Conspectus
The study of ordered mesoporous silica materials has exploded since their discovery by Mobil researchers twenty years ago. The ability to make uniformly sized, porous, and dispersible nanoparticles using colloidal chemistry and evaporation-induced self-assembly has led to many applications of mesoporous silica nanoparticles (MSNPs) as ‘nanocarriers’ for delivery of drugs and other cargos to cells. The exceptionally high surface area of MSNPs, often exceeding 1000 m2/g, and ability to independently modify pore size and surface chemistry, enables the loading of diverse cargos and cargo combinations at levels exceeding those of other common drug delivery carriers such as liposomes or polymer conjugates. This is because non-covalent electrostatic, hydrogen-bonding and van der Waals interactions of the cargo with the MSNP internal surface cause preferential adsorption of cargo to the MSNP, allowing loading capacities to surpass the solubility limit of a solution or that achievable by osmotic gradient loading. The ability to independently modify the MSNP surface and interior makes possible engineered bio-functionality and bio-compatibility.
In this Account, we detail our recent efforts to develop MSNPs as biocompatible nanocarriers that simultaneously display multiple functions including: 1) high visibility/contrast in multiple imaging modalities, 2) dispersibility, 3) binding specificity to a particular target tissue or cell type, 4) ability to load and deliver large concentrations of diverse cargos, and 5) triggered/controlled release of cargo. Toward (1), we chemically conjugated fluorescent dyes or incorporated magnetic nanoparticles to enable in-vivo optical or magnetic resonance imaging. For (2), we have made MSNPs with polymer coatings, charged groups or supported lipid bilayers, which decrease aggregation and improve stability in saline solutions. For (3) and (4), we have enhanced passive bioaccumulation via the enhanced permeability and retention effect by modifying the MSNP surfaces with positively charged polymers. We have also chemically attached ligands to MSNPs that selectively bind to receptors over-expressed in cancer cells. We have used encapsulation of MSNPs within reconfigurable supported lipid bilayers to develop new classes of responsive nanocarriers that actively interact with the target cell. Toward (4), we exploit the high surface area and tailorable surface chemistry of MSNPs to retain hydrophobic drugs. Finally, for (5), we have engineered dynamic behaviors by incorporating molecular machines within or at the entrances of MSNP pores and by using ligands, polymers, or lipid bilayers. These provide a means to seal-in and retain cargo and to direct MSNP interactions with and internalization by target cells.
MSNPs’ application as nanocarriers requires biocompatibility and low toxicity. Here the intrinsic porosity of the MSNP surface reduces the extent of hydrogen bonding and/or electrostatic interactions with cell membranes as does surface coating with polymers or lipid bilayers. Furthermore, the high surface area and low extent of condensation of the MSNP siloxane framework promote a high rate of dissolution into soluble silicic acid species, which are found to be non-toxic. Potential toxicity is further mitigated by the high drug capacity of MSNPs, which greatly reduces needed dosages compared to other nanocarriers. We anticipate that future generations of MSNPs incorporating molecular machines and encapsulated by membrane-like lipid bilayers will achieve a new level of controlled cellular interactions.
1. Introduction
History of Mesoporous Silica Nanoparticles (MSNP)
In their classic paper, Kresge and co-workers1 described a means of combining sol-gel chemistry with liquid-crystalline templating to create new classes of ordered porous molecular sieves characterized by periodic arrangements of uniformly sized mesopores (defined by IUPAC as pores with diameters between 2 and 50-nm) incorporated within an amorphous silica matrix. Controlled synthesis of spherical and shaped mesoporous silica nanoparticles (MSNP) has since been achieved by solution routes or by an aerosol-based evaporation induced self-assembly2 (EISA) process (Gallery Figure 2), and the pore surfaces have been modified with a wide range of chemical moieties based mainly on silane coupling chemistries. Now there exist MSNPs with varied internal and external surface chemistries and quite sophisticated, environmentally responsive characteristics including optical or pH modulation of molecular transport. This ACR will review our recent efforts to develop biocompatible MSNPs with engineered functionalities and dynamic behaviors needed for the emerging field of nanoparticle based drug delivery and diagnostics. A successful biocompatible nanocarrier must exhibit low toxicity combined with size uniformity, large capacity for diverse cargos, high traceability, colloidal stability, selective cell specific binding and internalization, and triggered cargo release. We show how specific, engineered chemical modifications of MSNPs result in functional and biocompatible nanocarriers. Both covalent modification and non-covalent encapsulation of MSNPs within polymers or supported lipid bilayers (SLB) enable nanocarrier imaging in both in vitro and in vivo systems, dispersion stability in bio-relevant media, directed and cell specific uptake and internalization, and storage and delivery of both hydrophilic and hydrophobic drugs.
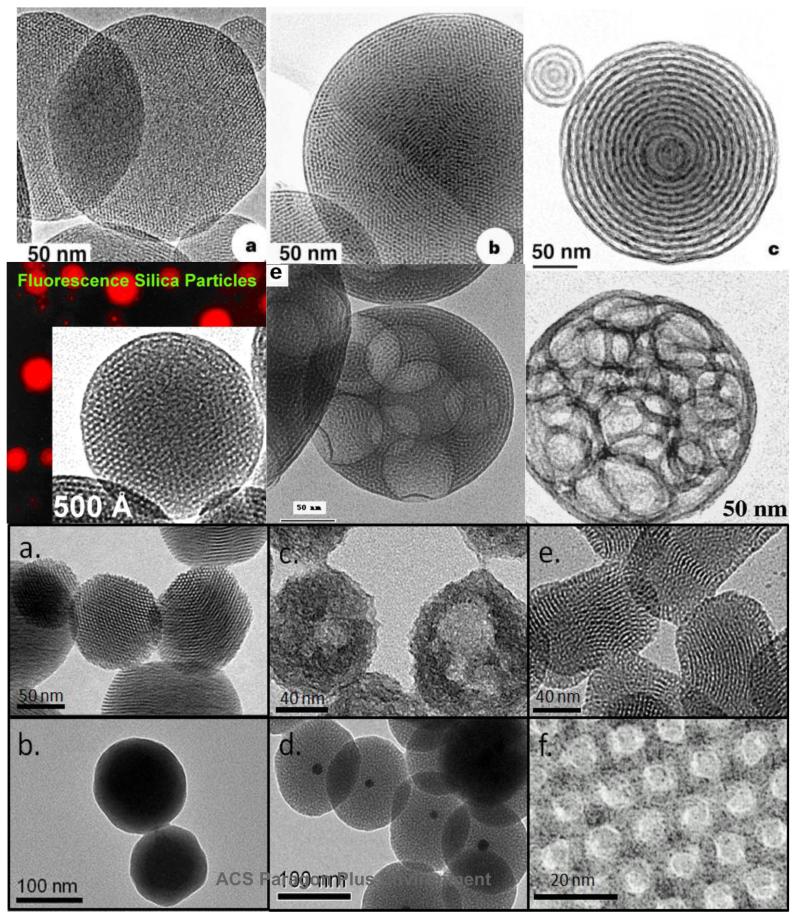
FIGURE 2.
Gallery of mesoporous silica nanoparticles. Particles in a, b, c, and d are formed by EISA. Lower panel are solution prepared MSNPs.7
2. Synthetic Routes to Mesoporous Silica Solution based synthesis of MSNPs
The most widely used type of MSNP is MCM-41, composed of ordered hexagonally arranged cylindrical mesopores.1, 3 Since its introduction by the Mobil company as micrometer sized, amorphous aggregates, improved synthetic procedures enabled the formation of MCM-41 as successively smaller particles with controlled shape: sub-μm spheres,4 100-150 nm nanoparticles suitable for in-vitro studies,5 mono-disperse 50-75 nm nanoparticles,6 and recently, 25 nm MCM-41 like MSNPs.7 The rapid progression of MCM-41 into a mono-disperse, <100 nm size MSNP reflects the emerging demand for biocompatible, functional nanocarriers for biological applications.
The synthesis of MCM-41 involves liquid crystal templating using an alkylammonium salt, commonly cetyl trimethylammonium bromide (CTAB). In aqueous solution, above the critical micelle concentration, amphiphilic surfactant molecules self-assemble into spherical micelles and at higher concentrations, into periodic liquid crystal mesophases. Conducted in the presence of water and hydrophilic, soluble silica precursors (e.g. silicic acid, Si(OH)4 or polysilicic acids), surfactant self-assembly results in hybrid nanocomposites. Through electrostatic and hydrogen bonding interactions, the silica precursors are concentrated at hydrophilic interfaces and condense to form an amorphous silica mold of the ordered periodic mesophase. Subsequent removal of the surfactant template by extraction or calcination results in the mesoporous product.
Evaporation Induced Self-Assembly (EISA)
EISA was established in 1997 as a means to direct the formation of continuous thin film mesophases via dip-coating.8 EISA starts with a homogeneous solution of soluble silica and surfactant in ethanol/water with an initial surfactant concentration of co << cmc. Solvent evaporation during dip-coating or any evaporative process2, 8-11 progressively increases surfactant concentration, driving self-assembly of silica-surfactant micelles and their further organization into liquid-crystalline mesophases. A logical extension of the EISA thin film process was to use aerosol processing to direct the formation of spherical mesoporous nanoparticles.10 Compared to solution routes, a potential advantage of EISA is that any non-volatile component that can be introduced into an aerosol droplet is inevitably incorporated within the MSNP, where the liquid-crystalline nature of the silica-surfactant mesophase allows the foreign object to be conformally encapsulated.
3. Methods of MSNP Modification Chemical Modification
MSNP functionality can be introduced by modifying silanol groups present both within the pore interiors and the outer surface. These groups are chemically accessible and can be facilely reacted with alkoxysilane derivatives to introduce organic functionality. Chemical moieties can also be adsorbed onto MSNP, especially facilitated by negatively charged SiO− groups, resulting from deprotonation of surface silanol groups at neutral pH, which result in attractive electrostatic interactions with positively charged mioieties (Figure 1). Generally, two main routes of modification exist: co-condensation, and post-synthetic grafting.
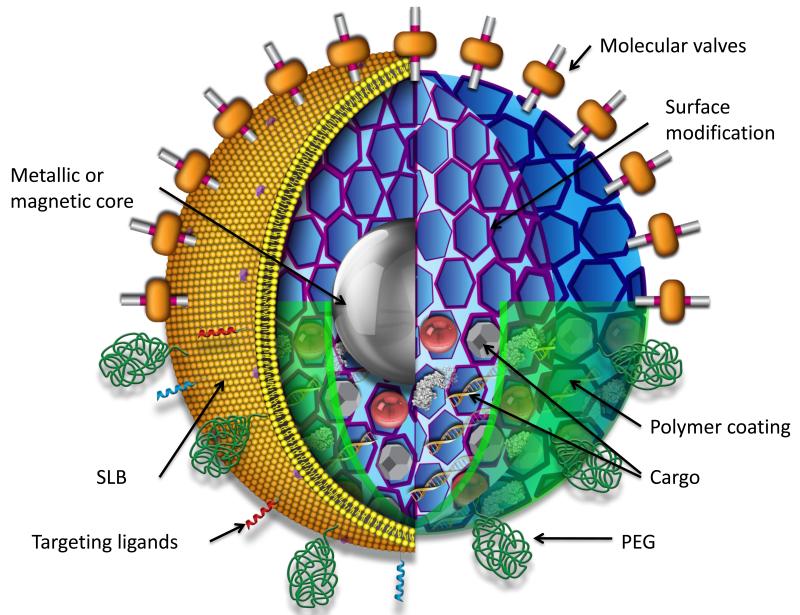
FIGURE 1.
Schematic of multifunctional mesoporous silica nanoparticle showing possible core/shell design, surface modifications, and multiple types of cargos. cargos
Co-condensation
The co-condensation method involves co-condensing hydrolyzed alkoxysilanes with organoalkoxysilanes (R’xSi(OR)4-x) which results in a directed modification of the interior pore surface.12 The amphiphilic nature of the hydrolyzed organosilane allows it to serve as a cosurfactant that is incorporated into the surfactant micelle. As silica is condensed, the organoalkoxysilane is co-condensed positioning the organic moiety directly onto the pore walls.
An extension of the co-condensation method is the synthesis of MSNP surrounding a metal or metal oxide nanoparticles in a core-shell architecture (Figure 2).13 Core-shell MSNPs have seen many recent applications in theranostics – the combination of therapy and diagnostics.14
Post-synthetic grafting
A second method, post-synthetic grafting, involves modification of MSNPs after synthesis. This method employs surface accessible silanol groups both within the mesopore network as well as on the exterior MSNP surfaces. Maximal surface coverage of interior mesopores is achieved via condensation with trifunctional organosilanes R’Si(OR)3 in an organic solvent and produces a self-assembled monolayer.15 To restrict/bias the deposition to the exterior surface of the MSNP, the modification can be performed prior to extracting the templating agent. The templating agent can then be removed, and the protected, unreacted silanol groups in the pore interiors can be further modified. In this way, functionalization can be directed. The various types of MSNP modifications and their strategies for synthesis are summarized in Figure 3. Using these strategies, MSNPs can be engineered with functionalities to achieve specific bio-relevant properties.
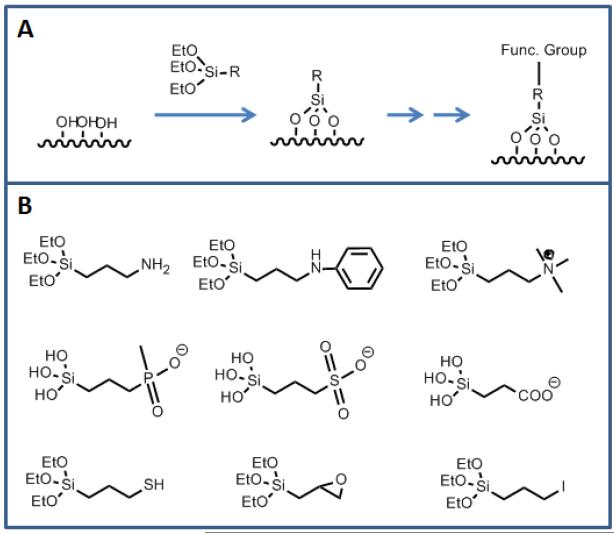
FIGURE 3.
(a) Schematic showing reaction of surface silanol groups with an alkoxysilane linker to introduce functionality. (b) Various linkers to attach bio-molecules or to change the surface properties.
Surface Coating
Introducing functional groups on the MSNP exterior surface gives rise to additional surface properties. They can be further reacted as linkers to attach larger molecules or used to adsorb coatings through non-covalent interactions (Figures 1, 4). For the latter case, polymers are commonly employed on MSNP.6, 16-18 Due to the intrinsic negative charge of the silica surface resulting from deprotonation of surface silanols, bare nanoparticles can be electrostatically functionalized with a positively charged polymer. Polymers or other surface bound functional groups can also be used to retain cargo within the MSNP.
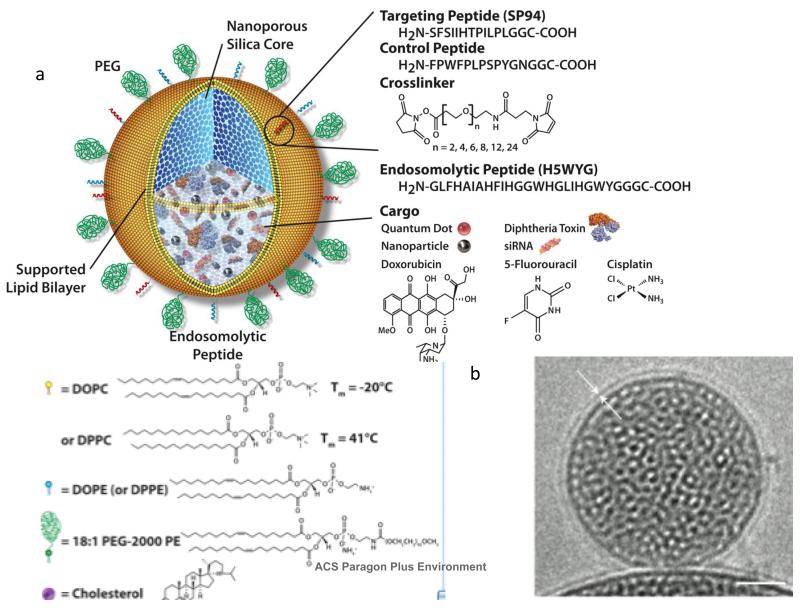
FIGURE 4.
Schematic (a) and cryo-TEM image (b) of protocells.
An alternative means of surface coating MSNP is by the fusion with phospholipid bilayers to form a construct referred to as a protocell (Figure 4).19-21 The cryo-TEM image (Figure 4b) shows a mesoporous silica particle core prepared by EISA enveloped by a conformal, 4-nm thick supported lipid bilayer (SLB). The properties of the SLB can be varied widely using lipids with differing fluidities/melting transition temperatures and headgroup chemistries that dictate charge and chemical reactivity. Membrane bound components like cholesterol and PEG can be introduced to control the fluidity and stability of the SLB, and it can be chemically conjugated with ligands to effect targeting and internalization (vide infra). As with polymer coatings, the SLB can serve to retain cargo introduced into the MSNP interior.
4. Modified MSNP for Biological Applications
A burgeoning area of MSNP research has been their use as nanocarriers in biology.28 Consequently, much of our present work has been directed towards tailoring MSNP properties in order to improve their bio-functionality and bio-compatibility. To be effective and universally applicable, nanocarriers must simultaneously demonstrate multiple functions / characteristics including: 1) ease of imaging, 2) dispersibility, 3) specificity, 4) ability to load and deliver large concentrations of diverse cargos, and 5) biocompatibility/low toxicity. Their inherent high surface area, versatile surface chemistry and low toxicity, confers to MSNPs the characteristics of an ideal nanocarrier platform.
Imaging
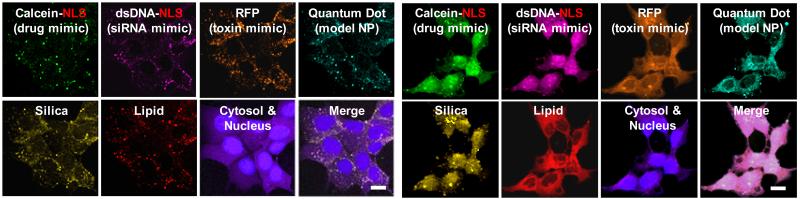
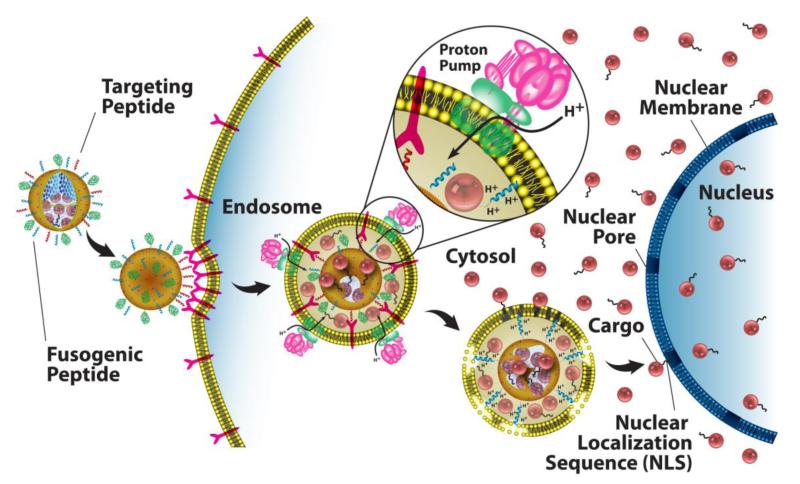
Direct imaging of MSNP under bio-relevant conditions can be used to follow biodistribution, cancer cell targeting efficiency, internalization pathways, cytotoxicity, and the progress of therapy. MSNPs are multifunctional in that the core can be loaded or derivatized with fluorescent dyes, quantum dots, as well as therapeutic agents. Fluorescein isothiocyanate (FITC) and Rhodamine B isothiocyanate (RITC) are the most common fluorescent compounds that are incorporated into the MSNP core. Near-IR dyes, such as AlexaFluor 700 and DyLight 680, have also been used in MSNP for in vivo imaging. This type of fluorescent labeling is fairly robust and can be easily achieved. The resulting fluorescent MSNPs are capable of generating high-resolution, multi-channel images and can also provide quantitative data using flow cytometry techniques.16 As an example, Figure 5a shows eight channel hyperspectral confocal images of four fluorescently labeled cargo classes delivered using targeted protocells. Colocalization of the cargo, silica, and lipid after fifteen minutes is consistent with a receptor-mediated endocytotic pathway (Figure 6).19 After twelve hours (Figure 5b), pH-triggered cargo release and endosomal disruption delivers the cargo into the cytosol.
FIGURE 5.
Hyperspectral confocal imaging of targeted delivery of multicomponent cargos in protocells. Alexa Fluor 532-labelled nanoporous silica cores (yellow) were loaded with calcein (green), an Alexa Fluor 647-labelled dsDNA oligonucleotide (magenta), RFP (orange) and CdSe/ZnS quantum dots (teal). Cargos were sealed in the cores by fusion of Texas Red-labeled DOPC liposomes (red). (Adapted from ref. 17).
FIGURE 6.
Schematic diagram depicting the successive steps of multivalent binding and internalization of targeted MSNP supported lipid bilayers, followed by endosomal escape and nuclear localization of MSNP-encapsulated cargo. (Adapted from ref. 17).
Another relatively new method of imaging MSNP is to incorporate magnetic nanoparticles, i.e. iron oxide, as cores of MSNP, which allows T2-weighted MRI imaging.16 Alternatively, T1-weighted imaging can be achieved using a chelated gadolinium compound.22
Dispersibility
For their use in biomedical applications, MSNP must remain highly dispersed requiring colloidal stability. If aggregated, cell internalization suffers, biodistribution is difficult to control and larger effective particle sizes may lead to potentially higher toxicity (see MSNP Toxicity). Particle agglomeration can be reduced by chemically modifying the surfaces,23 introducing surface coatings with proteins and/or polymers,6 and by coating with a supported lipid bilayer.19-21 These methods provide steric hindrance and electrostatic repulsion to achieve stable saline dispersions of MSNP.
Targeting Specificity
To limit the degree of nonspecific binding while enhancing specific internalization by the target cell or tissue, MSNPs can be actively targeted towards an intended region. Despite the success in developing MSNPs that passively accumulate at the site of interest, active targeting also plays an important role in enhancing overall bioavailability. Passive targeting schemes rely on the enhanced permeability of tumor vasculature (the so-called enhanced permeability and retention (EPR) effect)6 to direct accumulation of nanocarriers at tumor sites, but the lack of cell-specific interactions needed to induce nanocarrier internalization decreases therapeutic efficacy and can result in drug expulsion and induction of multiple drug resistance (MDR).24 Selective targeting strategies employ ligands that specifically interact with receptors on the cell surface of interest to promote nanocarrier binding and internalization.25 This strategy requires that receptors are highly overexpressed by cancer cells (104–105 copies/cell) relative to normal cells.
In terms of passive targeting, Xia et al demonstrated that cationic polymer (PEI) coating of MSNPs significantly facilitates their uptake.17 Meng et al showed that through combined size-control and PEI/PEG copolymer coating, an enhanced EPR effect can be observed on a xenograft model.6
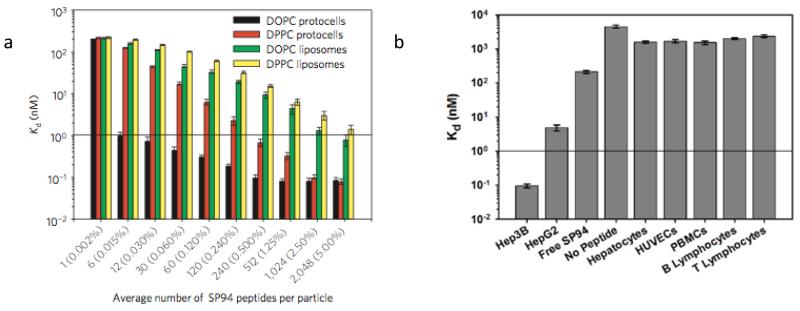
Active targeting employs ligands that bind specifically to receptors over-expressed on the cancer cell surface. Bio-active ligands, such as folate, RGD peptide and transferrin have been employed26 due to their respective receptors being over-expressed on many different cancer cell types. In general, high specificity and binding affinity require a high concentration of surface conjugated ligands to promote multivalent binding effects, which result in more efficient drug delivery through receptor-mediated internalization pathways (Figure 6). However, high ligand densities can promote nonspecific interactions with endothelial and other non-cancerous cells and increase immunogenicity, resulting in opsonization-mediated clearance of nanocarriers. In this regard, the MSNP supported lipid bilayer construct (protocell) provides some potential advantages as its fluid SLB enables targeting ligand recruitment to target cell surface receptors, promoting high avidity with a low overall peptide concentration (Figure 7).
FIGURE 7.
Selective and non-selective binding characteristics of peptide (SP94)-targeted protocells. a) Dissociation constants (Kd) of SP94-targeted protocells and liposomes for the target hepatocarcinoma cell, Hep3B, as a function of the average number of SP94 peptides per particle (average SP94 wt% is in parentheses). b) Dissociation constants (Kd) of SP94-targeted protocells for the target Hep3B and selected non-target control cells. Peptide density is 0.015wt%. All surface-binding experiments were conducted at 4° C to prevent internalization of targeted protocells and liposomes. All error bars in a and b represent 95% confidence intervals (1.96 σ) for n = 5.17 and supplemental information
Cargo Loading and Delivery
The high surface area and controllable chemistry of the MSNPs allow for simple loading of high concentrations12, 19, 20 of diverse classes and combination of cargos that can be delivered by endocytosis (Figure 6) or macropinocytosis. Early studies of drug delivery using MSNP focused on drugs exhibiting low solubility in water, in particular, Ibuprofen and Aspirin.27 Lu and Liong et al later demonstrated the delivery of a hydrophobic chemotherapeutic agent, Camptothecin, into cancer cells using MSNP.23 The high interior volume and surface area of MSNPs allows hydrophobic compounds to be loaded into the pores from a non-aqueous solution and be retained in aqueous environments. When MSNP are internalized through endocytosis (Figure 6), it is envisioned that lipid membrane components facilitate the phase-transfer of the stored hydrophobic payload enabling it to be gradually released.
When a hydrophilic cargo is involved, further modification of the MSNP is often required. Meng and Liong et al attached negatively charged groups onto MSNP to enable the loading and retention of the positively charged hydrophilic drug, Doxorubicin (DOX).18 Compared to FDA-approved Doxil, the Doxorubicin capacity in MSNPs can be nearly 1000-times greater due to the high surface area and attractive electrostatic interactions.19
For most nanocarrier delivery strategies, the cargo must be retained within the nanocarrier and released only upon delivery to the target cell. For MSNPs this generally requires that either the cargo be strongly adsorbed, as for DOX described above, or that the pores be ‘sealed’ after cargo loading. A method where quantum dots and small nanoparticles (caps) were used to block the entrance of pores was developed.28 Numerous strategies have been demonstrated where, by constructing a supramolecular nanovalve on the outer surface of MSNP, one can achieve controlled release via a variety of stimuli, such as pH change, enzymatic ligand cleavage, light, and externally applied magnetic fields.29-32 Polymer coatings33 or supported lipid bilayers19-21 have also been proven effective in sealing cargos within MSNP, where in the latter case endosome-disrupting peptides were used to achieve pH-triggered cargo release into the cytosol.
5. Toxicity
MSNP Toxicity
A critical issue for any nanocarrier application is nanoparticle toxicity. The toxicity of silicon dioxide, both crystalline and amorphous, has been studied for more than a century, especially as it relates to silicosis,34-37 and recently, the toxicity of silica nanoparticles has been extensively investigated, because the high surface to volume ratio of nanoparticles could lead to enhanced cellular interactions and different pathways of toxicity compared to coarse-grained silica.6 In spite of hundreds of studies of the toxicity of amorphous and crystalline silicas,34-37 the mechanism(s) by which silica exposure leads to silicosis remain unclear, and literature reports are at times contradictory. Here it should be emphasized that all silica is not created equal. The amorphous silica framework and surface chemistry, in particular hydroxyl coverage38 and size and distribution of siloxane rings that comprise the framework structure39 can exhibit a wide range of configurations depending on processing conditions and environmental exposure. Consequently, there have been widely differing reports concerning the toxicity of MSNP and amorphous silica in general.7, 40-42 There is however, a general consensus that toxicity is associated in part with surface silanol (≡SiOH) groups,43 which can hydrogen bond to membrane components44 or when dissociated to form SiO− (above the isoelectric point of silica ~pH 2-3), interact electrostatically with the positively charged tetraalkylammonium-containing phospholipids,44 both processes leading to strong interactions, and possibly membranolysis. Such a process occurring at the cell surface could cause lysis, e.g. hemolysis shown for red blood cells.7, 42, 44 Within a phagosome, damage to the membrane could cause release of hydrolytic enzymes from lysosomes into the cytoplasm. In support of the importance of silanols, it is known that treating the silica surface with polyvinylpyridine-N-oxide, aluminium salts, or surfactants can reduce or even switch-off hemolysis of red blood cells.44 Based on the high surface to volume ratio of silica NPs, it might be anticipated that they would show in general higher toxicity compared to their bulk counterparts. However in the case of mesoporous silica NPs, the reduced solid fraction of the MSNP surface serves to reduce the surface area normalized hydroxyl coverage, and therefore the extent of hydrogen bonding and electrostatic interactions between the MSNP and the cell membrane.43 Additionally, based on membrane curvature arguments, very small NPs are less likely to disrupt and/or become internalized by the cell membrane43 because the membrane binding energy needed for the cell membrane to contact and fully envelop the NP scales quadratically with the NP curvature (1/iameter).
A second contribution to toxicity can be the reaction of radicals present on the silica surface with water to yield reactive oxygen species (ROS), in particular the hydroxy radical HO•, one of the most reactive species in nature.45 ROS can cause cell death by disrupting cell membranes (necrosis) or initiating programmed cell death (apoptosis). In sublethal concentrations, ROS can upregulate production of cytokines and other inflammatory mediators and can promote mutagenesis and carcinogenesis. Although the ability of freshly ground crystalline silicas to serve as generators of ROS is generally recognized and has been linked to traces of iron suggesting a Fenton-like mechanism, the ability of iron-free and amorphous silicas to generate ROS is just beginning to be appreciated.46 Here it is noteworthy that depending on processing conditions, amorphous silicas can contain significant populations of strained three-membered siloxane rings.47, 39Strained siloxane rings could undergo homolytic cleavage to form surface associated radicals including the non-bridging oxygen hole center,48 which react exothermically with water to form hydroxy radicals.45 Whether, like defects generated by grinding of crystalline silica, strained 3-membered rings in amorphous silica could serve as ROS generators is a question we are currently addressing.39 Notably, as-synthesized and surfactant-extracted MSNP have a negligible concentration of three-membered rings.39 For amorphous silica nanoparticles in general, dissolution results in monosilicic acid or oligosilicic acid which have been shown to have no intrinsic toxicity.49
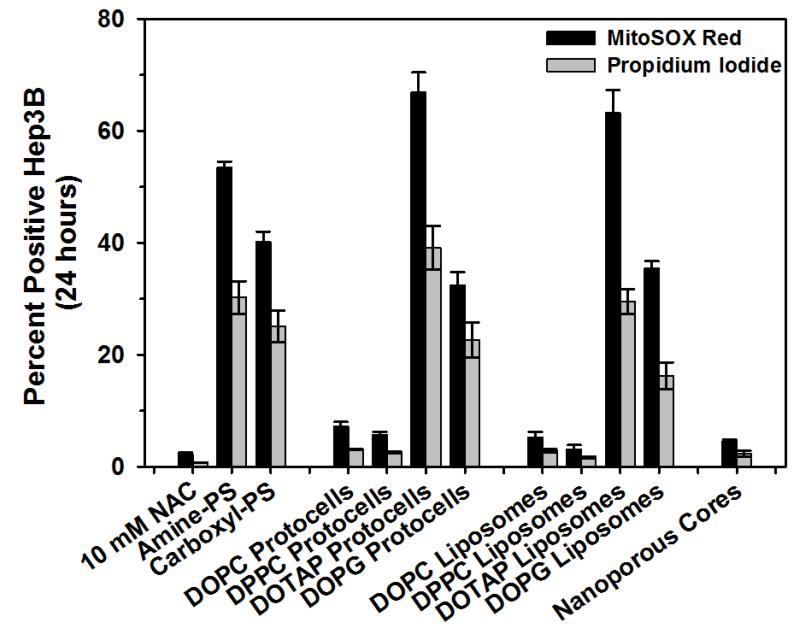
Although based on numerous recent studies it is generally observed that MSNPs have much lower toxicity than corresponding non-porous silica colloids (Stöber silica or LUDOX), presumably due to the mesoporosity reducing the effective MSNP/membrane contact area, conclusions concerning the role of particle size, shape, and charge are mixed. Notably Slowing et al43 report reduced hemolysis of red blood cells, whereas Lin and Haynes7 report increased hemolysis with decreasing MSNP sizes over the range 25-260 nm diameters. Toxicity effects are also reported to be highly cell specific. Yu et al42 show RAW264.7 macrophage cells to be more sensitive to ~115 nm solid or MSNPs than A549 cells, especially at very high doses of 250-500 μg/ml, and that solid Stöber silica colloids were more toxic and taken up into macrophage cells to a much greater extent than the corresponding MSNPs. Rationalization and unification of these varied findings requires a detailed understanding of possible internalization pathways. For endocytosis, membrane curvature arguments discussed above indicate that individual 50-60 nm diameter NPs should be most efficiently internalized as shown experimentally for targeted MSNP.19 Using spherical and rod-shaped MSNP Meng et al50 showed for HeLa and A549 cells that particle shape (aspect ratio) is actively sensed and can stimulate efficient NP uptake by macropinocytosis in which multiple NPs are incorporated at once within pinosomes. Toxicity associated with both membrane damage and the fate of endocytosed or pinocytosed MSNPs must be clearly established and the role of NP aggregation on effective particle shape and membrane association or internalization elucidated. In an effort to benchmark MSNP toxicity against control NPs, Figure 8 compares oxidative stress and cell viability for bare MSNP with neutral, positively, and negatively charged MSNP-supported lipid bilayers, liposomes, and polystyrene NPs at 109 particles/mL corresponding to ~1-2 μg/ml. A trend of cationic > anionic > neutral NPs is observed, where cationic NPs in general are shown to have high ROS associated toxicity.
FIGURE 8.
Reactive oxygen species generation (MitoSox Red) and % cell death (Propidium Iodide) of MSNP (nanoporous core), neutral, cationic, or anionic (DOTAP or DOPG) protocells, liposomes, and polystyrene beads. The antioxidant, N-acetylcysteine (NAC), was used as a negative control. (C.E. Ashley, unpublished data).
The relevance of many recent MSNP toxicity studies to in vivo applications of MSNP may be questionable however as coating the MSNP with polymers, lipid bilayers, or proteins (as could immediately occur in vivo) screens electrostatic interactions with surface silanols and is observed to effectively eliminate toxicity as demonstrated in vivo.51 Additionally when considering drug delivery, we must bear in mind potential therapeutically administered doses. For example, for the hepatocellular carcinoma cell line Hep3B, the LC50 and LC90 values of free DOX are 150 ng/mL and 500 ng/mL, respectively. Those quantities could be delivered in 400 ng/mL and 1.3 ug/mL of MSNP. As shown by Ashley et al,19 when using targeted MSNPs, these values fall to 6 ng/mL and 20 ng/mL due to the MSNP capacity, stability, and internalization efficiency. If only a few percent of MSNP are delivered to the tumor microenvironment, relevant concentrations for the study of MSNP toxicity are less than about 100 μg/ml, where most studies have shown insignificant toxicity.
Future Directions
The multifunctional modular design of MSNP shown in Figure 1 suggests next generation nanocarriers where multicomponent cargos are delivered to target cells through the active recruitment of targeting ligands combined with biologically triggered responses that actuate molecular valves. The combination of molecular machines and increasingly complex and biomimetic SLBs, e.g. those derived from red blood cells,52 may allow unprecedented engineering of NP/cellular interactions and provide a universal platform for theranostics and personalized medicine.
Acknowledgments
Funding Sources
The research described in this Account was supported in part by National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number EF 0830117; the NCI Cancer Nanotechnology Platform Partnership grant U01CA151792-01; the Laboratory Directed Research and Development program and the Truman Fellowship in National Security Science and Engineering at Sandia National Laboratories (CEA); and the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering. Sandia is a multiprogram laboratory operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Company, for the United States Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000. The work at UCLA is supported by the US Public Health Service Grants (RO1 ES016746, RO1 CA133697 and U19 ES019528) and by the National Science Foundation and the Environmental Protection Agency to the UC Center for the Environmental Impact of Nanotechnology under Cooperative Agreement Number EF 0830117.
BIOGRAPHICAL INFORMATION
Derrick Tarn graduated in 2008 with a BSc in Chemistry from UCLA. Currently, he is pursuing his PhD under the supervision of Prof. Jeffrey I. Zink at UCLA, where his research is focused on the design and synthesis of nanomachines which function on both meso-and microporous silica nanoparticles.
Carlee E. Ashley graduated from UNM with a PhD in chemical engineering in 2010 and is now a Truman Post-Doctoral Fellow at Sandia National Laboratories. Her work focuses on the development of targeted protocells and virus-like particles for applications in infectious disease and cancer therapy.
Min Xue graduated in 2008 from Nanjing University with a BS in Physical Chemistry. He is now pursuing his PhD under the supervision of Prof. Jeffrey I. Zink at UCLA. Currently his research focus is on silica nanoparticle based drug delivery systems.
Eric C. Carnes graduated from UNM with a PhD in chemical engineering in 2008 and is now a Lecturer and Research Professor at UNM where he studies the engineering of bio-nano interfaces and for controlling cellular behavior and for development of targeted nanoparticle based drug delivery.
Jeffrey I. Zink is a Distinguished Professor of Chemistry at UCLA. He received his Ph.D. degree from the University of Illinois at Urbana-Champaign. His research interests included excited state properties of metal-containing molecules and multifunctional mechanized nanomaterials.
C. Jeffrey Brinker is a Fellow at Sandia National Laboratories and Distinguished Professor of Chemical Engineering at UNM, where he studies nanoscale materials made by self-and directed assembly.
Footnotes
The authors declare no competing financial interest.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
REFERENCES
- (1).Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. [Google Scholar]
- (2).Brinker CJ, Lu YF, Sellinger A, Fan HY. Evaporation-induced self-assembly: Nanostructures made easy. Advanced Materials. 1999;11:579–585. [Google Scholar]
- (3).Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW. A new family of mesoporous molecular sieves prepared with liquid crystal templates. Journal of the American Chemical Society. 1992;114:10834–10843. [Google Scholar]
- (4).Grün M, Lauer I, Unger KK. The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Advanced Materials. 1997;9:254–257. [Google Scholar]
- (5).Huh S, Wiench JW, Yoo J-C, Pruski M, Lin VSY. Organic Functionalization and Morphology Control of Mesoporous Silicas via a Co-Condensation Synthesis Method. Chemistry of Materials. 2003;15:4247–4256. [Google Scholar]
- (6).Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink JI, Nel AE. Use of Size and a Copolymer Design Feature To Improve the Biodistribution and the Enhanced Permeability and Retention Effect of Doxorubicin-Loaded Mesoporous Silica Nanoparticles in a Murine Xenograft Tumor Model. ACS Nano. 2011;5:4131–4144. doi: 10.1021/nn200809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lin Y-S, Haynes CL. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. Journal of the American Chemical Society. 2010;132:4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- (8).Lu YF, Ganguli R, Drewien CA, Anderson MT, Brinker CJ, Gong WL, Guo YX, Soyez H, Dunn B, Huang MH, Zink JI. Continuous formation of supported cubic and hexagonal mesoporous films by sol gel dip-coating. Nature. 1997;389:364–368. [Google Scholar]
- (9).Huang MH, Dunn BS, Zink JI. Journal of the American Chemical Society. 2000;122:3739–3745. [Google Scholar]
- (10).Lu YF, Fan HY, Stump A, Ward TL, Rieker T, Brinker CJ. Aerosol-assisted self-assembly of mesostructured spherical nanoparticles. Nature. 1999;398:223–226. [Google Scholar]
- (11).Fan HY, Lu YF, Stump A, Reed ST, Baer T, Schunk R, Perez-Luna V, Lopez GP, Brinker CJ. Rapid prototyping of patterned functional nanostructures. Nature. 2000;405:56–60. doi: 10.1038/35011026. [DOI] [PubMed] [Google Scholar]
- (12).Li Z, Nyalosaso JL, Hwang AA, Ferris DP, Yang S, Derrien G, Charnay C, Durand J-O, Zink JI. Measurement of Uptake and Release Capacities of Mesoporous Silica Nanoparticles Enabled by Nanovalve Gates. The Journal of Physical Chemistry C. 2011;115:19496–19506. doi: 10.1021/jp2047147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angewandte Chemie International Edition. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- (14).Thomas CR, Ferris DP, Lee J-H, Choi E, Cho MH, Kim ES, Stoddart JF, Shin J-S, Cheon J, Zink JI. Noninvasive Remote-Controlled Release of Drug Molecules in Vitro Using Magnetic Actuation of Mechanized Nanoparticles. Journal of the American Chemical Society. 2010;132:10623–10625. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- (15).Feng X, Fryxell GE, Wang L-Q, Kim AY, Liu J, Kemner KM. Functionalized Monolayers on Ordered Mesoporous Supports. Science. 1997;276:923–926. [Google Scholar]
- (16).Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Hanna TN, Liu J, Phillips B, Carter MB, Carroll NJ, Jiang X, Dunphy DR, Willman CL, Petsev DN, Evans DG, Parikh AN, Chackerian B, Wharton W, Peabody DS, Brinker CJ. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature Materials. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ashley CE, Carnes EC, Epler KE, Padilla DP, Phillips GK, Castillo RE, Wilkinson DC, Wilkinson BS, Burgard CA, Kalinich RM, Townson JL, Chackerian B, Willman CL, Peabody DS, Wharton W, Brinker CJ. Delivery of Small Interfering RNA by Peptide-Targeted Mesoporous Silica Nanoparticle-Supported Lipid Bilayers. ACS Nano. 2012;6:2174–2188. doi: 10.1021/nn204102q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Liu JW, Stace-Naughton A, Jiang XM, Brinker CJ. Porous Nanoparticle Supported Lipid Bilayers (Protocells) as Delivery Vehicles. Journal of the American Chemical Society. 2009;131:1354–1355. doi: 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hsiao J-K, Tsai C-P, Chung T-H, Hung Y, Yao M, Liu H-M, Mou C-Y, Yang C-S, Chen Y-C, Huang D-M. Mesoporous Silica Nanoparticles as a Delivery System of Gadolinium for Effective Human Stem Cell Tracking. Small. 2008;4:1445–1452. doi: 10.1002/smll.200701316. [DOI] [PubMed] [Google Scholar]
- (23).Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small. 2007;3:1341–1346. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- (24).Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Reviews Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- (25).Sapra P, Allen TM. Internalizing Antibodies Are Necessary for Improved Therapeutic Efficacy of Antibody-targeted Liposomal Drugs. Cancer Research. 2002;62:7190–7194. [PubMed] [Google Scholar]
- (26).Ferris DP, Lu J, Gothard C, Yanes R, Thomas CR, Olsen J-C, Stoddart JF, Tamanoi F, Zink JI. Synthesis of Biomolecule-Modified Mesoporous Silica Nanoparticles for Targeted Hydrophobic Drug Delivery to Cancer Cells. Small. 2011;7:1816–1826. doi: 10.1002/smll.201002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Vallet-Regi M, Rámila A, del Real RP, Pérez-Pariente J. A New Property of MCM-41: Drug Delivery System. Chemistry of Materials. 2000;13:308–311. [Google Scholar]
- (28).Trewyn BG, Slowing II, Giri S, Chen H-T, Lin VSY. Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release. Accounts of Chemical Research. 2007;40:846–853. doi: 10.1021/ar600032u. [DOI] [PubMed] [Google Scholar]
- (29).Ambrogio MW, Thomas CR, Zhao Y-L, Zink JI, Stoddart JF. Mechanized Silica Nanoparticles: A New Frontier in Theranostic Nanomedicine. Accounts of Chemical Research. 2011;44:903–913. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chemical Society Reviews. 2012;41:2590–2605. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- (31).Angelos S, Yang Y-W, Khashab NM, Stoddart JF, Zink JI. Dual-Controlled Nanoparticles Exhibiting AND Logic. Journal of the American Chemical Society. 2009;131:11344–11346. doi: 10.1021/ja9042752. [DOI] [PubMed] [Google Scholar]
- (32).Meng H, Xue M, Xia T, Zhao Y-L, Tamanoi F, Stoddart JF, Zink JI, Nel AE. Autonomous in Vitro Anticancer Drug Release from Mesoporous Silica Nanoparticles by pH-Sensitive Nanovalves. Journal of the American Chemical Society. 2010;132:12690–12697. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- (34).Castranova V, Vallyathan V. Silicosis and coal workers’ pneumoconiosis. Environ Health Perspectives. 2000;108:675–684. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Donaldson K, Borm PJA. The Quartz Hazard: A Variable Entity. Annals of Occupational Hygiene. 1998;42:287–294. doi: 10.1016/s0003-4878(98)00044-1. [DOI] [PubMed] [Google Scholar]
- (36).Elias Z, Poirot O, DaniÃre MC, Terzetti F, Marande AM, Dzwigaj S, Pezerat H, Fenoglio I, Fubini B. Cytotoxic and transforming effects of silica particles with different surface properties in Syrian hamster embryo (SHE) cells. Toxicology In Vitro. 2000;14:409–422. doi: 10.1016/s0887-2333(00)00039-4. [DOI] [PubMed] [Google Scholar]
- (37).Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Current Opinion in Pulmonary Medicine. 2005;11:169–173. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- (38).Zhuravlev LT. Concentration of hydroxyl groups on the surface of amorphous silicas. Langmuir. 1987;3:316–318. [Google Scholar]
- (39).Zhang H, Dunphy DR, Jiang X, Meng H, Sun B, Tarn D, Xue M, Wang X, Lin S, Ji Z, Li R, Garcia FL, Yang J, Kirk ML, Xia T, Zink JI, Nel A, Brinker CJ. Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs. pyrolytic. Journal of the American Chemical Society. 2012;134:15790–15804. doi: 10.1021/ja304907c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Fubini B, Fenoglio I, Elias Z, Poirot O. Variability of biological responses to silicas: effect of origin, crystallinity, and state of surface on generation of reactive oxygen species and morphological transformation of mammalian cells. Journal of Environmental Pathology, Toxicology and Oncology. 2001;20:109–118. [PubMed] [Google Scholar]
- (41).Hudson SP, Padera RF, Langer R, Kohane DS. The biocompatibility of mesoporous silicates. Biomaterials. 2008;29:4045–4055. doi: 10.1016/j.biomaterials.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yu T, Malugin A, Ghandehari H. Impact of Silica Nanoparticle Design on Cellular Toxicity and Hemolytic Activity. ACS Nano. 2011;5:5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Slowing II, Wu C-W, Vivero-Escoto JL, Lin VSY. Mesoporous Silica Nanoparticles for Reducing Hemolytic Activity Towards Mammalian Red Blood Cells. Small. 2009;5:57–62. doi: 10.1002/smll.200800926. [DOI] [PubMed] [Google Scholar]
- (44).Nash T, Allison AC, Harington JS. Physico-Chemical Properties of Silica in Relation to its Toxicity. Nature. 1966;210:259–261. doi: 10.1038/210259a0. [DOI] [PubMed] [Google Scholar]
- (45).Schoonen MAA, Cohn CA, Roemer E, Laffers R, Simon SR, O’Riordan T. Mineral-Induced Formation of Reactive Oxygen Species. Reviews in Mineralogy and Geochemistry. 2006;64:179–221. [Google Scholar]
- (46).Ghiazza M, Polimeni M, Fenoglio I, Gazzano E, Ghigo D, Fubini B. Does Vitreous Silica Contradict the Toxicity of the Crystalline Silica Paradigm? Chemical Research in Toxicology. 2010;23:620–629. doi: 10.1021/tx900369x. [DOI] [PubMed] [Google Scholar]
- (47).Brinker CJ, Kirkpatrick RJ, Tallant DR, Bunker BC, Montez B. NMR Confirmation of Strained Defects in Amorphous Silica. Journal of Non-Crystalline Solids. 1988;99:418–428. [Google Scholar]
- (48).Griscom DL, Brinker CJ, Ashley CS. Electron-Spin-Resonance Studies of Irradiated O-17-Enriched Sol-Gel Silicas - Organic Impurity Effects and the Structure of the Nonbridging-Oxygen Hole Center. Journal of Non-Crystalline Solids. 1987;92:295–301. [Google Scholar]
- (49).He Q, Zhang Z, Gao Y, Shi J, Li Y. Intracellular localization and cytotoxicity of spherical mesoporous silica nano-and microparticles. Small. 2009;23:2722–2729. doi: 10.1002/smll.200900923. [DOI] [PubMed] [Google Scholar]
- (50).Meng H, Yang S, Li Z, Xia T, Chen J, Ji Z, Zhang H, Wang X, Lin S, Huang C, Zhou ZH, Zink JI, Nel AE. Aspect Ratio Determines the Quantity of Mesoporous Silica Nanoparticle Uptake by a Small GTPase-Dependent Macropinocytosis Mechanism. ACS Nano. 2011;5:4434–4447. doi: 10.1021/nn103344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Malvindi MA, Brunetti V, Vecchio G, Galeone A, Cingolani R, Pompa PP. SiO2 nanoparticles biocompatibility and their potential for gene delivery and silencing. Nanoscale. 2012;4:486–495. doi: 10.1039/c1nr11269d. [DOI] [PubMed] [Google Scholar]
- (52).Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]