Abstract
The immune system is under strong circadian control, and circadian desynchrony is a risk factor for metabolic disorders, inflammatory responses and cancer. Signaling pathways that maintain circadian rhythms (CRs) in immune function in vivo, and the mechanisms by which circadian desynchrony impairs immune function, remain to be fully-identified. These experiments tested the hypothesis that the hypothalamic circadian pacemaker in the suprachiasmatic nucleus (SCN) drives CRs in the immune system, using a non-invasive model of SCN circadian arrhythmia. Robust CRs in blood leukocyte trafficking, with a peak during the early light phase (ZT4) and nadir in the early dark phase (ZT18), were absent in arrhythmic hamsters, as were CRs in spleen clock gene (per1, bmal1) expression, indicating that a functional pacemaker in the SCN is required for the generation of CRs in leukocyte trafficking and for driving peripheral clocks in secondary lymphoid organs. Pinealectomy was without effect on CRs in leukocyte trafficking, but abolished CRs in spleen clock gene expression, indicating that nocturnal melatonin secretion is necessary for communicating circadian time information to the spleen. CRs in trafficking of antigen presenting cells (CD11c+ dendritic cells) in the skin were abolished, and antigen-specific delayed-type hypersensitivity skin inflammatory responses were markedly impaired in arrhythmic hamsters. The SCN drives robust CRs in leukocyte trafficking and lymphoid clock gene expression; the latter of which is not expressed in the absence of melatonin. Robust entrainment of the circadian pacemaker provides a signal critical to diurnal rhythms in immunosurveilliance and optimal memory T-cell dependent immune responses.
Keywords: leukocyte trafficking, delayed-type hypersensitivity, entrainment, SCN, inflammation
Introduction
In periodic environments, circadian clocks permit synchrony of the internal milieu and anticipation of predictable changes in the environment (Pittendrigh, 1960; Bronson, 1989). Daily temporal organization of physiology and behavior is provided by entrainment of the circadian pacemaker in the hypothalamic suprachiasmatic nucleus (SCN) to the environmental light:dark cycle. The mammalian circadian system is composed of a network of hierarchically-interacting tissue-specific circadian clocks (Baggs et al., 2009). In order to generate coherent physiological rhythms, the phases of these clocks are regularly reset by the output of the master circadian pacemaker in the SCN (Schibler, 2007). Perhaps the most conspicuous circadian rhythm (CR) driven by the SCN is the activity/rest cycle, but scores of physiological processes occur in a circadian manner, including endocrine function, cellular and organismal metabolism, body temperature, and countless behaviors (Takahashi et al., 2008).
Immune function is under strong circadian control. Virtually all immunological variables investigated to date exhibit circadian cycles. CRs are evident in circulating leukocyte numbers and phenotypes, lymphocyte metabolism and function, and cytokine production in vitro and in vivo (Esquifino et al., 2007). Brain-immune interactions that govern the expression of infection-induced sickness behaviors fluctuate across the circadian cycle (Arjona and Sarkar, 2005; Arjona and Sarkar, 2006; Guan et al., 2005; Marpegan et al., 2005), and mortality from sepsis varies markedly with time-of-day (Franklin et al., 2003; Franklin et al., 2007; Liu et al., 2006; Marpegan et al., 2009; Morrow and Opp, 2005; Weil et al., 2009). Furthermore, immunocompetence varies with the stability of the circadian system: extensive nighttime shift work or transmeridian travel induces chronic circadian disruption, leading to higher rates of various cancers (Pukkala et al., 2002; Reynolds et al., 2002; Schernhammer et al., 2001; Schernhammer et al., 2003), and stably-entrained behavioral and endocrine rhythms predict improved survival time in cancer patients (Mormont et al., 2000; Sephton et al., 2000). Mice with disrupted circadian phenotypes exhibit diverse immunological disorders, including enhanced tumorigenesis (Fu et al., 2002), disrupted lymphoid development (Kurebayashi et al., 2000; Sun et al., 2000), impaired T cell function and autoimmune disease (Seimiya et al., 2004), and exacerbated innate inflammatory responses (Castanon-Cervantes et al., 2010).
One major obstacle to addressing the functional significance of the circadian system in immunity has been the lack of an adequate and appropriate model system. SCN ablation reliably eliminates rhythms, but also damages substantial adjacent hypothalamic tissue, and causes nonspecific production of stress hormones (Bittman et al., 1991; Buijs et al., 1993), along with glial scars and CNS immune responses that continue for months after the insult (Logan et al., 1992; Silver and Miller, 2004). Constant light (LL) can induce arrhythmia, but such effects are transient and chronically elevate glucocorticoid secretion (Daan and Pittendrigh, 1975; Eastman and Rechtschaffen, 1983; Welberg et al., 2006). Combinations of clock gene knockouts eliminate CRs (Reppert and Weaver, 2002), but clock genes are present in all tissues, and are pleiotropic in function (Baggs et al., 2009; Greenspan, 2001; Meyer-Bernstein and Sehgal, 2001). The pleiotropy issue is of major logical importance for understanding reports of disease states in clock gene knockout mice: such effects may be due to circadian disruption (centrally or at tissue level), but may instead be due to direct roles of clock genes in cellular metabolic processes (Baggs et al., 2009; Bur et al., 2009; Kosaka and Bass, 2007; Male et al., 2012). In a single relevant lesion study, diurnal rhythms in blood leukocytes were moderately dampened (not abolished) and growth rates of implanted tumors were accelerated in mice with complete bilateral lesions of the SCN, suggesting that in the absence of circadian organization mechanisms inhibiting tumor growth are impaired (Filipski et al., 2003).
Here we use a model of chronic SCN arrhythmia generated noninvasively, thereby avoiding the pitfalls and confounds of CNS lesions, bright LL, and genetic mutations (Grone et al., 2011; Ruby et al., 2009). CRs in sleep/wake, body temperature, melatonin secretion and locomotor activity of hamsters can be eliminated within a few days in Siberian hamsters by light treatments administered once (see Materials and Methods). This noninvasive method for permanently eliminating CRs has the distinct advantage of allowing animals to remain undisturbed in the presence of a standard light:dark cycle and was used to investigate functional consequences of complete circadian desynchrony on multiple aspects of immune function.
Materials and Methods
Animals
Siberian hamsters were born and raised in a 15L:9D (lights off at 18:00 h CST) breeding colony maintained at the University of Chicago. Hamsters were housed in polypropylene cages, with food (Harlan, Teklad) and filtered tap water provided ad libitum; cotton nesting material was also available in the cage. Ambient temperature and relative humidity were held constant at 19 ±2°C and 53 ±10%, respectively. All procedures conformed to the USDA Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Experiment 1. Effects of circadian and ultradian arrhythmia on blood leukocyte trafficking
Male and female hamsters (3-5 months of age; (n=60; 24 females, 36 males) were single-housed and transferred to 16L:8D (lights off at 18:00) for 4 weeks prior to the implementation of a circadian disrupting phase-shift (DPS) protocol (Ruby et al., 2004; see DPS Procedure). A group of control hamsters (n=8) was subjected to a sham-DPS procedure. CRs in locomotor activity were monitored over 10 consecutive days in all hamsters 1-3 months after the phase-shift was administered; this allowed identification of the presence/absence of significant CRs, and measurement of quantitative aspects of the CR waveform (see Locomotor activity monitoring).
Circadian leukocyte trafficking
Rhythmic aspects of blood leukocyte trafficking were assessed in 25 μl blood samples collected at 4 time points (ZT4, ZT11, ZT18 and ZT23) across the circadian cycle, over the course of 3 successive days, according to methods described elsewhere (Prendergast et al., 2003).
Stress-induced leukocyte trafficking
To elicit stress responses, hamsters (ENTR, n=8; ARR, n=8) were placed in restraint stress tubes (RST; dimensions; Bilbo et al., 2002) at ZT3 (3 h after light onset); hamsters remained in the RST for 2 h, thus the midpoint of the stressor occurred at ZT4. Control (non-stressed) hamsters remained in their home cages in a separate room. Blood (150 μl) was obtained from RS hamsters via the retroorbital sinus under isoflurane anesthesia prior to the onset of the stress/control procedure (baseline), and again 15, 60 and 120 min later; blood was obtained from controls at baseline and again 120 min later. Following each blood draw, hamsters were administered 0.2 ml warm sterile physiological saline (s.c.) to facilitate rehydration. Leukocyte concentrations were determined in a 25 μl aliquot of heparinized whole blood (see Blood Leukocyte Quantification).
Experiment 2. Effects of circadian arrhythmia on leukocyte trafficking and lymphoid clock gene expression under constant conditions
Female hamsters (n=93) were subjected to the DPS procedure; 25 (27%) reentrained to the shifted photocycle, whereas 56 (60%) became behaviorally-arrhythmic; the remainder exhibited free-running locomotor activity and were excluded from subsequent investigation. From this population, 24 ENTR and 24 ARR hamsters were subjected to an Aschoff Type II manipulation (Aschoff, 1965), which eliminates masking effects of the light-dark cycle by allowing rhythms to persist in constant conditions (continuous darkness; DD) for >2 cycles prior to sample collection. At the onset of darkness (ZT16), ENTR and ARR hamsters were transferred from 16L:8D to DD. A dim (1 lux) red light remained on at all times in DD. Retroorbital blood (25 μl) was obtained for blood leukocyte determination, and hamsters were killed by rapid decapitation at projected ZT17 (49 h after the onset of DD) or projected ZT1 (58 h after the onset of DD). Spleens were rapidly dissected (<2 min) onto dry ice and stored at -80°C until RNA extraction.
Experiment 3. Effects of pinealectomy on blood leukocyte trafficking and lymphoid clock gene expression
Adult female hamsters (n=36) were pinealectomized (PINx) or sham-pinealectomized according to methods described by Carter and Goldman (1983; see Pinealectomy Procedure). Six weeks after surgery, retroorbital blood was collected at projected ZT1 and ZT17 using an Aschoff Type II design, as described above. Blood samples were obtained under isoflurane anesthesia in a pseudo-random order and leukocytes were enumerated from a 25 μl sample of whole heparinized blood. Hamsters were killed by rapid decapitation, and spleens were rapidly dissected as described for Experiment 2.
Experiment 4. Effects of circadian arrhythmia on DTH reactions
A subset of hamsters from Experiment 1 (n=35) remained housed in 16L:8D for two weeks after blood collections were completed. In these hamsters (ENTR, n=15; ARR, n=20), delayed-type hypersensitivity skin inflammatory responses were induced by application of the antigen, 2,4-dinitro-1-fluorobenzene (DNFB; Sigma), to the pinnae of each hamster after initial DNFB sensitization. Ear inflammatory responses were monitored for 7 days following final treatment (see DTH Procedure).
Skin dendritic cells were quantified in a separate group of hamsters from Experiment 1 (ENTR, n=16; ARR, n=16). Under isoflurane anesthesia, skin samples from the dorsum were obtained using a sterile 4 mm punch biopsy tool, via methods described in detail elsewhere (Kinsey et al., 2003). Tissue biopsies were rinsed in sterile PBS and ground against a 40 μm cell strainer continuously for 3 minutes. Cellular exudate was strained again at 40 μm, rinsed and centrifuged (800 × g, 10 min) 2× in PBS, resuspended in PBS, and fixed with 1.5% paraformaldehyde. Before fixation, the concentration and viability of cell suspensions were assessed using trypan blue exclusion. Samples were stored at 4°C until dendritic cells were quantified via flow cytometry (see Flow Cytometry).
General Methods
Disruptive phase shift (DPS) procedure
The disruptive phase shift (DPS) procedure destabilizes the hamster circadian pacemaker via treatment with phase-resetting light stimuli (Ruby et al., 2004). After ≥4 weeks in a 16L:8D photoperiod, a 2 h light pulse was administered during the 5th through 7th h of the dark phase. On the next day, the 16L:8D photocycle was phase-delayed by 3 h, via extension of the light phase (new lights off: 21:00 h). The DPS protocol typically renders ∼50% of hamsters permanently circadian arrhythmic (“ARR”; Ruby et al., 2004). Control hamsters were subjected to the 3 h phase-delay but did not receive the 2 h light pulse on the preceding night (sham-DPS); 75-90% of control hamsters re-entrain to the new photocycle (“ENTR”; Ruby et al., 2004; Prendergast et al., 2012). CRs in locomotor activity were monitored in all hamsters 1-3 months after the phase-shift was administered (cf. Ruby et al., 2009; see Locomotor Activity Monitoring).
Control hamsters received the 3 hour phase-delay but not the 2 h light pulse (sham-DPS). In all measures of immune function, circadian-arrhythmic hamsters that received the DPS treatment did not differ from those that received the sham-DPS treatment (blood leukocytes: F1,31=0.06, P>0.80; DTH: F1,31=1.32, P>0.20) and were collapsed into a single circadian arrhythmic (ARR) group (n=34). Similarly, entrained hamsters receiving the DPS treatment and those receiving the sham-DPS exhibited comparable blood leukocyte concentrations (F1,27=1.54, P>0.20) and DTH reactions (F1,28<0.01, P>0.90) and were combined into a single circadian entrained (ENTR) treatment group (n=30).
Locomotor activity monitoring
In all experiments, locomotor activity data were collected in the home cage for a minimum of 10 consecutive days with passive infrared motion detectors (Coral Plus, Visonic, Bloomfield, CT) positioned 22 cm above the cage floor. Motion detectors registered activity when 3 of 27 zones were crossed. Activity triggered closure of an electronic relay, recorded by a computer running ClockLab software (Actimetrics, Evanston, IL). Cumulative activity counts were collected at 6 min intervals for quantitative analyses of CRs.
Circadian waveform analyses
The presence/absence of CRs was determined using criteria identical to those described in prior reports of DPS-induced arrhythmia in this species (Ruby et al., 2004; Ruby et al., 2009). χ2 periodogram analyses (ClockLab; Actimetrics) were performed on 10-day blocks of activity data. Peaks in the χ2 periodogram were considered statistically significant if they exceeded the 99.9% confidence interval limit (P<0.001). Hamsters were considered arrhythmic (ARR) if there were no significant peaks in the periodogram in the circadian range, activity was distributed throughout the LD cycle, and daily rhythm onsets and offsets could not be identified via visual inspection of the periodogram (Ruby et al., 2009). Hamsters with significant circadian activity in the χ2 periodogram were considered entrained (ENTR). Supplemental analyses after completion of χ2 periodogram analyses were adopted as recommended by Refinetti et al. (2007); to this end, the cosinor periodogram (Bingham et al., 1982), a reliable curve-fitting tool to quantify rhythm parameters, was used (Refinetti et al., 2007). Cosinor analyses were used to calculate the mesor and amplitude of the best-fit cosinor waveform in the circadian range (22-26 h). Relative amplitude of the CR was calculated as the quotient of CR amplitude divided by CR mesor (Prendergast et al., 2012).
RNA extraction, cDNA synthesis and quantitative PCR
per1 (period 1) and bmal1 (brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like) mRNA expression was measured in spleen samples via qPCR. Total RNA was extracted using RNeasy (Qiagen) according to the kit instructions. Extracted RNA was suspended in 30 μl RNase-free water and RNA concentration and quality for each sample were assessed using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). cDNA was created via RT of 2 μg RNA using MMLV RT enzyme (Invitrogen, Carlsbad, CA, USA).
In Experiment 2, primers and probes for the mRNAs of interest were designed using PrimerExpress based on Siberian hamster-specific sequences for these 3 genes published in Genbank: per1 (AY316535), per2 (EU (AY316536) and bmal1 (AY316534). Primers and probes were synthesized as follows, with probes labeled with 6-FAM and MGB (non-fluorescent 5′- AACTGCTTTTTCTATTGCAGACAATG-3′, per2 probe 5′-AGCCACCAGTGAGGAT-3′ bmal1 forward 5′- GGCAGCGATGGCTGTCA -3′, bmal1 reverse 5′- TCCACCCAGGCCTGCAT -3′, bmal1 probe 5′-CATGAGCCTATTGGAAGC-3′. A TaqMan 18S Ribosomal RNA primer and probe set (labeled with VIC; Applied Biosystems) was used as the control gene for quantification. Amplification was performed on an ABI 7900HT Sequencing Detection System by using Taqman® Universal PCR Master Mix. The universal two-step RT-PCR cycling conditions used were: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Relative gene expression of individual samples run in duplicate was calculated by comparison to relative standard curve consisting of serial dilutions of pooled P. sungorus hypothalamic cDNA (1:1, 1:10, 1:100, 1:1000, 1:10,000) followed by normalization to 18S rRNA gene expression; 6 samples were excluded due to low 18S rRNA (ENTR: ZT13, n=2; ARR: ZT1, n=2; ZT13, n=2).
In Experiment 3, primers for per1 and bmal1 were identical to those used in Experiment 2. Reporter probes were not required because qPCR was performed using iQ Sybr Green Supermix (BioRad, Hercules, CA USA). qPCR reactions used an initial denaturation at 95°C for 30 s, followed by 39 cycles of 95°C for 10 s, annealing at 60°C for 30 s and extension at 72°C for 30 s. A melting curve analysis was added to determine the quality and specificity of each reaction. Quantification of mRNA expression levels was accomplished with iQ Sybr Green Supermix (BioRad). PCR Miner (Zhao & Fernald, 2005) was used to calculate reaction efficiencies (E) and cycle thresholds (CTs). The expression of each target gene of interest was calculated relative to gapdh using 2-(delta-deltaCt). Four samples were identified as outliers (±2 SD from mean) and were excluded from subsequent analyses.
Blood collection
Blood (270 μl) was obtained under light anesthesia from the right retro-orbital sinus using heparinized Natelson collection tubes. A dim (1 lux) red light was used to facilitate dark-phase (ZT18, ZT23) collections. Blood collections were performed in pseudo-random order, in a room separate from the general animal colonies, and following the procedure, hamsters were isolated from the colony until all collections were completed. Animal handling during the blood collection was kept to a minimum (<1 min). Blood samples were deposited into heparinized (50 units) microcentrifuge tubes, mixed gently, and a 25 μl aliquot of whole heparinized blood was removed for leukocyte determination.
Leukocyte redeployment out of the blood at each of the 3 post-peak sampling intervals (ZTx, where x =11, 18 or 23) was calculated for each individual hamster as a proportion of its baseline (peak; ZT4) blood leukocyte concentration according to methods described by (Dhabhar and McEwen, 1997):
Blood Leukocyte Quantification
Leukocyte counts from whole blood were obtained via hemolysis with 3% acetic acid at a 1:20 dilution, and enumeration in duplicate on a hemacytometer at 400× magnification by an experimenter blind to the hamster's ultradian and circadian phenotype. Lysed samples were kept at room temperature for < 3 h before leukocyte counts were determined. Although distinct leukocyte subtypes are not identifiable with this method, this procedure reliably identifies environmentally-induced (e.g., photoperiod, stress) changes in total leukocyte number in this, and other, rodent species. In several studies of Siberian hamster leukocytogenesis, total leukocyte counts correlates positively with photoperiodic changes in specific leukocyte subtypes, including total lymphocytes, T-cells, and NK cells (Bilbo et al., 2002; Wen et al., 2007). Circadian leukocyte trafficking requires clock-driven leukocyte migration out of the circulation to skin, organs, and lymph nodes. The measure thus provides a rapid and omnibus indicator of effects of circadian rhythmicity on the capacity for immunosurveilliance in the blood. Applied longitudinally, this measure provides an accurate indicator of leukocyte trafficking (Dhabhar et al., 1995).
Pinealectomy Procedure
Anesthetized hamsters were immobilized in a stereotaxic apparatus and a midline skin incision exposed the skull from bregma to approximately 3 mm caudal to lambda. A trephine bit was centered on lambda used to remove a portion of the skull. The pineal gland was then removed with microdissecting forceps. PINx was verified via direct histological examination of the excised tissue under a dissecting microscope. Gelfoam was inserted into the wound site, and the skin closed with wound clips. Topical antibiotic ointment was applied externally. Hamsters received buprenorphine analgesic (0.01 mg/kg, sc) 2×/day for 2 days after surgery. The sham-PINx and PINx procedures were similar, except the pineal gland was not removed.
DTH Procedure
DTH skin inflammatory responses were induced by application of the antigen, 2,4-dinitro-1-fluorobenzene (DNFB; Sigma), to the pinna of each hamster after initial immunization (“sensitization”) by application of DNFB to the dorsum (Bilbo et al., 2002). Sensitization was induced and DTH elicited as follows: on all hamsters (DNFB naïve) were anesthetized with isoflurane vapor, and an area of 2 × 3 cm was shaved on the dorsum. Twenty-five μl of DNFB [0.5% (wt/vol) in 4:1, acetone/olive oil vehicle] was applied to the dorsal skin on 2 consecutive days. Six days later, baseline thickness of both pinnae was measured under light anesthesia using a constant-loading dial micrometer (±0.01 mm; Mitutoyo, Tokyo). Immediately after baseline pinna measurements were obtained, the DTH reaction was elicited by applying 20 μl of DNFB [0.2% (wt/vol) in 4:1, acetone/olive oil] to the dorsal skin of the right pinna. Left pinnae were treated with vehicle and served as a control treatment. Pinna thicknesses were measured every day for the next 7 days. All measurements were performed by the same experimenter who was blind to ultradian/circadian phenotype. Pinna thickness values obtained on each day following challenge were expressed as a percentage of baseline thickness for statistical calculations. All DNFB treatments and pinna measurements were performed between 14:00-15:00 h (ZT12-13), and all measurements were made on the same relative region of the pinna. This regimen of sensitization and challenge uses concentrations of DNFB that differ from those used in rats (Dhabhar and McEwen, 1999), but have been optimized for use in Siberian hamsters (Bilbo et al., 2002; Prendergast and Pyter, 2009). DTH reactions require antigen capture, drainage of antigen-presenting cells from the skin to the lymphatic system, antigen presentation, clonal T cell expansion and, following antigen challenge, a rapid deployment of leukocytes out of the blood and infiltration into the epidermis and dermis (Dhabhar, 2000). DTH is a standard in vivo measure of T cell mediated immunity (Turk, 1980).
Flow Cytometry
Appropriate dilutions of each monoclonal antibody (Ab) were established to use saturating antibody concentrations. Extracted cells from the entire skin biopsy were centrifuged at 800 × g for 10 min, resuspended PBS with 0.5% BSA, and incubated with Ab (at appropriate concentrations as determined by titration, see below) for 30 min at 20°C. Stained cells were then washed 2× in PBS, and resuspended in 200 μl PBS/BSA plus 50 μl counting beads. Negative-controls and single-stained controls were included for use in gating and compensation, using FlowJo version 9.5.4 (Tree Star Inc., Palo Alto, CA). All samples were analyzed using the same gates. For each molecule studied, all samples were analyzed with the same conditions of voltage on the cytometer.
Lymphocytes and granulocytes were identified based on forward and side scatter properties. Leukocytes were identified as CD45+ (using FITC-conjugated anti-mouse-CD45; clone 30-F11, 1:200; BioLegend, San Diego, CA). Dendritic cells (DCs) were identified as CD11c+ (using PE-conjugated, anti-mouse-CD11c; clone BU15, 1:5; BioLegend). CD11c is a standard marker for DCs (Brocker, 1997; Metlay et al., 1990); mature dendritic cells were identified as CD45+/CD11+ lymphocytes with high SSC, indicative of extensive dendritic outgrowth (Wang et al., 2009). Absolute numbers of cells per μl of skin extract were determined via the addition of 50 μl polystyrene microbeads (3.4 μm diameter, 43500 beads; Spherotech, Lake Forest, IL) to each sample. Following cytometry, the formula: (# CD1 1c+ events/# microbead events) × (217.5 beads/μl initial sample) was used to calculate the number of DCs per μl of sample (Ben-Eliyahu et al., 2000; Neeman et al., 2012). The quotient of total DCs divided by the area skin obtained by the 4 mm diameter biopsy tool (12.57 mm2) provided a measure of skin DC density. Due to unequal variances, data for all groups were log10 transformed for statistical evaluation.
Plasma cortisol
Cortisol was measured in plasma (diluted 1:40) in a single EIA (Correlate-EIA; Assay Designs, Ann Arbor, MI, USA) that has been validated for this species (Demas et al., 2004) according to the manufacturer's instructions. The cortisol EIA had a sensitivity of < 57 pg/ml, an intra-assay CV of <10.5% and an inter-assay CV of <8.6%.
Statistical analyses
Cosinor analyses were performed using software written by R. Refinetti (available at http://www.circadian.org/softwar.html). Repeated measures ANOVAs were used to evaluate the effects of circadian phenotype on diurnal rhythms in blood leukocytes and on the evolution of the DTH reaction. ANOVAs were used to evaluate the effects of circadian phenotype, circadian phase and surgical treatment on blood leukocyte values, spleen clock gene expression, and skin dendritic cell concentrations. A priori planned pairwise comparisons were performed using t tests. Differences were only considered significant if P≤0.05.
Results
Circadian arrhythmia abolishes light-entrained rhythms in leukocyte trafficking
Circadian rhythms in behavior
Hamsters in a 16L:8D photocycle were subjected to a disruptive phase shift (DPS) treatment, consisting of a 2 h pulse of light at night, followed by a 3 h phase-delay of the photocycle (see Materials and Methods), and home-cage locomotor activity of each hamster was monitored for 10 days beginning >1 month after DPS treatment. Among DPS-treated hamsters, 52% (31/60) became circadian arrhythmic (ARR), 40% (24/60) continued to express entrained CRs (ENTR), and 8% (5/60) exhibited free-running locomotor activity rhythms (Fig. S1). Free-running hamsters were excluded from subsequent analyses. Among hamsters subjected to a control treatment (a 3 h phase delay without the 2 h light pulse), 75% (6/8) re-entrained normally, and 25% (2/8) became arrhythmic (cf. Ruby et al., 2004).
CRs in blood leukocyte trafficking
Rhythmic aspects of blood leukocyte trafficking were assessed in blood samples from ENTR (n=29) and ARR (n=33) hamsters collected at 4 time points across the circadian cycle: Zeitgeber time 4 (ZT4; 4 h after the onset of the light phase), ZT11, ZT18 and ZT23). Circadian phenotype significantly affected the daily pattern of blood leukocyte concentrations (F3,180=11.1, P<0.001; Fig. 1A). Among ENTR hamsters, blood leukocytes peaked early in the light phase (ZT4) and reached a nadir shortly after the onset of darkness (ZT18). The diurnal waveform of blood leukocytes was markedly dampened among ARR relative to ENTR hamsters (P<0.001). Leukocyte concentrations were significantly lower in ENTR relative to ARR hamsters at ZT18 (t60=9.22, P<0.001) and ZT23 (t60=6.54, P<0.001).
Figure 1. Circadian arrhythmia eliminates diurnal rhythms in blood leukocyte trafficking.
(A) Mean ± SEM concentration of blood leukocytes at 4 time points over the circadian day in hamsters that were entrained to the light-dark cycle (ENTR, n=29) or were rendered behaviorally arrhythmic via DPS treatment (ARR, n=33; see Methods). Data are double-plotted. (B) Mean ± SEM blood leukocyte trafficking (as % ZT4 value) responses of ENTR and ARR hamsters over the circadian cycle. (C) Mean ± SEM blood leukocyte trafficking responses of ENTR (n=8) and ARR (n=8) hamsters to a 2 h restraint stress manipulation delivered from ZT3 - ZT5. ***P<0.001 vs. ARR value at similar time point. #P<0.01 vs. pre-stress value, within phenotype.
Circadian phenotype also affected the pattern of leukocyte redeployment out of the blood (F2,120=9.85, P<0.001; Fig. 1B). When calculated relative to peak (ZT4) blood leukocyte values, significant leukocyte redeployment was evident in ENTR (F2,56=21.4, P<0.001) but not in ARR (F2,64=2.60, P>0.05) hamsters. Leukocytes deployment out of the blood was greater in ENTR relative to ARR hamsters at ZT18 (t60=-7.63, P<0.001) and ZT23 (t60=-5.10, P<0.001; Fig. 1B)
Stress-induced blood leukocyte trafficking
To evaluate the effect of circadian arrhythmia on stress-induced leukocyte trafficking, blood leukocyte concentrations were measured in ARR (n=8) and ENTR (n=8) hamsters prior to, and during, a 2 h restraint stress (RS) manipulation from ZT3 - ZT5. ENTR and ARR hamsters exhibited comparable leukocyte trafficking responses to RS (F1,12=0.01, P>0.90; Fig. 1C). In both phenotypes, RS caused a significant redeployment of leukocytes out of the blood (+60 min: P<0.01; +120 min: P<0.01 vs. pre-stress values). Leukocyte trafficking responses to RS were comparable in ENTR and ARR hamsters at all time points post-stress (P>0.20, all comparisons; Fig. 1C).
In hamsters (ARR, n=6; ENTR, n=7) not exposed to RS, plasma cortisol concentrations at ZT8 (around the cortisol nadir, at the midpoint of the light phase) did not differ between circadian phenotypes (ENTR: 85.0 ±5.8 ng/ml; ARR: 82.5 ±6.2; mean ±SEM; t11=0.3, P>0.70).
Circadian arrhythmia eliminates endogenous rhythms in leukocyte trafficking and lymphoid clock gene expression
CRs in blood leukocyte trafficking
The next experiment examined whether CRs in blood leukocytes reflect endogenous rhythms in leukocyte trafficking, and whether circadian oscillations in clock gene expression in peripheral lymphoid tissues require a functional SCN. ENTR (n=24) and ARR (n=24) hamsters were transferred to constant darkness; blood and spleen samples were collected after >48 h in constant darkness (DD) at projected ZT1 and ZT17 (Aschoff et al., 1965). Circadian phenotype significantly affected blood leukocyte values in DD (F1,44=13.2, P<0.001; Fig. 2A): CRs in blood leukocyte trafficking were sustained in DD among ENTR hamsters, with concentrations approximately 2-fold higher at ZT1 relative to ZT17 (t22=4.92, P<0.001). In contrast, among ARR hamsters leukocyte concentrations were comparable at ZT1 and ZT17 (t22=-0.635, P>0.50; Fig. 2A).
Figure 2. The SCN regulates tissue-level synchrony in splenic clock gene expression.
Mean ± SEM (A) concentration of blood leukocytes, (B) spleen per1 and bmal1 mRNA expression, and (C) ratio of per1:bmal1 mRNA of ENTR (n=24) and ARR (n=24) hamsters at projected ZT1 and ZT17, 48 h after transfer to continuous darkness (DD). **P<0.01, ***P<0.001. ###P<0.001 vs. ZT1 value.
Spleen clock gene expression
Circadian clock gene (per1, bmal1) expression in the spleen of ENTR and ARR hamsters was also measured after 48 h in DD using qPCR. Among ENTR hamsters, per1 mRNA was significantly lower at ZT1 relative to ZT17 (t18=-2.82, P=0.01), and bmal1 mRNA was significantly higher at ZT1 relative to ZT17 (t18=2.02, P<0.05; Fig. 2B). In contrast, among ARR hamsters ZT1/ZT17 differences in per1 (t16=1.14, P>0.20) and in bmal1 (t16=1.05, P>0.30) were not statistically significant (Fig. 2B).
Total daily mRNA expression (collapsed across ZTs) was comparable in ENTR and ARR hamsters (per1: F1,36=2.15, P>0.10; bmal1: F1,36=0.06, P>0.80; not illustrated), but circadian phenotype altered the ratio of per1:bmal1 mRNA in the spleen (F1,34=21.3, P<0.001; Fig. 2C). ENTR hamsters exhibited robust circadian variation in per1:bmal1 (per1:bmal1: t18=-4.86, P<0.001), whereas in ARR hamsters per1:bmal1 values remained low and did not vary over the subjective circadian cycle (t16=1.03, P>0.30; Fig. 2C).
Melatonin imparts circadian time information onto peripheral oscillators in lymphoid tissues
CRs in blood leukocyte trafficking
Pineal melatonin (MEL) secretion is a high-amplitude circadian endocrine signal and melatonin signaling exerts diverse immunomodulatory effects (Carrillo-Vico et al., 2005). Thus, the next experiment examined whether nocturnal MEL secretion is necessary to maintain CRs in blood leukocyte trafficking and in spleen clock gene expression. ENTR hamsters were pinealectomized (PINx; n=16) or sham-operated (n=20), and six weeks later blood and spleens were collected >48 h after transfer to DD. CRs in blood leukocytes were not affected by PINx (F1,32=0.72, P>0.70; Fig. 3A): circadian leukocyte trafficking was evident in both groups, with greater blood leukocyte concentrations at ZT1 relative to ZT17 (sham-PINx: t18=6.44, P<0.001; PINx: t14=3.69, P<0.001; Fig. 3A).
Figure 3. Circadian output to the spleen requires melatonin secretion.
Mean ± SEM (A) concentration of blood leukocytes, (B) spleen per1 and bmal1 mRNA expression, and (C) ratio of per1:bmal1 mRNA of pinealectomized (PINx, n=16) and pineal-intact (sham-PINx, n=20) entrained (ENTR) hamsters at projected ZT1 and ZT17, 48 h after transfer to continuous darkness (DD). *P<0.05, **P<0.01).
Spleen clock gene expression
In control hamsters spleen per1 expression was higher at ZT17 relative to ZT1 (t15=-1.79, P<0.05, one tailed), and bmal1 expression was higher at ZT1 relative to ZT17 (t18=2.35, P<0.05; Fig. 3B); PINx eliminated ZT1/ZT17 differences in per1 and bmal1 expression in DD (P>0.90 and P>0.70, respectively). In sham-PINx hamsters, the per1:bmal1 mRNA ratio in spleen changed markedly with circadian time: per1:bmal1 was higher at ZT17 relative to ZT1 (t15=-2.64, P<0.05; Fig. 3C). Circadian changes in per1:bmal1 were eliminated by PINx (t12=-0.57, P>0.50; Fig. 3C).
Total per1 mRNA expression (collapsed across ZTs) was significantly greater in PINx relative to sham-PINx hamsters (F1,29=8.87, P>0.01; not illustrated); omnibus bmal1 expression was not affected by surgical treatment (F1,32=0.22, P>0.60; not illustrated).
Effects of circadian arrhythmia on DTH reactions and skin leukocyte trafficking
DTH reactions
Delayed-type skin hypersensitivity (DTH) reactions were elicited in a subset of ENTR (n=15) and ARR (n=20) hamsters from Expt 1. DTH was induced by application of the antigen, 2,4-dinitro-1-fluorobenzene (DNFB; Sigma), to the pinna of each hamster after initial immunization (sensitization, by application of DNFB to the dorsum); sensitization and challenge both occurred between ZT12 and ZT13. Circadian phenotype significantly altered the magnitude and waveform of the DTH inflammatory reaction over several days (F7,231=6.53, P<0.001): ear thicknesses were greater in ENTR relative to ARR hamsters on day +2 (P<0.001) and day +3 (P<0.005) after challenge (Fig. 4A).
Figure 4. Circadian arrhythmia impairs organismal-level inflammatory responses and trafficking of antigen-presenting cells.
(A) Mean ± SEM delayed-type hypersensitivity (DTH) responses (pinna thickness) during the week post-antigen (DNFB) challenge of ENTR (n=15) and ARR (n=20) Siberian hamsters. DNFB sensitization, challenge, and all ear measurements were performed between ZT12 and ZT13. (B) Mean ± SEM number of dendritic cells (CD45+/SSChigh/CD11+) in the skin of ENTR and ARR hamsters at ZT3 and ZT18. **P<0.005, ***P<0.001 vs. ARR value on same day. #P<0.05 vs. ZT3 value, within phenotype.
Skin dendritic cells
To examine if impaired DTH reactions in ARR hamsters are accompanied by changes in mechanisms that participate in antigen presentation, activated skin dendritic cells (CD45+/CD11c+/SSChigh) were quantified in ARR (n=8) and ENTR hamsters (n=8) from punch biopsies obtained between ZT2-ZT3 and between ZT17-ZT18 (hereafter, ‘ZT3’ and ‘ZT18’; n=8/phenotype/time). In ENTR hamsters, CD45+/CD11c+ skin dendritic cells were 3-fold higher at ZT18 relative to ZT3 (t14=-2.50, P<0.025; Fig. 4B); diurnal variation in skin dendritic concentrations were not evident in ARR hamsters (t14=0.93, P>0.30).
Quantitative relations between circadian amplitude and immune function
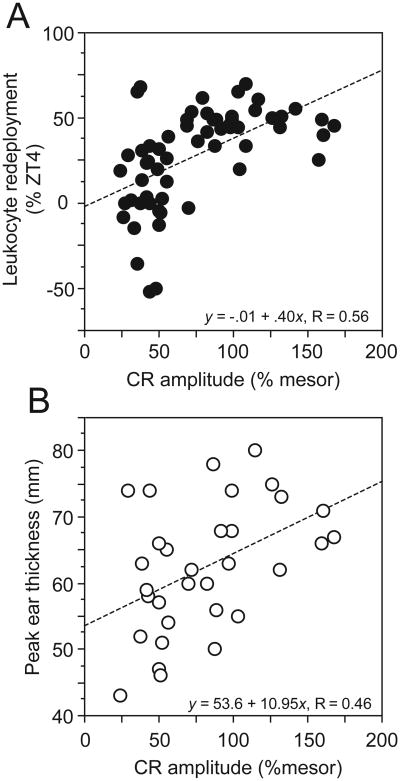
Linear regression analyses identified a significant positive correlation between amplitude of the circadian locomotor activity rhythm and the amplitude of the CR in blood leukocytes (β=0.56, R2=0.32, P<0.001). CR amplitude also predicted the amplitude of the peak (day +2) DTH response (β=0.46, R2=0.21, P<0.01; Fig. 5A, 5B).
Figure 5. Amplitude of the circadian waveform predicts the magnitude of leukocyte trafficking and adaptive immune responses.
Linear regression between amplitude of the circadian locomotor activity waveform and (A) peak leukocyte redeployment, and (B) peak skin inflammatory responses. Both regression coefficients were statistically different from zero (leukocyte trafficking: P<0.001; DTH inflammatory response: P<0.01).
Discussion
Disruptive phase shifts (DPS) induced behavioral arrhythmia (Fig. S1), abolished circadian control of leukocyte trafficking (Fig. 1), rendered peripheral clocks in lymphoid tissues arrhythmic (Fig. 2), and impaired organismal-level antigen-specific immune responses (Fig. 4). Critical to the interpretation of the present data is that DPS-induced arrhythmic hamsters, unlike SCN ablation models of arrhythmia, did not sustain trauma or injury to the CNS. Brain lesions result in CNS inflammation that persists for months after surgery (Norenburg, 1994; Suzuki et al., 2012), and bone remodeling and cutaneous wound healing that continues for the remainder of the lifespan (Lalani et al., 1998)— processes which permanently alter immune function and confound interpretation of measures of immunity. In contrast to lesion models, DPS-induced ARR hamsters differ from ENTR controls only in that the pacemaker in the SCN is driven to a state of near zero amplitude (Steinlechner et al., 2002); expression of core circadian clock genes in the SCN is low-amplitude and arrhythmic (Grone et al., 2011), giving rise to profound arrhythmia in locomotor activity, sleep, and body temperature (Larkin et al., 2004; Ruby et al., 2009) and eliminating nocturnal melatonin secretion (Steinlechner et al., 2002). Circadian clock genes are increasingly recognized as important to cellular immune processes (clock: [Spengler et al., 2012]; cry: [Narasimamurthy, 2012]; reverbα: [Gibbs et al., 2012]; per: [Liu et al., 2006]; e4bp4: [Male et al., 2012]). Unlike transgenic and mutation models of arrhythmia, however, DPS-arrhythmic hamsters possess the full complement of clock genes. Thus, the present data suggest that, among the pleiotropic effects of clock genes and their protein products, the conferral of time information per se is of functional significance towards organismal-level immune function.
Immune function is under strong circadian control: CRs in blood leukocytes are well-documented (see Dhabhar et al., 1994), and innate (Marpegan et al., 2009) and adaptive (Pownall et al., 1979) inflammatory responses vary over circadian time. Here we show that the functional integrity of the circadian pacemaker in the SCN is required to impart temporal organization to leukocyte trafficking and to tissue-level circadian clocks in secondary lymphoid tissues, the sites of lymphocyte activation by antigen (Mebius and Kraal, 2005). In addition, rhythmic clock gene expression in the spleen was absent in hamsters without nocturnal pineal melatonin secretion, but free-running rhythms in leukocyte trafficking persisted in the absence of melatonin. The present work also shows that the role of the circadian system in immune function goes beyond merely providing temporal phase information. In addition to losing diurnal rhythms in skin antigen (DTH) responses. Together, the data indicate that circadian organization is essential for coordination of organismal-level immune function and identify a novel neuroendocrine mechanism by which circadian time information is communicated from the central pacemaker to the peripheral immune system.
SCN arrhythmia abolishes circadian rhythms in leukocyte trafficking
In ARR hamsters, CRs in leukocyte trafficking were eliminated. Notably, this did not manifest as leukopenia, which would be indicative of immunosuppression; rather, blood leukocyte concentrations were maintained throughout the day near peak levels, with little evidence of masking effects of light. In ENTR hamsters, blood leukocytes peaked at the beginning of the rest phase (ZT4) and reached a nadir shortly after the beginning of the active phase (ZT18). In rats, blood leukocytes exhibit a similar waveform (Dhabhar et al., 1994, and circadian trafficking out of the blood is primarily accomplished by CD4+ T cells, B cells, NK cells and monocytes (Dhabhar et al., 1994). The present data indicate that a functional pacemaker in the SCN is required for the expression of diurnal rhythms in leukocyte trafficking; indeed, even in the presence of a full light-dark cycle CRs in blood leukocyte trafficking were absent in ARR hamsters. Other models of circadian arrhythmia have suggested the existence of non-dampening circadian oscillators in the immune system, capable of sustaining CRs in lymphopoesis or trafficking in the absence of a functional central pacemaker: CRs in blood leukocytes persisted in rats after 8 weeks of exposure to LL (Deprés-Brummer et al., 1997), and after 11 weeks in LL, low-amplitude CRs in CD4+ and CD8+ T cells persisted, despite the loss of CRs in total lymphocytes (Deprés-Brummer et al., 1997). Chronic LL reliably eliminates behavioral and thermoregulatory CRs, but CRs in SCN activity (clock gene expression, c-fos) are not consistently eliminated by LL treatments (Beaulé et al., 2003; Edelstein and Amir, 1996; Nováková et al., 2011). Residual, low-amplitude CRs in the SCN, while inadequate to drive behavioral or thermoregulatory rhythms, may be of sufficient amplitude to sustain rhythms in leukocyte trafficking under chronic LL treatment.
In contrast to circadian-driven leukocyte trafficking, trafficking in response to an acute stressor was normal in ARR hamsters (Fig. 1C), indicating that the capacity for leukocyte trafficking may be unimpaired by the loss of central rhythmicity; decrements in leukocyte trafficking in ARR hamsters are selective-- specific to trafficking responses to circadian time cues.
The SCN regulate tissue-level synchrony in clock gene expression
Lesions of the SCN eliminate CRs in clock gene expression in multiple peripheral organs (Akhtar et al., 2002; Guo et al., 2006; Sakamoto et al., 1998). The spleen is a key organ for immunological homeostasis: both innate and adaptive immune responses can be initiated and mounted in the spleen (Mebius and Kraal, 2005). In the present study, ZT1/ZT17 differences in splenic per1 and bmal1 expression were absent in ARR hamsters. Such an outcome may arise from either tissue-level arrhythmia or asynchronous rhythmicity between individuals. If tissue-level rhythmicity persists in individual ARR hamsters, then per1 and bmal1 mRNAs levels should remain in antiphase, as in ENTR hamsters (cf. Fig. 2C). In addition, if individual hamsters within a treatment group retain free-running tissue-level rhythms, but are merely desynchronized at a population level, then per1:bmal1 variance should be considerably greater among ARR relative to ENTR hamsters across the circadian day. Neither of these outcomes was obtained. Spleen per1:bmal1 ratios in ARR hamsters were low and invariant over circadian time in DD, and the SEM of this ratio was consistently smaller in ARR relative to ENTR hamsters (Fig. 2C). This outcome is consistent with an arrest of free-running rhythms in cellular circadian clocks in the spleen following the induction of SCN arrhythmia (Guo et al., 2006). The data corroborate previous reports in SCN-lesioned Syrian hamsters (Guo et al., 2006) and mice (Guo et al., 2005) which demonstrated peripheral arrhythmia following loss of SCN-derived time information, suggesting that the SCN is a pacemaker, rather than a phase-coordinator, of peripheral oscillators (Guo et al., 2006). Importantly, the present data demonstrate peripheral immunological arrhythmia in neurologically-intact, unoperated hamsters, permitting the inference that neither the loss of fibers of passage, nor pathological inflammatory (Norenburg, 1994; Suzuki et al., 2012) or neuroendocrine (Bartness et al., 1991; Bittman et al., 1991) sequelae common to postoperative physiology are responsible for the loss of peripheral tissue oscillations. Rather, the mere dampening of rhythms in clock gene expression in the SCN is sufficient to disengage immunological peripheral oscillators from the central pacemaker.
Circadian output to the spleen depends on nocturnal melatonin secretion
Data from ARR hamsters imply that, under conditions of normal entrainment, signals emanate from the SCN to impose temporal order on leukocyte trafficking and spleen clock gene expression. We tested the hypothesis that the high-amplitude nocturnal rhythm in pineal melatonin (MEL) secretion constituted a coupling mechanism between the central pacemaker in the SCN and circadian events in the immune system. The SCN drives nocturnal MEL secretion via a polysynaptic central-sympathetic pathway (Moore, 1996); SCN lesions abolish MEL secretion (Bittman et al., 1991), and MEL secretion is likewise absent in behaviorally-arrhythmic Siberian hamsters (Schöttner et al., 2011). PINx did not affect leukocyte trafficking (Fig. 3A), but eliminated CRs in splenic per1 and bmal1 mRNA expression (Fig. 3B). As in the previous experiment, per1:bmal1 ratios were less variable in PINx relative to sham-PINx hamsters, consistent with the interpretation that the absence of nocturnal MEL secretion led to a loss of phase coherence among cells in the spleen and tissue-level arrhythmia of the splenic clock. Importantly, PINx does not alter the pattern of SCN entrainment to the external photocycle (Prendergast and Freeman, 1999); PINx hamsters lack only MEL secretion concurrent with the nocturnal active phase (Bartness et al., 1993). Specification of the molecular mechanisms by which PINx abolishes clock gene rhythms was beyond the scope of the present investigation, but MEL is sufficient to set the phase of Rev-erba α expression (Wagner et al., 2007), and Rev-erba α repression of bmal1 expression plays a critical role in driving CRs in macrophage function (IL-6 responses to endotoxin; [Gibbs et al., 2012]). In the absence of MEL, rhythmic inhibition of bmal1 by Rev-erb α may be sufficiently diminished in ARR hamsters to give rise to tissue-specific arrhythmia.
Circadian integrity is essential for coordinating organismal-level inflammatory responses
Antigen-specific T cell responses vary markedly over the circadian day in vivo and in vitro, and functional T cell rhythms are eliminated in Clock-/- mice, suggesting important roles for CNS and cellular clocks in T cell function (Fortier et al., 2011). The present study identified the importance of CNS-derived circadian time information in the genesis of T cell-dependent immune responses. Both ENTR and ARR hamsters exhibited skin inflammatory (DTH) reactions, but DTH was markedly reduced in ARR hamsters. This suggests that the mechanisms underlying memory T cell dependent skin inflammatory responses are impaired following loss of CNS circadian rhythmicity. This outcome stands in contrast with a report of enhanced contact hypersensitivity (CHS) responses in Clock-/- mice (Takita et al., 2013). However, plasma corticosterone (B) concentrations are markedly reduced in Clock-/- mice, and effects of this genotype on CHS are eliminated by B or dexamethasone treatment (Takita et al., 2013), suggesting that the immunophenotype is likely mediated by a pleiotropic effect of CLOCK on neuroendocrine function.
Transient circadian desynchrony potentiates innate inflammatory responses (Castanon-Cervantes et al., 2010), but the present data indicate that chronic circadian desynchrony inhibits adaptive inflammatory responses. Either the formation, retention or recall of immunological memory is diminished in the absence of circadian time in the internal milieu. The mediators of this immunosuppression are not yet known. The SCN regulates rhythms in corticosterone secretion (Buijs et al., 1999) and in leukocyte trafficking (Figs. 1, 2). Chronically-elevated glucocorticoids inhibit skin DTH reactions (Dhabhar and McEwen, 1997); however, the present data indicate that daytime (resting) cortisol is not elevated in ARR relative to ENTR hamsters. Skin dendritic cells (DCs) play a critical role in antigen presentation during inflammatory responses (Varona et al., 2001); and antibodies that block DC CD11c binding markedly inhibit DTH reactions (Sadhu et al., 2007). The absence of diurnal rhythms in skin DC trafficking, and in omnibus leukocyte trafficking, in ARR hamsters may decrease the efficiency of multiple stages of the DTH reaction.
Together, the present data indicate widespread impairments in immunity following the loss of central circadian rhythmicity. Understanding how the circadian pacemaker affects the immune system may afford novel insights into how interactions with the circadian environment (lighting, shift work, transmeridian travel) affect health and disease. Indeed, recent work in this species indicates that dim illumination at night (5 lux) inhibits DTH and plasma bactericidal activity (Bedrosian et al., 2011), suggesting that either CR disruption or non-circadian masking effects of light are sufficient to inhibit immune responses. Importantly, in the present study, significant relations were evident between the degree of circadian disruption and decrements in leukocyte trafficking (Fig. 5A) and DTH responses (Fig. 5B). Together, these data suggest that immunological deficits may not be solely revealed under conditions of complete circadian arrhythmia. Abundant epidemiological data support an association between non-traditional work schedules or transmeridian travel and increased risks of multiple cancers, inflammatory diseases and metabolic disorders (Erren et al., 2008; Karlsson et al., 2005). Poor circadian hygiene (Mill et al., 2004), even in the absence of arrhythmia, may lead to impairments in immune responses that require the formation of immunological memory (e.g., DTH, antibody production, autoimmunity) or precise circadian control of leukocyte trafficking (e.g., skin inflammation, wound healing).
Supplementary Figure 1 (S1)
Representative double-plotted activity records of ENTR (A-C) and ARR (D-F) Siberian hamsters. All actograms are drawn to the same scale (counts/bin). Clock time is indicated on the horizontal axis at the top of each actogram, along with light (white) and dark (black) phases of the shifted (post-DPS) 16 h light:8 h dark photocycle (lights off from 20:00 h to 04:00 h). Chi-square periodograms to the right of each record indicate robust and significant (P<0.001, green line) CRs in ENTR actograms, and the absence of detectable CRs in ARR actograms.
Acknowledgments
The authors thank Ela Sehic, Omkar Kelkar, August Kampf-Lassin, Ryan Duggan, and Dr. Betty Theriault for expert technical assistance. This work was supported by Grant AI-67406 from the National Institute of Allergy and Infectious Diseases.
References
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Grant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20:469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Circadian Clocks. North Holland; Amsterdam, NL: 1965. [Google Scholar]
- Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol. 2009;7:e52. doi: 10.1371/journal.pbio.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD, Bittman EL. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol. 1991;260:R102–R112. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Beaulé C, Houle LM, Amir S. Expression profiles of PER2 immunoreactivity within the shell and core regions of the rat suprachiasmatic nucleus: lack of effect of photic entrainment and disruption by constant light. J Mol Neurosci. 2003;21:133–147. doi: 10.1385/JMN:21:2:133. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol Lett. 2011;7:468–471. doi: 10.1098/rsbl.2010.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci USA. 2002;99:4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- Bittman EL, Bartness TJ, Goldman BD, DeVries GJ. Suprachiasmatic and paraventricular control of photoperiodism in Siberian hamsters. Am J Physiol. 1991;260:R90–R101. doi: 10.1152/ajpregu.1991.260.1.R90. [DOI] [PubMed] [Google Scholar]
- Brocker T, Riedinger M, Karjalainen K. Driving gene expression specifically in dendritic cells. Adv Exp Med Biol. 1997;417:55–57. doi: 10.1007/978-1-4757-9966-8_9. [DOI] [PubMed] [Google Scholar]
- Bronson F. Mammalian Reproductive Biology. University of Chicago Press; Chicago, IL: 1989. [Google Scholar]
- Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264:R1186–R1192. doi: 10.1152/ajpregu.1993.264.6.R1186. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Bur IM, Cohen-Solal AM, Carmignac D, Abecassis PY, Chauvet N, Martin AO, van der Horst GT, Robinson IC, Maurel P, Mollard P, Bonnefont X. The circadian clock components CRY1 and CRY2 are necessary to sustain sex dimorphism in mouse liver metabolism. J Biol Chem. 2009;284:9066–9073. doi: 10.1074/jbc.M808360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents, III. Heavy water and constant light: Homeostasis of frequency? J Comp Physiol. 1975;106:267–290. [Google Scholar]
- Demas GE, Polacek KM, Durazzo A, Jasnow AM. Adrenal hormones mediate melatonin-induced increases in aggression in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2004;46:582–91. doi: 10.1016/j.yhbeh.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Deprés-Brummer P, Bourin P, Pages N, Metzger G, Lévi F. Persistent T lymphocyte rhythms despite suppressed circadian clock outputs in rats. Am J Physiol. 1997;273:R1891–R1899. doi: 10.1152/ajpregu.1997.273.6.R1891. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann NY Acad Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- Eastman C, Rechtschaffen A. Circadian temperature and wake rhythms of rats expos to prolonged continuous illumination. Physiol Behav. 1983;31:417–427. doi: 10.1016/0031-9384(83)90061-6. [DOI] [PubMed] [Google Scholar]
- Edelstein K, Amir S. Constant light induces persistent Fos expression in rat intergeniculate leaflet. Brain Res. 1996;731:221–225. doi: 10.1016/0006-8993(96)00691-9. [DOI] [PubMed] [Google Scholar]
- Erren TC, Pape HG, Reiter RJ, Piekarski C. Chronodisruption and cancer Naturwissenschaften. 2008;95:367–382. doi: 10.1007/s00114-007-0335-y. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Cano P, Jimenez-Ortega V, Fernandez-Mateos P, Cardinali DP. Neuroendocrine-immune correlates of circadian physiology: studies in experimental models of arthritis, ethanol feeding, aging, social isolation, and calorie restriction. Endocrine. 2007;32:1–19. doi: 10.1007/s12020-007-9009-y. [DOI] [PubMed] [Google Scholar]
- Filipski E, King VM, Li X, Granda TG, Mormont MC, Claustrat B, Hastings MH, Lévi F. Disruption of circadian coordination accelerates malignant growth in mice. Pathol Biol (Paris) 2003;51:216–219. doi: 10.1016/s0369-8114(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Fortier EE, Rooney J, Dardente H, Hardy MP, Labrecque N, Cermakian N. Circadian variation of the response of T cells to antigen. J Immunol. 2011;187:6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- Franklin AE, Engeland CG, Kavaliers M, Ossenkopp KP. Lipopolysaccharide-induced hypoactivity and behavioral tolerance development are modulated by the light-dark cycle in male and female rats. Psychopharmacology (Berl) 2003;170:399–408. doi: 10.1007/s00213-003-1554-3. [DOI] [PubMed] [Google Scholar]
- Franklin AE, Engeland CG, Kavaliers M, Ossenkopp KP. The rate of behavioral tolerance development to repeated lipopolysaccharide treatments depends upon the time of injection during the light-dark cycle: a multivariable examination of locomotor activity. Behav Brain Res. 2007;180:161–173. doi: 10.1016/j.bbr.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ. The flexible genome. Nat Rev Genet. 2001;2:383–387. doi: 10.1038/35072018. [DOI] [PubMed] [Google Scholar]
- Grone BP, Chang D, Bourgin P, Cao V, Fernald RD, Heller HC, Ruby NF. Acute light exposure suppresses circadian rhythms in clock gene expression. J Biol Rhythms. 2011;26:78–81. doi: 10.1177/0748730410388404. [DOI] [PubMed] [Google Scholar]
- Guan Z, Vgontzas AN, Omori T, Peng X, Bixler EO, Fang J. Interleukin-6 levels fluctuate with the light-dark cycle in the brain and peripheral tissues in rats. Brain Behav Immun. 2005;19:526–529. doi: 10.1016/j.bbi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J Neurosci. 2006;26:6406–6412. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson BL, Alfredsson A, Knutsson E, Andersson K, Torén Total mortality and cause-specific mortality of Swedish shift- and dayworkers in the pulp and paper industry in 1952–2001. Scand J Work Environ Health. 2005;31:30–35. doi: 10.5271/sjweh.845. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Prendergast BJ, Nelson RJ. Photoperiod and stress affect wound healing in Siberian hamsters. Physiol Behav. 2003;78:205–11. doi: 10.1016/s0031-9384(02)00967-8. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani T, Bhol K, Khan IU, Ahmed AR. The scarring processes in mucosal tissues after immune injury. Semin Arthritis Rheum. 1998;27:371–381. doi: 10.1016/s0049-0172(98)80017-6. [DOI] [PubMed] [Google Scholar]
- Larkin JE, Yokogawa T, Heller HC, Franken P, Ruby NF. Homeostatic regulation of sleep in arrhythmic Siberian hamsters. Am J Physiol. 2004;287:R104–R111. doi: 10.1152/ajpregu.00676.2003. [DOI] [PubMed] [Google Scholar]
- Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Frautschy SA, Gonzalez AM, Baird AA. Time course for the focal elevation of synthesis of basic fibroblast growth factor and one if its high-affinity receptors (flg) following a localized cortical brain injury. J Neurosci. 1992;12:3828–3837. doi: 10.1523/JNEUROSCI.12-10-03828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male V, Nisoli I, Gascoyne DM, Brady HJ. E4BP4: an unexpected player in the immune response. Trends Immunol. 2012;33:98–102. doi: 10.1016/j.it.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Marpegan L, Bekinschtein TA, Costas MA, Golombek DA. Circadian responses to endotoxin treatment in mice. J Neuroimmunol. 2005;160:102–109. doi: 10.1016/j.jneuroim.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Sehgal A. Molecular regulation of circadian rhythms in Drosophila and mammals. Neuroscientist. 2001;7:496–505. doi: 10.1177/107385840100700606. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS. Psychological stress and antibody response to influenza vaccination: when is the critical period for stress, and how does it get inside the body? Psychosom Med. 2004;66:215–223. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- Moore RY. Neural control of the pineal gland. Behav Brain Res. 1996;73:125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, Lellouch J, Misset JL, Touitou Y, Lévi F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–3045. [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman E, Shaashua L, Benish M, Page GG, Zmora O, Ben-Eliyahu S. Stress and skin leukocyte trafficking as a dual-stage process. Brain Behav Immun. 2012;26:267–276. doi: 10.1016/j.bbi.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD. Astrocyte responses to CNS injury. J Neuropathol Exp Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- Nováková M, Polidarová L, Sládek M, Sumová A. Restricted feeding regime affects clock gene expression profiles in the suprachiasmatic nucleus of rats exposed to constant light. Neuroscience. 2011;197:65–71. doi: 10.1016/j.neuroscience.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living organisms. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Pownall R, Kabler PA, Knapp MS. The time of day of antigen encounter influences the magnitude of the immune response. Clin Exp Immunol. 1979;36:347–354. [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Cisse YM, Cable EJ, Zucker I. Dissociation of ultradian and circadian phenotypes in female and male Siberian hamsters. J Biol Rhythms. 2012;27:287–298. doi: 10.1177/0748730412448618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Freeman DA. Pineal-independent regulation of photo-nonresponsiveness in the Siberian hamster (Phodopus sungorus) J Biol Rhythms. 1999;14:62–71. doi: 10.1177/074873099129000452. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Nelson RJ. Photoperiodic regulation of circulating leukocytes in juvenile Siberian hamsters: mediation by melatonin and testosterone. J Biol Rhythms. 2003;18:473–480. doi: 10.1177/0748730403258486. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Pyter LM. Photoperiod history differentially impacts reproduction and immune function in adult Siberian hamsters. J Biol Rhythms. 2009;24:509–522. doi: 10.1177/0748730409349714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hrafkelsson J, Kyyrönen P, Linnersjö A, Rafnsson V, Storm H, Tveten U. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ. 2002;325:567. doi: 10.1136/bmj.325.7364.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R, Cornélissen G, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. Cancer incidence in California flight attendants. Cancer Causes Control. 2002;13:317–324. doi: 10.1023/a:1015284014563. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Barakat MT, Heller HC. Phenotypic differences in reentrainment behavior and sensitivity to nighttime light pulses in Siberian hamsters. J Biol Rhythms. 2004;19:530–541. doi: 10.1177/0748730404268055. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci USA. 2009;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu C, Ting HJ, Lipsky B, Hensley K, Garcia-Martinez LF, Simon SI, Staunton DE. CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J Leukoc Biol. 2007;81:1395–1403. doi: 10.1189/jlb.1106680. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, Ishida N. Multi-tissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem. 1998;273:27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- Schibler U. The daily timing of gene expression and physiology in mammals. Dialogues Clin Neurosci. 2007;9:257–272. doi: 10.31887/DCNS.2007.9.3/uschibler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttner K, Simonneaux V, Vuillez P, Steinlechner S, Pévet P. The daily melatonin pattern in Djungarian hamsters depends on the circadian phenotype. Chronobiol Int. 2011;28:873–882. doi: 10.3109/07420528.2011.622328. [DOI] [PubMed] [Google Scholar]
- Seimiya M, Wada A, Kawamura K, Sakamoto A, Ohkubo Y, Okada S, Hatano M, Tokuhisa T, Watanabe T, Saisho H, Tagawa M, O-Wang J. Impaired lymphocyte development and function in Clast5/Stra13/DEC1-transgenic mice. Eur J Immunol. 2004;34:1322–1332. doi: 10.1002/eji.200324700. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, Antoch MP. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc Natl Acad Sci USA. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlechner S, Stieglitz A, Ruf T. Djungarian hamsters: a species with a labile circadian pacemaker? Arrhythmicity under a light-dark cycle induced by short light pulses. J Biol Rhythms. 2002;17:248–258. doi: 10.1177/074873040201700308. [DOI] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sakata H, Kato C, Connor JA, Morita M. Astrocyte activation and wound healing in intact-skull mouse after focal brain injury. Eur J Neurosci. 2012;36:3653–3664. doi: 10.1111/j.1460-9568.2012.08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takita E, Yokota S, Tahara Y, Hirao A, Aoki N, Nakamura Y, Nakao A, Shibata S. Biological clock dysfunction exacerbates contact hypersensitivity in mice. Br J Dermatol. 2013;168:39–46. doi: 10.1111/j.1365-2133.2012.11176.x. [DOI] [PubMed] [Google Scholar]
- Turk JL. Delayed Hypersensitivity Research Monographs in Immunology. Elsevier; Amsterdam, NL: 1980. [Google Scholar]
- Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutiérrez J, Torres M, Martínez-A C, Márques G. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–R45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Johnston JD, Tournier BB, Ebling FJ, Hazlerigg DG. Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus) Eur J Neurosci. 2007;25:485–490. doi: 10.1111/j.1460-9568.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- Wang YC, Hu XB, He F, Feng F, Wang L, Li W, Zhang P, Jia ZS, Liang YM, Han H. Lipopolysaccharide-induced maturation of bone marrow-derived dendritic cells is regulated by notch signaling through the up-regulation of CXCR4. J Biol Chem. 2009;284:15993–16003. doi: 10.1074/jbc.M901144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Karelina K, Su AJ, Barker JM, Norman GJ, Zhang N, Devries AC, Nelson RJ. Time-of-day determines neuronal damage and mortality after cardiac arrest. Neurobiol Dis. 2009;36:352–360. doi: 10.1016/j.nbd.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L, Thrivikraman KV, Plotsky PM. Combined pre- and postnatal environmental enrichment programs the HPA axis differentially in male and female rats. Psychoneuroendocrinology. 2006;31:553–564. doi: 10.1016/j.psyneuen.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Wen JC, Dhabhar FS, Prendergast BJ. Pineal-dependent and -independent effects of photoperiod on immune function in Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;51:31–39. doi: 10.1016/j.yhbeh.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative double-plotted activity records of ENTR (A-C) and ARR (D-F) Siberian hamsters. All actograms are drawn to the same scale (counts/bin). Clock time is indicated on the horizontal axis at the top of each actogram, along with light (white) and dark (black) phases of the shifted (post-DPS) 16 h light:8 h dark photocycle (lights off from 20:00 h to 04:00 h). Chi-square periodograms to the right of each record indicate robust and significant (P<0.001, green line) CRs in ENTR actograms, and the absence of detectable CRs in ARR actograms.