Abstract
Loss of control eating confers risk for excess weight gain and exacerbated disordered eating. Affect theory proposes that loss of control eating is used to cope with negative mood states. Self-report data suggest that negative affect may contribute to the etiology of loss of control eating, but this theory has not been well-tested using laboratory paradigms. We examined associations between pre-meal affective states and intake during a laboratory test meal. One-hundred and ten adolescent girls with reported loss of control eating whose body mass index fell between the 85th and 97th percentile for age and sex completed state mood ratings prior to a test-meal. Results indicated that pre-meal state negative affect was associated with greater carbohydrate and less protein consumption, as well as greater snack and dessert and less fruit and dairy intake. All girls experienced significant decreases in negative affect from pre- to post- meal, but intake during the meal was unassociated with post-meal affect. In support of affect theory, negative affective states reported among girls with loss of control may be a driving factor for increased energy-dense food intake, which may play a role in excess weight gain.
Keywords: adolescence; loss of control eating,; negative affect; binge eating
Loss of control (LOC) eating, defined as the subjective experience of being unable to stop eating or control what or how much one is eating, is prevalent among overweight youth (Tanofsky-Kraff, 2008). In contrast with classic binge eating episodes that require the consumption of a large amount of food, LOC eating encompasses episodes during which a lack of control is experienced but the amount consumed is ambiguously large. LOC eating in youth is associated with emotional distress including more depressive symptoms, greater internalizing symptoms, and the use of less adaptive emotion regulation strategies (Czaja, Rief, & Hilbert, 2009; Hilbert & Czaja, 2009; Johnson, Rohan, & Kirk, 2002; Tanofsky-Kraff et al., 2004). Children with LOC are heavier (Neumark-Sztainer et al., 1997; Tanofsky-Kraff et al., 2004) and gain excess weight and body fat over time (Sonneville et al., 2012; Tanofsky-Kraff et al., 2006; Tanofsky-Kraff, Yanovski, et al., 2009). Furthermore, LOC prospectively predicts worsening eating pathology, including a greater likelihood of developing partial or full-syndrome binge eating disorder (BED) (Tanofsky-Kraff et al., 2011).
One model that has been proposed to explain LOC eating behavior is affect theory (Hawkins & Clement, 1984; Kenardy, Arnow, & Agras, 1996). Affect theory suggests that negative mood states trigger episodes of uncontrolled eating in order to escape from, or alleviate, adverse emotions. This process may constitute a maladaptive strategy because relief is reinforcing, yet oftentimes produces only temporary abatement of the negative mood state (Stein et al., 2007), leading to repeated bouts of eating in order to reduce negative mood.
In support of affect theory are self-report data in adults (Eldredge & Agras, 1996; Masheb & Grilo, 2006; Ricca et al., 2009; Wolfe, Baker, Smith, & Kelly-Weeder, 2009) and children (Czaja et al., 2009; Goossens, Braet, & Decaluwe, 2007; Tanofsky-Kraff et al., 2007) linking negative mood states to episodes of LOC. In a laboratory test meal among adults with BED, pre-meal negative affect was found to act as a precipitant to eating episodes accompanied by LOC and labeled as binges (Telch & Agras, 1996). Similarly, ecological momentary assessment studies in adults with BED (Greeno, Wing, & Shiffman, 2000; Stein et al., 2007) and sub-threshold binge eating (Deaver, Miltenberger, Smyth, Meidinger, & Crosby, 2003; Wegner et al., 2002) suggest that negative affect is a common precipitant of binge episodes in the natural environment. Affect theory may also be relevant to understanding LOC eating behavior in children. In a large sample of non-treatment-seeking boys and girls (8–18 years), those with LOC reported worse mood prior to and after eating in the laboratory compared to their counterparts without LOC (Tanofsky-Kraff, McDuffie, et al., 2009).
Hypotheses surrounding the specific eating patterns of adults and youth with binge and LOC eating pathology include 1. individuals with binge/LOC eating consume more overall energy compared to their non-binge eating, non-LOC counterparts, and 2. despite consuming a similar overall amount of food compared to those without binge or LOC eating, those reporting these behaviors exhibit a shift in food preferences toward more highly palatable foods, defined as foods with the following properties: highly energy-dense, and containing relatively high amounts of sugar and fat, and low amounts of protein. The latter hypothesis suggests that even if individuals with binge or LOC eating do not consume more overall energy than non-binge/LOC eating peers at a specific “moment in time,” they may be more prone to weight- and body fat-gain as a result of consuming a diet comprised of a greater proportion of highly-palatable foods (Tanofsky-Kraff, McDuffie, et al., 2009).
Among adults, laboratory studies have generally established that those with BED consume more energy compared to their counterparts without BED (Walsh & Boudreau, 2003; Yanovski et al., 1992). Additionally, women with BED were found to consume a greater proportion of calories from fat, and less from protein (Yanovski et al., 1992). Among children, laboratory findings lend mixed support to the hypotheses that binge eating is associated with greater total and highly-palatable food intake. In a sample of overweight treatment-seeking boys and girls ages 6–12 years, binge eating was associated with greater overall, but not macronutrient, intake (Mirch et al., 2006). Among 8–13 year olds, those with LOC consumed more overall energy, protein, and fat compared to non-LOC peers during a post-meal “snack” array (Hilbert, Tuschen-Caffier, & Czaja, 2010). In a third study among children and adolescents (8–18 years old), LOC youth exhibited a pattern of eating in which they consumed more snack and dessert type foods, greater percent calories from carbohydrates, and fewer percent calories from protein (Tanofsky-Kraff, McDuffie, et al., 2009). In this study, only among overweight girls did those with LOC demonstrate greater overall caloric intake (Tanofsky-Kraff, McDuffie, et al., 2009). Taken together, findings suggest a possible relationship between LOC eating and overall and palatable food intake, yet such associations may be moderated by developmental stage (i.e. adolescence), female sex, or overweight status.
Despite findings indicating that negative affect often precedes “binge” or “LOC” episodes, as well as findings suggesting that individuals with LOC eating may consume more energy-dense, highly palatable foods compared to those without LOC eating, less is known about the foods consumed in response to negative affect among individuals who experience LOC. Among adult women with recurrent binge eating, exposure to a negative mood induction resulted in increased consumption of a highly palatable food, chocolate (Chua, Touyz, & Hill, 2004). Among youth, there is a relative dearth of knowledge in this area, especially as assessed under controlled laboratory paradigms. Among 8–13 year olds, there was minimal evidence to suggest that negative mood contributed to the prediction of total or macronutrient intake, yet analyses included youth with and without LOC (Hilbert et al., 2010). In contrast, one study suggests that among girls ages 6–12 years who experience LOC, exposure to a sad (versus neutral) mood induction was associated with increased fat intake during a laboratory test meal, despite no relationship between negative affect and overall intake (Goldschmidt, Tanofsky-Kraff, & Wilfley, 2011).
Taken together, findings point to the possibility that negative affect may be associated with increased total intake and/or increased intake of highly-palatable foods. No study has yet examined the relationship between pre-meal affect and intake among adolescent girls reporting LOC eating in the absence of a mood manipulation. The current study aims to add to a dearth of literature among children and adolescents by measuring eating behavior in response to self-reported negative affect among adolescents who endorse the presence of LOC eating. Our objective is to examine the associations between pre-meal state affect and total energy intake and intake of highly palatable foods in a sample of adolescent girls at risk for excess weight gain and BED.
Consistent with affect theory (Hawkins & Clement, 1984; Kenardy et al., 1996), we hypothesized that greater state negative affect prior to a laboratory test meal designed to facilitate disinhibited eating would be associated with greater total energy intake and greater intake of highly-palatable foods. Consistent with data suggesting that intake of palatable foods activates physiological reward pathways that reduce negative feelings (Adam & Epel, 2007), we expected that greater energy intake and greater intake of palatable foods would be associated with greater reductions in negative affect.
Method
Participants
Participants were adolescent girls ages 12 through 17 years who were recruited for participation in a randomized clinical trial examining the efficacy of group interventions for excess weight gain prevention (Clinical Trials.gov ID: NCT00680979) at the Uniformed Services University of the Health Sciences (USUHS) and the National Institutes of Health (NIH) in Bethesda, Maryland. All participants were deemed at risk for excess weight gain by virtue of a body mass index (BMI, kg/m2) between the 75th and 97th percentiles and the report of at least one episode of LOC eating in the month prior to assessment. Individuals were excluded if they had a major medical condition (e.g., diabetes), current or lifetime diagnosis of an eating disorder (other than BED), a current severe psychiatric condition (e.g., major depressive disorder, psychosis), were simultaneously participating in a structured weight loss program or psychotherapy, or were taking medications known to affect body weight or appetite. Additionally, participants were excluded if they were pregnant or had lost more than 5% of their body weight in the three months prior to assessment.
Adolescents were recruited through the NIH clinical trials website, local area community flyer postings, and direct mailings to homes within a 50-mile radius of Bethesda, Maryland. The study was approved by the USUHS and Eunice Kennedy Shriver National Institute of Child Health and Human Development institutional review boards. Parents provided written consent for study participation, and all girls provided written assent.
Procedure
Families participated in a preliminary screening visit at USUHS during which LOC eating was assessed. Participants were then seen at the NIH Mark O. Hatfield Clinical Research Center for a second screening visit following an overnight fast beginning at 10 p.m. on the night before the visit. The test meal was administered at 11:00 a.m. Mood ratings were completed immediately before and after the meal. For purposes of the current study, all data were collected at baseline, prior to initiation of any component of the intervention programs.
Measures
Physical assessments included children’s height measured three times to the nearest millimeter by a calibrated electronic stadiometer and weight measured to the nearest 0.1 kg by a calibrated digital scale. Body weight and the average of the three heights were used to calculate BMI (kg/m2). The Center for Disease Control and Prevention growth charts were used to convert BMI to BMI-Z scores (Kuczmarski et al., 2002). Total lean body mass (kg) and percent body fat mass were assessed by dual-energy X-ray absorptiometry using a Hologic QDR-4500A or Discovery instrument (Waltham, MA).
LOC eating was evaluated using the Eating Disorder Examination (EDE) interview version 14 OD/C.2 (Fairburn & Cooper, 1993). The EDE is used to measure eating disordered attitudes and cognitions as well as DSM-IV-TR eating disorders (American Psychiatric Association, 2000). In the current study, the EDE was used to determine the presence of objective binge episodes, characterized by experiencing LOC during consumption of an unambiguously large amount of food, as well as subjective binge episodes, (the experience of LOC during consumption of an ambiguously excessive amount of food). The EDE has demonstrated excellent internal consistency in adolescents (Glasofer et al., 2007).
Immediately before and after the test meal, girls completed the Brunel Mood Rating Scale (Terry, Lane, Lane, & Keohane, 1999), a 24-item self-report measure of a variety of state emotions. Adolescents reported the degree to which they felt 24 feelings “right now,” rated on a 5-point Likert scale ranging from 1=‘Not at all’ to 5=‘Extremely.’ Six subscales, five of which assess negative affective states (depression, anger, tension, confusion and fatigue) and one positive affect state (vigor), are derived by summing the respective items. An overall measure of pre-meal state negative affect was calculated by summing individual items from the five negative affective states subscales (Vannucci et al., 2012). The Brunel Mood Scale has demonstrated good construct and concurrent validity in adolescents (Terry, Lane, & Fogarty, 2003; Terry et al., 1999).
Depressive symptoms were assessed using the Beck Depression Inventory, second version (BDI-II) (Beck, Steer, & Brown, 1996), a 21-item self-report measure that assesses depressive symptoms. Each response is assigned a score between zero and three, with total scores ranging from 0–63. Scores from zero to nine represent minimal depressive symptoms, scores of 10 to 16 indicate mild depression, scores of 17 to 29 indicate moderate depression, and scores of 30 to 63 indicate severe depression. The BDI-II has well-established psychometric properties with adolescents (Krefetz, Steer, & Kumar, 2003). Adolescents were also assessed for Major Depressive Disorder using the Schedule for Affective Disorders and Schizophrenia. If an adolescent met criteria for Major Depressive Disorder, she was excluded from study participation.
Total energy intake, macronutrient composition, and intake of food types including dairy, meat, snacks and desserts, drinks, condiments, fruits and vegetables, were measured during a lunchtime laboratory test meal. The test meal consisted of a 9,835 kcal buffet with a wide assortment of individual items that varied in macronutrient composition (12% protein, 51% carbohydrate, 37% fat) (Mirch et al., 2006; Tanofsky-Kraff, McDuffie, et al., 2009). Participants were given the tape-recorded instruction to “let yourself go and eat as much as you want.” Each adolescent ate alone and was instructed to inform a research assistant when she was finished eating. Amounts of food and beverage consumed were calculated by weighing each food or beverage item once before and once after the meal on digital Mettler Toledo and Ohaus balances to the nearest tenth of a gram. The balances were calibrated with external weights monthly. Energy content and macronutrient composition for each item were determined according to data from the U.S. Department of Agriculture Nutrient Database for Standard Reference as well as nutrient information supplied by food manufacturers.
The construct of highly-palatable foods was operationalized by examining proportion of carbohydrate, fat and protein intake, as well as intake of ‘snack and dessert’ foods. ‘Snack and dessert’ foods included both ‘sweet snacks’ (vanilla wafer cookies, sandwich cookies, jellybeans, chocolate candy) and ‘salty snacks’ (tortilla chips and pretzels) (Tanofsky-Kraff, McDuffie, et al., 2009), thought to best represent highly palatable foods, that are energy dense, high in sugar and fat, and low in protein. Greater carbohydrate, fat, ‘snack and dessert,’ and less protein, intake reflected highly-palatable food intake.
A post-meal rating of the degree of similarity between the laboratory eating episode and a ‘typical’ episode of LOC outside of the laboratory was used to assess LOC during the laboratory test meal. Adolescents rated degree of similarity on a 5-point Likert scale ranging from 1 = “extremely” through 5 = “not at all.” Participants also had the option to select “Not applicable/Never feel LOC” (6).
Data Analyses
Analyses were performed with SPSS 16.0 (SPSS, Chicago, IL). Data were screened for normality. Logarithmic transformations were applied to total energy intake (kcal), and arcsine transformations were made for percentage of macronutrient content intake. Square root transformations were applied to Brunel Mood total scales (pre- and post-meal). Outliers were defined as values 1.5 times the interquartile range below or above the 25th and 75th percentile, and were adjusted to fall 1.5 times the interquartile range below or above the 25th or 75th percentile, respectively (Behrens, 1997).
A series of linear regressions were conducted to examine the main effects of pre-meal state negative affect and state vigor on the dependent variables of total energy, macronutrient content (carbohydrate, fat, and protein) and specific food group (dairy, meat, snacks and desserts, drinks, condiments, fruits, and vegetables) intake at the test meal. The Brunel Mood total score (with the exception of the ‘vigor’ subscale) was used to represent negative affect because the relationships between negative affect and food consumption were similar for each negative affect subscale (depression, anger, tension, confusion, fatigue), and a more parsimonious number of analyses reduced the likelihood of type I error. Similarly, ‘sweet snacks’ and ‘salty snacks’ were examined as a singular category because relationships between affect and food consumption were similar for these two categories examined separately. Covariates included in all models were age (y), race/ethnicity (non-Hispanic White versus Other), height (cm), and body composition (total lean mass, kg and percent fat mass). For models examining macronutrient composition and specific food group intake, total energy intake was included as a covariate.
Linear regressions were also used to examine the main effects of total energy, macronutrient, and specific food group intake on the dependent variable of post-meal negative affect, controlling for pre-meal negative affect. The same covariates as described previously were considered. In models examining macronutrient and specific food group intake, total energy intake was not included as a covariate due to a high degree of multicollinearity.
Results
Outlier Screenings
Across all intake and affect variables, forty-six outliers were present. Outliers fell between -5 and 6 standard deviations outside of the mean. Outlier values were distributed across subjects. Although analyses yielded similar findings when outliers were left unadjusted, findings of adjusted analyses are presented.
Participant Characteristics
Participants were 110 adolescent girls, with a mean age of 14.51 (SD = 1.69) and mean BMI-Z of 1.54 (SD = 0.33) [Table 1]. Girls experienced an average of 4.72 (SD = 6.26, Median = 2, Range = 1 – 39) LOC episodes in the month prior to assessment on 4.39 (SD = 5.21, Median = 2, Range = 1 – 25) days. Ninety-three percent of girls endorsed presence of subjective binge episodes, while just over a quarter (27%) reported classic (objective) binge episodes. Nearly one-third (32%) of girls reported both objective and subjectively large binge episodes.
Table 1.
Energy of Meal Items by Food Groups Presented at the Buffet Test Meals
| Item | Energy (kcal) |
Item | Energy (kcal) |
|---|---|---|---|
| Dairy | Fruit | ||
| 240g American cheese | 901.1 | 3 medium bananas | 325.7 |
| 850g 2% milk | 422.2 | 250g grapes | 177.5 |
| Desserts and snacks | 3 medium oranges | 184.7 | |
| 12 sandwich cookies† | 566.4 | Bread | |
| 12 Vanilla wafer cookies | 206.3 | 12 slices white bread | 801.0 |
| 120g tortilla chips | 601.2 | Condiments | |
| 150g pretzels | 571.5 | 120g peanut butter | 711.6 |
| 120g jellybeans | 440.4 | 120g grape jelly | 339.6 |
| 120g chocolate candy‡ | 590.4 | 90g mayonnaise | 645.1 |
| Meats | 90g mustard | 59.4 | |
| 180g ham | 216.0 | 90g light ranch dressing | 240.0 |
| 180g turkey | 282.6 | 90g barbeque sauce | 67.5 |
| 200g chicken nuggets | 550.8 | 250g mild salsa | 70.0 |
| Vegetables | Drinks | ||
| 200g tomatoes | 42.0 | 850g bottled water | 0.0 |
| 50g lettuce | 6.0 | 850g apple juice | 400.0 |
| 200g baby carrots | 76.0 | 850g lemonade | 340.0 |
| Total | 9,835 | ||
Oreo®,
M&M’s®
Loss of Control during Laboratory Test Meal
The mean typicality score was 3.9 (SD = 1.1, Median = 4 (slightly) Range = 1 – 5), on a scale of 1 through 5 (1 = “extremely similar;” 5 = “not at all similar”). The majority (54.5%) of adolescents reported that the laboratory eating episode was slightly, moderately, very much or extremely similar to a typical LOC eating episode. Thirty-five percent of adolescents reported that the meal was “not at all” similar, and 11% reported “Not Applicable/I never feel Loss of Control.”
Pre-meal Negative Affect and Intake
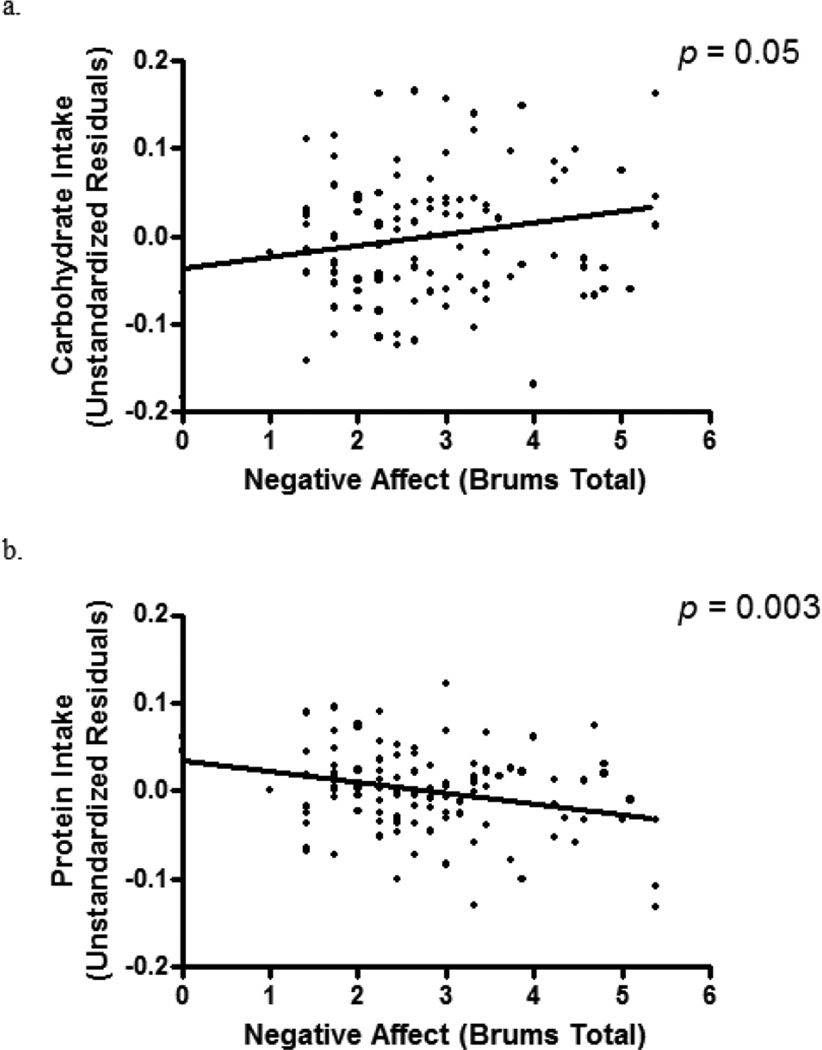
Pre-meal state negative affect was not significantly associated with overall energy intake (p = .65) after accounting for age, race, and body composition. However, controlling for the same covariates and total intake, there was a significant relationship between state negative affect and carbohydrate intake (β = 0.19, p = 0.05), such that greater negative affect prior to the meal was associated with consuming a greater proportion of intake from carbohydrate [Figure 1]. Further, an inverse relationship was found between state negative affect and protein intake (β = −0.29, p = 0.003), such that greater state negative affect was associated with lower proportion of intake from protein [Figure 1]. Pre-meal state negative affect was unassociated with proportion of fat intake (p = 0.36).
Figure 1.
Pre-meal state negative affect, measured by the Brunel Mood Scale total score, in relation to (a) carbohydrate intake (b) protein intake, adjusted for race, age, height, total lean mass (kg), percent fat mass, and total energy intake.
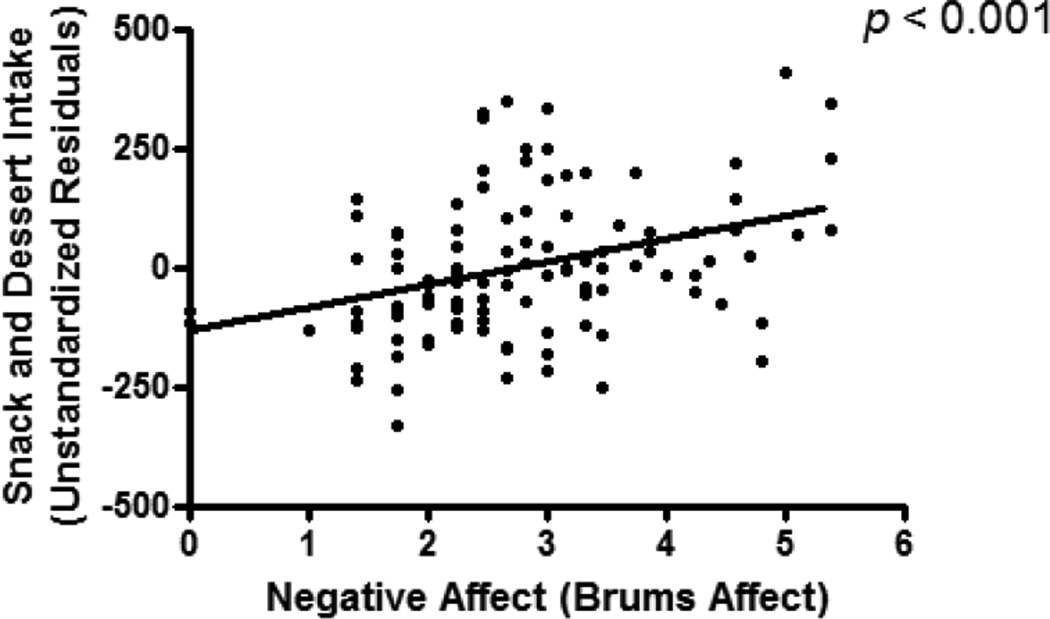
There was a significant relationship between pre-meal state negative affect and snack and dessert intake (β = 0.29, p < 0.001), such that higher state negative affect predicted greater snack and dessert intake after controlling for all covariates [Figure 2]. Further, state negative affect was inversely associated with both dairy intake (β = −0.20, p = 0.03) and fruit intake (β = −0.24, p = 0.02). There were no associations between statenegative affect and intake of meat, condiments, vegetables, or drinks (p’s > 0.20).
Figure 2.
Pre-meal state negative affect, measured by the Brunel Mood Scale total score, in relation to snacks and desserts, adjusted for race, age, height, total lean mass (kg), percent fat mass, and total energy intake.
Pre-Meal Positive Affect and Intake
Total caloric intake was unrelated to pre-meal state positive affect (“vigor,” p = 0.78). Non-significant positive relationships were found between pre-meal state vigor and proportion protein intake (p = 0.09) and intake of vegetables (p = 0.09). No other relationships were found between pre-meal positive affect and proportion macronutrient intake or intake of any specific food group (ps > 0.15).
Intake and Post-meal Negative Affect
There was a significant decrease in negative affect from pre- (M = 9.13, SD = 7.49) to post-meal (M = 5.83, SD = 6.63; p < 0.001). Controlling for pre- meal state negative affect, total energy intake did not predict post-meal negative affect (p = 0.59). Similarly, controlling for pre-meal negative affect, there were no associations between intake from any macronutrient group (ps > 0.49), or specific food group (ps > 0.25) and post-meal negative affect.
Secondary Analysis accounting for LOC during Laboratory Meal
All models were tested excluding adolescents (n =50) who indicated that the meal was “Not similar at all” to a typical LOC eating episode or that they never experienced LOC. Although limited by inadequate power, most results remained significant. The positive association between negative affect and snack intake (p = 0.01) and the inverse association between negative affect and dairy intake (p = 0.02), were significant. The inverse association between negative affect and protein intake was marginally significant (p = 0.09). Significant relationships were no longer found between carbohydrate intake (p = 0.18) and fruit intake (p = 0.19). All findings regarding positive affect and post-meal affect remained the same when this subset of adolescents was excluded from analyses.
Discussion
To test affect theory’s prediction that negative mood states lead to increased total intake and intake of highly palatable foods, we studied pre-meal state negative affect and food intake during a laboratory test meal designed to facilitate disinhibited eating in a sample of adolescent girls at high risk for excess weight gain and binge eating disorder (BED). Findings suggested that greater pre-meal negative affect was associated with a specific pattern of eating characterized by consumption of a greater proportion of calories from carbohydrate, a smaller proportion of calories from protein, greater snack food intake, and less dairy and fruit intake. By contrast, there was generally no relationship between pre-meal state affect and overall energy intake, proportion of fat intake, or intake of any other specific food group. Adolescents experienced significant reductions in negative affect from pre- to post-meal. In contrast to affect theory’s prediction, there was no relationship between intake during the test meal and adolescents’ mood following the meal.
Our findings among girls with LOC eating are partially in concert with adult (Oliver, Wardle, & Gibson, 2000; Zellner et al., 2006) and child (Goldschmidt et al., 2011) data suggesting that negative emotional states may impact food consumption by promoting intake of highly palatable foods. In adults reporting emotional eating, types of foods consumed during adverse mood states are generally palatable and higher in fat compared to foods consumed during neutral mood states (Habhab, Sheldon, & Loeb, 2009; Oliver et al., 2000). Goldschmidt and colleagues also reported that young girls with LOC consumed a significantly greater proportion of fat in response to negative affect. Although adolescents in our study consumed more carbohydrates, snacks and desserts, and less protein, whereas children in the study by Goldschmidt and colleagues consumed more fat, findings from both studies reflect a general pattern of eating in which girls consumed more highly palatable foods following negative affect. It is possible that variability across findings may reflect developmental differences in individuals’ eating patterns in response to negative mood. Additional studies will help elucidate whether meaningful differences exist in the types of palatable foods consumed by youth of varying ages, when feeling upset.
Whether individuals identify negative mood as a precipitant of LOC may also follow a developmental trajectory. Across several childhood studies (Hilbert, Rief, Tuschen-Caffier, de Zwaan, & Czaja, 2009; Hilbert et al., 2010), negative mood was not found to precede LOC eating. In two laboratory paradigms, however, children ate more highly palatable food following negative experiences (Goldschmidt et al., 2011; Hilbert et al., 2010). It is possible that preadolescent children with LOC have difficulty identifying negative mood states, which may explain why negative affective theories of LOC are generally not supported when children are simply asked to self-report their mood. Adolescence may be a developmental period accompanied by a growing ability for youth with LOC eating to identify and report mood preceding LOC episodes.
In a prior report (Tanofsky-Kraff, McDuffie, et al., 2009), we found that children and adolescents with LOC reported greater negative affect before meals and consumed more carbohydrates, snacks, and desserts, and less protein during meals, compared to non-LOC youth. The current study extends on these findings by suggesting that food intake may be an attempt made by LOC youth, whether successful or not, to reduce negative affect. Indeed, for all girls, negative affect was significantly reduced following the laboratory meal. However, in contrast to our hypothesis, neither total intake, nor intake of highly palatable foods, was associated with improvement in mood.
Our finding that adolescents’ mood improves following a large meal is in contrast with results from a meta-analysis examining post-state affect among adults with bulimic and BED symptomatology, which reported that across 36 studies including over 900 adult participants, negative affect increases following binge episodes (Haedt-Matt & Keel, 2011). Discrepancies in study findings may involve differences in study methodology (laboratory versus ecological momentary assessment) and the timing of measurements of negative affect. In the majority of studies of affect and eating among individuals who experience binge eating, affect is measured only during and following a meal, however, it is possible, and affect theory might predict, that the course of negative affect first improves during eating, and then worsens following eating. Thus, measurements of affect taken immediately following a meal might capture improvement in mood, whereas measurements taken a short time following a meal might capture worsening of mood. This hypothesis is supported by a study in which women with binge eating rated their mood throughout the course of a binge meal, and found that episodes of binge eating were characterized improvement in mood during, but not following, the episode (Deaver et al., 2003).
Alternatively, discrepancies in findings regarding post-meal affect across ours versus some adult studies may reflect differences between affective experiences among individuals with sub-threshold disinhibited eating symptomatology versus those with full-syndrome BED. Among adults, individuals’ post-meal affective experiences varied based on diagnostic status: for those with BED, experience of LOC was associated with greater post-meal negative affect, whereas for obese controls without BED, greater food intake and experience of LOC predicted lower post-meal negative affect (Goldschmidt et al., 2012). Post-meal negative affect following LOC episodes, as described in those with BED, may in part reflect distress surrounding binge eating behavior. This pattern may be less common among those with sub-threshold pathology, such as adolescents in the current study.
The reinforcing value of palatable food while eating may be, in part, physiologically driven, grounded in genetic and resulting neurobiological underpinnings. Potential mechanisms for the observed link between LOC and palatable-food consumption in response to negative affect may include abnormalities in stress-responsiveness (Gibson, 2006) and/or differences in reward activation pathways (Adam & Epel, 2007; Davis et al., 2008). Specifically, youth with LOC eating may be differentially sensitive to the effects of palatable foods on opioid (reward) pathways, stemming from genetic polymorphisms that encode dopamine receptor genes (e.g. primary dopamine receptor deficiency). Burger and Stice (Burger & Stice, 2011) propose several mechanisms by which this might occur: One possibility is that hypo-responsive reward circuitry produce overeating as a compensatory mechanism. Alternatively, hyper-responsive reward circuitry may place overweight youth or those with LOC eating at risk for overeating. An extension on the latter model suggests that hyper-responsive reward circuitry results in down-regulation in D2 receptor density, further exacerbating overeating in a feed-forward fashion (Burger & Stice, 2011). Both models propose that overeating, in turn, sensitizes regions in the brain that encode the incentive salience of food (“wanting”) through conditioning which occurs during and following overeating episodes. Indeed, adults with BED and related symptoms have genotypes reflecting enhanced D2 transmission in neural reward circuitry (Davis et al., 2012).
Alternatively, it is possible that LOC eating and subsequent weight gain actually initiate neurobiological changes in neural reward circuitry, such as dopamine receptor down-regulation, leading to a state of reward-deficiency and further promoting overeating. Lending support to this hypothesis, a study among women who underwent bariatric surgery found clinically significant increases in dopamine receptor availability six weeks following surgery (Steele et al., 2010). Such findings suggest that regulation of dopamine receptor density within neural reward circuitry may be responsive to specific eating behaviors, such as LOC eating. Taken together, identification of LOC eating precipitants may be an important step in reducing the likelihood of neurobiological sequelae, such as reduced receptor density or enhanced sensitization of brain regions which encode the incentive salience of food, which may follow repeated episodes of LOC eating.
Data from the current study suggest a potential mechanism whereby pediatric LOC may lead to excess weight gain—namely, via a pattern of eating characterized by frequently consuming highly palatable, calorie-dense “comfort foods” in response to negative affect. Adolescents with LOC who respond to negative affect by consuming foods that are energy-dense and high in sugar and fat and low in protein may be at risk for gaining excess weight over time, even in the absence of consuming more overall intake at a specific “moment in time.” Indeed, among children and adolescents, proportion of one’s intake from “snacking” is positively related to overweight status and to intake of snack foods and sugar (Cusatis & Shannon, 1996; Francis, Lee, & Birch, 2003; Nicklas, Yang, Baranowski, Zakeri, & Berenson, 2003).
Study strengths include the racially-diverse sample of adolescents, the use of a well-validated interview to assess LOC eating, and the administration of a well-controlled laboratory test meal. Conversely, the large buffet-style meal, although used effectively in prior studies (Mirch et al., 2006; Tanofsky-Kraff, McDuffie, et al., 2009; Walsh & Boudreau, 2003), may not capture eating behavior in the natural environment. Ecological momentary assessment studies may help elucidate whether such associations persist in the naturalistic environment. An additional limitation of the large laboratory test meal paradigm is that the physical and sensory properties of the macronutrient and specific food categories may differentially contribute to adolescents’ intake of specific foods. For example, foods comprising the snack and dessert group such as cookies and chips require little preparation and are generally easy to chew. It is possible that the ease with which such foods are able to be consumed, compared to, for example, building a sandwich, might cause adolescents to preferentially choose these foods. Although other macronutrient and specific food group categories also contained items that were easy to prepare and chew, such as chicken fingers and grapes, future studies might aim to match foods across groups, based on such properties.
Although not necessarily a limitation, the nature of our study sample may limit generalizability. By design, the sample was restricted in body composition variability and was homogenous for the presence of LOC eating. Therefore, our findings may not be relevant to children of lower or higher BMI percentiles, boys, and those without reports of LOC. The study did not include one or more control groups of overweight or normal weight adolescents without LOC. Therefore, the results cannot be attributed to adolescents’ LOC eating status. Adolescents’ reports that the meal was at least somewhat similar to an LOC eating episode suggests that results reflect LOC eating patterns. However, querying participants about how similar a laboratory eating episode is to a LOC episode experienced in the natural environment may not accurately capture their experience of LOC during the test meal (e.g., some participants may have consumed similar types/quantities of food in the laboratory as in the natural environment, but not have experienced the same sense of LOC). In future studies, momentary assessment of LOC during the laboratory meal will elucidate this question.
Of note, when excluding the adolescents who described the meal as entirely dissimilar from a typical LOC episode, the relationship between pre-meal negative affect and snack and dessert (highly-palatable food) intake persisted. However, other key relationships (e.g. between negative affect and reduced intake of protein, and between negative affect and increased intake of carbohydrates) were no longer significant. Whether the discrepancies in study findings are attributable to reduced power, or to true differences in the eating patterns of all adolescents with reported LOC eating versus only those who actually experience LOC during the laboratory meal, requires additional investigation.
The present study contributes to our current understanding of LOC eating and supports affect theory by demonstrating an increase in intake of highly palatable foods in response to negative affect. That affect theory is applicable to explaining mechanisms underlying LOC eating suggests that it may be fruitful to target negative mood states as a potential point of intervention to reduce highly palatable food intake in youth who report out-of-control eating.
Table 2.
Participant Demographics
| M | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age (y) | 14.51 | 1.69 | 12.02 | 17.83 |
| Race (% Non-Hispanic White) | 55.5 | -- | -- | -- |
| BMI (kg/m2) | 27.03 | 2.51 | 22.55 | 33.52 |
| BMI-Z scorea | 1.54 | 0.33 | 0.68 | 2.06 |
| Fat Mass (%) | 35.11 | 5.65 | 20.00 | 47.40 |
| Fat Free Mass (kg) | 43.91 | 5.65 | 29.95 | 56.94 |
| Depression (Beck Depression Inventory) | 10.78 | 6.72 | 0 | 30 |
| Pre Meal State Negative Affect (Brunel Mood Total Score) | 9.21 | 7.50 | 0 | 38 |
| Laboratory Test Meal Intake | ||||
| Total Intake (kcal) | 1175.74 | 455.60 | 158.46 | 158.46 |
| Protein (%) | 12.91 | 3.26 | 5.19 | 20.86 |
| Carbohydrate (%) | 50.79 | 7.79 | 33.24 | 73.51 |
| Fat (%) | 36.26 | 6.66 | 12.33 | 51.31 |
| Dairy (kcal) | 72.21 | 86.23 | 0 | 548.30 |
| Snacks and Desserts (kcal) | 297.07 | 209.73 | 0 | 1216.91 |
| Meats (kcal) | 343.66 | 200.34 | 0 | 820.78 |
| Condiments (kcal) | 128.44 | 119.71 | 0 | 665.12 |
| Drinks (kcal) | 109.21 | 102.87 | 0 | 456.77 |
| Vegetables (kcal) | 15.99 | 15.26 | 0 | 64.94 |
| Fruits (kcal) | 43.88 | 41.81 | 0 | 170.90 |
Note: Values are means ± standard deviation unless otherwise indicated;
BMI-Z score, BMI accounting for age and sex according to Centers for Disease Control and Prevention (Kuczmarski, et al., 2002).
Table 3.
Linear Regression of Reported Pre-Meal Negative Affect Predicting Carbohydrate Intake
| Predictor variables | Standardized Beta |
t | P |
|---|---|---|---|
| Constant | 3.704 | .000 | |
| Race | 0.075 | 0.771 | .443 |
| Age | 0.084 | 0.823 | .413 |
| Height | 0.168 | 1.096 | .275 |
| Lean Mass | −0.238 | −1.463 | .147 |
| Percent Fat Mass | −0.168 | −1.679 | .096 |
| Total Caloric Intake | −0.205 | −2.167 | .033 |
| Pre-Meal State Negative Affect | 0.192 | 2.012 | .047 |
Note: Energy intake (kcal) was log transformed; Percentage carbohydrate intake was arcsine transformed; N = 110; Race coded: 0 = other, 1 = non-Hispanic Caucasian
Table 4.
Linear Regression of Reported Pre-Meal Negative Affect Predicting Protein Intake
| Predictor variables | Standardized Beta |
T | P |
|---|---|---|---|
| Constant | 2.112 | 0.036 | |
| Race | −0.049 | −0.499 | 0.619 |
| Age | 0.089 | 0.864 | 0.390 |
| Height | −0.018 | −0.118 | 0.906 |
| Lean Mass | 0.052 | 0.320 | 0.749 |
| Percent Fat Mass | 0.146 | 1.459 | 0.148 |
| Total Caloric Intake | −0.013 | −1.380 | 0.891 |
| Pre-Meal State Negative Affect | −0.287 | −2.992 | 0.003 |
Note: Energy intake (kcal) was log transformed; Percentage protein intake was arcsine transformed; N = 110; Race coded: 0 = other, 1 = non-Hispanic Caucasian
Table 5.
Linear Regression of Reported Pre-Meal Negative Affect Predicting Snack and Dessert Intake
| Predictor variables | Standardized Beta |
t | P |
|---|---|---|---|
| Constant | −2.712 | .008 | |
| Race | .203 | 2.715 | .008 |
| Age | −.051 | −.652 | .516 |
| Height | −.104 | −.883 | .379 |
| Lean Mass | −.017 | −.137 | .891 |
| Percent Fat Mass | −.059 | −.768 | .444 |
| Total Caloric Intake | .602 | 8.286 | .000 |
| Pre-Meal State Negative Affect | .292 | 3.988 | .000 |
Note: Energy intake (kcal) was log transformed; Percentage protein intake was arcsine transformed; N = 110; Race coded: 0 = other, 1 = non-Hispanic Caucasian
Acknowledgments
Research support: NIDDK grant 1R01DK080906-01A1 (to MTK), USUHS grant R072IC (to MTK) and NICHD Intramural Research Program ZIA-HO-00641 (to JAY). J. Yanovski and M. Kozlosky are commissioned officers in the U.S. Public Health Service (PHS). M. Stephens is active duty in the US Navy.
Footnotes
Publisher's Disclaimer: Disclaimer: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS, the Department of the Navy, USUHS or the U.S. Department of Defense.
References Cited
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition, test revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Behrens JT. Principles and procedures of exploratory data analysis. Psychological Methods. 1997;2(2):131–160. [Google Scholar]
- Burger KS, Stice E. Variability in reward responsivity and obesity: evidence from brain imaging studies. [Review] Curr Drug Abuse Rev. 2011;4(3):182–189. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua JL, Touyz S, Hill AJ. Negative mood-induced overeating in obese binge eaters: an experimental study. Int J Obes Relat Metab Disord. 2004;28(4):606–610. doi: 10.1038/sj.ijo.0802595. 0802595 [pii] [DOI] [PubMed] [Google Scholar]
- Cusatis DC, Shannon BM. Influences on adolescent eating behavior. J Adolesc Health. 1996;18(1):27–34. doi: 10.1016/1054-139X(95)00125-C. 10.1016/1054-139X(95)00125-C. [DOI] [PubMed] [Google Scholar]
- Czaja J, Rief W, Hilbert A. Emotion regulation and binge eating in children. Int J Eat Disord. 2009;42(4):356–362. doi: 10.1002/eat.20630. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, Kennedy JL. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):620–628. doi: 10.1016/j.pnpbp.2007.09.024. 10.1016/j.pnpbp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Yilmaz Z, Kaplan AS, Carter JC, Kennedy JL. Binge eating disorder and the dopamine D2 receptor: genotypes and sub-phenotypes. [Research Support, Non-U.S. Gov't] Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):328–335. doi: 10.1016/j.pnpbp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Deaver CM, Miltenberger RG, Smyth J, Meidinger A, Crosby R. An evaluation of affect and binge eating. Behavior Modification. 2003;27(4):578–599. doi: 10.1177/0145445503255571. [DOI] [PubMed] [Google Scholar]
- Eldredge KL, Agras WS. Weight and shape overconcern and emotional eating in binge eating disorder. Int J Eat Disord. 1996;19(1):73–82. doi: 10.1002/(SICI)1098-108X(199601)19:1<73::AID-EAT9>3.0.CO;2-T. 10.1002/(SICI)1098-108X(199601)19:1<73::AID-EAT9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, editors. The Eating Disorder Examination (12th ed.) 12 ed. New York: Guilford Press; 1993. [Google Scholar]
- Francis LA, Lee Y, Birch LL. Parental weight status and girls' television viewing, snacking, and body mass indexes. Obes Res. 2003;11(1):143–151. doi: 10.1038/oby.2003.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89(1):53–61. doi: 10.1016/j.physbeh.2006.01.024. 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Glasofer DR, Tanofsky-Kraff M, Eddy KT, Yanovski SZ, Theim KR, Mirch MC, Yanovski JA. Binge eating in overweight treatment-seeking adolescents. Journal of Pediatric Psychology. 2007;32(1):95–105. doi: 10.1093/jpepsy/jsl012. 10.1093/jpepsy/jsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Engel SG, Wonderlich SA, Crosby RD, Peterson CB, Le Grange D, Mitchell JE. Momentary affect surrounding loss of control and overeating in obese adults with and without binge eating disorder. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Obesity (Silver Spring) 2012;20(6):1206–1211. doi: 10.1038/oby.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Tanofsky-Kraff M, Wilfley DE. A laboratory-based study of mood and binge eating behavior in overweight children. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Eat Behav. 2011;12(1):37–43. doi: 10.1016/j.eatbeh.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Braet C, Decaluwe V. Loss of control over eating in obese youngsters. Behav Res Ther. 2007;45(1):1–9. doi: 10.1016/j.brat.2006.01.006. 10.1016/j.brat.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. J Consult Clin Psychol. 2000;68(1):95–102. [PubMed] [Google Scholar]
- Habhab S, Sheldon JP, Loeb RC. The relationship between stress, dietary restraint, and food preferences in women. Appetite. 2009;52(2):437–444. doi: 10.1016/j.appet.2008.12.006. 10.1016/j.appet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. [Comparative Study Meta-Analysis Research Support, N.I.H., Extramural] Psychol Bull. 2011;137(4):660–681. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RC, Clement PF. Binge eating: Measurement problems and a conceptual model. In: Hawkins RC, Fremouw WJ, Clement PF, editors. The binge purge syndrome: Diagnosis, treatment, and research. New York, NY: Springer; 1984. pp. 229–251. [Google Scholar]
- Hilbert A, Czaja J. Binge eating in primary school children: towards a definition of clinical significance. Int J Eat Disord. 2009;42(3):235–243. doi: 10.1002/eat.20622. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Rief W, Tuschen-Caffier B, de Zwaan M, Czaja J. Loss of control eating and psychological maintenance in children: an ecological momentary assessment study. Behav Res Ther. 2009;47(1):26–33. doi: 10.1016/j.brat.2008.10.003. 10.1016/j.brat.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Tuschen-Caffier B, Czaja J. Eating behavior and familial interactions of children with loss of control eating: a laboratory test meal study. Am J Clin Nutr. 2010;91(3):510–518. doi: 10.3945/ajcn.2009.28843. 10.3945/ajcn.2009.28843. [DOI] [PubMed] [Google Scholar]
- Johnson WG, Rohan KJ, Kirk AA. Prevalence and correlates of binge eating in white and African American adolescents. Eating Behaviors. 2002;3(2):179–189. doi: 10.1016/s1471-0153(01)00057-5. [DOI] [PubMed] [Google Scholar]
- Kenardy J, Arnow B, Agras WS. The aversiveness of specific emotional states associated with binge-eating in obese subjects. [Comparative Study] Aust N Z J Psychiatry. 1996;30(6):839–844. doi: 10.3109/00048679609065053. [DOI] [PubMed] [Google Scholar]
- Krefetz DG, Steer RA, Kumar G. Lack of age differences in the Beck Depression Inventory-II scores of clinically depressed adolescent outpatients. Psychology Reprints. 2003;92:489–497. doi: 10.2466/pr0.2003.92.2.489. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- Masheb RM, Grilo CM. Emotional overeating and its associations with eating disorder psychopathology among overweight patients with binge eating disorder. Int J Eat Disord. 2006;39(2):141–146. doi: 10.1002/eat.20221. [DOI] [PubMed] [Google Scholar]
- Mirch MC, McDuffie JR, Yanovski SZ, Schollnberger M, Tanofsky-Kraff M, Theim KR, Yanovski JA. Effects of binge eating on satiation, satiety, and energy intake of overweight children. Am J Clin Nutr. 2006;84(4):732–738. doi: 10.1093/ajcn/84.4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Story M, French SA, Hannan PJ, Resnick MD, Blum RW. Psychosocial concerns and health-compromising behaviors among overweight and nonoverweight adolescents. Obesity Research. 1997;5(3):237–249. doi: 10.1002/j.1550-8528.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Nicklas TA, Yang SJ, Baranowski T, Zakeri I, Berenson G. Eating patterns and obesity in children. The Bogalusa Heart Study. Am J Prev Med. 2003;25(1):9–16. doi: 10.1016/s0749-3797(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62(6):853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Ricca V, Castellini G, Lo Sauro C, Ravaldi C, Lapi F, Mannucci E, Faravelli C. Correlations between binge eating and emotional eating in a sample of overweight subjects. Appetite. 2009;53(3):418–421. doi: 10.1016/j.appet.2009.07.008. 10.1016/j.appet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Sonneville KR, Horton NJ, Micali N, Crosby RD, Swanson SA, Solmi F, Field AE. Longitudinal Associations Between Binge Eating and Overeating and Adverse Outcomes Among Adolescents and Young Adults: Does Loss of Control Matter? Arch Pediatr Adolesc Med. 2012:1–7. doi: 10.1001/2013.jamapediatrics.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Wong DF. Alterations of central dopamine receptors before and after gastric bypass surgery. [Research Support, N.I.H., Extramural] Obes Surg. 2010;20(3):369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- Stein RI, Kenardy J, Wiseman CV, Dounchis JZ, Arnow BA, Wilfley DE. What's driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. Int J Eat Disord. 2007;40(3):195–203. doi: 10.1002/eat.20352. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M. Binge eating among children and adolescents. In: Jelalian E, Steele R, editors. Handbook of Child and Adolescent Obesity. New York: Springer Publishers; 2008. pp. 41–57. [Google Scholar]
- Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, Keil M, Yanovski JA. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117(4):1203–1209. doi: 10.1542/peds.2005-1329. 10.1542/peds.2005-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, Yanovski JA. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr. 2009;89(3):738–745. doi: 10.3945/ajcn.2008.26886. 10.3945/ajcn.2008.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, Yanovski JA. A prospective study of pediatric loss of control eating and psychological outcomes. [Research Support, N.I.H., Extramural] J Abnorm Psychol. 2011;120(1):108–118. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Theim KR, Yanovski SZ, Bassett AM, Burns NP, Ranzenhofer LM, Yanovski JA. Validation of the emotional eating scale adapted for use in children and adolescents (EES-C) Int J Eat Disord. 2007;40(3):232–240. doi: 10.1002/eat.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord. 2009;42(1):26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. Journal of Consulting and Clinical Psychology. 2004;72(1):53–61. doi: 10.1037/0022-006X.72.1.53. 2004-10364-005 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch CF, Agras WS. Do emotional states influence binge eating in the obese? Int J Eat Disord. 1996;20(3):271–279. doi: 10.1002/(SICI)1098-108X(199611)20:3<271::AID-EAT6>3.0.CO;2-L. 10.1002/(SICI)1098-108X(199611)20:3<271::AID-EAT6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Terry PC, Lane AM, Fogarty GJ. Construct Validity of the Profile of Mood States-Adolescents for use with Adults. Psychology of Sport and Exercise. 2003;4:125–139. [Google Scholar]
- Terry PC, Lane AM, Lane HJ, Keohane L. Development and validation of a mood measure for adolescents. J Sports Sci. 1999;17(11):861–872. doi: 10.1080/026404199365425. [DOI] [PubMed] [Google Scholar]
- Vannucci A, Tanofsky-Kraff M, Shomaker LB, Ranzenhofer LM, Matheson BE, Cassidy OL, Yanovski JA. Construct validity of the Emotional Eating Scale Adapted for Children and Adolescents. Int J Obes (Lond) 2012;36(7):938–943. doi: 10.1038/ijo.2011.225. 10.1038/ijo.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT, Boudreau G. Laboratory studies of binge eating disorder. [Research Support, U.S. Gov't, P.H.S. Review] Int J Eat Disord. 2003;34(Suppl):S30–S38. doi: 10.1002/eat.10203. [DOI] [PubMed] [Google Scholar]
- Wegner KE, Smyth JM, Crosby RD, Wittrock D, Wonderlich SA, Mitchell JE. An evaluation of the relationship between mood and binge eating in the natural environment using ecological momentary assessment. Int J Eat Disord. 2002;32(3):352–361. doi: 10.1002/eat.10086. [DOI] [PubMed] [Google Scholar]
- Wolfe BE, Baker CW, Smith AT, Kelly-Weeder S. Validity and utility of the current definition of binge eating. Int J Eat Disord. 2009;42(8):674–686. doi: 10.1002/eat.20728. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ, Leet M, Yanovski JA, Flood M, Gold PW, Kissileff HR, Walsh BT. Food selection and intake of obese women with binge-eating disorder. Am J Clin Nutr. 1992;56(6):975–980. doi: 10.1093/ajcn/56.6.975. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiol Behav. 2006;87(4):789–793. doi: 10.1016/j.physbeh.2006.01.014. 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]