar]