Abstract
The nutritional profile of an individual can influence the toxicity of persistent environmental toxicants. Polychlorinated biphenyls (PCBs), prevalent environmental pollutants, are highly lipid soluble toxic compounds that biomagnify through trophic levels and pose cancer, neuro-cognitive, and atherosclerotic risk to human populations. There is a growing body of knowledge that PCBs can initiate inflammatory responses in vivo, and this inflammation can be either exacerbated or ameliorated by nutrition. Data indicate that diets high in certain dietary lipids such as omega-6 fatty acids can worsen PCB-induced vascular toxicity while diets enriched with bioactive food components such as polyphenols and omega-3 polyunsaturated fatty acids can improve the toxicant-induced inflammation. There is evidence that bioactive nutrients protect through multiple cell signaling pathways, but we have shown that lipid raft caveolae and the antioxidant defense controller Nrf2 both play a predominant role in nutritional modulation of PCB-induced vascular toxicity. Interestingly, there appears to be intimate cross-talk between caveolae-related proteins and cellular Nrf2, and focusing on the use of specific bioactive food components that simultaneously alter both pathways may produce a more effective and efficient cytoprotective response to toxicant exposure. The use of nutrition as a protective tool is an economically beneficial means to address the toxicity of persistent environmental toxicants and may become a sensible means to protect human populations from PCB-induced vascular inflammation and associated chronic diseases.
Keywords: PCBs, POPs, nutrition, caveolae, Nrf2, PUFA, flavonoid
Introduction
Polychlorinated biphenyls are a diverse class of manmade chemicals that have been outlawed in the United States since the 1970’s, but still pose toxicological risks due to their persistence to environmental degradation (Arsenescu et al. 2008). Marketed for their effective thermal and electrical properties, PCBs became commonplace in capacitors, caulking, and hydraulic fluids but have become notorious for their correlations to multiple human toxicities ranging from endocrine disruption to vascular inflammation (Lundqvist et al. 2006). Although PCBs are detrimental to multiple target organ systems and tissue types (Crinnion 2011), the breadth of this review will encompass only vascular related toxicity. Of the 209 individual congeners that were manufactured, coplanar PCBs, which lack chlorine substitutions on ortho positions of both phenyl rings, are most toxic to the endothelium and associated vasculature (Giesy et al. 2000). These non-ortho substituted PCBs such as PCB 77 and PCB 126 are able to, much like dioxin, bind to the aryl-hydrocarbon receptor (AhR) found within vascular endothelial cells and increase reactive oxygen species (ROS) through cytochrome P450 1A1 (CYP1A1)-mediated uncoupling (Lim et al. 2008). Deregulation of cellular redox status can lead to upregulation of nuclear factor kappa B (NFκB) and subsequently the induction of multiple pro-inflammatory gene products including chemokines, cytokines, and cellular adhesion molecules (Majkova et al. 2009). Through these described pro-inflammatory pathways, PCBs have been shown to activate the endothelium and promote cellular dysfunction and ultimately atherosclerosis (Kuehn 2011; Toborek et al. 1995).
Polychlorinated biphenyls have been linked via human correlation studies to multiple vascular inflammatory complications and diseases including myocardial infarction, diabetes, stroke, and hypertension (Uemura 2012; Carpenter 2011; Goncharov et al. 2008). Historically, epidemiological studies have been focused on occupational exposures such as those that occurred with Swedish capacitor workers in the mid 20th century, but it is becoming more clear that chronic low dose exposure may pose the most risk for the general public (Schettgen et al. 2012; Gustavsson and Hogstedt 1997). For example, populations most at risk for chronic exposure are those that ingest high levels of fatty fish due to the fact that PCBs preferentially bioaccumulate in adipose tissue and thus can be transported vertically through the food chain (Goncharov et al. 2008; Butler Walker et al. 2003). Although Inuit populations have been observed to have 3.4-fold higher plasma PCB levels than Caucasians, low ppb concentrations are common for the general United States population (Hopf et al. 2009). Importantly, according to National Health and Nutrition Examination Survey (NHANES) there appears to be an association with plasma levels of PCBs and cardiovascular disease in women in the United States (Ha et al. 2007). Although these association studies are far from causative and can only correlate levels of PCBs with disease outcome, there are human studies that show PCB exposure can alter blood lipid profiles and increase total blood cholesterol and triglycerides, lending merit to the paradigm that environmental toxicants can promote or exacerbate vascular pathologies in humans (Goncharov et al. 2008).
Although exposure duration and congener type play a major role in overall PCB toxicity, there is increasing evidence that additional factors must be taken into consideration including individual genetic variability and lifestyle choices. For example, researchers have shown a higher incidence of PCB-associated breast and lung cancers in individuals with a single nucleotide polymorphism (SNP) in their CYP1A1 gene (Wang et al. 2011; Moysich et al. 1999). Although correlations between one’s genome and susceptibility to toxicants can be distinguished, obvious questions arise regarding the feasibility and cost/benefits of utilizing personalized medical techniques such as genome-wide arrays and sequencing technologies as preventative measures. Mutations in detoxifying-, antioxidant- or excretion-related genes may result in exacerbated PCB toxicity, but we argue that factors under the control of the individual, such as poor nutritional status, are more directly related to PCB-induced vascular toxicity.
Diet can exacerbate PCB-induced vascular toxicity
There is a large collection of evidence pointing to the role that a person’s dietary makeup and eating habits can play in the promotion of chronic inflammation, metabolic disorders and vascular diseases (Ganguly and Pierce 2012; Baum et al. 2012; Kuipers et al. 2011). Additionally, emerging data show that exposure to persistent organic pollutants such as PCBs may work in concert with unhealthy diets to promote cumulative or synergistic negative effects (Hennig et al. 1999). Newly coined “obesogen research,” which deals with the study of toxicants that can promote obesity and related syndromes, is a compound-driven area of study that is an important addition to the toxicological sciences, but we suggest that it does not focus enough on the interactions between diet and pollutant (Dirinck et al. 2011). Our data support the paradigm that a poor nutritional state can exacerbate toxicity associated with exposure to PCBs and other persistent organic pollutants (POPs), and taking into account the interactions between diet and toxicants will help to better elucidate the impacts that PCBs and related compounds have on human health.
Multiple research groups have shown negative interactions between unhealthy or refined diets and environmental pollutants ranging from heavy metals to PCBs. For example, high fat diets appear to intensify arsenic-induced inflammation, hepatofibrogenesis, and cancer initiation (Wu et al. 2008), while diets high in saturated fats exacerbate polycyclic aromatic hydrocarbon-induced adenomas (Harris et al. 2009). PCBs are prime pollutant candidates to study the interactions between diet and toxicants because they can sequester in adipose for long durations, and exposures are due often to ingestion of contaminated foods. Our research has focused on the interactions of PCBs and proinflammatory omega-6 fatty acids. We have shown that omega-6 fatty acids, and in particular linoleic acid, can cross-amplify the detrimental effects of coplanar PCBs and produce increases in oxidative stress, CYP1A1 induction, and endothelial permeability (Hennig et al. 1999). This work was expanded in vivo as we observed increased proinflammatory cytokine levels and lipid staining in mice fed oils rich in omega-6 fatty acids and exposed to PCB compared to mice exposed to PCB alone (Hennig et al. 2005b). Mechanistically, synergism between unhealthy diets (e.g., high-fat/high caloric diets) and toxicants makes logical sense because both factors act upon many of the same receptors and cell signaling pathways. For example, omega-6 fatty acids and PCBs have both been implicated in inflammatory initiation through AhR, cytochrome P450’s, and toll-like receptors (TLRs) (Fresno et al. 2011; Nebert and Karp 2008; Tompkins and Wallace 2007). With the growing rates of obesity and chronic oxidative and inflammatory stress, it is important to more thoroughly elucidate interactions between proinflammatory diets and toxicants. Although it does appear that unhealthy nutrition can work in concert with PCBs and related toxicants to create an increasingly proinflammatory phenotype, a new paradigm has emerged that implicates certain bioactive food components in protection against persistent organic pollutant-induced vascular toxicity.
Diet can improve PCB-induced vascular toxicity
Utilizing nutrition to bolster physiological health is a sensible and responsible way to protect against toxicity of environmental pollutants. Diets rich in bioactive food components such as polyphenols and omega-3 fatty acids have been correlated with decreased inflammation, metabolic syndrome, and atherosclerosis (Hennig et al. 2005a; Hennig et al. 2007). These diets, which are rich in antioxidant and anti-inflammatory agents, are well known as a part of French and Mediterranean diets (Nadtochiy and Redman 2011). It should not be a surprise then that heart disease and related pathologies are significantly lower in these areas when compared to similarly industrialized western regions (Urpi-Sarda et al. 2012). Although it is extremely difficult to determine a unique causative protective agent, bioactive nutrients such as resveratrol, quercetin, tea catechins, and docosahexaenoic acid (DHA) may all work independently or synergistically to decrease oxidative stress, inflammation, and vascular diseases (Choi et al. 2010; Moore et al. 2009; Poudyal et al. 2011). Since the hallmark of coplanar PCB vascular toxicity is also increased oxidative stress and inflammation, polyphenols (e.g., flavonoids) and omega-3 polyunsaturated fatty acids are prime nutritional candidates as biomodulators of PCB-mediated cytotoxicity.
Food phytochemicals have been shown to protect against POP-related toxicity. Polyphenols, for example, are an abundant and diverse class of bioactive compounds found in many fruits and vegetables and have been linked to decreased toxicity from dioxin and dioxin-like PCBs (Terao 2009). These ROS scavenging compounds can also increase PCB excretion rates (Morita et al. 1997), prevent AhR-induced inflammation (Han et al. 2012), limit body wasting (Ciftci and Ozdemir 2011) and decrease cellular dysfunction (Zheng et al. 2010). Resveratrol, a well-studied polyphenol, has been shown to interact with the primary receptor of coplanar PCBs, AhR, and to limit its activation and subsequent proinflammatory signaling cascade (Tutel’yan et al. 2003; Wu et al. 2001). A goal of our research is to determine the efficacy of causative compounds found in everyday healthy diets, such as Vitamin E, that can bolster protective physiological mechanisms and prevent against PCB-induced vascular toxicity (Slim et al. 1999). We understand that pharmacological drugs can be created that mimic the observed protective effects of nutrients, but we argue that due to costs, compliance issues and possible side-effects, utilizing healthy nutrition centered on bioactive food components will be most successful. Obviously, most western diets are not fruit and vegetable focused, so it is important to identify and classify lipid-based bioactive compounds as well.
Omega-3 polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are the main components of fish oil and have been shown to decrease inflammation, reduce vascular diseases, and protect against dioxin and PCB-mediated toxicity (Watkins et al. 2007; Turkez et al. 2012; Mozaffarian and Wu 2011). Unfortunately, most western diets lack adequate levels of omega-3 PUFAs, and the ratio of unhealthy omega-6 fats to omega-3 fats is very high (Watkins et al. 2007; Gomez Candela et al. 2011). Experimentally, it was determined that diets predominantly made up of linoleic acid (omega-6) increased PCB-induced cellular dysfunction, but this negative effect was blunted as the ratio favored protective omega-3’s (Wang et al. 2008). Interestingly, we have shown that mice fed a DHA-supplemented diet and subsequently exposed to coplanar PCB 126 exhibited a more profound antioxidant response as observed by higher expression levels of protective heme-oxygenase 1 (HO-1) and NAD(P)H:quinoneoxidoreductase 1 (NQO1) (unpublished data). This preliminary work illustrates that omega-3 PUFAs may help to protect against PCB-induced vascular toxicity by allowing for a more efficient and intense endogenous protective response that utilizes multiple physiological cell signaling pathways.
Importantly, PCBs have been shown to induce inflammation in cell types other than endothelial cells. Adipose tissue, and specifically adipocytes, has been shown in multiple settings as a target for PCB toxicity. For example, mice administered coplanar PCBs exhibit marked upregulation of proinflammatory adipokines, altered adipocyte differentiation and dislipidemia (Arsenescu et al. 2008). Although research illustrating the impact of phytochemicals on PCB-induced adipocyte toxicity is severely lacking, there is a large body of evidence describing the beneficial effects of bioactive nutrients on adipose inflammation. Bioactive food components such as curcumin, capsaicin, resveratrol and EGCG have all been shown to decrease adipose inflammation and adipocyte oxidative stress (Leiherer et al. 2013). Specifically, resveratrol has been shown to attenuate adipogenesis and adipose inflammation via the alteration of pro-inflammatory mediators, such as TLR4 (Kim et al. 2011). Physiological inflammation is a diverse phenomenon encompassing multiple tissue types, but, importantly, many cell types prone to inflammation have devised endogenous protective pathways to decrease oxidative stress and keep inflammation in check. Using bioactive food components that activate these pathways prior to toxicological insult may allow the body to more efficiently detoxify, eliminate or deal with environmental pollutants.
Protective cellular signaling pathways
Multiple signaling pathways have been attributed to nutritional modulation of environmental toxicants, but we have shown that caveolae and the antioxidant master controller nuclear factor (erythroid-derived 2)-like 2 (Nrf2) play integral protective roles. There is increasing evidence that lipid raft membrane microdomains, i.e. caveolae, may play an important role in atherosclerosis, the toxicity of coplanar PCBs, and nutritional protection (Sowa 2012; Han et al. 2010; Frank et al. 2004). Caveolae are flask shaped membrane invaginations that are important in cholesterol transport, nutrient and xenobiotic import into cells and cellular signaling (Pavlides et al. 2012). Due to the lipophilicity of PCBs, they may enter the cell through lipid raft mediated events or come into contact with caveolae-related signaling proteins. Caveolae are abundant in vascular endothelial cells and the cardiovascular system in general, which points to a highly probable role for caveolae in inflammation and atherosclerosis (Panneerselvam et al. 2012). Our laboratory has previously reported that coplanar PCBs promote the upregulation of genes related to the activation of endothelial cells and the initial stages of atherosclerosis and that the loss of functional caveolae ameliorates these detrimental effects (Majkova et al. 2010). Caveolin-1 (Cav-1), the major structural protein of caveolae, contains the “Cav-1 binding domain” that is known to bind multiple proteins including endothelial nitric oxide-synthase (eNOS), v-src sarcoma (SRC), protein kinase C (PKC), extracellular-signal-regulated kinase(ERk) and protein kinase B (Akt); many of which are involved in inflammatory pathways (Sowa 2012). For example, downregulation of Cav-1 can lead to eNOS activation and subsequent increases in diffusible nitric oxide, which has been shown to play a major role in healthy blood pressure and vessel tone (Lim et al. 2007). Many publications from our laboratory and others show downregulation and elimination of Cav-1 to be anti-atherosclerotic (Lim et al. 2008; Majkova et al. 2009). Importantly, eliminating Cav-1 prevents PCB-induced cellular dysfunction (Majkova et al. 2010). Our data point to Cav-1 as a possible anti-atherosclerotic therapeutic target and we hypothesize that nutritional intervention can downregulate Cav-1 and, in turn, protect against PCB-induced inflammation.
Nrf2 is a transcription factor that can upregulate cytoprotective genes in response to oxidative stress, xenobiotics, and bioactive food molecules (Singh et al. 2010; Itoh et al. 2010; Kim et al. 2010). Many nutrients, including resveratrol, sulforaphane, and epigallocatechin gallate (EGCG), have been shown to activate Nrf2 (Miao et al. 2012; Kang et al. 2012; Nair et al. 2010). There are multiple mechanisms of Nrf2 activation, including direct phosphorylation of Nrf2 by PKC delta and loss of contact between Nrf2 and inhibitory kelch-like ECH-associated protein 1 (Keap-1) (Niture et al. 2009). Upon activation, Nrf2 is able to evade ubiquitination, enter the nucleus, and bind cis-acting antioxidant response elements (ARE) in target genes such as heme-oxygenase 1 (HO-1) and NAD(P)H:quinoneoxidoreductase 1(NQO1) (Baird and Dinkova-Kostova 2011). Importantly, Nrf2 activation leads to decreased inflammation which is a hallmark of PCB toxicity (Kim et al. 2010). Activation of Nrf2 may lead to vascular protection from PCB-induced toxicity and we hypothesize that a diet rich in bioactive food components can activate Nrf2 and prevent PCB-induced inflammation. Nrf2 has been shown to cross-talk with multiple signaling partners, especially with the major player in PCB toxicity, AhR (Hayes et al. 2009). Although it has been known for decades that dioxin and dioxin-like compounds activate both AhR- and Nrf2-related genes, it was shown only recently that Nrf2 is required for induction of AhR genes such as CYP1A1 (Yeager et al. 2009). This observed cross-talk can be explained mechanistically at the genetic level by the fact that the promoter region for Nrf2 contains AhR binding regions and the gene promoter for AhR contains multiple Nrf2 binding elements (Hayes et al. 2009). Our laboratory previously determined that AhR is a binding partner of Cav-1, so it is plausible that cross-talk between caveolae and Nrf2 signaling pathways also exists (Lim et al. 2008).
Little is known about the cross-talk between Nrf2 and caveolae signaling and how bioactive compounds such as omega-3 lipids, flavonoids and other polyphenols interact to protect against environmental insults. We have shown that decreasing cellular Cav-1 levels results in a more intense antioxidant response. Mechanistically, we attribute this to decreased AhR activity as well as increased Nrf2 activity (Han et al. 2012). Recently, an intimate example of cross-talk between Cav-1 and Nrf2 was illustrated (Li et al. 2012), and our published and unpublished data supports this phenomenon. We have shown that decreasing Cav-1 via siRNA technology or utilizing Cav-1 KO mice can result in decreased expression of inhibitory Keap1, which allows for increased Nrf2 activation. Importantly, we have shown in vitro that pretreatment with the polyphenol EGCG can cause decreased expression of Cav-1 and upregulation of the Nrf2 target cytoprotective genes glutathione S-transferase (GST) and NQO1 (unpublished data). We believe that an efficient nutritional biomodulator will work through the observed cross-talk between caveolae and Nrf2 and result in broad and diverse cellular protection. These data support the paradigm that utilizing bioactive food components can be an effective strategy to combat PCB-induced vascular toxicity. Using bioactive food components such as flavonoids and omega-3 PUFAs may be an economically and logistically beneficial method to counteract PCB-induced vascular inflammation. Although it does appear that both caveolae and Nrf2 signaling pathways play an important role in nutritional modulation of vascular inflammation, other signaling cascades may also prove to be involved.
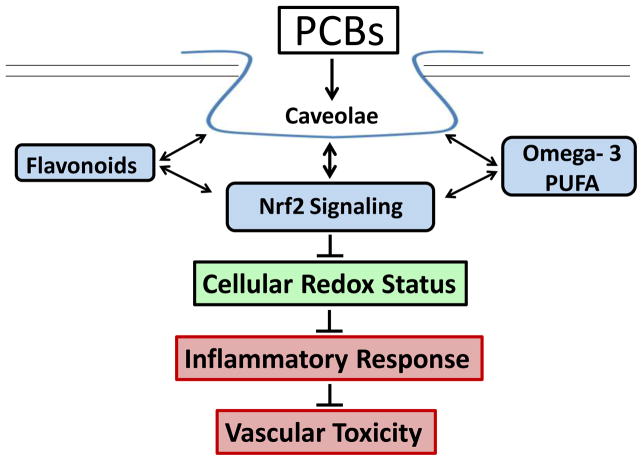
Phytochemicals may work through mechanisms that upregulate protective genes, downregulate pro-inflammatory genes and/or decrease overall oxidative stress (Fig. 1). For example, the green tea polyphenol EGCG has been shown to protect against inflammation via many of the previously discussed signaling mechanisms, but interesting new data also points to a role of the laminin receptor. The laminin receptor is a cell surface receptor that has been shown to play a role in inflammation and cancer metastasis, and multiple groups have shown EGCG to directly bind the receptor and subsequently decrease basophil activation, induce apoptosis in cancer cells, inhibit TLR4 and downregulate pro-inflammatory cytokines and chemokines (Muller and Pfaffl 2012; Byun et al. 2012). Studying the interactions between laminin receptor, nutrients and environmental toxicants may help to elucidate more effective nutritional modulation strategies to prevent vascular inflammation and disease.
Fig. 1.
Caveolae play a critical role in PCB-induced vascular toxicity. Protective bioactive nutrients such as omega-3 PUFAs and flavonoids can utilize caveolae Nrf2 cross-talk to decrease reactive oxygen species, limit inflammatory responses and ultimately prevent PCB-induced vascular toxicity
Emerging and unresolved issues
The paradigm of nutritional modulation of environmental toxicants is still new, thus many issues remain unresolved. One critically important area in need of further investigation involves the metabolism of protective nutrients. With the advent of more precise and high-resolution analytical techniques, researchers have finally begun to elucidate truly causative bioactive food components. In a physiological system, both nutrients and xenobiotics interact with similar cell signaling molecules and pathways. Both nutrients and toxicants are impacted by all aspects of absorption, distribution, metabolism and excretion, and understanding how bioactive compounds are altered or influenced at each step will allow for more efficient biomodulation. Specifically, the detection and identification of bioactive metabolites is of utmost importance due to the fact that many parent compounds are altered and modified in vivo. As mentioned previously, resveratrol is an extensively studied protective polyphenol, and exemplifies the necessity of further investigating the roles that bioactive metabolites play. For example, a major limitation with resveratrol supplementation involves poor gut absorption of the parent compound and fast metabolism to sulfate and glucuronide products (Wang et al. 2004; Urpi-Sarda et al. 2007). However, even with the short half-life of the parent compound, there is a large body of evidence associating resveratrol supplementation with protective phenotypes in vivo (Baur and Sinclair 2006). This has led some research groups to investigate the bioactivity of specific metabolites and observed protection via the activation of Sirtuin1(SIRT1) and inhibition of cyclooxygenase (Baur and Sinclair 2006). This is an important finding worthy of further exploration because bioactive metabolites, such as the glucuronide and sulfate forms of resveratrol, have much longer half-lives than the parent compound, which may qualify the metabolites as more appropriate and effective nutritional modulators (Rechner et al. 2002). Also, our laboratory has shown that oxidized metabolites of the bioactive omega-3 PUFA DHA are more protective against such PCB-induced vascular toxicity compared to the parent PUFA (Majkova et al. 2011). Mechanistically, oxidation of PUFAs can lead to multiple F-type isoprostanes with active cyclopentenone rings that have been shown to activate Nrf2, inhibit NFκB and decrease inflammation (Fam et al. 2002; Gao et al. 2007; Musiek et al. 2008). Also, other metabolically-relevant electrophilic fatty acid modifications, such as nitro-fatty acids, may play a role in combating inflammation and environmental toxicant-induced diseases (Khoo and Freeman 2010; Rudolph et al. 2010; Tsujita et al. 2011). Nitro-fatty acids are a newly discovered class of modified lipids that exhibit anti-inflammatory properties via multiple mechanisms (Freeman et al. 2008). Unsubstituted fatty acids such as oleic acid (18:1) and arachidonic acid (20:4) can be nitrated endogenously by multiple enzymatic and non-enzymatic reactions, resulting in the inhibition of pro-inflammatory mediators, induction of protective heme oxygenase-1 and the relaxation of blood vessels (Baker et al. 2009). This class of bioactive lipids can protect via multiple mechanisms including upregulation of PPARγ and Nrf2 as well as downregulation of pro-inflammatory NFκB (Kansanen et al. 2011; Borniquel et al. 2010). More work needs to be accomplished to elucidate the mechanistic impact of nitro and oxidized PUFAs on vascular inflammation and toxicant-induced diseases. It appears that both oxidized PUFAs and nitro-fatty acids are anti-inflammatory, which may implicate the importance of bioactive modified lipids in a clinical and therapeutic setting. Although research linking bioactive lipids, vascular inflammation and environmental toxicants is lacking, state of the art high-resolution mass spectroscopy technologies are beginning to allow researchers to explore the most efficient and physiologically-relevant bioactive compounds, which will in turn help further the use of nutrition in a clinical and preventative setting.
Conclusions
PCBs can cause vascular toxicity through ROS initiated inflammation, but the detrimental effects can be counteracted by nutritional modulation via bioactive food components that have potent antioxidant and anti-inflammatory properties. Diets high in polyphenols and omega-3 PUFAs, such as the Mediterranean diet, are fruit, nut, vegetable and fish based. Conversely, many western diets rely heavily on omega-6 fatty acids, saturated fats and refined carbohydrates. These comparably unhealthy diets can promote cellular dysfunction, inflammation and vascular diseases. Importantly, many people who are exposed to environmental pollutants, such as those surrounding Superfund sites, display poor nutritional profiles and lack protective bioactive food compounds in their diets (Hennig et al. 2012). Compromised physiological health, unhealthy diets and exposure to toxicants, such as PCBs, can cause exacerbated negative effects and increase the impact of environmental contaminants on populations. Our work aims to utilize nutritionally relevant compounds to ameliorate toxicant-induced disease phenotypes and to determine the intricate molecular mechanisms in play. We have shown that caveolae and Nrf2 signaling pathways are integral to vascular protection from PCBs and that newly discovered crosstalk between the two pathways may be extremely important physiologically (see Figure 1 for overview). New tools have arisen such as high-resolution mass spectroscopy that allow investigators to identify novel and modified bioactive compounds such as polyphenols, omega-3 PUFAs, and their metabolites. To bolster endogenous cellular defenses and protect against toxicant-induced vascular disorders we recommend a diet high in fruits, vegetables and omega-3 PUFAs, and low in refined foods rich in saturated fats and omega-6 fatty acids. Such positive nutritional lifestyle changes will lead to overall better health and thus reduce the vulnerability to negative risks associated with exposure to environmental stressors. Moreover, the use of healthy nutrition as a tool against the toxicity of environmental pollutants is an economically and socially viable alternative to expensive and logistically challenging ecological remediation.
Acknowledgments
This work was supported by NIH/NIEHS grant P42ES007380.
References
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environmental health perspectives. 2008;116(6):761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Archives of toxicology. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- Baker PR, Schopfer FJ, O’Donnell VB, Freeman BA. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free radical biology & medicine. 2009;46(8):989–1003. doi: 10.1016/j.freeradbiomed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, Rudel LL, Maki KC, Whelan J, Ramsden CE, Block RC. Fatty acids in cardiovascular health and disease: a comprehensive update. Journal of clinical lipidology. 2012;6(3):216–234. doi: 10.1016/j.jacl.2012.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews Drug discovery. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel disease. Free radical biology & medicine. 2010;48(4):499–505. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler Walker J, Seddon L, McMullen E, Houseman J, Tofflemire K, Corriveau A, Weber JP, Mills C, Smith S, Van Oostdam J. Organochlorine levels in maternal and umbilical cord blood plasma in Arctic Canada. The Science of the total environment. 2003;302(1–3):27–52. doi: 10.1016/s0048-9697(02)00319-4. [DOI] [PubMed] [Google Scholar]
- Byun EB, Choi HG, Sung NY, Byun EH. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor on lipopolysaccharide-stimulated dendritic cells. Biochemical and biophysical research communications. 2012;426(4):480–485. doi: 10.1016/j.bbrc.2012.08.096. [DOI] [PubMed] [Google Scholar]
- Carpenter D. Exposure to Polychlorinated Biphenyls Is Associated With an Increased Risk of Hypertension and Cardiovascular Disease. Epidemiology. 2011;22(1):S147–S147. doi: 10.1097/01.ede.0000392123.34740.fc. [DOI] [Google Scholar]
- Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environment international. 2010;36(8):931–934. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci O, Ozdemir I. Protective effects of quercetin and chrysin against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induced oxidative stress, body wasting and altered cytokine productions in rats. Immunopharmacology and immunotoxicology. 2011;33(3):504–508. doi: 10.3109/08923973.2010.543686. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Alternative medicine review: a journal of clinical therapeutic. 2011;16(1):5–13. [PubMed] [Google Scholar]
- Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, Van Gaal L. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring, Md) 2011;19(4):709–714. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- Fam SS, Murphey LJ, Terry ES, Zackert WE, Chen Y, Gao L, Pandalai S, Milne GL, Roberts LJ, Porter NA, Montine TJ, Morrow JD. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. The Journal of biological chemistry. 2002;277(39):36076–36084. doi: 10.1074/jbc.M205638200. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(1):98–105. doi: 10.1161/01.atv.0000101182.89118.e5. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. The Journal of biological chemistry. 2008;283(23):15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Archives of physiology and biochemistry. 2011;117(3):151–164. doi: 10.3109/13813455.2011.562514. [DOI] [PubMed] [Google Scholar]
- Ganguly R, Pierce GN. Trans fat involvement in cardiovascular disease. Molecular nutrition & food research. 2012;56(7):1090–1096. doi: 10.1002/mnfr.201100700. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. The Journal of biological chemistry. 2007;282(4):2529–2537. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K, Blankenship AL, Jones PD, Hilscherova K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Central European journal of public health. 2000;8(Suppl):43–45. [PubMed] [Google Scholar]
- Gomez Candela C, Bermejo Lopez LM, Loria Kohen V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: nutritional recommendations. Nutricion hospitalaria: organo oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral. 2011;26(2):323–329. doi: 10.1590/s0212-16112011000200013. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G, McCaffrey RJ, Rej R, Carpenter DO. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environmental research. 2008;106(2):226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) American journal of industrial medicine. 1997;32(3):234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environmental health perspectives. 2007;115(8):1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicology and applied pharmacology. 2010;246(1–2):74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Han SS, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicology and applied pharmacology. 2012;261(2):181–188. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DL, Niaz MS, Morrow JD, Mary K, Ramesh A. Diet as a Modifier of Benzo (a) pyrene Metabolism and Benzo (a) pyrene—Induced Colon Tumors in ApcMin mice. Interdisciplinary Studies on Environmental Chemistry—Environmental Research in Asia. 2009;2:227–238. [Google Scholar]
- Hayes JD, Dinkova-Kostova AT, McMahon M. Cross–ztalk between transcription factors AhR and Nrf2: lessons for cancer chemoprevention from dioxin. Toxicological sciences: an official journal of the Society of Toxicology. 2009;111(2):199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- Hennig B, Oesterling E, Toborek M. Environmental toxicity, nutrition, and gene interactions in the development of atherosclerosis. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2007;17(2):162–169. doi: 10.1016/j.numecd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hennig B, Ormsbee L, McClain CJ, Watkins BA, Blumberg B, Bachas LG, Sanderson W, Thompson C, Suk WA. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environmental health perspectives. 2012;120(6):771–774. doi: 10.1289/ehp.1104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Majkova Z, Oesterling E, Meerarani P, Toborek M. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovascular toxicology. 2005a;5(2):153–160. doi: 10.1385/ct:5:2:153. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environmental health perspectives. 2005b;113(1):83–87. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Slim R, Toborek M, Robertson LW. Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells. Journal of biochemical and molecular toxicology. 1999;13(2):83–91. doi: 10.1002/(sici)1099-0461(1999)13:2<83::aid-jbt4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hopf NB, Ruder AM, Succop P. Background levels of polychlorinated biphenyls in the U.S. population. The Science of the total environment. 2009;407(24):6109–6119. doi: 10.1016/j.scitotenv.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxidants & redox signaling. 2010;13(11):1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Hong YB, Kim HJ, Wang A, Bae I. Bioactive food components prevent carcinogenic stress via Nrf2 activation in BRCA1 deficient breast epithelial cells. Toxicology letters. 2012;209(2):154–160. doi: 10.1016/j.toxlet.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. The Journal of biological chemistry. 2011;286(16):14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo NK, Freeman BA. Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Current opinion in pharmacology. 2010;10(2):179–184. doi: 10.1016/j.coph.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutation research. 2010;690(1–2):12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochemical pharmacology. 2011;81(11):1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Environmental pollutants tied to atherosclerosis. JAMA: the journal of the American Medical Association. 2011;306(19):2081. doi: 10.1001/jama.2011.1666. [DOI] [PubMed] [Google Scholar]
- Kuipers RS, de Graaf DJ, Luxwolda MF, Muskiet MH, Dijck-Brouwer DA, Muskiet FA. Saturated fat, carbohydrates and cardiovascular disease. The Netherlands journal of medicine. 2011;69(9):372–378. [PubMed] [Google Scholar]
- Leiherer A, Mundlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascular pharmacology. 2013;58(1–2):3–20. doi: 10.1016/j.vph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Li W, Liu H, Zhou JS, Cao JF, Zhou XB, Choi AM, Chen ZH, Shen HH. Caveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2) The Journal of biological chemistry. 2012;287(25):20922–20930. doi: 10.1074/jbc.M112.352336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Majkova Z, Xu S, Bachas L, Arzuaga X, Smart E, Tseng MT, Toborek M, Hennig B. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chemico-biological interactions. 2008;176(2–3):71–78. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. American journal of physiology Heart and circulatory physiology. 2007;293(6):H3340–3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Zuurbier M, Leijs M, Johansson C, Ceccatelli S, Saunders M, Schoeters G, ten Tusscher G, Koppe JG. The effects of PCBs and dioxins on child health. Acta paediatrica (Oslo, Norway: 1992) Supplement. 2006;95(453):55–64. doi: 10.1080/08035320600886257. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicology and applied pharmacology. 2011;251(1):41–49. doi: 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicology and applied pharmacology. 2009;237(1):1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Toborek M, Hennig B. The role of caveolae in endothelial cell dysfunction with a focus on nutrition and environmental toxicants. Journal of cellular and molecular medicine. 2010;14(10):2359–2370. doi: 10.1111/j.1582-4934.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X, Bai Y, Su W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its down-stream antioxidants. Nutrition & metabolism. 2012;9(1):84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Jackson KG, Minihane AM. Green tea (Camellia sinensis) catechins and vascular function. The British journal of nutrition. 2009;102(12):1790–1802. doi: 10.1017/s0007114509991218. [DOI] [PubMed] [Google Scholar]
- Morita K, Matsueda T, Iida T. Effect of green tea (matcha) on gastrointestinal tract absorption of polychlorinated biphenyls, polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxins in rats. Fukuoka igaku zasshi = Hukuoka acta medica. 1997;88(5):162–168. [PubMed] [Google Scholar]
- Moysich KB, Shields PG, Freudenheim JL, Schisterman EF, Vena JE, Kostyniak P, Greizerstein H, Marshall JR, Graham S, Ambrosone CB. Polychlorinated biphenyls, cytochrome P4501A1 polymorphism, and postmenopausal breast cancer risk. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1999;8(1):41–44. [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Muller J, Pfaffl MW. Synergetic downregulation of 67 kDa laminin receptor by the green tea (Camellia sinensis) secondary plant compound epigallocatechin gallate: a new gateway in metastasis prevention? BMC complementary and alternative medicine. 2012;12:258. doi: 10.1186/1472-6882-12-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Brooks JD, Joo M, Brunoldi E, Porta A, Zanoni G, Vidari G, Blackwell TS, Montine TJ, Milne GL, McLaughlin B, Morrow JD. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. The Journal of biological chemistry. 2008;283(29):19927–19935. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman EK. Mediterranean diet and cardioprotection: the role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition (Burbank, Los Angeles County, Calif) 2011;27(7–8):733–744. doi: 10.1016/j.nut.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Barve A, Khor TO, Shen GX, Lin W, Chan JY, Cai L, Kong AN. Regulation of Nrf2- and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta pharmacologica Sinica. 2010;31(9):1223–1240. doi: 10.1038/aps.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Karp CL. Endogenous functions of the aryl hydrocarbon receptor (AHR): intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. The Journal of biological chemistry. 2008;283(52):36061–36065. doi: 10.1074/jbc.R800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. Journal of cell science. 2009;122(Pt 24):4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Panneerselvam M, Patel HH, Roth DM. Caveolins and heart diseases. Advances in experimental medicine and biology. 2012;729:145–156. doi: 10.1007/978-1-4614-1222-9_10. [DOI] [PubMed] [Google Scholar]
- Pavlides S, Gutierrez-Pajares JL, Danilo C, Lisanti MP, Frank PG. Atherosclerosis, caveolae and caveolin-1. Advances in experimental medicine and biology. 2012;729:127–144. doi: 10.1007/978-1-4614-1222-9_9. [DOI] [PubMed] [Google Scholar]
- Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Progress in lipid research. 2011;50(4):372–387. doi: 10.1016/j.plipres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free radical biology & medicine. 2002;33(2):220–235. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NK, Hasty AH, Baldus S, Freeman BA. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(5):938–945. doi: 10.1161/atvbaha.109.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettgen T, Gube M, Esser A, Alt A, Kraus T. Plasma polychlorinated biphenyls (PCB) levels of workers in a transformer recycling company, their family members, and employees of surrounding companies. Journal of toxicology and environmental health Part A. 2012;75(8–10):414–422. doi: 10.1080/15287394.2012.674905. [DOI] [PubMed] [Google Scholar]
- Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free radical research. 2010;44(11):1267–1288. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- Slim R, Toborek M, Robertson LW, Hennig B. Antioxidant protection against PCB-mediated endothelial cell activation. Toxicological sciences: an official journal of the Society of Toxicology. 1999;52(2):232–239. doi: 10.1093/toxsci/52.2.232. [DOI] [PubMed] [Google Scholar]
- Sowa G. Caveolae, caveolins, cavins, and endothelial cell function: new insights. Frontiers in physiology. 2012;2:120. doi: 10.3389/fphys.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao J. Dietary flavonoids as antioxidants. Forum of nutrition. 2009;61:87–94. doi: 10.1159/000212741. [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. Journal of biochemical toxicology. 1995;10(4):219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Tompkins LM, Wallace AD. Mechanisms of cytochrome P450 induction. Journal of biochemical and molecular toxicology. 2007;21(4):176–181. doi: 10.1002/jbt.20180. [DOI] [PubMed] [Google Scholar]
- Tsujita T, Li L, Nakajima H, Iwamoto N, Nakajima-Takagi Y, Ohashi K, Kawakami K, Kumagai Y, Freeman BA, Yamamoto M, Kobayashi M. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes to cells: devoted to molecular & cellular mechanisms. 2011;16(1):46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Yousef MI. Ameliorative effect of docosahexaenoic acid on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced histological changes, oxidative stress, and DNA damage in rat liver. Toxicology and industrial health. 2012;28(8):687–696. doi: 10.1177/0748233711420475. [DOI] [PubMed] [Google Scholar]
- Tutel’yan VA, Gapparov MM, Telegin LY, Devichenskii VM, Pevnitskii LA. Flavonoids and resveratrol as regulators of Ah-receptor activity: protection from dioxin toxicity. Bulletin of experimental biology and medicine. 2003;136(6):533–539. doi: 10.1023/b:bebm.0000020196.18460.99. [DOI] [PubMed] [Google Scholar]
- Uemura H. Associations of exposure to dioxins and polychlorinated biphenyls with diabetes: based on epidemiological findings. Nihon eiseigaku zasshi Japanese journal of hygiene. 2012;67(3):363–374. doi: 10.1265/jjh.67.363. [DOI] [PubMed] [Google Scholar]
- Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martinez P, Arranz S, Andres-Lacueva C, Llorach R, Medina-Remon A, Lamuela-Raventos RM, Estruch R. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacological research: the official journal of the Italian Pharmacological Society. 2012;65(6):577–583. doi: 10.1016/j.phrs.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Urpi-Sarda M, Zamora-Ros R, Lamuela-Raventos R, Cherubini A, Jauregui O, de la Torre R, Covas MI, Estruch R, Jaeger W, Andres-Lacueva C. HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clinical chemistry. 2007;53(2):292–299. doi: 10.1373/clinchem.2006.071936. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Zheng Y, Sun L, Wang L, Yu PB, Li HL, Tian XP, Dong JH, Zhang L, Xu J, Shi W, Ma TY. CYP1A1 Ile462Val polymorphism and susceptibility to lung cancer: a meta-analysis based on 32 studies. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 2011;20(6):445–452. doi: 10.1097/CEJ.0b013e328345f937. [DOI] [PubMed] [Google Scholar]
- Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chemico-biological interactions. 2008;172(1):27–38. doi: 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Heredia A, Song H, Zhang Z, Yu B, Davis C, Redfield R. Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity. Journal of pharmaceutical sciences. 2004;93(10):2448–2457. doi: 10.1002/jps.20156. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Hannon K, Ferruzzi M, Li Y. Dietary PUFA and flavonoids as deterrents for environmental pollutants. The Journal of nutritional biochemistry. 2007;18(3):196–205. doi: 10.1016/j.jnutbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu J, Waalkes MP, Cheng ML, Li L, Li CX, Yang Q. High dietary fat exacerbates arsenic-induced liver fibrosis in mice. Experimental biology and medicine (Maywood, NJ) 2008;233(3):377–384. doi: 10.3181/0710-rm-269. [DOI] [PubMed] [Google Scholar]
- Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review) International journal of molecular medicine. 2001;8(1):3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicological sciences: an official journal of the Society of Toxicology. 2009;111(2):238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Toborek M, Hennig B. Epigallocatechin gallate-mediated protection against tumor necrosis factor-alpha-induced monocyte chemoattractant protein-1 expression is heme oxygenase-1 dependent. Metabolism: clinical and experimental. 2010;59(10):1528–1535. doi: 10.1016/j.metabol.2010.01.018. [DOI] [PubMed] [Google Scholar]