Abstract
It has been suggested that oxidative stress may play a role in the pathogenesis of Autism Spectrum Disorders (ASD), but the literature reports somewhat contradictory results. To further investigate the issue, we evaluated a high number of peripheral oxidative stress parameters, and some related issues such as erythrocyte membrane functional features and lipid composition. Twenty-one autistic children (Au) aged 5 to 12 years, were gender and age-matched with 20 typically developing children (TD). Erythrocyte thiobarbituric acid reactive substances, urinary isoprostane and hexanoyl-lysine adduct levels were elevated in Au, thus confirming the occurrence of an imbalance of the redox status of Au, whilst other oxidative stress markers or associated parameters (urinary 8-oxo-dG, plasma radical absorbance capacity and carbonyl groups, erythrocyte superoxide dismutase and catalase activities) were unchanged. A very significant reduction of Na+/K+-ATPase activity (−66%, p<0.0001), a reduction of erythrocyte membrane fluidity and alteration in erythrocyte fatty acid membrane profile (increase in monounsaturated fatty acids, decrease in EPA and DHA-ω3 with a consequent increase in ω6/ω3 ratio) were found in Au compared to TD, without change in membrane sialic acid content. Some Au clinical features appear to be correlated with these findings; in particular, hyperactivity score appears to be related with some parameters of the lipidomic profile and membrane fluidity. Oxidative stress and erythrocyte membrane alterations may play a role in the pathogenesis of ASD and prompt the development of palliative therapeutic protocols. Moreover, the marked decrease in NKA could be potentially utilized as a peripheral biomarker of ASD.
Introduction
Autism spectrum disorders (ASD) are complex neuro-developmental disorders characterized by impairment in social interaction and communication, and exhibition of repetitive and stereotypic behaviours. Diagnosis of ASD is based on clinical features only, and at present there are no validated biomarkers for diagnostic and/or screening purposes [1]. Genetic susceptibility, immunologic alterations, and environmental factors have been proposed to play an etio-pathogenic role in ASD [2]. It has been suggested that oxidative stress may play a role in the etio-pathogenesis of ASD [3]–[5]. Oxidative stress is defined as the disruption of the normal intracellular balance between reactive oxygen species (ROS), produced either during aerobic metabolism or as a consequence of pathologic processes and antioxidant defence mechanisms [6]. Oxidative stress, in turn, induces the secretion of numerous vasoactive and pro-inflammatory molecules [7] leading to neuroinflammation [2]. Oxidative stress has been suggested to underlie several other mental disorders, including schizophrenia and bipolar disorder [8]–[10], and neurodegenerative pathologies such as Alzheimer disease [11]. Oxidative stress is the result of increased production of pro-oxidant species or decreased antioxidant defences; glutathione redox status has indeed been found to be decreased in autistic patients, also in the post-mortem analysis of Autistic brain tissues [12].
Oxidative stress can be detected by studying a panel of different markers [13], some of which, such as DNA, proteins and polyunsaturated fatty acid (PUFA) residues, are pathognomonic of oxidative damage of biomolecules. It is worth mentioning that lipid peroxidation was found to be elevated in autism [14] and that PUFA are important for neurodevelopment [15]. Noteworthy, the imbalance of membrane fatty acid composition and PUFA loss can affect ion channels and receptors [16]. In particular, Ca2+ channel deficiency was found in Au [17], but never correlated to membrane parameters.
The aim of our study was to evaluate an integrated biomarker panel in Autistic (Au) children, in order to assess the possible imbalance of their redox status. The rationale for the choice of the parameters we examined was based on the strong correlation between: a) erythrocyte fatty acid membrane profile and preservation/degeneration of brain functions in aging and in neurodegenerative diseases [18], [19]; b) erythrocyte membrane ω6/ω3 balance and inflammation markers [20]; c) peripheral and central nervous system markers of oxidative stress [21]. All these biomarkers are components of an intertwined biological system, wherein erythrocyte membrane functional and structural characteristics act as a sensor of pathological changes. The recognition of biochemical alterations occurring in ASD subjects may also result in therapeutic methods aimed at reducing some of the symptoms. Also, the examined parameters are a potentially useful biomarker of ASD.
Materials and Methods
Ethics Statement
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by Local Ethical Committee (Azienda USL Bologna, CE 10020- n.30, 06/04/2010 prot 45424/10-03). Written consent was obtained from all parents and also from children through pictures and simplified information.
Subjects
A total of 48 children were approached as part of the present case–control study. Of these, 25 had a diagnosis of Autism (Au) and 23 were classified as Typically Developing (TD) children. Of these, 21 were recruited for inclusion in the study from the autism group (4 F and 17 M), and 20 in the TD group (6 F and 14 M). Reasons for rejection included: taking fish oil supplements (two subjects in the Au group), taking vitamins and/or other substance known to have antioxidant properties (two subjects in Au group and 3 subjects in TD group).
Au group mean age was 6.8 years (SD = 2.23 years, median = 6 years, range 5–12 years); in TD group mean age was 7.6 years (SD = 1.96 years, median = 7 years, range 5–12 years). Both the non-parametric comparison of the average age in the two groups and the comparison by gender (chi-square test), were not significant, confirming the comparability between cases and controls.
All the patients were admitted to Child Neuropsychiatric Unit of the Maggiore Hospital of Bologna (Neurological Sciences Institute IRCCS-Bologna), for assessment by a comprehensive diagnostic- neurological workup and regular follow-ups. None of the autistic patients had active epilepsy at the time of blood and urine sampling. One patient experienced a first and (at the moment of writing this paper) single benign rolandic seizure six months after the blood and urine collection (this patient was the only one with a normal intellectual level). Any medical and neurological comorbidity was excluded by electroencephalography (recorded during awake and sleep), cerebral magnetic resonance imaging, standard clinical and neurological examination, neurometabolic and genetic investigations (including 550 band karyotype, and molecular assay for Fragile X and MECP2). No infective or inflammatory disease was detected at the time of blood collection. No subject underwent any surgery intervention in the four months prior to blood and urine collection.
Autism diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV TR [22]) criteria, Autism Diagnostic Observation Schedule (ADOS) [23] and Childhood Autism Rating Scale (CARS) [24] by two clinicians (a child neuro-psychiatrist and a child psychologist) experienced in the field of autism (P.V., F.R.). Developmental and cognitive levels were assessed by Psychoeducational Profile-3 (PEP-3) [25] and Leiter International Performance Scale–Revised (Leiter-R) [26]. Parents were questioned regarding the age of onset of early autistic signs. Demographic and clinical features of Au group are summarized in Table 1. Control group children were healthy TD children, recruited in the local community, with no sign of cognitive, learning and psychiatric involvement, as clinically and anamnestically determined by three experienced clinicians (A.G., P.V., F.R.). All TD were attending mainstream school and had not been subjected to stressful events. Dietary habits were assessed by a Food Questionnaire. All patients and controls were on a typical Mediterranean diet.
Table 1. Demographic and clinical features of the autistic children group.
| No. | Gender | Age (months) | Age of onset (months) | Cognitive/developmental impairment | DSM IV-TRdiagnosis | ADOSscore | ADOSdiagnosis | CARSglobalscore | CARS activity level item score (hyperactivity) | CARS body use item score (stereotypes) |
| 1 | M | 66 | ≤12 | Moderate | PDD | 18 | Au | 40.5 | 2.5 | 3.5 |
| 2 | M | 74 | ≤12 | Severe | PDD | 21 | Au | 42 | 2.5 | 2.5 |
| 3 | M | 64 | ≤12 | Moderate | PDD | 16 | Au | 35 | 2.5 | 2 |
| 4 | M | 103 | 13–18 | Moderate | PDD | 19 | Au | 44.5 | 3 | 3 |
| 5 | M | 61 | ≤12 | Severe | PDD | 21 | Au | 46 | 3.5 | 4 |
| 6 | M | 71 | 13–18 | Severe | PDD | 22 | Au | 41 | 2 | 3 |
| 7 | M | 142 | ≤12 | Severe | PDD | 22 | Au | 44.5 | 2.5 | 3 |
| 8 | M | 131 | 13–18 | Severe | PDD-NOS | 16 | Au | 38 | 3 | 3 |
| 9 | M | 66 | 13–18 | Moderate | PDD | 22 | Au | 40.5 | 3 | 3 |
| 10 | F | 66 | 13–18 | Borderline IQ | PDD-NOS | 15 | Au | 41.5 | 2.5 | 2 |
| 11 | M | 74 | 13–18 | Severe | PDD | 22 | Au | 42.5 | 3 | 2.5 |
| 12 | F | 66 | 13–18 | Severe | PDD | 22 | Au | 43.5 | 3.5 | 3.5 |
| 13 | M | 66 | 13–18 | Mild | PDD-NOS | 14 | Au | 34 | 2 | 2 |
| 14 | M | 89 | 19–24 | Mild | PDD | 19 | Au | 40 | 3 | 3 |
| 15 | M | 102 | 13–18 | Mild | PDD | 22 | Au | 36.5 | 2 | 2.5 |
| 16 | F | 110 | 25–30 | Moderate | PDD | 15 | Au | 47.5 | 3.5 | 3 |
| 17 | M | 79 | 13–18 | Moderate | PDD | 19 | Au | 37 | 2.5 | 3 |
| 18 | M | 144 | 13–18 | Severe | PDD-NOS | 20 | Au | 39 | 3 | 3.5 |
| 19 | M | 80 | ≤12 | Normal IQ | PDD | 19 | Au | 36.5 | 2.5 | 2 |
| 20 | M | 79 | ≤12 | Severe | PDD | 21 | Au | 40.5 | 2.5 | 3 |
| 21 | F | 65 | ≤12 | Mild | PDD-NOS | 17 | Au | 31.5 | 2 | 2 |
PDD: Pervasive Developmental Disorder; PDD-NOS: Pervasive Developmental Disorder-Not Otherwise Specified; Au: Autism.
Biochemical Evaluations
Blood samples, obtained from Au and TD children, were collected in Na2-EDTA (∼9 mL) and heparin (∼5 mL) vacutainers. Some hematological parameters were carried out by routine laboratory techniques. One ml Na2-EDTA whole blood was set apart for lipidomics evaluation. The remaining blood was centrifuged (10 min. at 1000×g) in order to separate the plasma, which was frozen at −80°C in 1 mL eppendorf sterile tubes. Na2-EDTA and heparinised plasma was used for a radical absorbance capacity (ORAC) test and protein carbonyl evaluation, respectively. After diluting (1∶1) the cell suspension with sterile Phosphate Buffered Saline (PBS), mononuclear white blood cells were separated from red cells by Ficoll (Histopaque 1077, Sigma, St.Louis, MO, USA) density gradient centrifugation. Cells were lysed in 1 mL Trizol® Reagent (Invitrogen, Milan, Italy) and stored at −80°C for other evaluations. In order to remove all Ficoll residue red blood cells were washed three times with PBS. Erythrocytes in Na2-EDTA were stored at 4°C and then used for the evaluation of Na+/K+-ATPase activity (NKA) and cell membrane fluidity. Heparinised red blood cells (RBC) were used for the evaluation of superoxide dismutase (SOD) and catalase activity. In particular, for SOD activity measurement, heparinised RBC were lysed in 4 volumes of ice-cold water and then stored at −80°C. The remaining heparinised RBC were diluted 30-fold in PBS and subsequently lysed in 10 mM potassium phosphate buffer pH 7.2. Lysates were stored at −80°C and subsequently were used for catalase activity evaluation. Spot urine samples (10 mL) from Au and TD were collected. Proteinuria and creatinine determinations were carried out by laboratory techniques. The remaining urine was centrifuged at 1200 g for 10 min in order to remove insoluble materials. Five mL of clear urine were aliquoted and stored at −80°C for hexanoyl-lysine adduct (HEL) and 8-isoprostane evaluations. The remaining urine was filtered with 0,45 µm filter, supplemented with 0.05% sodium azide and stored at −80°C for 8-hydroxy-2′-deoxyguanosine (8-oxo-dG) analysis.
Urinary 8-isoprostane
Urinary 8-isoprostane (also known as 8-epi-PGF2α, 8-iso-PGF2α or 15-isoprostane F2t) was determined by the use of a competitive ELISA kit (Oxford Biomedical Research Inc., Oxford, MI, USA). As suggested by the manufacturer, urine samples are diluted 1∶5 with a buffer provided in the kit. The 15- isoprostane F2t in the samples competes with 15-isoprostane F2t conjugated to horseradish peroxidase (HRP) for binding to a polyclonal antibody specific for 15-isoprostane F2t coated on the microplate. A substrate was added and the absorbance was measured at 450 nm in a microplate reader. The 15-isoprostane F2t concentration was expressed in ng per milligram of creatinine.
Urinary hexanoyl-lysine adduct
Hexanoyl-lysine adduct (HEL) concentration was measured by a competitive ELISA kit (JaICA, Fukuroi, Shizuoka, Japan) in unfiltered urine of autistic and control children. According to the manufacturer's instructions, urine samples were diluted five times with PBS. Some urine samples containing proteins were treated with 14 mg/mL alpha-chymotrypsin in PBS (pH 7.4) and incubated at 37°C O.N. Samples were filtered using ultra filters with cut-off molecular weight 10 kDa (Amicon Ultra, Millipore, Cork, Ireland). The absorbance was measured at 450 nm using a microplate reader. The HEL concentration was expressed in nmol per milligram of creatinine (nmol/mg creatinine).
Urinary 8-oxo-dG
Urinary 8-hydroxy-2′-deoxyguanosine (8-oxo-dG) was measured using the HT 8-oxo-dG ELISA Kit (Trevigen Inc. Gaithersburg, MD, USA) according to the manufacturer’s instructions. Briefly, filtered urine was diluted 1∶20 with a buffer provided by the kit and added to a plate pre-bounded with 8-oxo-dG. Bound and sample 8-oxo-dG compete for binding to the anti-8-oxo-dG which was then added to the plate; the antibody fraction captured by the immobilized 8-oxo-dG in the plate was then detected by means of a HRP-conjugated secondary antibody. The assay was developed with tetramethylbenzidine substrate (TMB) and the absorbance was measured in a microplate reader at 450 nm. The 8-oxo-dG concentration was expressed in ng per milligram of creatinine.
Protein carbonyl determination
Protein carbonyls were determined in plasma samples using the Protein Carbonyl ELISA kit (Enzo Life Sciences Inc. Farmingdale, NY, USA) following the manufacturer’s instructions. Plasma (5 µL) was derivatized with dinitrophenylhyidrazine (DNPH); derivatized proteins were then adsorbed to an ELISA plate. The adsorbed protein was then probed with biotinylated anti-DNP antibody followed by streptavidin-linked horseradish peroxidase. The absorbance was read at 450 nm using a spectrophotometer plate reader (Victor II, Pelkin-Elmer, Waltham, MA, USA). Plasma samples were assayed in duplicate, and protein carbonyl concentration was expressed as nanomoles of carbonyl groups per milligram of protein in the sample (nmol/mg).
Plasma radical absorbance capacity (ORAC)
The ORAC assay was carried out on a Fluoroskan FL® ascent (Thermo Fisher Scientific, Inc. Waltham, MA, USA) with fluorescent filters (excitation wavelength: 485 nm; emission filter: 538 nm). following a previously published procedure [27].
Briefly, in the final assay mixture (0.2 mL total volume), fluorescein sodium salt (85 nM) was used as a target of free radical attack with 2,2′-azobis(2-amidino-propane) dihydrochloride (AAPH) as a peroxyl radical generator. Trolox, a water-soluble analogue of vitamin E, was used as a standard control and calibration curves were determined for 10, 20, 30, 40, 50 µM solution. Fluorescence measurements, carried out at 37°C, were recorded at 5 min intervals, up to 30 min after the addition of AAPH. The ORAC values, calculated as difference of the areas under the quenching curves of fluoresceine between the blank and the sample, were expressed as Trolox equivalents (TE), pH = 7.4. All the assays were performed with three replicates.
Superoxide dismutase (SOD) activity
SOD activity was determined in erythrocyte lysates by a competitive colorimetric inhibition assay, as previously described [28], [29]. This method is based on water-soluble tetrazolium salt, WST-1 (2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) (Dojindo Laboratories Co., Kumamoto, Japan), which produces a water-soluble formazan dye upon reduction with the superoxide anion generated by xanthine and xanthine oxidase (Sigma-Aldrich, St. Louis, MO, USA). SOD activity reduces the superoxide concentration and inhibits formazan formation. A SOD standard curve was obtained; different dilutions of erythrocyte lysates were assayed in order to find a sample dilution that falls within the range of standard curve linearity. Samples or standards (10 µL) were incubated for 20 min at 37°C with 100 µL reaction mixture containing 500 µM WST-1 and 75 µM xanthine in 50 mM CHES (2-N-(Cyclohexylamino) ethanesulphonic acid, pH 8.0. Finally, 10 µL Xanthine Oxidase (350 mU/mL) (Sigma-Aldrich, St. Louis, MO, USA) was added. Formazan formation was measured at 450 nm using a 96-well plate reader (Victor2 Multilabel Counter, Perkin-Elmer, Waltham, MA, USA). SOD concentration, expressed in units per milligram of hemoglobin, was determined using the SOD standard curve.
Catalase activity
Catalase activity was determined in erythrocyte lysates using a method described by Ou and Wolff [30], based on the specific reaction of FOX-1 reagent (250 µM ammonium ferrous sulfate, 100 µM xylenol orange, 0,1 M sorbitol, 25 mM H2SO4) with H2O2 to yield a color complex having absorption maximum at 560 nm. The catalase causes decomposition of H2O2 such that residual H2O2 is inversely proportional to the activity of the catalase. One milliliter of erythrocyte lysates was incubated for 4 min. with 100 µL of 2.2 mM H2O2. Subsequently, 50 µL aliquots of the incubation mixtures were removed and rapidly mixed with 950 µL of FOX-1 reagent in eppendorf tubes, which were then incubated at room temperature for 30 min. Absorbance was measured at 560 nm. Catalase concentration was expressed in units per milligram of hemoglobin.
Erythrocyte plasma membrane fluidity
Erythrocytes plasma membrane fluidity was studied by determining the fluorescence anisotropy (reciprocal of fluidity) of two probes, TMA-DPH (1-(4-trimethylammoniophenyl)-6-phenyl-1,3,5-hexatriene), and DPH (1-6-phenyl-1,3,5-hexatriene); used to evaluate membrane fluidity of the outer and the inner leaflet of cell membrane, respectively [31]. The fluorescent probes were purchased from Molecular Probes Inc (Eugene, OR, USA). The incubation with TMA-DPH and DPH was performed as described by Sheridan and Block [32]. Briefly, 3 µl of TMA-DPH and DPH (10−3 mol/L) were incubated for 5 min and 45 min respectively, at room temperature (23°C) with 2 ml of erythrocyte membranes (final concentration of 100 µg/mL) in 50 mmol/L Tris-HCl buffer solution, pH 7.4. Fluorescence intensities (100 readings each) of the vertical and horizontal components of the emitted light were measured on a Perkin-Elmer MPF-66 spectrofluorometer equipped with two glass prism polarizers (excitation wavelength 365 nm, emission wavelength 430 nm). Sample temperature was maintained at 37°C using an external bath circulator (Haake F3). Steady-state fluorescence anisotropy (r) of TMA-DPH and DPH was calculated using the equation.
where G is an instrument factor correcting for unequal detection of vertically (Iv) and horizontally (Ih) polarized light.
Na+/K+-ATPase activity
Na+/K+-activated Mg2+-dependent ATPase activity was determined in cell membranes by the Kitao method [33]. ATPase activity was assayed by incubating 1 mL of erythrocyte plasma membrane after sonication (three bursts, 15 s each) at 37°C in a reaction medium containing MgCl2 (5 mmol/L), NaCl (140 mmol/L), KCl (14 mmol/L) in 40 mmol/L Tris-HCl, pH 7.7. The ATPase reaction was initiated with the addition of 3 mmol/L Na2ATP and stopped 20 min later by the addition of 1 mL of 15% trichloracetic acid. The tubes were then centrifuged at 1100 g for 10 min and the inorganic phosphate (Pi) hydrolysed from the reaction was measured in the supernatant by a colourimetric assay using a KH2PO4 standard [34]. ATPase activity, assayed in the presence of 10 mmol/L ouabain, was subtracted from the total Mg2+-dependent ATPase activity to calculate the activity of Na+/K+-ATPase. Protein concentration was determined as described by Bradford [35], using serum albumin as a standard. The interassay variation was 5.3%, while the intra-assay variation was 8.1%.
Lipoperoxide levels (TBARs) measurement
Lipoperoxide levels were evaluated using Cayman's thiobarbituric acid reactive substances (TBARs) assay kit. The product of fatty acid peroxidation, malondialdehyde (MDA), reacts with thiobarbituric acid (TBA) to yield a product that is measured fluorometrically. Membranes (100 mg of membrane proteins) were centrifuged at 3000 g for 15 min after the addition of 30% trichloroacetic acid, and 0.5 mL of the resulting supernatant was mixed with 1.1 mL of TBA reagent (equal volumes of 0.67% TBA aqueous solution and glacial acetic acid; v/v). The reaction mixture was heated for 60 min at 95°C in a sand bath. After cooling to room temperature, 5 mL of n-butanol was added and the mixture was shaken vigorously for 2 min. Thereafter, samples were centrifuged at 4000 g for 15 min, then 150 µL from each vial were loaded to the plate for fluorometric assay and the fluorescence of samples and standards was read at an excitation wavelength of 530 nm and an emission wavelength of 550 nm. The lipid peroxide level (Lp) was expressed in terms of MDA content (µM), using 1,1′,3,3′-tetramethoxypropane as a standard.
Sialic acid
Sialic acid content of RBC membranes was determined by the periodate thiobarbituric acid method of Denny et al. [36]. Briefly, membranes (1 mg membrane proteins/mL) were first hydrolyzed in 0.05-mol/L H2SO4 in a final volume of 0.1 mL for 1 hour at 80°C to release SA [37]. Standards and samples were both incubated with (assay samples) or without (correction samples) 0.25 mL periodate solution (0.025 mol/L periodic acid in 0.25 mol/L HCI) at 37°C for 30 minutes [38]. After reduction of excess periodate with 0.25 mL 0.32 mol/L sodium thiosulfate, the reaction was completed by addition of 1.25 mL 0.1-mol/L thiobarbituric acid. The samples were heated at 100°C for 15 minutes and then cooled to room temperature. The product was extracted with acidic butanol and colorimetrically assayed with a spectrophotometer at 549 nm. The readings of correction samples were subtracted from those of assay samples, thus corrected readings were obtained.
Protein content was determined by Bradford method to normalize the sialic acid content using BSA as standard [35].
Erythrocyte membrane lipidomic analysis
The erythrocyte fatty acid membrane profile analysis was carried out as previously described, using the erythrocyte membrane pellet obtained by standard methods [39]. For this study, selection of the erythrocyte fraction was made by modification of a literature procedure for the selection of aged erythrocytes (red blood cell age >3 months), with cells selected for high density and small diameter compared to the average erythrocyte population [40].
One mL of whole blood was first centrifuged at 2000 g for 5 min to eliminate the plasma, and a second round of centrifugation was then carried out at 4000 g at 4°C for 5 min in order to yield a stratification by cell density. The bottom layer (2.5 mm from the bottom of tube) consisted of erythrocyte cells, which were evaluated for their diameter using a Scepter™ 2.0 Cell Counter (Merck Millipore, Milan, Italy) to characterize the cell selection from each blood sample. The results were also compared with the cell population obtained from standard density gradient separation [41], [42].
Briefly, lipids were extracted from erythrocyte membranes according to the method of Bligh and Dyer [43]. The phospholipid fraction was controlled by TLC as previously described [39], then treated with KOH/MeOH solution (0.5 M) for 10 min at room temperature and under stirring [44].
Fatty acid methyl esters (FAME) were extracted using n-hexane; the hexane phase was collected and dried with anhydrous Na2SO4. After filtration, the solvent was eliminated by evaporation using a rotating evaporator, and the thin white film of the FAME was subsequently dissolved in a small volume of n-hexane. Approximately 1 µL of this solution was injected into the GC. A Varian CP-3800 gas chromatograph, with a flame ionization detector and an Rtx-2330 column (90% biscyanopropyl-10% phenylcyanopropyl polysiloxane capillary column; 60 m, 0.25 mm i.d., 0.20 µm film thickness) was used for the analysis. Temperature was held at 165°C held for the initial 3 min, followed by an increase of 1°C/min up to 195°C, held for 40 min, followed by a second increase of 10°C/min up to 250°C, held for 5 min. The carrier gas was helium, held at a constant pressure of 29 psi. Methyl esters were identified by comparison with the retention times of commercially available standards or trans fatty acid references, obtained as described elsewhere [45].
Statistics
All experiments were carried out in duplicate or triplicate and were usually repeated three times.
To compare Au and TD groups, normality tests were applied to all numeric variables, following which appropriate parametric tests (ANOVA, Student's t for independent data) or the nonparametric equivalent (Wilcoxon-Mann-Whitney) were used. Non-parametric correlation (Spearman's rho) was used to correlate clinical features and biochemical data in the Au group (non-parametric ANOVA for cognitive/developmental level). Differences were considered significant at p<0.05.
To account for multiple testing we used the Benjamini and Hochberg false discovery rate (FDR) [46]. FDR corrected p-values (pFDR) were evaluated separately for a) comparisons of biochemical parameters in Au and TD and b) correlations of clinical features and biochemical data in Au. In particular, the comparisons of biochemical parameters included a1) erythrocyte membrane features and molecules, oxidative stress markers (in urine and plasma) and antioxidant enzyme activities in erythrocytes (12 comparisons); a2) erythrocyte membrane fatty acids (19 comparisons). As for the correlations between Au clinical features and biochemical data, pFDR was calculated for CARS global score (31 comparisons), CARS activity level (hyperactivity) item (31 comparisons), CARS body use (stereotypes) item (31 comparisons), cognitive/developmental impairment levels (31 comparisons). Age was compared with all biochemical data (31 comparisons).
Even though it is usual to set at <0.05 the significance level of statistic tests, Benjamini & Hochberg [46], as well as others [47], have argued that a more liberal threshold (as high as 0.1 or even a bit higher) may be reasonable for pFDR.
Statistical analysis was performed using SAS v. 9.2.
Results
1. Comparisons between Au and TD
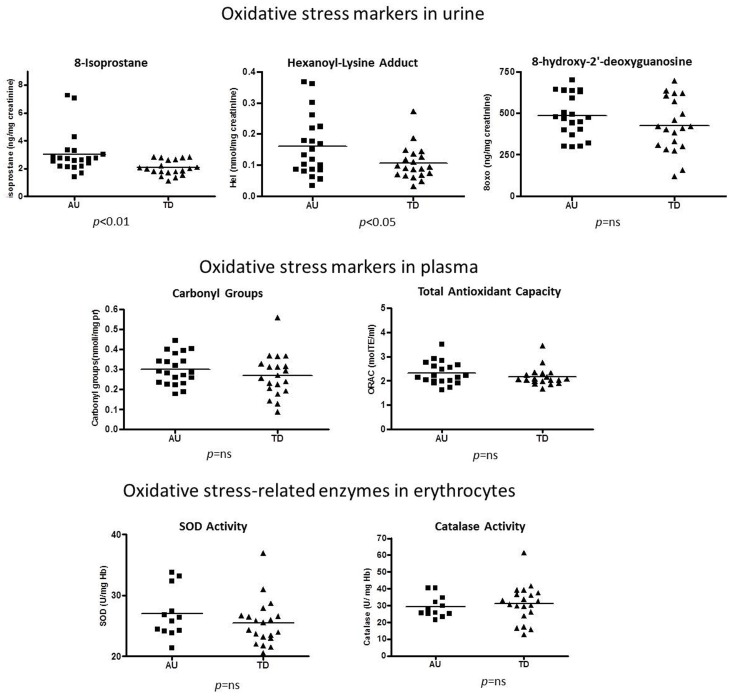
1.1 Oxidative stress markers in urine and plasma and antioxidant enzymes activities in erythrocytes (Fig. 1 and table 2)
Figure 1. Scatter plot showing oxidative stress markers in urine and plasma and antioxidant enzymes activities in erythrocytes.
Au = Autistic children; TD = typically developing children. Horizontal bars indicate means. Standard deviation values and whether parametric or not parametric statistic tests were applied, are reported in Tab. 2. p<0.01 highly significant; p<0.05 significant; ns, not significant.
Table 2. Erythrocyte membrane features and molecules, oxidative stress markers in urine and plasma, antioxidant enzymes activities in erythrocytes.
| Mean values ± St. Dev | % difference Au vsTD | Statistical significance | ||||
| Au (N = 21) | TD (N = 20) | p values | pFDR | |||
| Erythrocyte membrane features and molecules | ||||||
| Na+/K+-ATPase° | 2.54±0.58 | 7.39±1.62 | −66% | <0.0001 | <0.0001 | |
| TMA-DPH° | 0.27±0.02 | 0.25±0.03 | +8% | 0.0123 | 0.0368 | |
| DPH° | 0.27±0.02 | 0.25±0.03 | +8% | 0.0196 | 0.0469 | |
| TBARS° | 0.72±0.38 | 0.51±0.37 | +41% | 0.0021 | 0.0125 | |
| Sialic Acid° | 6.19±4.36 | 7.63±7.08 | −19% | 0.7248 | 0.7248 | |
| Oxidative stress markers in urine | ||||||
| 8-Isoprostane° | 3.04±1.50 | 2.07±0.54 | +47% | 0.0069 | 0.0278 | |
| HEL* | 0.16±0.09 | 0.11±0.05 | +45% | 0.0380 | 0.0760 | |
| 8-Oxo-dG* | 484.80±130.07 | 426.46±163.64 | +14% | 0.2127 | 0.346 | |
| Oxidative stress markers in plasma | ||||||
| Carbonyl Groups* | 0.30±0.08 | 0.27±0.11 | +11% | 0.2509 | 0.3763 | |
| ORAC° | 2.47±0.86 | 2.36±0.94 | +5% | 0.4573 | 0.5487 | |
| Antioxidant enzymes activities in erythrocytes | ||||||
| SOD activity° (Au N = 12) | 26.10±4.02 | 25.49±3.78 | +2% | 0.2960 | 0.3947 | |
| Catalase activity* (Au N = 12) | 29.28±6.34 | 31.25±11.06 | −6% | 0.5783 | 0.6309 | |
Au: Autistic children; TD: typically developing children; p values were calculated with non parametric Wilcoxon-Mann-Whitney test (°) or parametric ANOVA test (*); p<0.05: significant; p<0.01 highly significant; pFDR: Benjamini and Hochberg False Discovery Rate (FDR) corrected p-values.
Peroxidation of arachidonic acid causes membranes to release 8-isoprostane, a prostaglandin-F2-like compound. Oxidized arachidonic acid or other omega-6 fatty acids, such as linoleic acid, may also react with protein lysine residues, yielding HEL. Both 8-isoprostane (p<0.01; pFDR = 0.0278) and HEL (p<0.05; pFDR = 0.076) were found in higher amount in the urine of Au than in the urine of TD children (+47% and +45%, respectively). However, the amount of 8-oxo-dG, derived from the oxidation of nucleic acid bases by free radicals, did not significantly differ between the two groups.
Plasma levels of carbonyl groups (an oxidative modification of proteins) and plasma radical absorbance capacity (a measure of the antioxidant capacity, which is reduced by free radicals) did not differ between the two groups.
Similarly, neither SOD nor catalase enzymatic activity measured in erythrocytes were found to differ between the two groups.
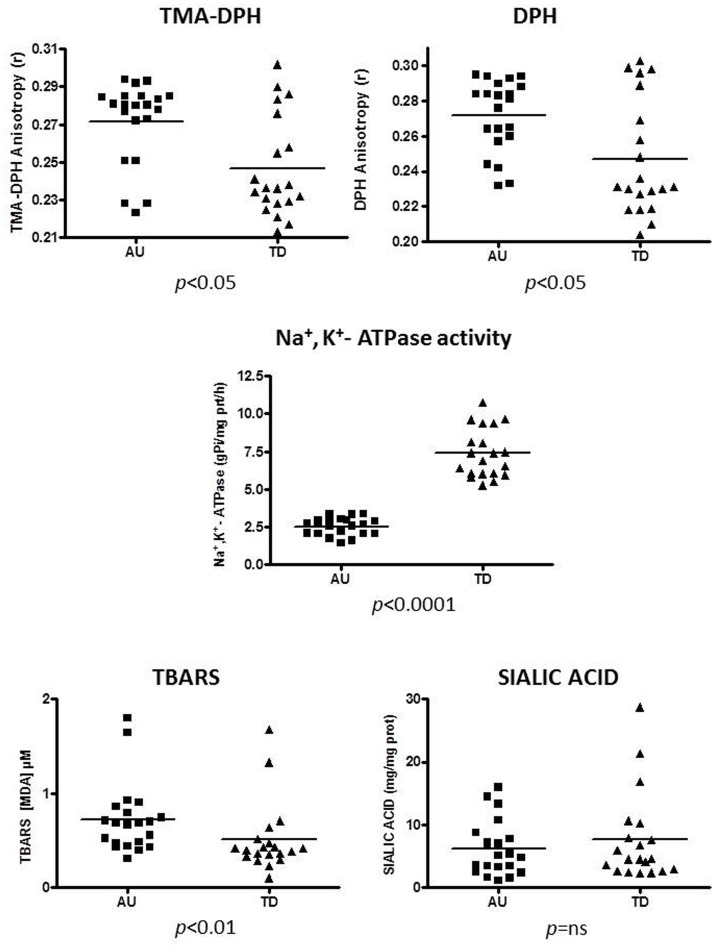
1.2 Erythrocyte membrane features and molecules (Fig. 2 and table 2)
Figure 2. Scatter plot showing erythrocyte membrane features and molecules.
Au = Autistic children; TD = typically developing children. TMA-DPH and DPH values are inversely correlated with the outer and the inner membrane fluidity, respectively. TBARS = Thiobarbituric Acid Reactive Substances. Horizontal bars indicate means. Standard deviation values and whether parametric or not parametric statistic tests were applied, are reported in Tab. 2. p<0.01 highly significant; p<0.05 significant ns, not significant.
TMA-DPH and DPH are two probes used to evaluate membrane fluidity of the outer and the inner leaflet of cell membrane, respectively. Taking into account that TMA-DPH and DPH fluorescence anisotropy is inversely related to the fluidity of the microenvironment where the probe is located, it was found that membrane fluidity was decreased in Au with respect to TD. The decrease reached the statistical significance (p<0.05) for both the outer and inner membrane (pFDR = 0.0368, pFDR = 0.0469, respectively).
The activity of Na+/K+-ATPase, an active ion transporter localized in the plasma membrane, was markedly decreased (−66%) in Au in comparison with TD (p<0.0001; pFDR<0.0001), with no overlapping values between Au and TD (Au min. 1.41, max. 3.38; TD min. 5.27, max. 10.75).
TBARS assay measures MDA generated from the decomposition of primary and secondary lipid peroxidation products. TBARS were found to be significantly higher (+41%) in the erythrocyte membrane from Au children in comparison with those from TD children (p<0.01; pFDR = 0.0125).
Sialic acid levels in erythrocyte did not differ between Au and TD.
1.3 Analysis of erythrocyte membrane fatty acids (Table 3)
Table 3. Erythrocyte membrane Fatty Acid profile.
| Mean values (± St. Dev) | % differencesAu vs TD | Statistical significance | |||
| Au (N = 21) | TD (N = 20) | p values | pFDR | ||
| DHA omega 3 (22∶6)* | 4.80±1.08 | 5.62±0.67 | −14% | 0.0065 | 0.07220 |
| Total Monounsaturated Fatty Acids (MUFA)* | 18.03±1.25 | 17.04±0.98 | +6% | 0.0076 | 0.07220 |
| Vaccenic acid (18∶1)° | 1.30±0.16 | 1.20±0.11 | +9% | 0.0220 | 0.07329 |
| Oleic acid (18∶1)* | 16.42±1.25 | 15.60±0.94 | +5% | 0.0228 | 0.07329 |
| SFA/MUFA° | 2.38±0.24 | 2.52±0.19 | −5% | 0.0232 | 0.07329 |
| Palmitoleic acid (16∶1)° | 0.3±0.08 | 0.24±0.09 | +25% | 0.0262 | 0.07329 |
| ω6/ω3 ratio* | 6.66±1.62 | 5.76±0.67 | +16% | 0.0270 | 0.07329 |
| EPA omega 3 (20∶5)° | 0.43±0.16 | 0.51±0.14 | −16% | 0.0434 | 0.10308 |
| Total Polyunsaturated Fatty Acids (PUFA)° | 39.40±1.80 | 40.18±1.74 | −2% | 0.1173 | 0.24763 |
| Trans 18∶1° | 0.11±0.05 | 0.14±0.07 | −21% | 0.1863 | 0.35397 |
| Eicosatrienoic acid omega 6 (20∶3)° | 2.25±0.45 | 2.13±0.34 | +6% | 0.3714 | 0.64151 |
| Stearic acid (18∶0)* | 18.58±1.04 | 18.78±0.88 | −1% | 0.4940 | 0.76378 |
| Total Saturated Fatty Acids (SFA)* | 42.31±1.96 | 42.60±1.53 | −1% | 0.6012 | 0.76738 |
| Linoleic omega 6 (18∶2)° | 12.2±0.96 | 12.66±1.34 | −4% | 0.6012 | 0.76738 |
| Total TRANS° | 0.23±0.08 | 0.24±0.06 | −1% | 0.6180 | 0.76738 |
| Trans-ARA° | 0.12±0.06 | 0.11±0.03 | +9% | 0.6756 | 0.76738 |
| Arachidonic acid omega 6 (20∶4)* | 19.57±1.67 | 19.39±1.13 | +1% | 0.6866 | 0.76738 |
| EFA deficiency° | 0.66±0.07 | 0.67±0.08 | −1% | 0.7961 | 0.84033 |
| Palmitic acid (16∶0) * | 23.73±1.94 | 23.82±1.48 | 0% | 0.8810 | 0.88100 |
Au: Autistic children; TD: typically developing children; ARA, arachidonic acid; DHA, docosahexaenoic acid; EFA, essential fatty acids; EPA, eicosapentaenoic acid; MUFA, monounsaturated fatty acids; PUFA; polyunsaturated fatty acids; SFA, saturated fatty acids; TRANS, transfatty acids; p values were calculated with non parametric Wilcoxon-Mann-Whitney test (°) or parametric ANOVA test (*); p<0.05: significant; p<0.01 highly significant; pFDR: Benjamini and Hochberg False Discovery Rate (FDR) corrected p-values.
The percentage of oleic, palmitoleic and vaccenic acids and, in general, total MUFA were increased in Au with respect to TD children. This caused also a decrease in SFA/MUFA ratio in Au with respect to TD children (p<0.05; pFDR = 0.07329).
The relative amount of the different PUFA was also altered, since EPA and DHA-ω3 acids were decreased in Au children (−16%, p<0.05, pFDR = 0.10308 and −14%, p<0.01, pFDR = 0.0722, respectively), causing an increase in ω6/ω3 ratio (+16%, p<0.05, pFDR = 0.07329). The results were interpreted using the fatty acid-based functional lipidomic approach [48].
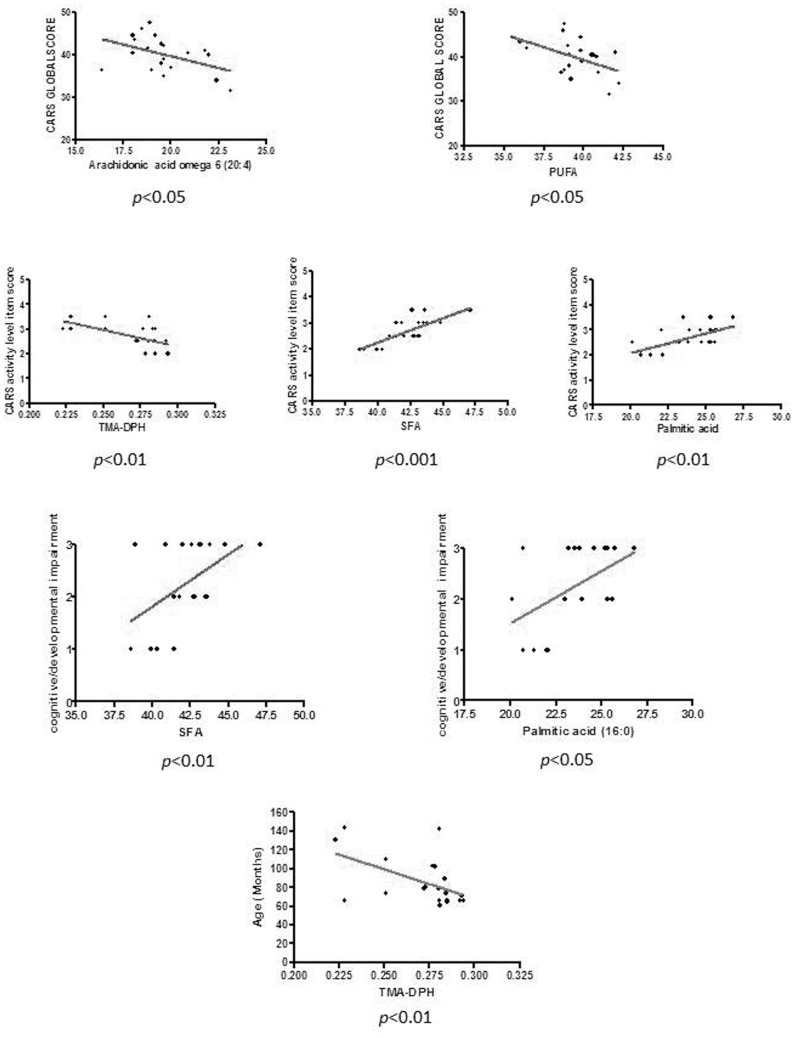
2. Correlation between Au Clinical Features and Biochemical Data (Main Results Reported in Fig. 3 and Table 4)
Figure 3. Relevant correlations between Au clinical features and biochemical data.
Au patients were divided into three levels of cognitive/developmental impairment as follows: 1: mild, 2: moderate, 3: severe. TMA-DPH values are inversely correlated with the outer membrane fluidity. SFA = Saturated Fatty Acids. CARS activity level item score denotes hyperactivity. p<0.01 highly significant; p<0.05 significant. More details are reported in Tab.4.
Table 4. Significant correlations of clinical features and biochemical data in Autistic children.
| CARS global scores | CARS activity item (Hyperactivity) | Cognitive/developmental impairment level | Age | |
| Total SFA | NS | r = 0.70834; p<0.001; pFDR = 0.00930 | p<0.05; pFDR = 0.33199 | NS |
| SFA/MUFA | NS | r = 0.57825; p<0.001; pFDR = 0.03720 | NS | NS |
| TMA-DPH | NS | r = − 0.58923; p<0.01; pFDR = 0.03720 | NS | r −0.6054; p<0.01; pFDR = 0.2376 |
| Total PUFA | r = −0.52589; p<0.05; pFDR = 0.18450 | r = −0.58719; p<0.01; pFDR = 0.03720 | p = 0.0553; pFDR = 0.33199 | NS |
| Palmitic acid (16∶0) | NS | r = 0.59763; p<0.01; pFDR = 0.03720 | p<0.05; pFDR = 0.33199 | NS |
| Arachidonic acidω6 (20∶4) | r = −0.432: p<0.05; pFDR = 0.31104 | r = −0.45377; p<0.05; pFDR = 0.15035 | p<0.05; pFDR = 0.33199 | NS |
| Total MUFA | NS | r = −0.49446; p<0.05; pFDR = 0.11728 | NS | NS |
| Oleic acid (18∶1) | NS | r = −0.46048; p<0.05; pFDR = 0.15035 | NS | NS |
| 8-isoprostane | NS | NS | p<0.05; pFDR = 0.33199 | NS |
Non-parametric correlation (Spearman's rho, r) was used to correlate clinical features (CARS, CARS activity item and Age) and biochemical data in the Autistic children group. Non-parametric ANOVA was used for Cognitive/developmental impairment level. MUFA, monounsaturated fatty acids; PUFA; polyunsaturated fatty acids; SFA, saturated fatty acids. p<0.05: significant; p<0.01 highly significant.
pFDR: Benjamini and Hochberg False Discovery Rate (FDR) corrected p-values.
Non-parametric correlation (Spearman's rho) was used to correlate clinical features and biochemical data in the Au group. CARS global scores were inversely related with ω6 arachidonic acid (p<0.05; pFDR = 0.31104) and PUFA (p<0.05; pFDR = 0.18450). CARS activity level item scores (hyperactivity) were negatively correlated with TMA-DPH (p<0.01; pFDR = 0.03720), oleic acid (p<0.05; pFDR = 0.15035), ω6 arachidonic acid (p<0.05; pFDR = 0.15035), MUFA (p<0.05; pFDR = 0.11728) and PUFA (p<0.01; pFDR = 0.03720), and directly correlated with SFA (p<0.001; pFDR = 0.00930), palmitic acid (p<0.01; pFDR = 0.03720), SFA/MUFA (p<0.001 pFDR = 0.03720). TMA-DPH was correlated with age (p<0.01 pFDR = 0.2376). CARS body use item scores (stereotypes) were not significantly related to any biochemical marker.
When only cognitive/developmental impaired Au children (n: 19) were considered, the non parametric ANOVA revealed that the level of cognitive/developmental impairment was inversely related with ω6 arachidonic acid (p<0.05; pFDR = 0.33199), and directly related with 8-isoprostane (p<0.05; pFDR = 0.33199), total SFA (p<0.05; pFDR = 0.33199) and palmitic acid (p<0.05; pFDR = 0.33199), while cognitive impairment and total PUFA showed only a trend of inverse correlation (p = 0.0553; pFDR = 0.33199).
Even if it was beyond the scope of this research, additional correlations were performed within Au clinical features. We found a significant correlation between CARS global score and other clinical features, such as cognitive/developmental delay (r = 0.52009, p<0.05; pFDR = 0.18450), hyperactivity (r = 0.61669, p<0.01; pFDR = 0.10440), CARS body use item scores (stereotypes) (r = 0.52009, p<0.01; pFDR = 0.18450). Moreover, the variable stereotypes was related to CARS activity levels item score (hyperactivity) (r = 0,60308, p<0.01; pFDR = 0.03060).
3. Statistics
FDR analysis confirmed the statistical significance of most uncorrected p values in both comparisons Au vs TD and in correlations between clinical features and biochemical parameters.
Discussion
There is increasing evidence that autistic patients show excessive ROS production and several studies reported the presence of different peripheral biomarkers of oxidative stress [13], [49], [50]. However, the great heterogeneity of the syndrome makes it difficult to assess whether this finding is occasional and whether it is restricted to a sub-group of patients. Moreover, not all oxidative stress markers appear to be altered in patients, and it is still unknown whether oxidative stress, if really present, is secondary to a generic inflammatory status or due to genetic alterations still to be recognized. In addition, most researchers addressing this problem have a tendency to evaluate few markers at a time, thus making it very difficult to compare data obtained in different patient’s subgroups [51], [52]. To our best knowledge, this is the first study, which evaluated, at the same time, a wide range of different but strongly related biological biomarkers in a group of Au children that underwent a rigorous clinical characterization. Among the oxidative stress parameters we evaluated, we found, in Au compared to TD, a significant increase in TBARS, 8-isoprostane and HEL, which are markers of lipid peroxidation. No significant differences were found in the oxidative biomarkers 8-oxo-dG and ORAC. This finding suggests that the oxidative stress-related phenomena are localized mainly at the cell surface. Systemic involvement is suggested by detection of these markers both in urine and in erythrocytes.
The fatty acid composition of the brain and neural tissues is characterized by high PUFA concentrations, which play a very important role in signal transduction [53], neuro-inflammation [54] and cellular repair and survival [55]. Erythrocyte membrane fatty acid composition is a very sensitive indicator of the status of different tissues and may reflect the fatty acid composition of brain [56]. In a number of neurodevelopmental conditions, including Attention Deficit Hyperactivity Disorder (ADHD) and dyslexia, reduced concentrations of erythrocyte membrane PUFA have been reported [57]. Moreover, a polymorphism in the gene cluster associated with the fatty acid desaturase-2 gene (FADS2) for Delta 6-desaturase (the rate-limiting step in PUFA synthesis) was described in patients with ADHD [58], [59], pointing to a possible correlation between membrane fatty acid composition and hyperactivity.
Table 5 summarizes published data about alterations in erythrocyte membrane fatty acid composition in ASD children. In our study, a significant increase of erythrocyte membrane MUFA and of ω6/ω3 ratio (due to a decrease in EPA and DHA) was shown. These results are partially superimposable to those reported by Bell et al. [60]. Alteration in membrane lipid composition was not related to dietary habits, since they did not significantly differ between Au and TD, as evidenced by the Food Questionnaire. On the other hand, oxidative stress is not a likely explanation for the specific decrease of the ω3, since this would have also affected the ω6 PUFA family. The observed imbalance in ω6/ω3 ratio may lead to the proinflammatory status reported previously in ASD children [12], [61], [62]. The significant increase in MUFA may be representative of a feedback remodelling of erythrocyte membrane lipid composition. It is interesting to note that a study on adipocyte membranes showed DHA loss coexistent with MUFA increase [63].
Table 5. Summary of published results on fatty acid composition of erythrocyte membrane.
| fatty acid composition of erythrocyte membrane | Patients | |
| Highly unsaturated fatty acids (HUFA) | decreased | One ASD patient [75] |
| Stearic acid (18∶0) | increased | 18 Au children with developmental regression [76] |
| Arachidic acid (24∶0) | increased | |
| Total SFA | increased | |
| Oleic acid (18∶1 n-9) | decreased | |
| Nervonic acid (24∶1) | increased | |
| Total MUFA | decreased | |
| Linoleic acid (18∶2 n-6) | increased | |
| Arachidonic acid (ARA) (20∶4 n-6) | decreased | |
| Docosapentaenoic acid (DPA) (22∶5 n-6) | increased | |
| Docosapentaenoic acid (DPA) (22∶5 n-3) | decreased | |
| Total ω3 | decreased | |
| ARA:EPA ratio (20∶4 n-6/20∶5n-3) | increased | |
| Stearic acid (18∶0) | increased | 11 children with classical autism or Asperger [76] |
| Arachidic acid (24∶0) | increased | |
| Nervonic acid (24∶1) | increased | |
| Docosapentaenoic acid (DPA) (22∶5 n-6) | increased | |
| Docosapentaenoic acid (DPA) (22∶5 n-3) | decreased | |
| Total ω3 | decreased | |
| ARA:EPA ratio (20∶4 n-6/20∶5n-3) | increased | |
| Eicosenoic acid (20∶1n9) | increased | 20 Au children with developmental regression (mean age 3.5 years) [77] |
| Erucic acid (22∶1n9) | increased | |
| total MUFA | increased | |
| α-Linolenic acid (18∶3 n-3) | decreased | 49 Au children (mean age 7.5 years) [78] |
| ARA:EPA ratio (20∶4 n-6/20∶5 n-3) | increased |
ARA, arachidonic acid; EPA, eicosapentaenoic acid; MUFA, monounsaturated fatty acids; PUFA; polyunsaturated fatty acids; SFA, saturated fatty acids.
It has not escaped our notice that the membrane fluidity decrease we observed cannot be directly explained on the ground of these alterations in fatty acid composition. Schengrund et al. [64] recently reported a decrease in cholesterol and a related increase in GM1 ganglioside in erythrocyte membranes from ASD children, which could affect membrane fluidity. However, we failed to observe any change in membrane sialic acid - a component of GM1 ganglioside, in Au patients.
Na+/K+-ATPase maintains intracellular gradients of ions that are essential for cellular activities. Despite the crucial role of NKA in cellular metabolism and the fact that it accounts for approximately 30% of the total body energy consumption and for 50% brain energy consumption, very little is known about NKA in autism. In a mouse model of Angelman Syndrome, a neurodevelopmental disorder associated with autism, an intrinsic alteration of membrane properties of pyramidal neurons in hippocampal area CA1 has recently been observed [65]. Alterations were also observed in resting membrane potential, threshold potential, and action potential amplitude correlated with significant increases in the expression of the α1 subunit of Na+/K+-ATPase [64]. In postmortem tissues from different brain regions of autistic subjects, a specific increase in NKA in the frontal cortex and cerebellum was found. The authors suggested that such increase might be due to compensatory responses to increased intracellular calcium concentration in autism [66].
On the contrary, we showed a very significant reduction of erythrocyte NKA in Au compared to TD, in keeping with a similar report by Kurup and Kurup [67]. There is no overlap between the range values of the two groups, suggesting that this parameter might be a biomarker of autism. Future work should be addressed at understanding how sensitive and specific is the decrease of NKA as far as autism is concerned. A number of other factors may affect NKA; for example, a positive correlation between the molecular activity of Na+/K+-ATPase units and the membrane content of DHA has been shown [68] and a reduction of NKA has also been related to oxidative stress [69], [70]. Changes in ATPase activities might stem from sub-conformational changes in the enzymes depending on their microenvironment, indirectly reflecting changes in surrounding lipids and in membrane fluidity [71].
Noteworthy, some clinical features were correlated with some parameters of the lipidomic profile. In our study, hyperactivity is the clinical aspect found to be most highly related to erythrocyte membrane features. The higher the fluidity of the erythrocyte membrane and the lower the PUFA concentration, the greater was the hyperactivity level. Also, the severity of hyperactivity was directly and highly correlated with erythrocyte SFA and palmitic acid concentration. These data not only suggest that such disequilibrium in membrane fatty acid composition may be a useful tool to assess the severity of the autistic clinical picture, but also suggest possible therapeutic interventions with a tailored and balanced fatty acid intake. Two distinct double blind trials showed an improvement in hyperactivity score in autistic children treated with ω3 supplementation [72], [73]. Despite these encouraging results, a recent Cochrane meta-analysis stated that “to date there is no high quality evidence that omega-3 fatty acids supplementation is effective for improving core and associated symptoms of ASD” [74]. Nevertheless, our data clearly show an imbalance of membrane fatty acids and their correlation with relevant clinical features, thus pointing to the importance of restoring the membrane equilibrium. However, the intake of ω3 should be accompanied by antioxidant protection. For example, since our data also show the alteration of the redox balance of Au, supplementation of PUFA in the absence of antioxidant protection might paradoxically worsen the picture, as, in oxidative milieu, PUFA undergo a peroxidation process and may become, in turn, pro-oxidant. Also, omega-6/omega-3 balance might modulate neurotransmitters of the central nervous system: increased omega-3 fatty acid concentrations in cell membranes have been shown to affect serotonin and dopamine neurotransmission, especially in the prefrontal cortex [75]. Taking into account that serotoninergic and dopaminergic systems are deeply involved in ASD [76], [77], cell membrane lipid profile restoration could play a significant therapeutic role in improving some ASD features.
Conclusions
Taken together, these results show significant erythrocyte membrane alterations in Au, at structural and functional levels, and an increase of lipid peroxidation markers. These alterations, and in particular the marked decrease in NKA, may play a role in the pathogenesis of ASD and potentially may be useful tools as peripheral biomarkers of ASD to be exploited for a more precise or an earlier diagnosis of ASD. Future work will be addressed at understanding the reason(s) for the impairment of the NKA and associated relevance to the pathogenesis of ASD. Finally, our data suggest the presence of systemic alterations in ASD, and emphasizes the possibility of an integrated approach aimed at correcting the membrane defects by means of nutraceutic tools.
Acknowledgments
The Authors are grateful to their colleague David Muehsam for his expert review of the English language.
Funding Statement
The present work was exclusively supported by a legacy of late Ms. Maria Luisa Cimadori, a member of ANGSA (Associazione Nazionale Genitori Soggetti Autistici). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Momeni N, Bergquist J, Brudin L, Behnia F, Sivberg B, et al. (2012) A novel blood-based biomarker for detection of autism spectrum disorders. Transl Psychiatry 2: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theoharides TC, Kempuraj D, Redwood L (2009) Autism: an emerging neuroimmune disorder in search of therapy. Expert Opin Pharmacother 10: 2127–2143. [DOI] [PubMed] [Google Scholar]
- 3. James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, et al. (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80: 1611–1617. [DOI] [PubMed] [Google Scholar]
- 4. Chauhan A, Chauhan V (2006) Oxidative stress in autism. Pathophysiology 13: 171–181. [DOI] [PubMed] [Google Scholar]
- 5. James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, et al. (2006) Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet 141B: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sies H (1985) Oxidative stress: introductory remarks. In: Sies H, editor. Oxidative Stress. London: Academic Press 1–7.
- 7. Frossi B, deCarli M, Daniel KC, Rivera J, Pupillo C (2003) Oxidative stress stimulates IL-4 and IL-6 production in mastcells by APE/Ref-1-dependent pathway. Eur J Immunol 33: 2168–2177. [DOI] [PubMed] [Google Scholar]
- 8. Andreazza AC, Kauer-Sant’Anna M, Frey BN, Bond DJ, Kapczinski F, et al. (2008) Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord 111: 135–144. [DOI] [PubMed] [Google Scholar]
- 9. Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11: 851–876. [DOI] [PubMed] [Google Scholar]
- 10. Tsaluchidu S, Cocchi M, Tonello L, Puri BK (2008) Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry 8 (Suppl 1)S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sultana R, Perluigi M, Butterfield DA (2010) Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: insight into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signaling 8: 2021–2037. [DOI] [PubMed] [Google Scholar]
- 12. Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, et al. (2012) Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry Jul 10 2: e134 doi: 101038/tp201261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, et al. (2012) Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic Biol Med 52: 2128–2141. [DOI] [PubMed] [Google Scholar]
- 14. ChauhanA, Chauhan V, Brown WT, Cohen I (2004) Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin – the antioxidant proteins. Life Sci 75: 2539–2549. [DOI] [PubMed] [Google Scholar]
- 15. Innis SM (2008) Dietary omega 3 fatty acids and the developing brain. Brain Res 1237: 35–43. [DOI] [PubMed] [Google Scholar]
- 16. Murphy MG (1990) Dietary fatty acids and membrane protein function. J Nutr Biochem 1: 68–78. [DOI] [PubMed] [Google Scholar]
- 17. Krey JF, Dolmetsch RE (2007) Molecular mechanisms of autism: a possible role for Ca2+ signalling. Curr Op.Neurobiol 17: 112–119. [DOI] [PubMed] [Google Scholar]
- 18. Kim M, Nam JH, Oh DH, Park Y (2010) Erythrocyte α-linolenic acid is associated with the risk for mild dementia in Korean elderly. Nutr Res 30: 756–761. [DOI] [PubMed] [Google Scholar]
- 19. Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, et al. (2012) Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology 78: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calder PC (2011) Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol 668 (Supplement 1) S50–S058. [DOI] [PubMed]
- 21. Skoumalová A, Mádlová P, Topinková E (2012) End products of lipid peroxidation in erythrocyte membranes in Alzheimer's disease. Cell Biochem Funct 30: 205–210. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders 4th ed Washington, DC: American Psychiatric Association (text revised).
- 23.Lord C, Rutter M, DiLavore PC, Risi S (1999) Autism Diagnostic Observation Schedule–Generic. Los Angeles: Western Psychological Service.
- 24.Schopler E, Reichler RJ, Renner BR (1988) The Childhood Autism Rating Scale (CARS). Los Angeles, CA: Western Psychological Services.
- 25.Schopler E, Lansing MD, Reichler RJ, Marcus LM (2005) Psychoeducational profile. Third edition (PEP-3), Torrance, CA: Western Psychological Services.
- 26.Roid GM, Miller LJ (1997) Leiter International Performance Scale-Revised: Examiners Manual Wood Dale, IL: Stoelting Co.
- 27. Pessina F, Marazova K, Ninfali P, Avanzi L, Manfredini S, et al. (2004) In vitro neuroprotection by novel antioxidants in guinea-pig urinary bladder subjected to anoxia-glucopenia/reperfusion damage. N-S Arch Pharmacol 370: 521–528. [DOI] [PubMed] [Google Scholar]
- 28. Peskin AV, Winterbourn CC (2000) A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin Chim Acta 293: 157–66. [DOI] [PubMed] [Google Scholar]
- 29. Abruzzo PM, di Tullio S, Marchionni C, Belia S, Fanó G, et al. (2010) Oxidative stress in the denervated rat muscle. Free Radic Res 44: 563–576. [DOI] [PubMed] [Google Scholar]
- 30. Ou P, Wolff SP (1996) A discontinuous method for catalase determination at ‘near physiological’ concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J Biochem Biophys Methods 31: 59–67. [DOI] [PubMed] [Google Scholar]
- 31. Fiorini R, Curatola G (1991) Membrane heterogeneity studied by fluorescence lifetime distributions of DPH and TMA-DPH. Appl Fluorescence Technol 3: 33–8. [Google Scholar]
- 32. Sheridan NP, Block ER (1988) Plasma membrane fluidity measurements in intact endothelial cells: effect of hyperoxia on fluorescence anisotropies of 1-[4-(trimethylamino)phenyl]-6-phenylhexa-1,3,5-triene. J Cell Physiol 134: 117–123. [DOI] [PubMed] [Google Scholar]
- 33. Kitao T, Hattori K (1983) Inhibition of erythrocyte ATPase activity by aclacinomycin and reverse effect of ascorbate on ATPase activity. Experientia 39: 1362–1364. [DOI] [PubMed] [Google Scholar]
- 34. Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66: 375–400. [Google Scholar]
- 35. Bradford M (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 36. Denny PC, DennyPA, Allerton SE (1983) Determination of sialic acid using 2-thiobarbituric acid in the absence of hazardous sodium arsenite. Clin Chim Acta 131: 333–336. [DOI] [PubMed] [Google Scholar]
- 37. Warren L (1959) The thiobarbituric acid assay of sialic acids. J Biol Chem 234: 1971–1975. [PubMed] [Google Scholar]
- 38. Sobenin IA, Tertov VV, Orekhov AN (1998) Optimization of the assay for sialic acid determination in low density lipoprotein. J Lipid Res. 39: 2293–9. [PubMed] [Google Scholar]
- 39. Viviani Anselmi C, Ferreri C, Novelli V, Roncarati R, Bronzini R, et al. (2010) Fatty acid percentage in erythrocyte membranes of atrial flutter/fibrillation patients and controls. J Interv Card Electrophysiol 27: 95–99. [DOI] [PubMed] [Google Scholar]
- 40. van der Vegt SG, Ruben AM, Werre JM, Palsma DM, Verhoef CW, et al. (1985) Counterflow centrifugation of red cell populations: a cell age related separation technique. Br J Hematol 61: 393–340. [DOI] [PubMed] [Google Scholar]
- 41. Rennie CM, Thompson S, Parker AC, Maddy A (1979) Human erythrocyte fractionation in “Percoll” density gradients. Clin Chim Acta 98: 119–125. [DOI] [PubMed] [Google Scholar]
- 42.Corash LM, Piomelli S, Chen C, Seaman C, Gross E (1974) Separation of erythrocytes according to age on a simplified density. gradient. J Lab Clin Med 84,147–151. [PubMed]
- 43. Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem Physiol 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 44. Kramer JKG, Fellner V, Dugan ME, Sauer FD, Mossob MM, et al. (1997) Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 32: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 45. Ferreri C, Kratzsch S, Brede, Marciniak B, Chatgilialoglu C (2005) Trans lipid formation induced by thiols in human monocytic leukemia cells. Free Rad Biol Med 38: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 46. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 57: 289–300. [Google Scholar]
- 47. Mosig MO, Lipkin E, Khutoreskaya G, Tchourzyna E, Soller M, et al. (2001) Whole genome scan for quantitative trait loci affecting milk protein percentage in Israeli-Holstein cattle, by means of selective milk DNA pooling in a daughter design, using an adjusted false discovery rate criterion. Genetics 157: 1683–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferreri C, Chatgilialoglu C (2012) The role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert Rev Mol Diagn 12: 767–780. [DOI] [PubMed] [Google Scholar]
- 49. Geier DA, Geier MR (2008) Autism spectrum disorder-associated biomarkers for case evaluation and management by clinical geneticists. Expert Rev Mol Diagn 8: 671–674. [DOI] [PubMed] [Google Scholar]
- 50. Bradstreet JJ, Smith S, Baral M, Rossignol DA (2010) Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Alternat Med Rev 15: 15–32. [PubMed] [Google Scholar]
- 51. Meguid NA, Dardir AA, Abdel-Raouf ER, Hashish A (2011) Evaluation of oxidative stress in autism: defective antioxidant enzymes and increased lipid peroxidation. Biol Trace Elem Res 143: 58–65. [DOI] [PubMed] [Google Scholar]
- 52.Pecorelli A, Leoncini S, De Felice C, Signorini C, Cerrone C, et al.. (2012) Non-protein-bound iron and 4- hydroxynonenal protein adducts in classic autism. Brain Dev Apr 23. [DOI] [PubMed]
- 53. Kim HY (2007) Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem 282: 18661–18665. [DOI] [PubMed] [Google Scholar]
- 54. Orr SK, Bazinet RP (2008) The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opinion Invest Drugs 9: 735–743. [PubMed] [Google Scholar]
- 55. Bazan NG (2005) Lipid signalling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol 32: 89–103. [DOI] [PubMed] [Google Scholar]
- 56. Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA (1994) Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 60: 189–194. [DOI] [PubMed] [Google Scholar]
- 57. Richardson AJ, Ross MA (2000) Fatty acid metabolism in neurodevelopmental disorder: a new perspective on associations between attention-deficit/hyperactivity disorder, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins Leukot Essent Fatty Acids 63: 1–9. [DOI] [PubMed] [Google Scholar]
- 58. Brookes KJ, Chen W, Xu X, Taylor E, Asherson P (2006) Association of fatty acid desaturase genes with attention deficit/hyperactivity disorder. Biol Psychiatry 60: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 59. Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, et al. (2006) Common genetic variants of the FADS1-FADS 2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Human Mol Genetics 15: 1745–1756. [DOI] [PubMed] [Google Scholar]
- 60. Bell JG, Miller D, MacDonald DJ, MacKinlay EE, Dick JR, et al. (2010) The fatty acid compositions of-erythrocyte and plasma polar lipids in children with autism, developmental delay or typically developing controls and-the effect of fish oil intake. Br J Nutr 103: 1160–1167. [DOI] [PubMed] [Google Scholar]
- 61. Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and-neuroinflammation in the brain of patients with autism. Ann Neurol 57: 67–8. [DOI] [PubMed] [Google Scholar]
- 62. Rossignol DA, Frye RE (2012) A review of research trends in physiological abnormalities in autism spectrum-disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant-exposures. Mol Psychiatry 17: 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pietiläinen KH, Róg T, Seppänen-Laakso T, Virtue S, Gopalacharyulu P, et al. (2011) Association of Lipidome-Remodeling in the Adipocyte Membrane with Acquired Obesity in Humans. PLoS Biol 9: e1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schengrund CL, Ali-Rahmani F, Ramer JC (2012) Cholesterol, GM1, and autism. Neurochem Res 37: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 65. Kaphzan H, Buffington SA, Jung JI, Rasband MN, Klann E (2011) Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of Angelman syndrome. J Neurosci 31: 17637–17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ji L, Chauhan A, Brown WT, Chauhan V (2009) Increased activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase in the frontal cortex and cerebellum of autistic individuals. Life Sci 16: 788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kurup RK, Kurup PA (2003) A hypothalamic digoxin-mediated model for autism. Int J Neurosci 113: 1537–1559. [DOI] [PubMed] [Google Scholar]
- 68. Turner N, Else PL, Hulbert AJ (2003) Docosahexaenoic acid (DHA) content of membranes determines molecular activity of the sodium pump: implications for disease states and metabolism. Naturwissenschaften 90: 521–523. [DOI] [PubMed] [Google Scholar]
- 69. Rodrigo R, Bächler JP, Araya J, Prat H, Passalacqua W (2007) Relationship between (Na+K)-ATPase activity, lipid peroxidation and fatty acid profile in erythrocytes of hypertensive and normotensive subjects. Mol Cell Biochem 303: 73–81. [DOI] [PubMed] [Google Scholar]
- 70. Vignini A, Buldreghini E, Nanetti L, Amoroso S, Boscaro M, et al. (2009) Free thiols in human spermatozoa: are Na+/K+-ATPase, Ca2+-ATPase activities involved in sperm motility through peroxynitrite formation? Reprod Biomed Online. 18: 132–40. [DOI] [PubMed] [Google Scholar]
- 71. Kamboj SS, Chopra K, Sandhir R (2009) Hyperglycemia-induced alterations in synaptosomal membrane fluidity and activity of membrane bound enzymes: beneficial effect of N-acetylcysteine supplementation. Neuroscience. 162: 349–58. [DOI] [PubMed] [Google Scholar]
- 72. Amminger GP, Berger GE, Schäfer MR, Klier C, Friedrich MH, et al. (2007) Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biol Psychiatry 61: 551–553. [DOI] [PubMed] [Google Scholar]
- 73. Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL (2011) A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. J Autism Dev Disord 41: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. James S, Montgomery P, Williams K (2011) Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst Rev 9: CD007992. [DOI] [PubMed] [Google Scholar]
- 75. Chalon S (2006) Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids 75: 259–269. [DOI] [PubMed] [Google Scholar]
- 76. Pardo CA, Eberhart CG (2007) The neurobiology of autism. Brain Pathology 17: 434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Farook MF, DeCuypere M, Hyland K, Takumi T, LeDoux MS, et al. (2012) Altered serotonin, dopamine and norepinepherine levels in 15q duplication and Angelman syndrome mouse models. PLoS One 7(8): e43030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bell JG, Sargent JR, Tocher DR, Dick JR (2000) Red blood cell fatty acid compositions in a patient with autistic spectrum disorder: a characteristic abnormality in neurodevelopmental disorders? Prostaglandins Leukot. Essent. Fatty Acids. 63: 21–25. [DOI] [PubMed] [Google Scholar]
- 79. Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, et al. (2004) Essential fatty acids and phospholipase A2 in autistic spectrum disorders Prostaglandins. Leukot. Essent. Fatty Acids. 71: 201–204. [DOI] [PubMed] [Google Scholar]
- 80. Bu B, Ashwood P, Harvey D, King IB, Water JV, et al. (2006) Fatty acid compositions of red blood cell phospholipids in children with autism. Prostaglandins. Leukot. Essent. Fatty Acids. 74: 215–221. [DOI] [PubMed] [Google Scholar]
- 81. Bell JG, Miller D, MacDonald DJ, MacKinlay EE, Dick JR, et al. (2010) The fatty acid compositions of erythrocyte and plasma polar lipids in children with autism; developmental delay or typically developing controls and the effect of fish oil intake. Br. J. Nutr. 103: 1160–1167. [DOI] [PubMed] [Google Scholar]