Abstract
Objectives
Identify risk factors of anastomotic leak (AL) after large bowel resection (LBR) for ovarian cancer (OC) and compare outcomes between AL and no AL.
Methods
All cases of AL after LBR for OC between 01/01/1994-05/20/2011 were identified and matched 1:2 with controls for age (+/−5years), sub-stage (IIIA/IIIB;IIIC;IV), and date of surgery (+/−4years). Patient-specific and intraoperative risk factors, use of protective stomas, and outcomes were abstracted. A stratified conditional logistic regression model was fit to determine the association between each factor and AL.
Results
42 AL cases were evaluable and matched with 84 controls. Two-thirds of the AL had stage IIIC disease and >90% of both cases and controls were cytoreduced to <1cm residual disease. No patient-specific risk factors were associated with AL (pre-operative albumin was not available for most patients). Rectosigmoid resection coupled with additional LBR was associated with AL (OR=2.73, 95%CI 1.13–6.59, P=0.025), and protective stomas were associated with decreased risk of AL (0% vs. 10.7%, P=0.024). AL had longer length of stay (P<0.001), were less likely to start chemotherapy (P=0.020), and had longer time to chemotherapy (P=0.007). Cases tended to have higher 90-day mortality (P=0.061) and were more likely to have poorer overall survival (HR=2.05, 95%CI 1.18–3.57, P=0.011).
Conclusions
Multiple LBRs appear to be associated with increased risk of AL and protective stomas with decreased risk. Since AL after OC cytoreduction significantly delays chemotherapy and negatively impacts survival, surgeons should strongly consider temporary diversion in selected patients (poor nutritional status, multiple LBRs, previous pelvic radiation, very low anterior resection, steroid use).
Keywords: risk factor, anastomotic leak, protective stoma, large bowel resection, ovarian cancer
Introduction
Ovarian cancer (OC) is widely recognized as a systemic disease given its propensity to disseminate along peritoneal surfaces, frequently involving the bowel and extending to the upper abdomen. Most patients (up to 70%) will present with advanced stage disease [1, 2]. Primary cytoreductive surgery followed by platinum- and taxane-based chemotherapy constitutes current standard treatment [3]. Despite advances in surgical techniques and systemic chemotherapy over the past 3 decades, ovarian cancer remains the leading cause of cancer death among women with gynecologic malignancies [4] with 5-year disease-free survival rates not exceeding 30% [5].
An increasing number of studies report a significant survival improvement with cytoreduction to microscopic residual disease (RD) compared to the current definition of “optimal” cytoreductive surgery (RD ≤1 cm) [6–10]. In order to achieve maximal cytoreduction, extensive surgery, including large bowel resection (LBR), may be required.
A well-recognized complication of LBR is anastomotic leak (AL) which, although infrequent, can be a catastrophic event associated with significant morbidity, mortality and increased hospital costs. Rates of AL range from 0.8% to 6.8% in the gynecologic oncology literature [11–15]. In colorectal literature, published mortality rates associated with AL range from 6% to 22% [16–21]. Historically, AL was thought to only impact 30-day mortality and not long-term colon cancer survival [22, 23], however, more contemporary studies indicate that an AL portends a significant reduction in long-term survival as well [24–28]. Although the impact of AL in the long-term survival of OC patients has not been previously studied, the consequences of AL and the resultant delay in chemotherapy in a cancer where approximately 95% of patients will require adjuvant chemotherapy may be substantially more detrimental than in colorectal cancer patients.
Patient-specific and intraoperative factors have been shown to independently predict AL after LBR in colon cancer patients and include poor nutritional status (preoperative albumin <3.0 g/dL), compromised physical status (ASA score 3 or 4), alcohol and steroid use, smoking, obesity, prior bevacizumab receipt, previous pelvic irradiation, operative time more than 2 hours, intra-operative septic conditions, peri-operative blood transfusion, and most importantly, distance of anastomosis from the anal verge [17, 29–38]. The limited number of studies in the OC patient population have shown that previous pelvic irradiation, poor nutritional status and distance of anastomosis from the anal verge are all important factors, with a very low anastomosis being the most reproducible and significant risk factor [14, 15, 39–42].
In comparison to colorectal literature, there is a relative paucity of data examining risk factors and short- and long-term outcomes of AL in OC. Given the profound impact that AL carries in OC patients additional information to guide peri-operative decision making on diverting stomas is needed. We thus sought to identify factors contributing to AL after LBR during cytoreductive surgery. Secondarily we aimed to compare short- and long-term outcomes between OC patients who suffered a post-cytoreduction AL versus matched control patients without AL.
Methods
After obtaining approval by the Institutional Review Board of Mayo Foundation, all patients who underwent LBR with primary anastomosis during cytoreductive surgery for primary or recurrent OC (including fallopian tube and primary peritoneal cancer, collectively referred to as OC for this study) between January 1, 1994 and May 20, 2011 at the Mayo Clinic were identified. Medical records including operative reports were reviewed by the authors and all cases of AL were identified. We defined AL as follows: 1) feculent fluid from drains, wound, or vagina, 2) definitive radiographic evidence of extravasation at the anastomotic site, or 3) AL found at reoperation. Cases of isolated pelvic abscesses near the anastomotic site with no proven communication with the bowel lumen were not included. AL cases were matched 1:2 with cases of LBR for OC without AL (controls) on date of birth (+/− 5 years), stage (IIIA/IIIB; IIIC; IV), and date of surgery (+/− 4 years).
Patient-specific risk factors (including age, body mass index (BMI), ASA score, diabetes mellitus, use of tobacco, preoperative albumin, preoperative hemoglobin, history of abdominal and/or pelvic surgery), intraoperative risk factors (including type of LBR (rectosigmoid resection (RSR) alone, RSR coupled with an additional LBR, isolated non-pelvic LBR), perioperative RBC transfusion, end-operative body temperature, operative time), creation of diverting protective stomas at initial surgery, and outcomes (including hospital length of stay (LOS), ability to start chemotherapy, time to chemotherapy (TTC), 30- and 90-day mortality, overall survival (OS)) were abstracted. Patients were followed until death or last follow-up. Patients were considered positive for tobacco use if they were a smoker at time of surgery or if they had quit less than 10 years prior to surgery. Continuous variables were dichotomized as follows: BMI≥35 kg/m2 (WHO class II and III obesity), ASA≥3, preoperative albumin <3.0 g/dL, preoperative hemoglobin <10 g/dL and end-operative body temperature ≤36°C.
The distribution of each factor was summarized using standard descriptive statistics, separately for the cases and controls. For each factor of interest, a separate stratified conditional logistic regression model was fit to evaluate the association between the factor and case/control status, thereby taking into account the matching between the cases and controls. The functional form of BMI, end-operative body temperature, and operative time were first evaluated using smoothing splines. Each was identified as having a linear relationship with the probability of being a case and was therefore analyzed as a continuous measure. A stratified Cox proportional hazards regression model was fit to compare OS between AL cases and controls. Statistical analysis to compare OC patients with LBR with protective stoma vs. patients with LBR with no stoma included Chi-square test for categorical variables and Wilcoxon rank sum test for continuous variable. OS between stoma vs. no stoma patients was compared using the Wilcoxon test. All calculated P-values were two-sided and P-values less than 0.05 were considered statistically significant. Statistical analyses were performed using the SAS version 9.2 software package (SAS Institute, Inc.; Cary, NC).
Results
There were 43 cases of AL among the 725 cases of LBR with primary anastomosis performed during the study period. Among the 43 AL cases, 42 were included in our study cohort and matched 1:2 with controls. The single case of excluded AL had no matched controls due to unique factors (age, stage, era of surgery). Distribution of stage and RD in cases vs. controls is summarized in Table 1A. Over 90% of the AL cases had stage IIIC or IV disease. More than 90% of both cases and controls were debulked to ≤1cm RD, with 33.3% of the cases and 34.2% of the controls having no gross RD. Specific data on RD were not available in 2 controls. Among those who had an AL, 54.8% underwent RSR alone (vs. 70.2% controls), 38.1% underwent RSR coupled with an additional LBR (vs. 19.1% of controls), and 7.1% underwent isolated non-pelvic LBR (vs. 10.7% of controls). The technique of anastomosis in AL cases was equally divided (21/42) between stapled (end-to-end) and hand-sewn; the stapled technique was slightly more common in controls (51/84, 62.2%). Details regarding the diagnosis and management of the AL cases can be found in a previously published manuscript [43]. In brief, the vast majority of AL underwent reoperation (37/42, 88.1%): 23 patients received a diverting stoma (loop ileostomy, n=13; loop colostomy, n=10), 12 received an end colostomy, one patient underwent excision and repair of primary anastomosis, and one patient was treated with placement of a deep drain in the pelvis as a definitive site of AL was not possible to be identified intraoperatively. The remaining 5 (11.9%) patients were treated conservatively with broad-spectrum antibiotics and drainage (CT drainage/drain left in place, n=4; deep drain from surgery, n=1).
Table 1.
Distribution of stage and residual disease; A. AL cases vs. controls; B. Stoma vs. no stoma patients
| A. | Cases (n=42) | Controls (n=84) | P-value* |
|---|---|---|---|
| Stage | - | ||
| III, IIIA, IIIB | 4 (9.5%) | 8 (9.5%) | |
| IIIC | 27 (64.3%) | 54 (64.3%) | |
| IV | 11 (26.2%) | 22 (26.2%) | |
| Residual disease† | 0.81 | ||
| 0 cm | 14 (33.3%) | 28 (34.2%) | |
| 0 – 1 cm | 24 (57.2%) | 47 (57.3%) | |
| >1 cm | 4 (9.5%) | 7 (8.5%) | |
|
| |||
| B. | Stoma (n=9) | No stoma (n=75) | P-value‡ |
|
| |||
| Stage | 0.56 | ||
| III, IIIA, IIIB | 0 (0%) | 8 (10.7%) | |
| IIIC | 6 (66.7%) | 48 (64%) | |
| IV | 3 (33.3%) | 19 (25.3%) | |
| Residual disease | 0.48 | ||
| 0 cm | 3 (33.3%) | 25 (39.1%) | |
| 0 – 1 cm | 6 (66.7%) | 32 (50%) | |
P-value from a univariate conditional logistic regression model
Missing data on residual disease in 2 controls
Categorical variables: Chi-square test
Patient-specific risk factors appear in Table 2. Preoperative albumin was not routinely measured during all eras of study which resulted in approximately half the cases and controls missing values such that we were not able to use this factor in subsequent analyses. There were no significant differences in evaluable patient factors between cases and controls (Table 2). In the case-control analysis of the intraoperative variables (Table 3), we observed that a significantly higher proportion of AL cases had RSR coupled with additional LBR compared to controls (38.1% vs. 19.1%, P=0.025). Multiple LBRs were thus associated with increased odds of AL; in fact, the odds of AL was 2.73 times greater in patients with multiple LBRs compared to patients with RSR only or other non-pelvic LBR (P=0.025). Surgical details on the type of LBR of the patients with multiple LBRs are depicted on Table 4. Perhaps not surprisingly we observed that 9 out of 84 (11%) controls had undergone prophylactic diverting stomas (loop ileostomy, n=6; loop colostomy, n=3), while no cases of AL had protective stomas, thus protective stomas were significantly associated with decreased risk of AL (P=0.024). Of the 9 patients who received prophylactic diverting stomas, 5 patients underwent a second reoperation to restore bowel continuity (reversal rate 55.6%; time to reversal in days: 174, 211, 213, 436, no data). The reasons for non-reversal were: 1. Interval death within 90 days after surgery (n=1); 2. Recurrence of disease before stoma takedown (n=2); and 3. No follow-up data available (n=1). Notably, 50% of cases and 54.8% of controls experienced an end-operating body temperature of ≤36°C. While this factor was not different between cases and controls, the rate of hypothermia is substantial and may be a modifiable risk factor for overall surgical complications [44].
Table 2.
Patient-specific variables
| Factor | Cases (n=42) | Controls (n=84) | P-value* |
|---|---|---|---|
| Age (mean, SD) | 63.9 (12.3) | 64 (11.6) | - |
| BMI ≥35 kg/m2 | 7 (16.7%) | 13/81 (16%) | 0.86 |
| ASA ≥3 | 24 (57.1%) | 40 (47.6%) | 0.28 |
| Diabetes mellitus | 4 (9.5%) | 6 (7.1%) | 0.66 |
| Current use of tobacco | 4/40 (10%) | 8/80 (10%) | 0.92 |
| Preop Albumin <3.0 g/dL | 2/21 (9.5%) | 4/47 (8.5%) | -† |
| Preop Hgb <10 g/dL | 0 (0%) | 6/83 (7.2%) | 0.18 |
| History abd/ pelvic surgery | 29 (69%) | 48 (57.1%) | 0.19 |
P-value from stratified conditional logistic regression models
Too many missing data
Table 3.
Intraoperative variables
| Factor | Cases (n=42) | Controls (n=84) | P-value* |
|---|---|---|---|
| RSR + additional LBR | 16 (38.1%) | 16 (19.1%) | |
| OR=2.73, 95% | CI 1.13–6.59 | 0.025 | |
| Protective stomas | 0 (0%) | 9 (10.7%) | 0.024 |
| PRBC | 35 (83.3%) | 72 (85.7%) | 0.72 |
| End-operative BT† ≤36°C | 21 (50%) | 46 (54.8%) | 0.63 |
| OT† in min (mean, SD) | 280 (88) | 293 (109) | 0.43 |
P-value from stratified conditional logistic regression models
Body temperature (BT); operative time (OT)
Table 4.
Characteristics of patients with multiple large bowel resections (LBRs): A. Cases; B. Controls
| Type of LBR | N | Number of anastomoses | Site of AL* |
|---|---|---|---|
| A. Cases with multiple LBRs | 16 | ||
|
| |||
| RSR† + L colon (extended resection) | 4 | 1 | Descending colorectostomy (n=3) |
| Transverse colorectostomy (n=1) | |||
|
| |||
| RSR + L colon (extended resection) + R colon | 1 | 2 | Ileoascending colostomy and descending colorectostomy |
|
| |||
| RSR + L colon (wedge) + T colon (wedge) + R colon | 1 | 3 + end colostomy | T and L colon wedge resection anastomotic sites |
| RSR + R colon | 10 | 2 | Sigmoidorectostomy (n=5) |
| Ileoascending colostomy (n=2) | |||
| Sigmoidorectostomy and Ileoascending colostomy (n=2) | |||
| Other (transverse colon defect) (n=1) | |||
|
| |||
| B. Controls with multiple LBRs | 16 | ||
|
| |||
| RSR + L colon | 4 | ||
| 1 extended resection | 3 | 1 | - |
| 2 resections | 1 | 1 + end colostomy | - |
| RSR + L colon (extended resection) + R colon | 1 | 2 | - |
| RSR + L colon + T colon + R colon (subtotal colectomy) | 1 | 1 | - |
| RSR + L colon + T colon (left hemicolectomy) | 1 | 1 | - |
| RSR + R colon | 6 | 2 | - |
| RSR + R colon + T colon | 2 | - | |
| RSR + right hemicolectomy | 1 | 2 | - |
| RSR + R colectomy + T colectomy | 1 | 3 | - |
Anastomotic Leak (AL)
Rectosigmoid resection (RSR)
Not surprisingly, AL cases had longer LOS (P<0.001), were less likely to start chemotherapy (P=0.020), less likely to start chemotherapy within 30 days after surgery (P=0.027), and had longer TTC (P=0.007) than controls (Table 5A). Cases tended to have higher 90-day mortality (P=0.061) and were significantly more likely to have poorer OS (HR=2.05, 95% CI 1.18–3.57, P=0.011) (Figure 1): fully 30% of AL cases were not able to start chemotherapy.
Table 5.
Perioperative outcomes: A. AL cases vs. controls; B. Stoma vs. no stoma patients
| A. | |||
|---|---|---|---|
| Factor | Cases (n=42) | Controls (n=84) | P-value* |
| LOS† in days (median, range) | 19.5 (5–84) | 9.5 (4–36) | <0.001 |
| Unplanned ICU admission | 15 (35.7%) | 10 (11.9%) | |
| OR=4.79, 95% | CI 1.70–13.48 | 0.003 | |
| Ability to start chemotherapy | 29/41 (70.7%) | 69/75 (92%) | |
| OR=0.31, 95% | CI 0.11–0.83 | 0.020 | |
| TTC ≤30 days | 5/41 (12.2%) | 25/72 (34.7%) | 0.027 |
| OR=0.28, 95% | CI 0.09–0.87 | ||
| TTC† (median, range) | 47.5 (25–92) | 35.0 (14–136) | 0.007 |
| 30-day mortality | 1 (2.4%) | 2 (2.4%) | -‡ |
| 90-day mortality | 8 (19.1%) | 6 (7.1%) | 0.061 |
|
| |||
| B. | |||
| Factor | Stoma (n=9) | No stoma (n=75) | P-value# |
|
| |||
| LOS† in days (median, range) | 11 (7.3) | 9 (6.0) | 0.26 |
| Unplanned ICU admission | 3 (33.3%) | 7 (9.3%) | 0.07 |
| Ability to start chemotherapy | 8/8 (100%) | 61/67 (91%) | 1.00 |
| TTC† (median, range) | 36.5 (30–54) | 34 (14–136) | 0.29 |
| 30-day mortality | 0 (0%) | 2 (2.7%) | 1.00 |
| 90-day mortality | 1 (11.1%) | 5 (6.8%) | 0.51 |
P-value from stratified conditional logistic regression models
Hospital length of stay (LOS); time to chemotherapy (TTC)
Too few events to compare
Continuous variables: Wilcoxon rank sum test; categorical variables: Chi-square test
Figure 1.
Overall survival: AL cases vs. controls
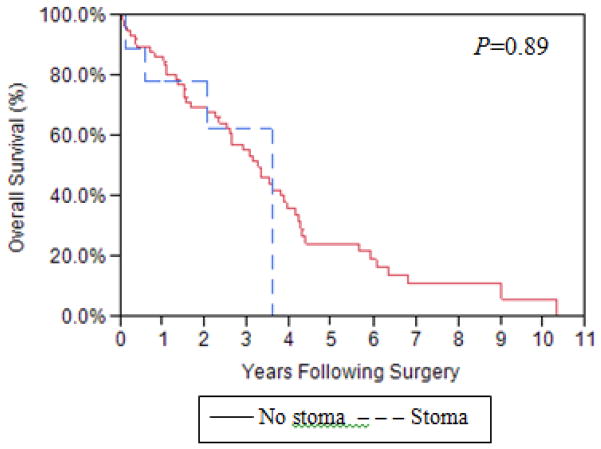
Given that protective stoma are thought to prevent devastating complications of AL, but can in and of itself contribute to complications, we examined the control group exclusively to compare characteristics and outcomes between stoma vs. no stoma patients. Stage and RD distribution were comparable between those who had a protective stoma and those who did not (Table 1B). Those with stomas tended to be older (mean (SD): 69.5 years (11.7) vs. 63.4 years (11.5), P=0.13) and have higher ASA scores (ASA≥3: 7/9 (77.8%) vs. 33/75 (44%), P=0.079), but these differences did not reach statistical significance; there were no statistically differences between these groups in the remaining of the patient-specific variables. With regards to intraoperative variables, there was a trend towards a higher frequency of multiple LBRs during OC cytoreduction in the stoma vs. no stoma patients (4/9 (44.4%) vs. 12/75 (16%), P=0.06) but small numbers limited power; there was no statistically significant difference between stoma vs. no stoma patients in percentage of patients receiving red blood cell transfusion (7/9 (77.8%) vs. 65/75 (86.7%), P=0.61), end-operative body temperature ≤36°C (6/9 (66.7%) vs. 40/75 (53.3%), P=0.50) or operative time (mean (SD): 332.7 min (143.4) vs. 288.7 min (103.9), P=0.68). We did not observe any statistically or clinically significant differences in the outcomes between stoma vs. no stoma patients (Table 5B). Importantly, placing a protective stoma did not impact the time to chemotherapy initiation (mean TTC 36.5 days vs. 34 days, P=0.29 respectively) or OS (Figure 2).
Figure 2.
Overall survival: Stoma vs. no stoma patients
Discussion
Aggressive OC cytoreduction frequently requires extensive pelvic and abdominal surgery which may include LBR. Despite advances in surgical techniques, AL continues to be a serious complication after LBR that adversely impacts postoperative morbidity and mortality, ICU admissions, hospital stay and healthcare costs. Given the gravity of AL and the increased risk profile encountered in OC patients (widely disseminated intra-abdominal disease, presence of ascites, tumor burden, poor nutritional status, marginal physical status) which makes extrapolation from colorectal literature less relevant, additional studies are needed to guide surgeons’ decision making at the time of LBR for OC.
We observed that LOS, ability to start chemotherapy, 90-day mortality and OS were negatively affected by AL. One out of 5 AL patients died within 90 days of primary surgery; this mortality rate is higher than the reported rate in the colorectal literature which ranges from 7.3% to 16% [26, 45, 46]. The marginal physical status reflected by the ASA score combined with the extent of the surgery may account for the higher postoperative mortality in the OC patient population compared to the colorectal cancer patients.
Unlike independent risk factors for AL in the colorectal literature, we did not find obesity, ASA status, diabetes mellitus, tobacco use, preoperative anemia, history of abdominal and/or pelvic surgery, peri-operative PRBC transfusion, intra-operative hypothermia, or operative time to impact AL in OC. However, we recognize the limitations of relatively small numbers of AL and specific risk factors in our population. We were unable to investigate the association between preoperative nutritional status and risk of AL in this cohort, but we and others have reported its independent importance [14, 15]. Despite small numbers we did find that RSR when combined with additional LBR was associated with a nearly 3-fold risk of AL. Salani et al. [47] reported the impact of multiple bowel resections in patients undergoing optimal primary cytoreductive surgery for advanced OC and observed a significantly higher rate of postoperative complications, including fistulas/anastomotic breakdown, in the patients undergoing 2 vs. 0–1 bowel resections (5.9% of anastomotic breakdown with 2 bowel resections vs. 4.4% with 0–1 bowel resections). However, they studied only cases of primary cytoreduction for OC and bowel resections included both large and small bowel; both of these factors may account for the difference in the magnitude of the association between multiple LBRs and AL rate differences between the two studies. This is the first time in the literature that multiple LBRs are shown to increase the risk of AL, and we believe the matched case-control approach may have helped to analyze this association. Given our findings, we suggest that multiple LBR should be added to the previously validated independent risk factors of AL in patients with gynecologic malignancies which include prior pelvic irradiation, poor nutritional status and short distance from anal verge [14, 15, 39–42].
The decision to perform a diverting stoma at primary surgery remains controversial with regards to the risk-benefit ratio attributed to the stoma. In the colorectal literature, while some investigators have shown a significant AL risk reduction with protective stomas [17, 45], others have observed no difference in AL when a stoma is utilized [39, 41, 42, 46, 48–54]. In a recent Cochrane systematic review and meta-analysis of colorectal literature, a 67% relative risk reduction of clinically significant AL was observed [55]; however, the authors cautioned that there were methodological drawbacks with their analyses. Whether the risk of detectable AL after colorectal surgery is reduced with diverting stoma remains debated; however, there is widespread consensus that protective stomas prevent the catastrophic consequences of AL and are associated with reductions in morbidity, emergency reoperation rates, ICU admissions and, importantly, mortality [41, 42, 49, 56–59]. In contrast to the abundance of data coming from colorectal literature, studies on the utility of protective stomas in the OC population are few and, as previously discussed, extrapolation from colorectal literature in OC patients carries less relevance not only when discussing risk factors but also management of AL. Historically, It has been thought that emergent diversion for AL after an isolated LBR is a less technically challenging procedure than after a comprehensive cytoreductive operation involving most of the peritoneal cavity; the morbidity associated with each scenario is quite different. Furthermore, AL after bowel resection is difficult to predict: a significant portion of the AL occurs in patients without previously established classic risk factors [14, 15, 39–42] such as poor nutrition, prior radiation and very low level of rectal resection. This underscores the need for more sensitive predictors and careful analysis of the alternative approach of more liberal use of diverting stomas. In our series, protective stomas were associated with a decreased risk of AL. Moreover, it is striking that none of the diverted patients experienced AL. If we were thus to assume that diverting stomas completely prevented AL, one third of AL cases would have been prevented had a stoma been done in all OC patients with multiple LBR (frequency of multiple LBRs in AL cases: 38.1%). Admittedly, one must weigh the risks and benefits associated with diverting stomas compared to risks of AL. Collectively, we found that we would need to perform prophylactic diverting stomas in 6.6 OC patients with multiple LBR to prevent one case of AL in this subgroup of patients with multiple LBR (Number Needed to Treat 6.6). Given the high cost of AL and the profound significance in terms of patient suffering and ability to complete recommended therapy this may be a reasonable trade-off but certainly one that should be considered with the patient in the context of other risk factors and goals.
Protective stomas do not come without potential intrinsic risks. In addition to increasing hospital costs, a second surgery is required for reversal and both the stoma itself (necrosis, retraction, hernia, electrolyte and fluid losses) as well as the reversal surgery can be associated with inherent morbidity ranging from 13% to 28%, albeit serious complications occur less then <5% of the time [60–64]. Moreover, an intended temporary stoma may become permanent with colorectal literature rates of non-reversal ranging from 15% to 50% [24, 30, 65]. Finally, given that stomas have been associated with adverse psychological effects and decreased patient quality of life, these aspects must be weighed when evaluating the risk-benefit ratio of stomas for women undergoing OC debulking [66]. However, given the substantial mortality following AL after OC cytoreduction and the fact that none of the diverted patients experienced AL, the benefit of a protective diverting stoma in well-counseled, appropriately selected patients should be clear. It is thus critical to risk-stratify OC patients undergoing cytoreductive surgery and seriously consider diverting stomas after LBR in high risk groups. Similarly the decision to perform LBR should be reserved for those patients we can achieve low RD or for palliation.
Our study is limited by the fact that it is retrospective. In addition, we were unable to analyze preoperative albumin as a risk factor of AL due to the large number of missing data. However, poor nutritional status is already a well-established independent predictor of AL [14, 15]. Many of the classic risk factors for AL are not present in the OC population so are not evaluable in most series, but will be occasionally present and should not be ignored. In addition, this study did not address the impact of AL in hospital costs compared to the impact of diverting stomas including takedown; however, a cost-analysis study is currently in progress to investigate these issues.
In conclusion, RSR when coupled with additional LBR appears to significantly increase the risk of AL. In agreement with the existing colorectal literature supporting the use of protective stomas after LBR as a way to mitigate the adverse sequelae following an AL, we provide evidence that protective stomas reduce and possibly prevent AL following OC cytoreduction. In addition to previously established indications for diverting stoma utilization, we suggest considering placement of a protective stoma when multiple LBRs have been performed during OC cytoreduction. Further cost-analysis and quality of life studies evaluating the impact of prophylactic bowel diversion are warranted.
Research Highlights.
Multiple large bowel resections increased the risk of anastomotic leak (AL) and protective diverting stomas decreased the risk.
AL patients had longer length of stay and were less likely to start chemotherapy.
AL patients tended to have higher 90-day mortality and were more likely to have poorer overall survival.
Acknowledgments
This work was partially supported by the National Institutes of Health Grant (R01CA148747 to WAC) and the Office of Women’s Health Research Building Interdisciplinary Careers in Women’s Health (BIRCWH award K12 HD065987 to JNB).
Abbreviations
- OC
Ovarian cancer
- LBR
large bowel resection
- AL
anastomotic leak
- BMI
body mass index
- LOS
hospital length of stay
- TTC
time to chemotherapy
- OS
overall survival
- RD
residual disease
- RSR
rectosigmoid resection
Footnotes
Presented at the 43rd Annual Meeting on Women’s Cancer of the Society of Gynecologic Oncology (SGO), March 23-27, 2012, Austin, Texas.
There are no conflicts of interest for this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heintz AP, Odicino F, Maisonneuve P, Beller U, Benedet JL, Creasman WT, Ngan HY, Pecorelli S. Carcinoma of the ovary. Int J Gynaecol Obstet. 2003;83 (Suppl 1):135–66. doi: 10.1016/s0020-7292(03)90118-4. [DOI] [PubMed] [Google Scholar]
- 2.Pecorelli S, Favalli G, Zigliani L, Odicino F. Cancer in women. Int J Gynaecol Obstet. 2003;82:369–79. doi: 10.1016/s0020-7292(03)00225-x. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 6.Bristow RE, Montz FJ. Complete surgical cytoreduction of advanced ovarian carcinoma using the argon beam coagulator. Gynecol Oncol. 2001;83:39–48. doi: 10.1006/gyno.2001.6344. [DOI] [PubMed] [Google Scholar]
- 7.Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998;69:103–8. doi: 10.1006/gyno.1998.4955. [DOI] [PubMed] [Google Scholar]
- 8.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–6. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 9.Le T, Krepart GV, Lotocki RJ, Heywood MS. Does debulking surgery improve survival in biologically aggressive ovarian carcinoma? Gynecol Oncol. 1997;67:208–14. doi: 10.1006/gyno.1997.4839. [DOI] [PubMed] [Google Scholar]
- 10.Makar AP, Baekelandt M, Trope CG, Kristensen GB. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. 1995;56:175–80. doi: 10.1006/gyno.1995.1027. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 12.Clayton RD, Obermair A, Hammond IG, Leung YC, McCartney AJ. The Western Australian experience of the use of en bloc resection of ovarian cancer with concomitant rectosigmoid colectomy. Gynecol Oncol. 2002;84:53–7. doi: 10.1006/gyno.2001.6469. [DOI] [PubMed] [Google Scholar]
- 13.Mourton SM, Temple LK, Abu-Rustum NR, Gemignani ML, Sonoda Y, Bochner BH, Barakat RR, Chi DS. Morbidity of rectosigmoid resection and primary anastomosis in patients undergoing primary cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2005;99:608–14. doi: 10.1016/j.ygyno.2005.07.112. [DOI] [PubMed] [Google Scholar]
- 14.Obermair A, Hagenauer S, Tamandl D, Clayton RD, Nicklin JL, Perrin LC, Ward BG, Crandon AJ. Safety and efficacy of low anterior en bloc resection as part of cytoreductive surgery for patients with ovarian cancer. Gynecol Oncol. 2001;83:115–20. doi: 10.1006/gyno.2001.6353. [DOI] [PubMed] [Google Scholar]
- 15.Richardson DL, Mariani A, Cliby WA. Risk factors for anastomotic leak after recto-sigmoid resection for ovarian cancer. Gynecol Oncol. 2006;103:667–72. doi: 10.1016/j.ygyno.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J. 1980;281:411–4. doi: 10.1136/bmj.281.6237.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karanjia ND, Corder AP, Bearn P, Heald RJ. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg. 1994;81:1224–6. doi: 10.1002/bjs.1800810850. [DOI] [PubMed] [Google Scholar]
- 18.Pakkastie TE, Luukkonen PE, Jarvinen HJ. Anastomotic leakage after anterior resection of the rectum. Eur J Surg. 1994;160:293–7. discussion 299–300. [PubMed] [Google Scholar]
- 19.Antonsen HK, Kronborg O. Early complications after low anterior resection for rectal cancer using the EEA stapling device. A prospective trial. Dis Colon Rectum. 1987;30:579–83. doi: 10.1007/BF02554801. [DOI] [PubMed] [Google Scholar]
- 20.Graf W, Glimelius B, Bergstrom R, Pahlman L. Complications after double and single stapling in rectal surgery. Eur J Surg. 1991;157:543–7. [PubMed] [Google Scholar]
- 21.Laxamana A, Solomon MJ, Cohen Z, Feinberg SM, Stern HS, McLeod RS. Long-term results of anterior resection using the double-stapling technique. Dis Colon Rectum. 1995;38:1246–50. doi: 10.1007/BF02049147. [DOI] [PubMed] [Google Scholar]
- 22.Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following ‘curative’ surgery for large bowel cancer: I. The overall picture. Br J Surg. 1984;71:12–6. doi: 10.1002/bjs.1800710104. [DOI] [PubMed] [Google Scholar]
- 23.Sauven P, Playforth MJ, Evans M, Pollock AV. Early infective complications and late recurrent cancer in stapled colonic anastomoses. Dis Colon Rectum. 1989;32:33–5. doi: 10.1007/BF02554722. [DOI] [PubMed] [Google Scholar]
- 24.Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. 2005;48:1021–6. doi: 10.1007/s10350-004-0869-4. [DOI] [PubMed] [Google Scholar]
- 25.Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11:8–15. doi: 10.1007/s11605-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 26.McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92:1150–4. doi: 10.1002/bjs.5054. [DOI] [PubMed] [Google Scholar]
- 27.Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255–9. doi: 10.1097/01.sla.0000133186.81222.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akyol AM, McGregor JR, Galloway DJ, Murray GD, George WD. Anastomotic leaks in colorectal cancer surgery: a risk factor for recurrence? Int J Colorectal Dis. 1991;6:179–83. doi: 10.1007/BF00341385. [DOI] [PubMed] [Google Scholar]
- 29.Sorensen LT, Jorgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jorgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86:927–31. doi: 10.1046/j.1365-2168.1999.01165.x. [DOI] [PubMed] [Google Scholar]
- 30.Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum. 2006;49:1719–25. doi: 10.1007/s10350-006-0703-2. [DOI] [PubMed] [Google Scholar]
- 31.Konishi T, Watanabe T, Kishimoto J, Nagawa H. Risk factors for anastomotic leakage after surgery for colorectal cancer: results of prospective surveillance. J Am Coll Surg. 2006;202:439–44. doi: 10.1016/j.jamcollsurg.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355–8. doi: 10.1046/j.1365-2168.1998.00615.x. [DOI] [PubMed] [Google Scholar]
- 33.Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, Valleur P. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg. 2002;26:499–502. doi: 10.1007/s00268-001-0256-4. [DOI] [PubMed] [Google Scholar]
- 34.Makela JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum. 2003;46:653–60. doi: 10.1007/s10350-004-6627-9. [DOI] [PubMed] [Google Scholar]
- 35.Golub R, Golub RW, Cantu R, Jr, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997;184:364–72. [PubMed] [Google Scholar]
- 36.Heinzerling JH, Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006;63:334–7. doi: 10.1016/j.cursur.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14:1860–9. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 38.Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, Strong SA, Oakley JR. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg. 1997;185:105–13. doi: 10.1016/s1072-7515(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 39.Hatch KD, Gelder MS, Soong SJ, Baker VV, Shingleton HM. Pelvic exenteration with low rectal anastomosis: survival, complications, and prognostic factors. Gynecol Oncol. 1990;38:462–7. doi: 10.1016/0090-8258(90)90092-y. [DOI] [PubMed] [Google Scholar]
- 40.Jurado M, Alcazar JL, Baixauli J, Hernandez-Lizoain JL. Low colorectal anastomosis after pelvic exenteration for gynecologic malignancies: risk factors analysis for leakage. Int J Gynecol Cancer. 2011;21:397–402. doi: 10.1097/IGC.0b013e31820b2df7. [DOI] [PubMed] [Google Scholar]
- 41.Mirhashemi R, Averette HE, Estape R, Angioli R, Mahran R, Mendez L, Cantuaria G, Penalver M. Low colorectal anastomosis after radical pelvic surgery: a risk factor analysis. Am J Obstet Gynecol. 2000;183:1375–9. doi: 10.1067/mob.2000.110908. discussion 1379–80. [DOI] [PubMed] [Google Scholar]
- 42.Moutardier V, Houvenaeghel G, Lelong B, Mokart D, Delpero JR. Colorectal function preservation in posterior and total supralevator exenteration for gynecologic malignancies: an 89-patient series. Gynecol Oncol. 2003;89:155–9. doi: 10.1016/s0090-8258(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 43.Kalogera E, Dowdy SC, Mariani A, Aletti G, Bakkum-Gamez JN, Cliby WA. Utility of closed suction pelvic drains at time of large bowel resection for ovarian cancer. Gynecologic oncology. 2012;126:391–6. doi: 10.1016/j.ygyno.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moslemi-Kebria M, El-Nashar SA, Aletti GD, Cliby WA. Intraoperative hypothermia during cytoreductive surgery for ovarian cancer and perioperative morbidity. Obstetrics and gynecology. 2012;119:590–6. doi: 10.1097/AOG.0b013e3182475f8a. [DOI] [PubMed] [Google Scholar]
- 45.Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004;6:462–9. doi: 10.1111/j.1463-1318.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 46.Wong NY, Eu KW. A defunctioning ileostomy does not prevent clinical anastomotic leak after a low anterior resection: a prospective, comparative study. Dis Colon Rectum. 2005;48:2076–9. doi: 10.1007/s10350-005-0146-1. [DOI] [PubMed] [Google Scholar]
- 47.Salani R, Zahurak ML, Santillan A, Giuntoli RL, 2nd, Bristow RE. Survival impact of multiple bowel resections in patients undergoing primary cytoreductive surgery for advanced ovarian cancer: a case-control study. Gynecologic oncology. 2007;107:495–9. doi: 10.1016/j.ygyno.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Fielding LP, Stewart-Brown S, Hittinger R, Blesovsky L. Covering stoma for elective anterior resection of the rectum: an outmoded operation? Am J Surg. 1984;147:524–30. doi: 10.1016/0002-9610(84)90016-3. [DOI] [PubMed] [Google Scholar]
- 49.Gastinger I, Marusch F, Steinert R, Wolff S, Koeckerling F, Lippert H. Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg. 2005;92:1137–42. doi: 10.1002/bjs.5045. [DOI] [PubMed] [Google Scholar]
- 50.Graffner H, Fredlund P, Olsson SA, Oscarson J, Petersson BG. Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument. A randomized study. Dis Colon Rectum. 1983;26:87–90. doi: 10.1007/BF02562579. [DOI] [PubMed] [Google Scholar]
- 51.Machado M, Hallbook O, Goldman S, Nystrom PO, Jarhult J, Sjodahl R. Defunctioning stoma in low anterior resection with colonic pouch for rectal cancer: a comparison between two hospitals with a different policy. Dis Colon Rectum. 2002;45:940–5. doi: 10.1007/s10350-004-6333-7. [DOI] [PubMed] [Google Scholar]
- 52.Mealy K, Burke P, Hyland J. Anterior resection without a defunctioning colostomy: questions of safety. Br J Surg. 1992;79:305–7. doi: 10.1002/bjs.1800790406. [DOI] [PubMed] [Google Scholar]
- 53.Pakkastie TE, Ovaska JT, Pekkala ES, Luukkonen PE, Jarvinen HJ. A randomised study of colostomies in low colorectal anastomoses. Eur J Surg. 1997;163:929–33. [PubMed] [Google Scholar]
- 54.Wexner SD, Alabaz O. Anastomotic integrity and function: role of the colonic J-pouch. Semin Surg Oncol. 1998;15:91–100. doi: 10.1002/(sici)1098-2388(199809)15:2<91::aid-ssu6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 55.Montedori A, Cirocchi R, Farinella E, Sciannameo F, Abraha I. Covering ileo- or colostomy in anterior resection for rectal carcinoma. Cochrane Database Syst Rev. 2010:CD006878. doi: 10.1002/14651858.CD006878.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Marusch F, Koch A, Schmidt U, Geibetaler S, Dralle H, Saeger HD, Wolff S, Nestler G, Pross M, Gastinger I, Lippert H. Value of a protective stoma in low anterior resections for rectal cancer. Dis Colon Rectum. 2002;45:1164–71. doi: 10.1007/s10350-004-6384-9. [DOI] [PubMed] [Google Scholar]
- 57.Matthiessen P, Hallbook O, Rutegard J, Simert G, Sjodahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207–14. doi: 10.1097/SLA.0b013e3180603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, Friess H. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. 2008;248:52–60. doi: 10.1097/SLA.0b013e318176bf65. [DOI] [PubMed] [Google Scholar]
- 59.Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009;96:462–72. doi: 10.1002/bjs.6594. [DOI] [PubMed] [Google Scholar]
- 60.Amin SN, Memon MA, Armitage NC, Scholefield JH. Defunctioning loop ileostomy and stapled side-to-side closure has low morbidity. Ann R Coll Surg Engl. 2001;83:246–9. [PMC free article] [PubMed] [Google Scholar]
- 61.Carlsen E, Bergan AB. Loop ileostomy: technical aspects and complications. Eur J Surg. 1999;165:140–3. doi: 10.1080/110241599750007324. discussion 144. [DOI] [PubMed] [Google Scholar]
- 62.Phang PT, Hain JM, Perez-Ramirez JJ, Madoff RD, Gemlo BT. Techniques and complications of ileostomy takedown. Am J Surg. 1999;177:463–6. doi: 10.1016/s0002-9610(99)00091-4. [DOI] [PubMed] [Google Scholar]
- 63.Khoo RE, Cohen MM, Chapman GM, Jenken DA, Langevin JM. Loop ileostomy for temporary fecal diversion. Am J Surg. 1994;167:519–22. doi: 10.1016/0002-9610(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 64.Senapati A, Nicholls RJ, Ritchie JK, Tibbs CJ, Hawley PR. Temporary loop ileostomy for restorative proctocolectomy. Br J Surg. 1993;80:628–30. doi: 10.1002/bjs.1800800529. [DOI] [PubMed] [Google Scholar]
- 65.Kairaluoma M, Rissanen H, Kultti V, Mecklin JP, Kellokumpu I. Outcome of temporary stomas. A prospective study of temporary intestinal stomas constructed between 1989 and 1996. Dig Surg. 2002;19:45–51. doi: 10.1159/000052005. [DOI] [PubMed] [Google Scholar]
- 66.O’Leary DP, Fide CJ, Foy C, Lucarotti ME. Quality of life after low anterior resection with total mesorectal excision and temporary loop ileostomy for rectal carcinoma. Br J Surg. 2001;88:1216–20. doi: 10.1046/j.0007-1323.2001.01862.x. [DOI] [PubMed] [Google Scholar]