Abstract
Citrus huanglongbing (HLB) is a highly destructive disease of citrus presumably caused by ‘ Candidatus Liberibacter asiaticus ’ (Las), a gram-negative, insect-transmitted, phloem-limited α-proteobacterium. Although almost all citrus plants are susceptible to HLB, reports have shown reduced susceptibility to Las infection in lemon ( Citrus limon ) plants. The aim of this study is to identify intra-species specific molecular mechanisms associated with Las-induced responses in lemon plants. To achieve this, comparative 2-DE and mass spectrometry, in addition to Inductively Coupled Plasma Spectroscopy (ICPS) analyses, were applied to investigate differences in protein accumulation and the concentrations of cationic elements in leaves of healthy and Las-infected lemon plants. Results showed a differential accumulation of 27 proteins, including an increase in accumulation of starch synthase but decrease in the production of photosynthesis-related proteins in Las-infected lemon plants compared to healthy plants. Furthermore, there was a 6% increase (P > 0.05) in K concentration in leaves of lemon plants upon Las infection, which support results from previous studies and might represent a common response pattern of citrus plants to Las infection. Interestingly, contrary to reports from prior studies, this study showed a general reduction in the production of defense-related pathogen-response proteins but a 128% increase in Zn concentration in lemon plants in response to Las infection. Taken together, this study sheds light on general and intra-species specific responses associated with the response of citrus plants to Las.
Introduction
Citrus Huanglongbing (HLB), previously known as citrus greening disease is arguably the most devastating disease threatening citrus production worldwide and all cultivated citrus species are susceptible [1–3]. The disease, which was discovered in Asian countries in the 1870s, has since been identified in other parts of the world including the U.S.A., Brazil, Saudi Arabia and South Africa [3]. ‘Ca. Liberibacter asiaticus ’ (Las), a Gram-negative, non-culturable, psyllid-transmissible and phloem-limited α-proteobacterium, is currently the most prevalent causal agent associated with the global presence of HLB [1,3]. Containment of HLB has remained elusive largely due to the paucity of information on the molecular and physiological processes involved in the plant–pathogen interactions associated with the disease.
When microbial pathogens invade a plant, a series of molecular responses are triggered by the plant through defense response pathways in an attempt to protect itself from the pathogenic activities of the microbe. Several reports on plant-microbe interactions suggest that there are highly conserved pathogen-derived molecules, such as flagellin, that generate a generalized or non-host specific molecular response in plants, referred to as nonhost response [4–6]. This response involves an increased production of defense-related proteins such as cysteine proteases, thaumatin-like proteins, chitinases, superoxide dismutase, peroxidases and catalase in the host plants [7]. Additionally, plant-microbe interactions also involves host-to-microbe specific responses in a gene-for-gene manner, whereby a specific pathogen-derived avirulent (Avr) protein is recognized by a specific plant resistant (R) protein as the case of Pseudomonas syringae AvrRPM1 and AvrRpt2 proteins which are respectively recognized by the products of the Arabidopsis RPM1 and RPS2 resistance genes [8,9]. However, a high-throughput transcriptional analysis study of innate responses of Arabidopsis plants showed a strong overlap in the production of nonhost and host-specific proteins in the presence of flagellin, highlighting the power of high-throughput technologies in identifying overall host responses to plant pathogens while noting that understanding plant-microbe interactions is still a complex subject [10].
Intra-species differences in the susceptibility or tolerance of plants to pathogens further complicates the difficulty in understanding plant-microbe interactions as was demonstrated in a transcriptomic study involving ‘Cleopatra’ mandarin ( Citrus reticulata ) plants and US-897 hybrid ( Citrus reticulata Blanco x Poncirus trifoliate ) plants, which are susceptible and tolerant to Las, respectively [11]. Folimonova et al. [12] classified 30 citrus genotypes ranging from sensitive to tolerant according to their response to HLB and two lemon ( Citrus limon ) varieties “Volkamer” and “Eureka” were classified as moderately tolerant and tolerant, respectively. Additionally, a study by Zhang et al. [13] demonstrated that HLB-affected lemon scions had a higher titer of Las, higher survival rate and pathogen transmission rate than pomelo ( Citrus maxima ) scions. Although several studies have investigated the molecular mechanisms associated with the response of citrus plants to Las, such studies have largely focused on sweet orange (Citrus sinensis) plants [14–19]. Thus, there is limited information in the literature about internal factors associated with the response of other citrus plants particularly those demonstrably tolerant to Las, such as lemon, which could be critical in the development of Las tolerant and resistant citrus varieties to combat HLB.
The importance of sustainable agriculture has become an important topic and there has been a lot of focus on moving away from the classical use of pesticides towards more environmentally-friendly strategies to control plant disease. In particular, it has been suggested that adopting strategic nutritional practices can help reduce plant susceptibility to disease if not completely but at least to a level at which there is a reduced need for other more expensive and less environmentally-friendly practices such as pesticide use [20,21]. Cationic plant nutrients such as Ca, K, Mg, Fe, Cu, Mn, and Zn have been shown to play important roles in plant susceptibility or tolerance to pathogen infection although the effects can sometimes be mixed largely due to the fact that the molecular processes involved in nutrient-disease relationships in plants are not well understood. For example Zn, which is important in the activation of Cu/Zn superoxide dismutase, has been shown to have mixed effects on disease severity by increasing in some cases, while decreasing in other cases, susceptibility to disease [22,23]. Importantly, physiological symptoms of HLB have been suggested to resemble that of Zn-deficiency [24] and the results from a recent study suggests that the availability of Zn will affect the growth of Las in citrus plants [25].
Although high-throughput transcriptional analysis have been successfully used to identify global effects of Las infection on gene expression in citrus plants [11,17,18,26], it is important to note that high-throughput proteomics studies represent the final stage of gene expression and are directly associated with gene expression at a functional level as demonstrated in a recent HLB-related study [15]. A recent proteomics study by our group showed that Las infection induces several defense-related proteins such as lectin-like proteins, miraculin-like proteins, Cu/Zn superoxide dismutase, and chitinases in grapefruit plants and also showed a novel Las-mediated correlated increased-accumulation of granule-bound starch synthase and K in same plants [27]. Since starch synthase requires K for its activation, it is our assumption that this might represent a unique biochemical response of citrus plants to Las infection. To confirm this, a proteomic approach involving 2-DE and mass spectrometry techniques was employed to analyze the protein expression profiles of leaves of healthy or Las-infected lemon plants. Additionally, ICP spectroscopy was applied to investigate the effect of Las-infection on the nutrient status of healthy or Las-infected lemon plants. The goal of this study was to identify a potential consensus pattern in the biochemical responses of citrus plants to Las as well as elucidate interrelationships between protein expression and nutrient concentration levels that could be specifically associated with HLB disease development. Such information would advance our understanding of the molecular and physiological mechanisms involved in host–pathogen interactions.
Results
Effect of Las-infection on protein expression
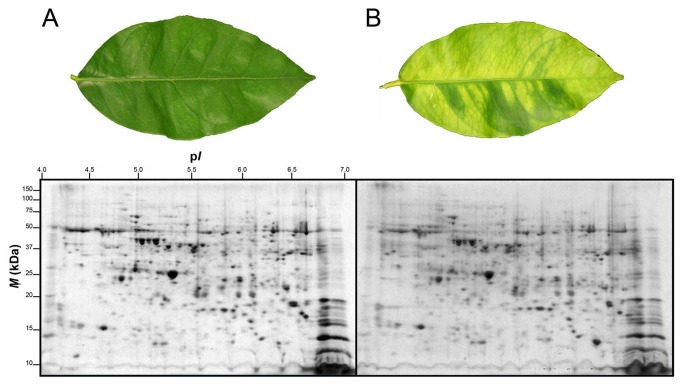
Leaves of lemon plants infected by Las showed visible HLB-associated symptoms such as blotchy mottling and chlorosis compared to healthy leaves (Fig. 1). However, Las infection had no significant effect on total protein yield in leaves of lemon plants and an average total protein yield of 12.2 mg g-1 or 13.9 mg g-1 was obtained from leaves of healthy or Las-infected leaves, respectively (Table 1). A high resolution of total protein separation in a pI range between 4 and 7 and molecular mass between 10 and 150 kDa was observed in 2-DE gels from extracted total leaf proteins of healthy or Las-infected lemon plants (Fig. 1). Using PDQuest analysis software we detected over 700 protein spots per gel and over 400 reproducible spots within replicate gels (Table 1). Mass spectrometry analysis via MALDI-TOF- or LC-MS, identified 42 out of 65 protein spots that were differentially produced in response to Las infection. Multiple protein spots matched to the same protein, especially spots around the 65 kDa region and within a pI range of 4.5 and 5.2, which all matched to granule-bound starch synthase. This could be due to a variety of factors including multimerism/protein isoforms, difference in maturation state, degradation and/or post-translational modifications [28,29]. Thus, based on identical protein matches and proximity of spots on gels, the 42 spots identified were summarized into 34 spots (Figure 2). Among these 34 spots, 10 showed higher-accumulation while 24 showed lower-accumulation in Las-infected lemon plants compared to healthy plants (Figure 3A) suggesting an up- or down-regulation of the 10 or 24 protein spots, respectively, due to Las infection. Categorization of proteins according to functional groups showed that majority of the Las-responsive protein spots (26.5%) matched to proteins involved in pathogen response whereas the functions of 14.7% of the identified Las-responsive proteins is unknown (Figure 3B). A magnified view of the profiles of identified spots in representative gels from each treatment group is shown in Figure 4.
Figure 1. 2-DE separation of proteins extracted from leaves of healthy of Las-infected lemon plants.
(A) Representative leaf and gel containing extracted proteins separated via 2-DE of a healthy lemon plant. (B) Representative leaf and gel containing extracted proteins separated via 2-DE of a Las-infected lemon plant. Two-year old healthy plants were either graft-inoculated with side shoots from PCR-confirmed Las-infected bud sticks or uninoculated and leaf samples were analyzed at six months post-inoculation. A sum of 200 µg of total protein was separated according to charge on a pH 4-7 IpG strip and according to mass on 8-16% gradient SDS-polyacrylamide Tris-HCl gels. Protein spots were visualized by staining with Coomassie Brilliant Blue (CBB). M r, relative molecular mass; pI, isoelectric point.
Table 1. Protein extraction and 2-DE separation result parameters for total leaf proteins from healthy or Las-infected lemon plants.
| Parameters | Lemon leaves |
|
|---|---|---|
| Healthy | Infected | |
| Protein yielda (mg g-1) | 12.2 ± 2.0 | 13.9 ± 2.8 |
| Number of detected spots per gel | 763 ± 26 | 806 ± 23 |
| Number of matched spots in replicate gels | 461 ± 38 | 496 ± 25 |
| Number of matched spots in all gels | 188 | 188 |
Data represents Means ± SD.
Protein extraction was repeated three times per sample with three replicate plant samples per treatment.
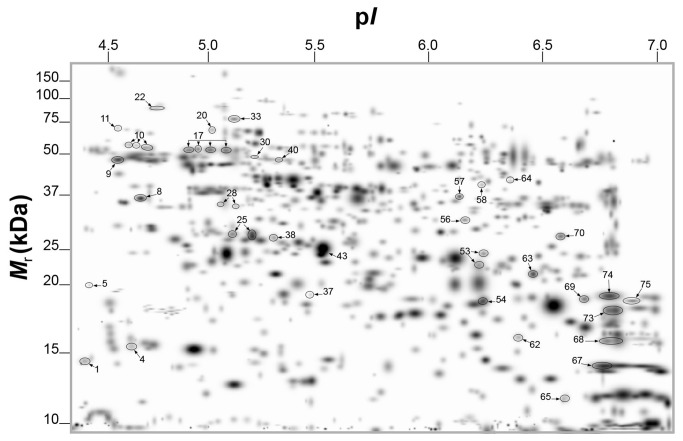
Figure 2. PDQuest-generated master gel image showing the general spot pattern of matched protein spots from the total leaf proteome of healthy or Las-infected lemon plants.
Labeled spots were differentially produced in response to Las-infection and described in Table 1. A sum of 200 µg of total protein was separated according to charge on a pH 4-7 IpG strip and according to mass on 8-16% gradient SDS-polyacrylamide Tris-HCl gels. Protein spots were visualized by staining with Coomassie Brilliant Blue (CBB). M r, relative molecular mass; pI, isoelectric point.
Figure 3. Categorization of differentially produced proteins in lemon plants in response to Las infection.
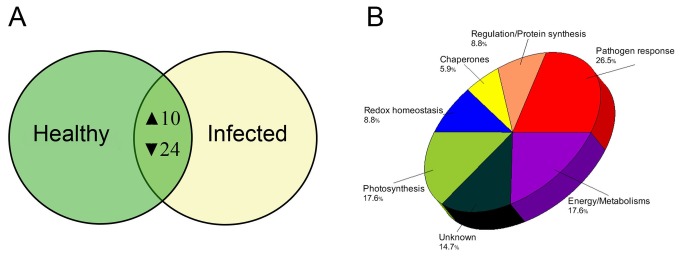
(A) Venn diagram showing the number of protein spots that showed higher-accumulation (▲) or lower-accumulation (▼) in infected lemon plants compared to healthy plants. (B) Functional category distribution of differentially produced protein spots from comparing 2-DE gel images of the total leaf proteome of healthy or Las-infected lemon plants.
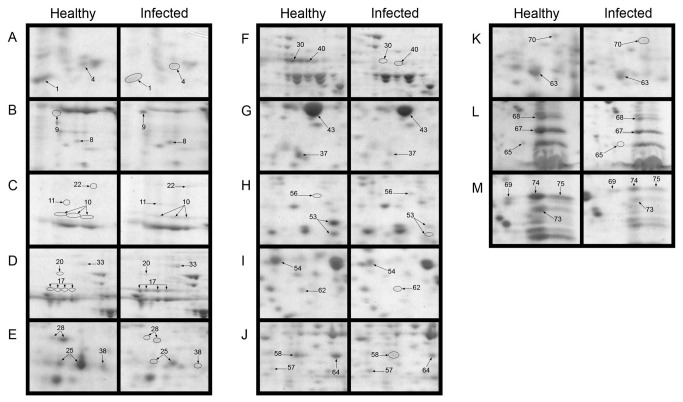
Figure 4. Differentially produced protein spots from 2-DE analysis of total leaf proteins from healthy or Las-infected lemon plants.
Panels A-M show magnified views of protein spots in representative 2-DE gels containing separated total proteins from leaves of healthy or Las-infected lemon plants. Labeled spots showed significant changes and correspond to the spots presented in in Figure 2 and Tables 2 and 3. Two-year old healthy plants were either graft-inoculated with side shoots from PCR-confirmed Las-infected bud sticks or uninoculated and leaf samples were analyzed at six months post-inoculation. A sum of 200 µg of total protein was separated according to charge on a pH 4-7 IpG strip and according to mass on 8-16% gradient SDS-polyacrylamide Tris-HCl gels. Protein spots were visualized by staining with Coomassie Brilliant Blue (CBB). M r, relative molecular mass; pI, isoelectric point.
The expression of stress response-related proteins such as chaperones, redox homeostasis-related proteins and pathogen response-related proteins was significantly affected by Las infection. Interestingly, pathogen response-related proteins such as lectin-related proteins (Table 2, spots 25, 37, and 38), class I chitinase (Table 2, spot 28), and miraculin-like proteins (Table 2, spots 53, 54, 65, 69 and 75) were all down-regulated upon Las infection. Results also showed that catalase (Table 2, spot 30) and a thioredoxin-like protein (Table 2, spot 70), which are involved in redox homeostasis, were down-regulated in Las-infected plants compared to healthy plants. In contrast, chaperones HSP 70 (Table 2, spot 20) and protein disulfide isomerase (spot 33) as well as an isoflavone reductase-related protein (Table 2, spot 56) were up-regulated in lemon plants in response to Las infection.
Table 2. Differentially produced proteins in leaves of lemon plants infected with ‘ Candidatus Liberibacter asiaticus ’ (Las).
| Spota | Δb | Protein function/namec | Accession #c | Theoreticald
|
Se | Mf | Pg | ||
|---|---|---|---|---|---|---|---|---|---|
| M r | pI | ||||||||
| Pathogenresponse | |||||||||
| 25 | -2.18 | Lectin-related protein precursor | gi|11596188 | 29272 | 5.10 | 90 | 8 | 38 | |
| 28h | -6.99 | Acidic class I chitinase | gi|23496445 | 36735 | 4.81 | 52 | 8 | 22 | |
| 37 | -4.01 | Lectin-related protein precursor | gi|11596188 | 20359 | 5.32 | 69 | 7 | 47 | |
| 38h | -2.70 | Lectin-related protein precursor | gi|11596188 | 29272 | 5.10 | 61 | 7 | 38 | |
| 53h | -5.17 | Miraculin-like protein 1 | gi|87299375 | 25585 | 8.11 | 61 | 6 | 32 | |
| 54 | -3.09 | Putative miraculin-like protein 2 | gi|119367468 | 23610 | 8.18 | 98 | 8 | 45 | |
| 65 | -2.87 | Miraculin-like protein 2 | gi|87299377 | 24525 | 6.88 | 93 | 6 | 32 | |
| 69 | -3.97 | Putative miraculin-like protein 2 | gi|119367468 | 23610 | 8.18 | 109 | 7 | 43 | |
| 75 | -10.71 | Putative miraculin-like protein 2 | gi|119367468 | 23610 | 8.18 | 110 | 8 | 49 | |
| Chaperones | |||||||||
| 20 | On | Heat shock protein 70 | gi|6911549 | 73678 | 5.10 | 70 | 15 | 29 | |
| 30 | -6.71 | Catalase | gi|32526568 | 57669 | 6.64 | 112 | 15 | 43 | |
| 33h | 1.52 | Protein disulfide isomerase | gi|255578860 | 65701 | 4.64 | 60 | 6 | 17 | |
| Redoxhomeostasis | |||||||||
| 56 | On | Isoflavone reductase-related protein | gi|3243234 | 33862 | 5.92 | 105 | 8 | 40 | |
| 70h | -3.38 | Thioredoxin-like protein, chloroplastic | gi|225459760 | 39336 | 8.59 | 56 | 9 | 25 | |
Protein spot numbers are arranged in chronological order and correspond to the numbers given in Figure 1
Protein fold change in Las-infected leaves compared to healthy leaves; minus sign (-), decrease; On, undetected in healthy leaves.
Protein function, name and accession number were determined by using http:/www.ncbi.nlm.nih.gov/BLAST/.
Theoretical molecular mass (M r) and isoelectric point (pI) were calculated by http:/www.expasy.org/.
Mascot score.
Number of matched peptide masses.
Percent sequence coverage.
Protein identification confirmed by LC-MS/MS.
i Protein identification confirmed by MALDI-TOF-MS/MS.
A general down-regulation was observed in proteins associated with photosynthesis such as ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) (Table 3, spots 1 and 67), oxygen evolving enhancer proteins (Table 3, spots 43, 68, and 74), and a Photosystem (PS) I reaction center subunit II (Table 3, spot 73). On the other hand, there was a significant up-regulation of granule-bound starch synthase (Table 3, spots10 and 17), which is important in starch metabolism. Other metabolism-related proteins that were differentially produced in response to Las infection were aconitate hydratase (Table 3, spot 22), which was up-regulated and a nucleoside diphosphate kinase (Table 3, spot 62), which was down-regulated. Furthermore, proteins potentially involved in gene regulation such as a nucleosome-binding protein (Table 3, spot 9) and a polyadenylate-binding protein (Table 3, spot 11) in addition to glutamine synthase (Table 3, spot 57), which is associated with amino acid synthesis, and an abhydrolase domain-containing protein (Table 3, spot 8) were up-regulated in response to Las infection. However, glutathione transferase (Table 3, spot 63) was down-regulated.
Table 3. Differentially produced proteins in leaves of lemon plants infected with Las (Cont’d).
| Spota | Δb | Protein function/namec | Accession #c | Theoreticald
|
Se | Mf | Pg | ||
|---|---|---|---|---|---|---|---|---|---|
| M r | pI | ||||||||
| Photosynthesis | |||||||||
| 1 | -2.89 | Ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit | gi|24940138 | 20521 | 9.16 | 98 | 8 | 42 | |
| 43 | -1.58 | Oxygen evolving enhancer protein 1 | gi|326467059 | 29262 | 5.32 | 109 | 13 | 54 | |
| 67 | -1.64 | Ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit | gi|24940138 | 20521 | 9.16 | 116 | 14 | 74 | |
| 68 | -1.64 | Oxygen-evolving enhancer protein 3-2, chloroplastic | gi|225469185 | 24927 | 9.60 | 109 | 9 | 41 | |
| 73 | -2.05 | PSI reaction center subunit II | gi|157678730 | 22606 | 9.76 | 104 | 8 | 47 | |
| 74 | -3.05 | Oxygen-evolving enhancer protein 2, chloroplastic | gi|225446775 | 26777 | 8.63 | 116 | 7 | 37 | |
| Energy/Metabolisms | |||||||||
| 10h | On | Granule-bound starch synthase | gi|223029784 | 67320 | 8.56 | ||||
| 17h | 8.48 | Granule-bound starch synthase | gi|223029784 | 67320 | 8.56 | ||||
| 22i | On | Aconitate hydratase 2 | gi|285309969 | 109307 | 7.34 | 41 | 16 | 19 | |
| 62 | -1.60 | Nucleoside diphosphate kinase, putative | gi|255540363 | 14819 | 6.92 | 89 | 8 | 68 | |
| Regulation/Proteinsynthesis | |||||||||
| 9i | On | Nucleosome-binding protein, putative | gi|255553181 | 36140 | 4.66 | ||||
| 11i | On | Polyadenylate-binding protein, putative | gi|255555393 | 70243 | 8.09 | ||||
| 57 | 2.63 | Glutamine synthase | gi|211906462 | 39404 | 5.78 | 103 | 11 | 45 | |
| 63 | -1.52 | DHAR class glutathione transferase | gi|283135906 | 23962 | 6.18 | 130 | 12 | 62 | |
| Unknown | |||||||||
| 4 | -2.10 | Uncharacterized protein | gi|225426158 | 44041 | 5.98 | 79 | 10 | 35 | |
| 5 | -4.63 | Uncharacterized protein | gi|225426158 | 44041 | 5.98 | 64 | 11 | 38 | |
| 8h | 1.94 | Abhydrolase domain-containing protein | gi|225456828 | 43339 | 9.16 | 47 | 6 | 29 | |
| 40 | -1.98 | Uncharacterized protein At5g39570-like | gi|356550983 | 46695 | 5.00 | 90 | 12 | 37 | |
| 58 | -2.53 | Conserved hypothetical protein | gi|255558602 | 80625 | 5.65 | 69 | 15 | 30 | |
| 64 | -2.72 | Conserved hypothetical protein | gi|255558602 | 80625 | 5.65 | 116 | 15 | 29 | |
Legend same as Table 2
Effect of Las-infection on nutrient status
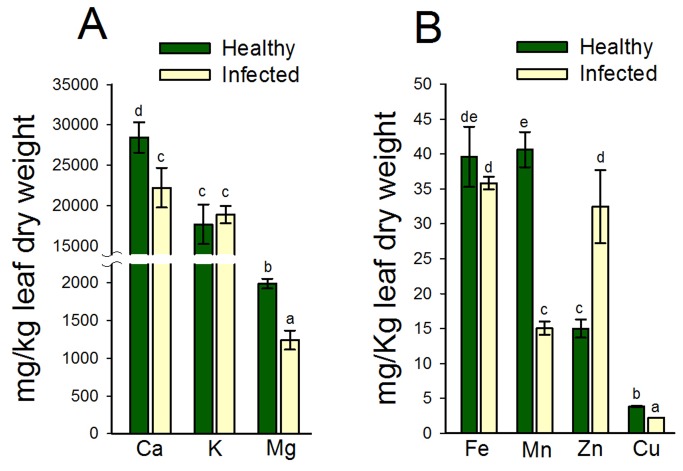
Plant cations are actively involved in gene regulation and several metabolically active proteins form co-enzymes with cations. This prompted an investigation on the effects of Las-infection on the nutrient status of lemon plants in tandem with our proteomic analyses. Results showed that there was a 21.8% and 37.6% reduction in Ca and Mg concentrations in leaves of Las-infected lemon plants compared to healthy plants (Figure 5). However, there was a 6% (P > 0.05) increase in K concentration in leaves of Las-infected lemon plants compared to healthy plants (Figure 5).
Figure 5. The leaf-nutrient concentrations of healthy or Las-infected lemon plants.
(A) Macronutrients: calcium (Ca), potassium (K) and magnesium (Mg); (B) Micronutrients: iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu). Two-year old healthy plants were either graft-inoculated or uninoculated with PCR-confirmed Las-infected bud sticks and leaf samples were analyzed 6 months post-inoculation. Bars within a plant group with the same lower case letter are not significantly different from each other (P > 0.05).
Further analysis of micronutrient concentrations showed that Las-infection resulted in a 9%, 62.9% and 41.3% decrease in the concentrations of Fe, Mn, and Cu in comparison to healthy plants (Figure 5). Interestingly, there was a 128% increase in the Zn concentration of leaves of Las-infected lemon plants compared to healthy plants (Figure 5).
Discussion
Huanglongbing is a highly destructive disease of citrus and all commercially grown cultivars of citrus species and citrus relatives are susceptible to the disease. Currently, no effective disease control is available largely due to the limited understanding of the molecular and physiological processes associated with the general response of citrus plants to Las infection especially those responsible for intra-species differences in susceptibility [12,13,30,31]. A recent study by our group elucidated novel molecular and nutritional responses of grapefruit plants upon Las infection [27]. Thus, the goal of this study was to identify a potential consensus pattern of biochemical response of citrus plants to HLB by elucidating the molecular and physiological responses of lemon plants to Las infection.
Las-mediated down-regulation of photosynthesis and pathogen response-related proteins
During periods of biotic and abiotic stress in plants, photosynthesis is typically inhibited and the regulation of gene expression is channeled towards the production of stress-response related factors at the expense of “housekeeping” proteins [32,33]. This is consistent with results from a previous study by Albrecht and Bowman on sweet orange plants [26] as well as results from our recent study on grapefruit plants [27], which showed a Las-mediated down-regulation of photosynthesis-related gene transcripts and photosynthesis-related proteins, respectively, as part of the molecular mechanisms associated with citrus response to HLB. These same studies and two others on citrus plants also showed a Las-mediated up-regulation of defense-related transcripts or proteins, such as catalase, chitinase, lectin-related proteins and miraculin-like proteins [15,17,26,27], suggesting that a Las-mediated down-regulation of photosynthesis but up-regulation of defense-response processes could represent a consensus pattern of response of citrus to HLB.
Thus, it was not surprising that all photosynthesis-related proteins identified in our current study were down-regulated in lemon plants in response to Las infection (Table 3). However, except for HSP70, protein disulfide isomerase and an isoflavone reductase-related protein, which were up-regulated, all other stress/pathogen-response related proteins identified in this study such as catalase, chitinase, lectin-related proteins and miraculin-like proteins were markedly down-regulated in lemon plants upon Las infection (Table 2). This might suggest a pathogenicity scheme for Las in lemon plants since prior studies have shown that microbial pathogens can suppress host defense response processes to facilitate invasion. Hauck et al. [34] demonstrated that Pseudomonas syringae type III secretion system-related AvrPto protein suppresses or down-regulates the expression of a set of Arabidopsis genes that encode putative cell wall and defense proteins in a salicylic acid-independent manner. Additionally, Bouarab et al. [35] showed that the fungal pathogen Septoria lycopersici produces tomatinase, which degrades saponin in Nicotiana benthamiana and suppresses the production of defense response proteins.
Catalase, chitinase, lectin-related proteins and miraculin-like proteins form part of the nonhost or innate response of plants to pathogens and it is unclear why all of these important defense-related proteins are down-regulated in lemon plants in response to Las infection. However, we suggest that since these proteins are not specific for Las, reducing their expression levels could be part of an energy conservation mechanism for an efficient utilization of resources by lemon plants upon Las-induced stress. Additionally, lectin-like proteins play a role in vascular tissue differentiation [36] and are involved in the plugging of phloem sieve plates in response to wounding and defense against pathogens and insects [37]. Thus, while lectin-mediated phloem-blockage might help limit bacterial spread, it could also limit the flow of photosynthates to other parts of the plant, potentially making those malnourished parts more vulnerable to disease. It appears that response to Las in lemon plants involves a scenario whereby the sieve-tubes are not clogged, which facilitates the flow of photosynthates. This is supported by a recent study by Fan et al. [16] that demonstrated that phloem transport was less inhibited in rough lemon leaves compared to sweet orange leaves. However, this could also enhance systemic spread and higher titers of Las in lemon tissues as was shown by Zhang et al. [13].
Las-mediated up-regulation of starch synthase and aconitate hydratase in lemon plants
This study identified 10 protein spots that were up-regulated in lemon plants in response to Las-infection, which included multiple isoforms of granule-bound starch synthase around the 65 kDa region and within a pI range of 4.5 and 5.2. The increased-accumulation of starch in plant tissues during HLB disease development has been previously demonstrated [38,39], but the molecular mechanisms involved are yet to be resolved.
Starch biosynthesis is controlled by four major enzymes namely: ADPase, starch synthase, granule-bound starch synthase, and starch debranching enzyme. ADPase catalyzes the rate limiting interconversion of glucose-1-phosphate to ADP-glucose. ADP-glucose is then polymerized into amylopectin by multiple isoforms of starch synthase or to amylose by granule-bound starch synthase [40]. Starch debranching enzyme together with starch branching enzyme regulates the extensive branching of amylopectin. Transcriptomics studies by Albrecht and Bowman [26] and Kim et al. [17] showed that the most up-regulated starch anabolism-related gene transcript in HLB-affected sweet orange (Citrus sinensis) plants was ADP-glucose pyrophosphorylase (ADPase). However, at the protein level, the fact that our recent study on grapefruit plants [27] and this study on lemon plants only showed a significant increase in the accumulation of granule-bound starch synthase compared to other starch biosynthesis-associated enzymes, suggests that the enzyme might play a key post-transcriptional role in HLB-mediated increase in starch accumulation in citrus plants. It is also important to mention that the occlusion of phloem tubes by aggregation of Las bacteria can result in the accumulation of starch. Additionally, the accumulation of starch could result in an inhibition of photosynthesis via a negative feedback mechanism.
Besides granule-bound starch synthase, another metabolism-related protein that was up-regulated in response to Las-infection was aconitate hydratase 2 (Table 3). Aconitate hydratase 2 is a mitochondrial enzyme with a citrate hydro-lyase catalytic activity, which is involved in several metabolic processes especially the reversible isomerization of citrate and isocitrate as part of the tricarboxylic acid (TCA) cycle. An up-regulation of aconitate hydratase was shown in rice (Oryza sativa) plants under Cd stress [41] and in Arabidopsis thaliana seedlings under phosphate deficiency [42]. Durand et al. [43] and Kieffer et al. [44] showed a patterned response to stress in poplar plants, whereby respiration and glucose catabolism-related proteins are up-regulated while proteins involved in photosynthesis are down-regulated. Consistent to their results, we observed a general down-regulation of photosynthesis-related proteins in addition to an up-regulation of aconitate hydratase in response to Las-infection (Table 3). Although the mechanisms involved in this patterned-response are not well understood, López-Millán et al. [45] suggested that a stress-mediated slowing down of photosynthetic carbon fixation would limit carbon availability, which might cause plants to induce processes involved in carbohydrate catabolism resulting in a remobilization of carbon/energy storage compounds.
Las-mediated up-regulation of regulatory and amino acid synthesis-related proteins in lemon plants
An up-regulation of a nucleosome-binding protein and a polyadenylate-binding protein was observed in Las infected plants compared to healthy plants (Table 3). Nucleosome-binding proteins and polyadenylate-binding proteins are regulatory proteins, which are directly involved in DNA replication, transcription, and transcriptional/post-transcriptional modification of gene expression [46,47]. Although reports on pathogen/biotic stress-mediated up-regulation of these proteins in plants is limited, under stress conditions, High Mobility Group B (HMGB) proteins, which are nuclear proteins that can bind to nucleosomes in a non-sequence specific manner, are known to be secreted into the extracellular milieu and function as pro-apoptotic proteins [47]. In addition, Minard et al. [48] showed that Asf1, a nucleosome-interacting protein in yeast, promotes the expression of DNA damage response genes during the S phase. Kozubowski et al. [49] showed that Pub1, a poly (A)-binding protein, is a subunit of the catalytic complex of calcineurin, which is a calcium-calmodulin-activated phosphatase important in cellular responses to stress. Thus, it should be promising to further investigate and characterize the putative nucleosome-binding protein and putative polyadenylate-binding protein identified in this study, especially when they represent the first observation of a potential involvement in the response of citrus plants to Las-infection.
Glutamine synthase, which catalyzes the production of glutamine from glutamate and ammonia and is actively involved in nitrogen metabolism, was also up-regulated in response to Las-infection. Purcino et al. [50] showed a significant up-regulation of glutamine synthase in sweet orange (Citrus sinensis cv. Pera) plants infected with the bacterium, Xyllela fastidiosa , and further showed that a disturbance in the nitrogen metabolism of the host plant could play a role in disease development.
Las-mediated modulation of nutrient homeostasis in lemon plants
Disease symptoms typically reflect the altered nutritional status of plants and nutrient-disease interactions in plant systems are well documented [20]. A malfunctioning or blocked vascular system such as that implicated in HLB-disease development [17,26] can induce a systemic or localized nutrient deficiency or sufficiency, respectively. Nonetheless, diseased plants have generally been shown to have reduced nutrient concentrations compared to healthy plants [20], which is supported by our observation of reductions in the concentrations of Ca. Mg, Fe, Mn and Cu in Las-infected lemon plants compared to healthy plants (Figure 5).
Plant nutrients are actively involved in gene regulation and several metabolically active proteins depend on the availability for specific nutrients for activation. Starch synthase depends on K for its activation and in this study, we observed a Las-mediated 6% increase (P > 0.05) in K concentration as well as an increase in the accumulation of starch synthase in lemon plants. This is consistent with results from our earlier study that showed a coordinated increase in K concentration and starch synthase accumulation in leaves of Las-infected grapefruit plants compared to healthy plants [27]. Pathogen-mediated disruption of membrane permeability could lead to electrolyte leakage [20], which could represent a virulence mechanism by Las to induce host K accumulation to sustain increased starch production, thus providing a steady source of carbon and other nutrition for this phloem-limited bacterium while the host cells are deprived of these nutrients.
Furthermore, symptoms of HLB are very similar to those of Zn-deficiency and citrus plants have generally shown reduced concentrations of Zn in tissues due to Las infection [3,24,51]. It was therefore surprising to see a 128% increase in Zn concentrations in lemon leaves in response to Las infection (Figure 5), especially when further investigations of the nutritional content of other citrus and citrus-related plants showed a reduction in leaf-Zn concentrations (unpublished data). Additionally, a recent study involving ‘Navel’ sweet orange scions on Cleopatra mandarin rootstocks, showed a slight increase (P > 0.05) in Zn concentration in leaves due to Las infection [19]. Together, these observations suggest that the effect of Las on the nutritional status of citrus plants remains unresolved and the intra-species differences in nutrient homeostatic response to Las infection could play a role in differences in susceptibility to Las [12,13].
The accumulation of metals, such as Zn, has been suggested to be part of an “elemental defense” mechanism in plants [52]. Fones et al. [53] showed a close correlation between the accumulation of Zn, nickel (Ni), and cadmium (Cd) in leaves of Thlaspi caerulescens plants and the resistance of such plants to bacterial leaf spot caused by Pseudomonas syringae pv. maculicola. Coleman et al. [54] investigated the relative toxicities of eight metals, including Zn, which are commonly accumulated or hyperaccumulated by plants and indicated that the elemental defenses provided by metal accumulation in plants can be effective at concentrations far lower than previously hypothesized. It seems that the Las-mediated increase in Zn concentration could be complementary to the observed and earlier discussed Las-mediated down-regulation of defense-related proteins in lemon plants. Thus, when we combine our observation of a lack of a Las-mediated up-regulation in defense-related proteins with that of our observation of a Las-mediated 128% increase in Zn concentration in lemon leaves, it is tempting to propose that the active defensive response of lemon plants to Las infection might be more focused at the nutritional level rather than at the proteomic level.
Conclusions
This study showed that Las infection resulted in a down-regulation of photosynthesis-related proteins but an up-regulation of granule-bound starch synthase accompanied by a 6% increase (P > 0.05) in K concentration of lemon plants. All of which is consistent with reports from prior studies and might be part of a consensus pattern of biochemical response of citrus plants to Las infection. Additionally, the study identified potential intra-species specific responses of citrus to Las, particularly an observation that Las-infection facilitates a down-regulation of defense-related proteins accompanied by a 128% increase in Zn concentration in lemon plants. An interesting find that might provide information on the potential biochemical mechanisms associated with the Las-induced responses in lemon plants, which have been previously shown to display a significant level of tolerance to Las. Thus, the information provided in this study has shed more light on the molecular and physiological mechanisms involved in host response to HLB, which could be applicable towards (i) development of citrus plants with reduced susceptibility to Las, (ii) development of more efficient nutritional management programs to control the disease, (iii) a broader understanding of plant-microbe interactions.
Materials and Methods
Growth conditions and treatments
Plant growth was performed under controlled conditions in an insect-proof greenhouse at the U.S. Horticulture Research laboratory, U.S. Department of Agriculture, Fort Pierce, Florida. Two-year old Lemon plants ( C . limon cv. ‘Todo del Ano’ grafted onto C . paradisi cv. ‘Duncan’ rootstock) from the same progeny were either uninoculated or inoculated by side-grafting with 3-4 cm long PCR-confirmed HLB-affected lemon bud sticks [13]. The absence or presence of Las in plants, pre- or post-inoculation, respectively, was confirmed by quantitative real-time PCR as previously described [55]. Inoculated plants with confirmed Las presence were henceforth described as Las-infected.
Plants were arranged randomly on the greenhouse bench and kept under natural light conditions at a temperature of 23–30 °C. Plants were irrigated as needed and fertilized every three weeks using a water-soluble fertilizer mix, 20N-10P-20K (Peters Professional, The Scotts Company, Marysville, OH). Micronutrients (Micro Key Palm and Ornamental Formulation, Brandt Consolidated, Springfield, IL) and additional iron (Sequestrene 138 Fe, Becker Underwood, Ames, IA) were applied. Plants were pruned immediately after graft-inoculation to promote new leaf growth and HLB disease development.
Six months post-inoculation, 10-15 fully expanded leaves were collected from three individual plants each from the healthy or infected group of plants. At this stage the infected plants were symptomatic (blotchy mottle and yellow shoots) and PCR-positive for Las. Harvested leaves were immediately frozen in liquid nitrogen and stored at -80 °C until further analysis.
Protein extraction and quantification
The method used for total leaf protein analysis was modified after Nwugo and Huerta [41]. Leaves from individual plants were pooled and ground to a fine powder in liquid nitrogen using a freezer mill (6850 Freezer/Mill, Wolf Laboratories Ltd., UK). Approximately 0.4 g of leaf powder was transferred to sterile 5 mL polyallomer centrifuge tubes (Beckman Instruments Inc., USA) and suspended in 4.5 mL of chilled solution A [90% (v/v) acetone, 9.9993% (v/v) trichloroacetic acid (TCA), 0.0007% (v/v) Beta-mercaptoethanol]. The mixture was incubated overnight at -80 °C followed by centrifugation at 4 °C for 20 min at 36,000 g (Optima L-70K Ultracentrifuge, Beckman Coulter Inc., USA). The supernatant was decanted, and the pellet was washed at least three times until the supernatant was clear (not greenish) by resuspension in 4.5 mL of chilled solution B [98.53% (v/v) acetone, 1 mM polymethylsulphonylfluoride (PMSF), 2 mM EDTA, 0.0007% (v/v) Beta-mercaptoethanol], incubation for 1 h at -80 °C followed by centrifugation at 4°C for 20 min at 36,000 g. The whitish pellet or crude protein extract was then transferred into sterile eppendorf tubes and vacuum-dried (Vacufuge™, Eppendorf, Germany). The dry pellet, which could be stored indefinitely at -80 °C, was suspended in 0.5mL of rehydration/isoelectric focusing (IEF) buffer [8 M Urea, 50 mM DTT, 4% (w/v) CHAPS, 0.2% (v/v) 3/10 ampholytes, 0.002% (w/v) bromophenol blue] and incubated at room temperature (RT) for 30 min to solubilize proteins. Insoluble material was removed by centrifugation at RT at 14,000 g for 15 min and 5µL of the supernatant was prepared using the Compat-Able™ Protein Assay Preparation Reagent Set (Pierce, Rockford, IL, USA) for total protein quantification via bicinchoninic acid (BCA) assay (Pierce, Rockford, IL, USA). Total protein extraction and quantification process was repeated three times generating three analytical replicates per plant.
2-DE separation and image analysis
For first dimension electrophoresis or IEF, 11-cm long pH 4-7 ReadyStrip IPG strips (Bio-Rad, Hercules, CA, USA) were passively rehydrated overnight at RT with 0.2 mL of IEF buffer containing 1mg/mL of total solubilized proteins. Rehydrated strips were placed in a PROTEAN IEF cell (Bio-Rad) and IEF was performed at a current limit of 50 µA/per IpG strip at 10 °C, in the following steps: active rehydration at 250 V for 9 h; 250 V (linear) for 15 min; 8 kV (linear) for 3 h; and 10 kV (rapid) until a total 60 kVh for a combined total of approximately 70 kVh. Each focused IPG strip was equilibrated by soaking, with mild stirring, in 4 ml of equilibration base buffer 1 (EBB1) [8M urea, 2% (w/v) sodium dodecyl sulphate (SDS), 50 mM Tris-HCl (pH 8.8), 20% (v/v) glycerol, 1% (w/v) DTT] for 10 min, followed by soaking in 4 ml of EBB2 [same content as EBB1 except DTT was replaced with 2.5% (w/v) iodoacetamide (IAA)]. Second dimension electrophoresis was performed in 8-16% gradient SDS-polyacrylamide Tris-HCl gels (Criterion precast gels, Bio-Rad) in a twelve-gel cell system (Criterion Dodeca Cell, Bio-Rad). Protein spots were visualized by staining with Biosafe Coomassie. Stained gels were scanned (ScanMaker 9800XL, Microtek, USA) under identical conditions and stored in 0.02% NaN3 at 4 °C.
Gel images were analyzed using the PDQuest software package (version 8.0, Bio-Rad, USA). A total of 18 gels were analyzed representing three analytical replicates per plant and three replicate plants per treatment. The gels were assigned to two groups namely: healthy or infected. Gel spots were detected and matched so that a given spot had the same number across all gels. A master gel image containing matched spots across all gels was auto-generated. Extensive analysis using the “Landmark” tool was used to resolve missed matches and spot volumes were normalized according to the total gel image density as suggested by the PDQuest software package. An average spot volume was determined for each spot per group and pair-wise quantitative as well as statistical analysis sets were generated by comparing the average volume of a given spot between both treatments. Only spots that had ≥10-fold increase over background and present in at least six of the nine gels per treatment as well as showed ˃1.5 fold change (P < 0.05) compared to the other treatment group were considered to be differentially produced and further analyzed.
Trypsin digestion and mass spectrometry
Protein spots were manually excised (OneTouch Plus Spotpicker, The Gel company, USA), reduced with DTT, alkylated with IAA, and digested with mass spectrometry grade trypsin in the presence of ProteaseMAX™ Surfactant according to the manufacturer’s protocol (Promega, USA). Acetonitrile extraction was used to enhance peptide recovery. Tryptic-digests were generally analyzed by MALDI-TOF- or LC-MS/MS.
For MALDI-TOF-MS or MS/MS analysis (QSTAR XL Hybrid Quadrupole TOF LC/MS/MS System, Applied Biosystems, USA), the target plate was spotted with 2 µL of a 1:1 (v/v) mixture of tryptic-digest and matrix solution [10 mg/mL α-cyano-4-hydroxycinnamic acid (CHCA) in 50% ACN/ 0.1% TFA]. Mass spectra were acquired in positive TOF MS mode over the mass range of 800–4000 Da using 300 one-second cycles with MCA on. A mixture of Des-Arg1-Bradykinin (904.47), Angiotensin I (1296.68), Neurotensin (1672.92) and ACTH (2093.09, 2465.20, 3657.92) monoisotopic [M+H]+ mass standards (Anaspec, USA) were used for external calibration. Monoisotopic peaks with S/N >5 were selected as the peptide mass fingerprint (PMF) per spot. Parent ion spectra (MS/MS) was acquired over a mass range of 50–4000 Da using 300 one-second cycles with MCA on.
For LC-MS/MS analysis (Ultimate 3000 RLSCnano System linked to Velos LTQ Orbitrap, Thermo, Fisher), peptides were solubilized in 0.1% TFA and loaded on to a self-made fused silica trap-column of 100 µm X 2 cm packed with Magic C18 AQ (5µm bead size, 200Å pore size Michrom Bioresources, Inc.) and washed with 0.2% formic acid at a flow-rate of 10 µL/min for 5 min. The retained peptides were separated on a fused silica column of 75 µm X 50 cm self-packed with Magic C18 AQ (3µm bead size, 200Å pore size, Michrom Bioresources, Inc.) using a linear gradient from 4 to 45% B (A: 0.1% formic acid, B: 0.08% formic acid, 80% ACN) in 30 min at a flow-rate of 300 nL/min. For each cycle, one full MS was scanned in the Orbitrap with resolution of 60000 from 300–2000 m/z followed by CID fragmentation of 20 most intense peaks. Data dependent acquisition was set for repeat count of 2 and exclusion of 60 sec.
Protein identification via database queries
Prior to database queries, the Peak Erazor software (v 2.01: Lighthouse data, Odense, Denmark) was used to process peptide mass fingerprints (PMFs) generated from MALDI-TOF-MS analysis as previously described [41]. The MASCOT search engine (Matrix Science, London, UK) was used to find matches of the PMF and MS/MS fragmentation spectra against a custom database containing entries for citrus (Citrus sinensis and Citrus Clementina) available at http://www.citrusgenomedb.org/ and entries for grape (Vitis vinifera) available in the NCBI nonreduntant database. The PAC nos. for citrus or Accession nos. for grape entries that matched to our protein/peptide queries at the moment of Mascot search was recorded. Fixed and variable modifications (Cys carbamidomethylation and Met oxidation, respectively) and one missed cleavage were considered. PMF database search was conducted using a maximum mass tolerance of ±100 ppm, while MS/MS ions search were conducted with a mass tolerance of ± 0.6 Da on the parent and 0.3-0.8 Da on fragments; in all cases the peptide charge was +1. Decoy search was done automatically by Mascot on randomized database of equal composition and size. For PMF analysis, the peptide mixtures that produced the highest statistically significant (P < 0.05) match scores and accounted for the majority of the peaks present in the mass spectra, were assumed to be positively identified proteins.
LC-MS/MS spectra were also searched via MASCOT against a custom citrus database using the following parameters: precursor mass tolerance 10 ppm, fragment mass tolerance: 0.6 Dalton, fixed modification of carbamidomethylaion on cysteine and variable modification of methionine oxidation. The peptide identification results were filtered using a False-Detection-Rate (FDR) of 1% and only the top match was reported. To gain functional information on identified proteins from MALDI-TOF and LC-MS/MS analysis, homology searches using BLASTP (http:www.ncbi.nlm.nih.gov/BLAST) was employed.
Nutrient Status analysis
The same samples used for proteomic analysis were assayed for major cationic elements, Ca, K, Mg, Fe, Cu, Mn, and Zn via Inductively-Coupled Plasma Optical Emission Spectroscopy (ICP-OES) as previously described [56]. Briefly, leaf tissues were oven-dried and 0.5g was ashed at 510 °C for 9hrs, allowed to cool, and digested in 10 mL of 1N HNO3 for 1 h. The filtered supernatant was brought to volume (25 mL) and the intensities of atomic emissions at 396.847nm for Ca, 766.491nm for K, 279.553nm for Mg, 238.204nm for Fe, 327.395nm for Cu, 257.610nm for Mn, and 213.857nm for Zn was measured on an ICP-OES System (Varian, Vista Pro CCD Simultaneous ICP-OES attached to Varian SPS 5 Sampler Preparation System, Agilent, USA). Samples were further diluted 1:100 in 1N HNO3 prior to analysis of macronutrients Ca, K, and Mg while the analysis of micronutrients Fe, Cu, Mn and Zn did not require further dilutions. All containers used for ICP Spectroscopy analysis were acid-washed by soaking overnight in 1N HNO3 before use.
Statistical analysis
The nutrient concentration data were subjected to analysis of variance (ANOVA) using SigmaPlot software Version 11 (Systat Software, Inc., Point Richmond, California, USA) and means were separated using the Fischer’s Least Significant Difference (FLSD) test at ˃ 95% confidence interval (P < 0.05). Pair-wise comparisons to determine significant differences in spot volumes between treatments were performed on standardized log10 values of protein spot volumes using the Student’s t-test analysis at ˃ 95% confidence interval (P < 0.05) as provided by the PDQuest software.
Acknowledgments
We are grateful to Parminder Sahota, Donnie Williams and Tom Pflaum for assistance with sample preparation and ICP spectroscopy. We also thank Dr. Will Jewel of the Campus Mass Spectrometry Facility at UC Davis for access to their local Mascot Server. Trade names or commercial products in this publication are mentioned solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture.
Funding Statement
Funding for this project was provided by the US Department of Agriculture, Agricultural Research Service. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bove JM (2006) Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88: 7-37. [Google Scholar]
- 2. Halbert SE, Manjunath KL (2004) Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Fla Entomol 87: 330-353. doi:10.1653/0015-4040(2004)087[0330:ACPSPA]2.0.CO;2. [Google Scholar]
- 3. Gottwald TR (2010) Current Epidemiological Understanding of Citrus Huanglongbing. In: VanAlfen NK, Bruening G, Leach JE. Annu Rev Phytopathol, Vol 48: 119-139. [DOI] [PubMed] [Google Scholar]
- 4. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265-276. doi:10.1046/j.1365-313X.1999.00265.x. PubMed: 10377992. [DOI] [PubMed] [Google Scholar]
- 5. Gómez-Gómez L, Bauer Z, Boller T (2001) Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13: 1155-1163. doi:10.2307/3871370. PubMed: 11340188. [PMC free article] [PubMed] [Google Scholar]
- 6. Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL et al. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977-983. doi:10.1038/415977a. PubMed: 11875555. [DOI] [PubMed] [Google Scholar]
- 7. Heath MC (2000) Nonhost resistance and nonspecific plant defenses. Curr Opin Plant Biol 3: 315-319. doi:10.1016/S1369-5266(00)00087-X. PubMed: 10873843. [DOI] [PubMed] [Google Scholar]
- 8. Dangl JL, Ritter C, Gibbon MJ, Mur LA, Wood JR et al. (1992) Functional homologs of the Arabidopsis RPM1 disease resistance gene in bean and pea. Plant Cell 4: 1359-1369. doi:10.1105/tpc.4.11.1359. PubMed: 1477552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Innes RW, Bent AF, Kunkel B-N, Bisgrove SR, Staskawicz BJ (1993) Molecular analysis of avirulence gene avrRpt2 and identification of a putative regulatory sequence common to all known Pseudomonas syringae avirulence genes. J Bacteriol 175: 4859–4869. PubMed: 8335641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S et al. (2004) The transcriptional innate immune response to flg22. interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol Rockv 135: 1113-1128. doi:10.1104/pp.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albrecht U, Bowman KD (2012) Transcriptional response of susceptible and tolerant citrus to infection with Candidatus Liberibacter asiaticus. Plant Sci 185–186: 118-130. PubMed; : 22325873 [DOI] [PubMed] [Google Scholar]
- 12. Folimonova SY, Robertson CJ, Garnsey SM, Gowda S, Dawson WO (2009) Examination of the responses of different genotypes of citrus to huanglongbing (citrus greening) under different conditions. Phytopathology 99: 1346-1354. doi:10.1094/PHYTO-99-12-1346. PubMed: 19900000. [DOI] [PubMed] [Google Scholar]
- 13. Zhang M, Powell CA, Guo Y, Doud MS, Duan Y (2012) A graft-based chemotherapy method for screening effective molecules and rescuing huanglongbing-affected citrus plants. Phytopathology 102: 567-574. doi:10.1094/PHYTO-09-11-0265. PubMed: 22568814. [DOI] [PubMed] [Google Scholar]
- 14. Fan J, Chen C, Brlansky RH, Gmitter FG Jr., Li ZG (2010) Changes in carbohydrate metabolism in Citrus sinensis infected with 'Candidatus Liberibacter asiaticus'. Plant Pathol 59: 1037-1043. doi:10.1111/j.1365-3059.2010.02328.x. [Google Scholar]
- 15. Fan J, Chen C, Yu Q, Brlansky RH, Li ZG et al. (2011) Comparative iTRAQ proteome and transcriptome analyses of sweet orange infected by "Candidatus Liberibacter asiaticus" Physiol Plant 143: 235-245. doi:10.1111/j.1399-3054.2011.01502.x. PubMed: 21838733. [DOI] [PubMed] [Google Scholar]
- 16. Fan J, Chen C, Yu Q, Khalaf A, Achor DS et al. (2012) Comparative transcriptional and anatomical analyses of tolerant rough Lemon and susceptible sweet orange in response to ‘Candidatus Liberibacter asiaticus’ infection. Mol Plant-Microbe Inter 25: 1396-1407. [DOI] [PubMed]
- 17. Kim JS, Sagaram US, Burns JK, Li JL, Wang N (2009) Response of sweet orange (Citrus sinensis) to 'Candidatus Liberibacter asiaticus' infection: microscopy and microarray analyses. Phytopathology 99: 50-57. doi:10.1094/PHYTO-99-1-0050. PubMed: 19055434. [DOI] [PubMed] [Google Scholar]
- 18. Liao H-L, Burns JK (2012) Gene expression in Citrus sinensis fruit tissues harvested from huanglongbing-infected trees: comparison with girdled fruit. J Exp Bot 63: 3307-3319. doi:10.1093/jxb/ers070. PubMed: 22407645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao H, Sun R, Albrecht U, Padmanabhan C, Wang A et al. (2013) Small RNA profiling reveals phosphorus deficiency as a contributing factor in symptom expression for Citrus Huanglongbing disease. Mol Plant 6: 301-310. [DOI] [PMC free article] [PubMed]
- 20. Huber DM, Haneklaus S (2007) Managing nutrition to control plant disease. Landbauforschung Völkenrode 57: 313-322. [Google Scholar]
- 21. Dordas C (2009) Role of Nutrients in Controlling Plant Diseases in Sustainable Agriculture: A Review. In: Lichtfouse E, Navarrete M, Debaeke P, Souchere V, Alberola C. J Sustain Agric: 443-460. [Google Scholar]
- 22. Graham DR, Webb MJ (1991) Micronutrients and disease resistance and tolerance in plants. In: Mortvedt JJ, Cox FR, Shuman LM, Welch RM. Micronutrients in Agriculture. 2 ed. Madison, WI, USA: Soil Science Society of America; Inc. pp. 329-370 [Google Scholar]
- 23. Grewal HS, Graham RD, Rengel Z (1996) Genotypic variation in zinc efficiency and resistance to crown rot disease (Fusarium graminearum Schw. Group 1 in wheat. Plant Soil 186: 219-226 [Google Scholar]
- 24. Cevallos-Cevallos JM, García-Torres R, Etxeberria E, Reyes-De-Corcuera JI (2011) GC-MS analysis of headspace and liquid extracts for metabolomic differentiation of citrus huanglongbing and zinc deficiency in leaves of ‘Valencia’ sweet orange from commercial groves. Phytochem Anal 22: 236-246. doi:10.1002/pca.1271. PubMed: 21046688. [DOI] [PubMed] [Google Scholar]
- 25. Vahling-Armstrong CM, Zhou H, Benyon L, Morgan JK, Duan Y (2012) Two plant bacteria, S. meliloti and Ca. Liberibacter asiaticus, Share Functional znuABC homologues that encode for a high affinity zinc uptake system. PLOS ONE 7: e37340. doi:10.1371/journal.pone.0037340. PubMed: 22655039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albrecht U, Bowman KD (2008) Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant Sci (Oxf) 175: 291-306. doi:10.1016/j.plantsci.2008.05.001. [Google Scholar]
- 27. Nwugo CC, Lin H, Duan Y-P, Civerolo EL (2013) The effect of ‘Candidatus Liberibacter asiaticus’ infection on the proteomic profiles and nutritional status of pre-symptomatic and symptomatic grapefruit (Citrus paradisi) plants. BMC Plant Biol . doi:10.1186/1471-2229-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH et al. (1999) Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol 17: 994-999. doi:10.1038/13690. PubMed; : 10504701 [DOI] [PubMed] [Google Scholar]
- 29. Washburn MP, Koller A, Oshiro G, Ulaszek RR, Plouffe D et al. (2003) Protein pathway and complex clustering of correlated mRNA and protein expression analyses in Saccharomyces cerevisiae . Proc Natl Acad Sci U S A 100: 3107-3112. doi:10.1073/pnas.0634629100. PubMed: 12626741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen C, Bowman KD, Choi YA, Dang PM, Rao MN et al. (2008) EST-SSR genetic maps for Citrus sinensis and Poncirus trifoliata. Tree Genet Gen 4: 1-10
- 31. Zhang M, Duan Y, Zhou L, Turechek WW, Stover E et al. (2010) Screening molecules for control of citrus huanglongbing using an optimized regeneration system for 'Candidatus Liberibacter asiaticus'-infected periwinkle (Catharanthus roseus) cuttings. Phytopathology 100: 239-245. doi:10.1094/PHYTO-100-3-0239. PubMed: 20128697. [DOI] [PubMed] [Google Scholar]
- 32. Bilgin DD, Zavala JA, Zhu J, Clough SJ, Ort DR et al. (2010) Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ 33: 1597-1613. doi:10.1111/j.1365-3040.2010.02167.x. PubMed: 20444224. [DOI] [PubMed] [Google Scholar]
- 33. Nwugo CC, Huerta AJ (2008) Silicon-induced cadmium resistance in rice (Oryza sativa). J Plant Nutr Soil Sci 171: 841-848. doi:10.1002/jpln.200800082. [Google Scholar]
- 34. Hauck P, Thilmony R, He SY (2003) A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci U S A 100: 8577-8582. doi:10.1073/pnas.1431173100. PubMed: 12817082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bouarab K, Melton R, Peart J, Baulcombe D, Osbourn A (2002) A saponin-detoxifying enzyme mediates suppression of plant defences. Nature 418: 889-892. doi:10.1038/nature00950. PubMed: 12192413. [DOI] [PubMed] [Google Scholar]
- 36. Dannenhoffer JM, Schulz A, Skaggs MI, Bostwick DE, Thompson GA (1997) Expression of the phloem lectin is developmentally linked to vascular differentiation in cucurbits. Planta (Heidelb) 201: 405-414. doi:10.1007/s004250050083. [Google Scholar]
- 37. Read SM, Northcote DH (1983) Subunit structure and interactions of the phloem proteins of Cucurbita maxima (pumpkin). Eur J Biochem 134: 561-570. doi:10.1111/j.1432-1033.1983.tb07603.x. PubMed: 6884347. [DOI] [PubMed] [Google Scholar]
- 38. Schneider H (1968) Anatomy of greening-disease sweet orange shots. Phytopathology 58: 1155-1160. [Google Scholar]
- 39. Etxeberria E, Gonzalez P, Achor D, Albrigo G (2009) Anatomical distribution of abnormally high levels of starch in HLB-affected Valencia orange trees. Physiol Mol Plant Pathol 74: 76-83. doi:10.1016/j.pmpp.2009.09.004. [Google Scholar]
- 40. Nakamura T, Yamamori M, Hirano H, Hidaka S, Nagamine T (1995) Production of waxy (amylose-free) wheats. Mol Gen Genet 248: 253-259. doi:10.1007/BF02191591. PubMed: 7565586. [DOI] [PubMed] [Google Scholar]
- 41. Nwugo CC, Huerta AJ (2011) The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium-stress. J Proteome Res 10: 518-528. doi:10.1021/pr100716h. PubMed: 21117708. [DOI] [PubMed] [Google Scholar]
- 42. Chevalier F, Rossignol M (2011) Proteomic analysis of Arabidopsis thaliana ecotypes with contrasted root architecture in response to phosphate deficiency. J Plant Physiol 168: 1885-1890. doi:10.1016/j.jplph.2011.05.024. PubMed: 21835495. [DOI] [PubMed] [Google Scholar]
- 43. Durand TC, Sergeant K, Planchon S, Carpin S, Label P et al. (2010) Acute metal stress in Populus tremula x P. alba (717-1B4 genotype): Leaf and cambial proteome changes induced by cadmium 2+. Proteomics 10: 349-368. doi:10.1002/pmic.200900484. PubMed: 20148406. [DOI] [PubMed] [Google Scholar]
- 44. Kieffer P, Dommes J, Hoffmann L, Hausman JF, Renaut J (2008) Quantitative changes in protein expression of cadmium-exposed poplar plants. Proteomics 8: 2514-2530. doi:10.1002/pmic.200701110. PubMed: 18563750. [DOI] [PubMed] [Google Scholar]
- 45. Lopez-Millan A-F, Sagardoy R, Solanas M, Abadia A, Abadia J (2009) Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot 65: 376-385. doi:10.1016/j.envexpbot.2008.11.010. [Google Scholar]
- 46. Yang J, Hunt AG (1994) Immunological characterization of plant polyadenylate-binding proteins. Plant Sci 99: 161-170. doi:10.1016/0168-9452(94)90173-2. [Google Scholar]
- 47. Stros M (2010) HMGB proteins: Interactions with DNA and chromatin. Biochim Biophys Acta 1799: 101-113. doi:10.1016/j.bbagrm.2009.09.008. PubMed: 20123072. [DOI] [PubMed] [Google Scholar]
- 48. Minard LV, Williams JS, Walker AC, Schultz MC (2011) Transcriptional regulation by Asf1: New mechanistic insights from studies of the DNA damage response to replication stress. J Biol Chem 286: 7082-7092. doi:10.1074/jbc.M110.193813. PubMed: 21190944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kozubowski L, Aboobakar EF, Cardenas ME, Heitman J (2011) Calcineurin colocalizes with p-bodies and stress granules during thermal stress in Cryptococcus neoformans . Eukaryot Cell 10: 1396-1402. doi:10.1128/EC.05087-11. PubMed: 21724937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Purcino RP, Medina CL, Martins D, Winck FV, Machado EC et al. (2007) Xylella fastidiosa disturbs nitrogen metabolism and causes a stress response in sweet orange Citrus sinensis cv. Pera J Exp Bot 58: 2733-2744. doi:10.1093/jxb/erm138. [DOI] [PubMed] [Google Scholar]
- 51. Masaoka Y, Pustika A, Subandiyah S, Okada A, Hanundin E et al. (2011) Lower concentrations of microelements in leaves of citrus infected with 'Candidatus Liberibacter asiaticus'. JARQ 45: 269-275. doi:10.6090/jarq.45.269. [Google Scholar]
- 52. Poschenrieder C, Tolrà R, Barceló J (2006) Can metals defend plants against biotic stress? Trends Plant Sci 11: 288-295. doi:10.1016/j.tplants.2006.04.007. PubMed: 16697693. [DOI] [PubMed] [Google Scholar]
- 53. Fones H, Davis CAR, Rico A, Fang F, Smith JAC et al. (2010) Metal hyperaccumulation armors plants against disease. PLOS Pathog 6: e1001093 PubMed: 20838462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coleman CM, Boyd RS, Eubanks MD (2005) Extending the elemental defense hypothesis: Dietary metal concentrations below hyperaccumulator levels could harm herbivores. J Chem Ecol 31: 1669-1681. doi:10.1007/s10886-005-5919-4. PubMed: 16222801. [DOI] [PubMed] [Google Scholar]
- 55. Li W, Hartung JS, Levy L (2006) Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J Microbiol Methods 66: 104-115. doi:10.1016/j.mimet.2005.10.018. PubMed: 16414133. [DOI] [PubMed] [Google Scholar]
- 56. Banuelos GS, Pasakdee S, Benes SE, Ledbetter CA (2007) Long-term application of biosolids on apricot production. Commun Soil Sci Plant Anal 38: 1533-1549. doi:10.1080/00103620701378474. [Google Scholar]