Abstract
Objectives
The relationship between thyroid dysfunction and mortality in elderly subjects is still undefined. In this population study we tested the hypothesis that in older subjects, living in a mildly iodine-deficient area, thyroid dysfunction may be associated with increased mortality independent of potential confounders.
Design
Longitudinal study
Setting
Community-based
Participants
Total of 951 subjects aged 65 years and older
Measurements
Plasma thyrotropin (TSH), free thyroxine (FT4), and free triiodothyronine (FT3) concentrations and demographic features were evaluated in participants of the Aging in the Chianti Area (InCHIANTI) study, aged 65 years or older. Participants were classified according to thyroid function test. Kaplan-Meier survival and Cox proportional hazards models adjusted for confounders were used in the analysis.
Results
A total of 819 participants were euthyroid, 83 had Subclinical hyperthyroidism (SHyper), and 29 had Subclinical hypothyroidism (SHypo). Overt Hypo- and Hyperthyroidism were found in 5 and 15 subjects, respectively. During a median of six-years of follow-up, N 210 deaths occurred (22.1 %) of which 98 (46.6%) due to cardiovascular causes. Kaplan–Meier analysis revealed higher overall mortality for SHyper (P<0.04) as compared to euthyroid subjects. After adjusting for multiple confounders, participants with SHyper (Hazard Ratio[HR]:1.65; 95% Confidence Interval [CI]: 1.02–2.69) had significantly higher all-cause mortality than those with normal thyroid function. No significant association was found between SHyper and cardiovascular mortality.
In euthyroid subjects, TSH was found to be predictive of a reduced risk of all-cause mortality (HR: 0.76; 95% CI, 0.57–0.99)
Conclusion
SHyper is an independent risk factor for all-cause mortality in the older population. Low-normal circulating TSH should be carefully monitored in euthyroid elderly individuals.
Keywords: subclinical hyperthyroidism, aging, mortality
INTRODUCTION
Thyroid function abnormalities are frequently observed in aging population (1, 2, 3) with the most prevalent types being represented by subclinical hyper-and hypothyroidism (4, 5). Whether subclinical thyroid dysfunction may negatively influence the aging processes or worsen the clinical course of other age-associated diseases is still a matter of debate.
Subclinical hyperthyroidism (SHyper) has been associated with several adverse clinical outcomes, mainly represented by abnormalities of cardiovascular system, bone metabolism, blood coagulation and cognition (5, 6). However, still undefined remains the relationship between SHyper and mortality, especially in elderly subjects. In 2001, Parle and co-workers demonstrated an increase in overall and cardiac mortality in subjects aged 60 years or older affected by SHyper (7). An increase in SHyper-associated all-cause mortality was also demonstrated in 2008 by Haentjens and coworkers who showed that the increased risk depends on the age at diagnosis, with a significant increase beginning at the age of 60 years (8). These data have been recently confirmed by Sgarbi et al. who, in a Japanese- Brazilian population of subjects aged 30 to 70 or older, demonstrated that SHyper is an independent risk factor for all-cause and cardiovascular mortality (9). However, these results were not confirmed by other Authors. For example, Cappola and coworkers demonstrated that SHyper is associated with atrial fibrillation but not with mortality in elderly subjects (10). The lack of association between SHyper and mortality in older subjects also emerges from the Longitudinal Aging Study Amsterdam (11) and has been recently confirmed in community-dwelling men participating in the Osteoporotic Fractures in Men Study (12). Recent metanalyses considered the association between Shyper and mortality as weak (13) or significantly positive (14) and, altogether, data have been considered to still remain undefined in terms of both risk estimation and need of treatment (15–17).
Subclinical hypothyroidism (SHypo) has been associated with negative clinical outcomes in older subjects, such as abnormalities in lipid profile, reduced cardiovascular function and worsening of bone metabolism (5, 6). However, the clinical relevance of these Shypo-associated negative findings is questionable. Previous studies found an association between SHypo and all-cause as well as cardiovascular mortality in adults (18, 19), but other studies did not (12). Experiences conducted in the oldest old found that a slightly decreased thyroid function characterized by high levels of thyrotropin as well as low level of thyroxine is associated with increased lifespan (6, 20, 21)
Differences in methodological procedures in the evaluation of thyroid function test, selection criteria, genetics of populations, environmental factors, may be considered among the main factors which are responsible of these discrepancies among different studies. For instance, some studies used radioimmunoassay for circulating TSH measurements (11) or included subjects with overt thyroid disease (22) or taking thyroid hormone medications (12, 23, 24) when categorizing subclinical thyroid dysfunctions and others failed to adjust for relevant confounders or initiation of hormone therapy (7, 23–25). Furthermore, it is possible that the effect of thyroid dysfunction on health and mortality may be different in older compared to younger persons. Using data from the InCHIANTI study, a large cohort study representative of community-dwelling individuals who live in Tuscany, Italy, we tested the hypothesis that hyper- or, hypothyroidism are associated with increased mortality in elderly subjects with unrecognized thyroid dysfunction during a 6-year follow-up.
METHODS
Study population
The study participants consisted of men and women, aged 65 and older, who participated in the Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in two small towns in Tuscany, Italy, a region which has been defined as mildly iodine deficient (26). The rationale, design, and data collection have been described elsewhere, (27). Briefly, in August 1998, 1,270 people aged 65 years and older were randomly selected from the population registry of Greve in Chianti (pop. 11,709) and Bagno a Ripoli (pop. 4,704), and of 1,256 eligible subjects, 1,155 (90.1%) agreed to participate. Of the 1,155 participants, 1,043 (90.3%) participated in the blood drawing. Plasma and serum were frozen in −80°C freezers for hormonal evaluations. Complete data on thyroid hormones were available in 968 (92.8%) participants. For the analyses presented here, we excluded participants who were on chronic treatment with drugs known to affect thyroid function, including thyroid hormone preparations, methimazole, propylthiouracil, amiodarone, and lithium, as well as those who were affected by low-T3 syndrome or non-thyroidal illness (total number of patients excluded = 17). The final study population included, at baseline, 951 subjects aged 65 years and older. Participants received an extensive description of the study and participated after written, informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee. Participants were evaluated again for a three-year follow-up visit from 2001–2003 (n = 926) and six-year follow-up visit from 2004–2006 (n = 844). At the end of the filed data collection, we collected data on mortality of the original InCHIANTI cohort, using data from the Mortality General Registry maintained by the Tuscany Region and the death certificates that were deposited immediately after the death at the Registry office of the Municipality of residence.
Laboratory measures
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were immediately obtained and stored at −80° C. Plasma concentrations of TSH, free triiodothyronine (FT3), and free thyroxine (FT4) were measured using a chemiluminescent immunoassay (Vitros Reagent, Ortho-Clinical Diagnostics, Johnson & Johnson Medical Section, Milan, Italy). Reference normal ranges were 0.46 to 4.68 mIU/L for TSH, 2.77 to 5.27 pg/mL for FT3, and 0.77 to 2.19 ng/dl for FT4. Assay sensitivities were 0.003 mIU/L for TSH, 0.39 pg/mL for FT3, and 0.03 ng/dL for FT4. Intra-assay coefficients of variation (CVs) were 3.9% to 5.3% over the range 0.06 – 80.11 mIU/L for TSH, 4.4% to 5.1% over the range 2.86 – 11.90 pg/mL for FT3, and 4.5% to 5.3% over the range 0.61 – 3.90 ng/dL for FT4. Interassay CVs were less than 9% for all three hormones.
Serum Interleukin 6 (IL-6) and Tumor Necrosis Factor-α (TNF-α) levels were measured by enzyme linked immunosorbent assay (ELISA) using ultrasensitive commercial kits (Human Ultrasensitive, BIOSOURCE International Inc., Camarillo California USA). Serum IL-6 concentrations were expressed in pg/mL, with a minimum detection limit of 0.10 pg/mL. Serum concentrations of TNF-α were expressed in pg/mL with a minimum detection limit of 0.09 pg/mL. Both IL-6 and TNF- α measurements had intra- and interassay CVs < 7%.
Other covariates
Demographic information and information on smoking and medication use were collected using standardized questionnaires. All participants were examined by a trained geriatrician. Diseases were ascertained according to standard, pre-established criteria and algorithms that combine information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations and blood tests (26). Diseases included in the current analysis were represented by congestive heart failure (CHF), stroke, and cancer.
Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Smoking status was assessed according to self-reported data. Pack-years, which is a measure of smoking exposure that combines intensity and duration, was calculated (28). Alcohol intake, expressed as g/day, was estimated using the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire, validated in the InCHIANTI population (29). Educational level was recorded as years of school. Cognitive function was evaluated using the Mini-Mental State Examination, and the total score was adjusted for educational level and age (30). Depressive symptoms were measured with the 20-item Center for Epidemiologic Studies–Depression scale. Participants with a Center for Epidemiologic Studies–Depression scale score of 16 or more were considered to be depressed (31). The total energy expenditure in leisure time of physical activity was expressed in METs by using a modified version of a standard questionnaire (32)
Definition of thyroid function
Participants were classified, according to TSH and free thyroid hormone concentrations into 5 categories: overt hypothyroidism (TSH >4.68 mIU/L and FT4<0.78 ng/dL), subclinical hypothyroidism (TSH >4.68 mIU/L and FT4 0.77–2.19 ng/dL), euthyroidism (TSH 0.46–4.68 mIU/L), subclinical hyperthyroidism (TSH <0.46 mIU/L, FT4 0.77–2.19 ng/dL and FT3 2.77–5.27 pg/mL), and overt hyperthyroidism (TSH <0.46 mIU/L, with FT4 >2.19 ng/dL and/or FT3 >5.27 pg/mL). Low-T3 syndrome or non-thyroidal illness was diagnosed when FT3 was < 2.77 pg /mL with normal FT4 and TSH or when FT3 was < 2.77 with FT4< 0.77 and normal TSH). Thyroid function test were measured in 2005. Thyroid function test results were not provided to participants.
Ascertainment of Events
Information about deaths was obtained from reviews of medical records, registers of municipality, autopsy reports. Ascertainment of mortality in the InCHIANTI study was 100%. The incident events in this report occurred after baseline and through May 30, 2006, with a mean duration of follow-up of six years. Cardiovascular deaths were defined as those due to atherosclerosis, including peripheral vascular disease, cerebrovascular events, coronary heart disease, and other cardiovascular causes, including CHF.
Statistical analysis
Survival analysis (Kaplan-Meier) and Cox proportional hazards models adjusted for age, BMI, log IL-6, TNF-alpha, educational level, depression, smoking, coronary heart disease (including angina and myocardial infarction), CHF, stroke and cancer were used to assess the effects of SHyper or Shypo on mortality. Covariates for adjustment were included in the model if in univariate analysis they were statistically associated with mortality. Kaplan Meier survival curves were compared using log-rank test. All analyses were performed with the SAS statistical package, version 9.1 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Of the 951 subjects who were considered for this study, 819 were euthyroid, 83 had SHyper. Of these subjects, 16 had TSH values lower than 0.1 mIU/L. Twenty-nine subjects had SHypo. Overt hypothyroidism and overt hyperthyroidism were found in 5 and 15 subjects, respectively. Due to the low number of subjects, these two types of thyroid dysfunction were excluded from all analyses. Therefore, since the two most representative groups of thyroid dysfunction were represented by SHyper and SHypo, the relationship between thyroid dysfunction and mortality was calculated for both these two groups of subjects in comparison with the euthyroid group. During the six years of follow-up, 210 deaths occurred (22.1 %) of which 98 (46.6%) due to cardiovascular causes. The baseline demographic and general characteristics of the participants who were alive versus those who died during the six years of follow-up are shown in Table 1. Subjects who died were older, more likely to be male and had higher baseline prevalence of congestive heart failure and stroke compared with those who survived over six years of follow up. Also, at baseline, serum FT4 levels were significantly higher (P<0.04) among dead individuals than among those who were alive at the end of the follow-up, but no differences in TSH levels were found between groups.
Table 1.
Characteristics of the study population at enrolment
| Characteristics (All Participants) | Survived (n = 741) | Dead (n = 210) | P2 |
|---|---|---|---|
| SHypo (%) | 23 (3.1) | 6 (3.0) | 0.65 |
| Euthyroidism (%) | 654 (89.3) | 165 (82.9) | 0.48 |
| SHyper (%) | 55 (7.5) | 28 (14.1) | 0.02 |
| Age (year)1 | 73.6 (6.2) | 82.2 (7.7) | <.0001 |
| Sex (%male) | 306 (41.3) | 103 (49.3) | 0.003 |
| CES-D Score1 | 12.6 (8.7) | 14.0 (8.9) | 0.86 |
| Body mass index (kg/m2)1 | 27.5 (4.1) | 27.0 (3.9) | 0.44 |
| IL-6 (pg/mL)1 | 1.9 (3.4) | 3.6 (6.9) | 0.11 |
| TNF-alpha (pg/mL)1 | 3.7 (5.3) | 4.3 (6.1) | 0.51 |
| FT4 (ng/dL)1 | 1.43 (0.3) | 1.59 (0.5) | 0.008 |
| FT3 (pg/mL)1 | 4.31 (0.6) | 4.15 (0.9) | 0.41 |
| TSH (mIU/L)1 | 1.78 (3.0) | 2.16 (7.9) | 0.65 |
| Congestive heart failure (%) | 122 (16.5) | 63 (30.1) | 0.001 |
| Cancer (%) | 47 (6.3) | 19 (9.1) | 0.17 |
| Stroke (%) | 37 (5.0) | 38 (17.8) | 0.0009 |
| Smoking (pack-years)1 | 11.9 (20.3) | 13.4 (22.1) | 0.47 |
| Alcohol Intake (g/day)1 | 16.8 (16.0) | 14.1 (15.1) | 0.08 |
| Physical Activity (METs)1 | 82.2 (30.6) | 72.7 (25.5) | 0.88 |
Mean (SD) for continuous variables or percentages as noted;
Age- and sex-adjusted ANCOVA
SHypo, Subclinical Hypothyroidism; SHyper, Subclinical Hyperthyroidism
Normal ranges: FT3, 2.77 to 5.27 pg/mL; FT4, 0.77 to 2.19 ng/dl; TSH, 0.46 to 4.68 mIU/L.
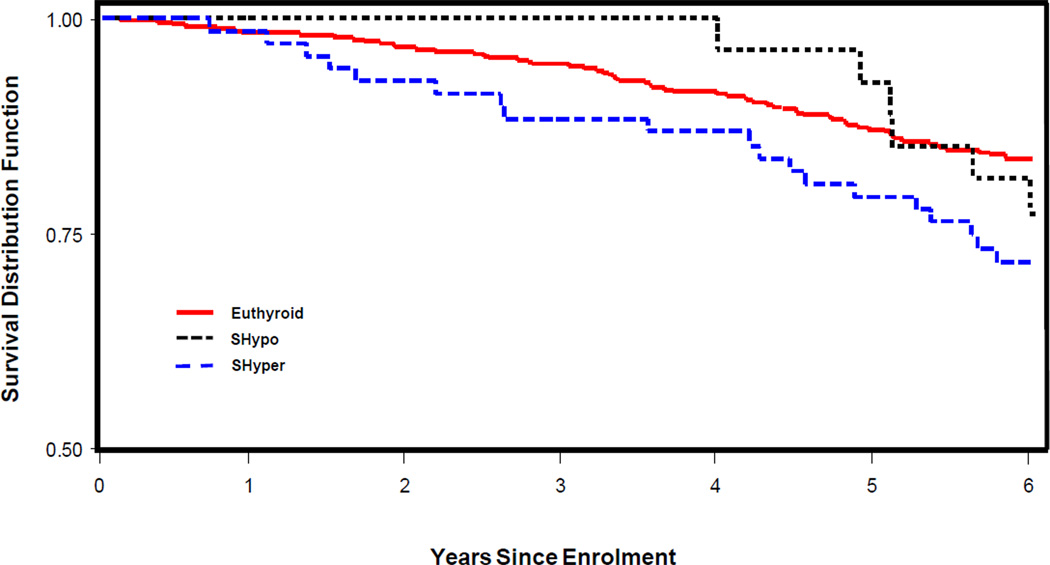
Kaplan–Meier analysis (Figure 1) revealed higher overall mortality for SHyper (P<0.04) group in comparison to the euthyroid group. No difference in survival was found between euthyroid and SHypo groups.
Figure 1.
Kaplan-Meyer survival curves of older adults showing the relation between overall survival and thyroid dysfunction.
SHypo, Subclinical Hypothyroidism; SHyper, Subclinical Hyperthyroidism
After adjustment for confounders in a Cox proportional hazard model, SHyper compared with euthyroidism was a significant independent risk factor for all-cause mortality (Model 2: HR, 1.65; 95% CI 1.02–2.69, P< 0.05; Table 2). When the association between SHyper and cardiovascular mortality was evaluated, a lack of a significant relationship was found (Model 2: HR, 1.42; 95% CI, 0.59–3.41, P= 0.43; Table 3). Within the SHyper group, all-cause mortality was statistically increased in subjects (N. 67) with TSH above 0.1 mIU/L (Model 2: H.R., 2.12; 95% CI, 1.41–3.20, P=0.005) as compared to euthyroid subjects. In Shyper subjects with TSH <0.1 mIU/L, all-cause mortality was found to be increased in comparison to euthyroid subjects with an increment which was close to, but did not reach, the statistical significance (Model 2: H.R., 2.04; 95% CI, 0.96–4.34, P=0.06). For this reason, we could not compare all-cause mortality between these two groups of SHyper elderly subjects. No association with cardiovascular mortality was found in SHyper subjects with TSH>0.1 mIU/L (Model 2: H.R., 1.38; 95% CI, 0.51–3.73, P=0.52) as compared to euthyroid subjects. Due to the low number of events, we could not perform such analysis in SHyper subjects with TSH<0.1mIU/L.
Table 2.
Multivariate relationship between Subclinical Hyperthyroidism and all-cause mortality in older adults
| Characteristic | H.R. | 95% C.I. | P |
|---|---|---|---|
| Model 1 | |||
| SHyper vs Euthyroidism | 2.018 | 1.351–3.014 | <.0001 |
| Age (years) | 1.156 | 1.136–1.178 | <.0001 |
| Sex (female) | 0.530 | 0.398–0.706 | <.0001 |
| Model 2 | |||
| SHyper vs Euthyroidism | 1.659 | 1.021–2.696 | <.008 |
| Age (years) | 1.153 | 1.127–1.180 | <.0001 |
| Sex (female) | 0.475 | 0.339–0.665 | <.0001 |
| BMI (Kg/cm2) | 1.021 | 0.978–1.065 | 0.34 |
| Cancer (%) | 1.626 | 0.891–2.965 | 0.11 |
| Congestive Heart Failure (%) | 1.427 | 1.152–1.649 | 0.005 |
| Stroke (%) | 1.502 | 1.208–1.868 | 0.002 |
SHyper, Subclinical Hyperthyroidism
Table 3.
Multivariate relationship between cardiovascular mortality and thyroid status in older adults
| Characteristic | H.R. | 95% C.I. | P |
|---|---|---|---|
| SHyper vs Euthyroidism | |||
| Model 1 | 1.72 | 0.82–3.64 | 0.15 |
| Model 2 | 1.42 | 0.59–3.41 | 0.43 |
| SHypo vs Euthyroidism | |||
| Model 1 | 0.51 | 0.10–2.47 | 0.39 |
| Model 2 | 0.50 | 0.10–2.55 | 0.41 |
SHyper, Subclinical Hyperthyroidism; SHypo, Subclinical Hypothyroidism
We found no evidence of an association between SHypo compared to euthyroidism for all-cause (Model 2: H.R., 0.98; 95% CI, 0.43–2.26, P=0.98; data not shown) and cardiovascular (Model 2: HR, 0.50; 95% CI, 0.10–2.55; p=0.41; Table 3) mortality.
With regard to the cohort of 819 euthyroid elderly subjects of our population, a multivariate analysis adjusted for multiple confounders, demonstrated that plasma TSH concentrations was independently associated with a reduced risk for all-cause, but not cardiovascular, mortality (HR, 0.76, 95% CI, 0.57–0.99, P<0.05; Table 4). An inverse association between FT3 and all-cause mortality was found, which was close to the statistical significance (Model 2: H.R., 0.60, 95% CI, 0.35–1.02; P=0.06). No significant association between FT4 and all-cause mortality was documented (Model 2: H.R., 1.85, 95% CI, 0.88–3.89; P=0.11). No significant association between FT3 and/or FT4 plasma concentrations and cardiovascular mortality was found.
Table 4.
Multivariate relationship between plasma TSH levels and all-cause mortality in euthyroid elderly subjects
| Characteristic | H.R. | 95% C.I. | P |
|---|---|---|---|
| Model 1 | |||
| TSH (mIU/mL) | 0.787 | 0.611–1.013 | 0.06 |
| Age (years) | 1.216 | 1.178–1.254 | <.0001 |
| Sex (female) | 0.414 | 0.270–0.633 | <.0001 |
| Model 2 | |||
| TSH (mIU/mL) | 0.746 | 0.564–0.985 | 0.03 |
| Age (years) | 1.201 | 1.159–1.244 | <.0001 |
| Sex (female) | 0.416 | 0.261–0.663 | 0.0002 |
| BMI (Kg/cm2) | 0.997 | 0.941–1.057 | 0.93 |
| Cancer (%) | 1.905 | 0.840–4.319 | 0.93 |
| Congestive Heart Failure (%) | 0.840 | 1.028–1.699 | 0.03 |
| Stroke (%) | 1.867 | 1.296–1.969 | 0.122 |
DISCUSSION
The results of this study demonstrate that six-year all-cause mortality is higher in older patients with endogenous SHyper compared with those with normal thyroid function. So far, only a few studies have examined the risk of mortality in elderly patients of both sexes with endogenous SHyper (7, 10, 11) with contrasting results. Our data add one more evidence to support this association. Interestingly, our population is from a mild to moderate iodine deficient area (26) and, although the cause of SHyper are not known in our database, autonomous functioning nodular thyroid disease accounts most likely for the thyrotoxicosis of subjects residing in this area. Mild hyperfunctioning thyroid nodules may progress, over time, toward more pronounced activity (33). It is possible, therefore, that the increased six-year mortality we have observed in our elderly SHyper subjects may be the consequence of a tendency toward an increase, over time, in nodular thyroid hyperfunction.
Another important aspect of our study is represented by sample size of our elderly SHyper subjects. The majority of the studies so far undertaken to explore the association between SHyper and mortality in elderly people are characterized by a number of subjects which is lower than that reported in the present study (i.e., we examined N. 83 SHyper subjects at baseline, who accounted for 8% prevalence of this thyroid disorders in our population). This might have caused those studies to be underpowered. A notable strength of our study is that all participants had measured FT3, in addition to FT4 and TSH, which allows a more appropriate classification into thyroid function categories and a better diagnosis of nonthyroidal illness which, in some cases, could be misclassified as SHyper based on a low TSH but normal FT4 levels. Also, the measurements of IL-6 and TNF-α gave further support to the exclusion of nonthyroidal illness in our data set, again underlining that subjects with endogenous SHyper were carefully selected in our study.
An important issue to be taken into account in the clinical characterization of SHyper in the elderly, is represented by comorbidities. The effects of SHyper may vary depending on comorbidities and SHyper is associated with a higher mortality rate in chronically ill geriatric patients (34). Comorbidities represent an important clinical characteristic of aging subjects and may vary between studies, thus potentially influencing the different reports on the clinical impact of SHyper, including mortality. However, in this study we cannot draw any conclusion on this issue, since we did not explore this hypothesis in our analysis.
The association between SHyper and mortality of cardiovascular type is controversial (14–16). We did not find any statistically significant correlation between SHyper and cardiovascular mortality in our population, our results being in agreement with other reports (10). However, our analyses suggests an increase in cardiovascular mortality in SHyper group, although with a lower point estimate than that of total mortality. Of interest, one of the most important independent covariates we found to be significantly associated with cardiovascular mortality, was represented by CHF (data not shown) which could be included in the causal pathway between hyperthyroidism and cardiovascular mortality. This could have affected, in our Cox regression model, the association with cardiovascular mortality which was found to be significant for CHF but not for SHyper. However, we cannot exclude that patients with SHyper who might have been also diagnosed with a cardiovascular event might have been treated with thyrostatic drugs, thus reducing the likelihood of an association between this thyroid dysfunction and cardiovascular mortality. Subclinical hyperthyroidism has been considered to be more harmful if TSH circulating concentrations are lower than 0.1 mIU/L (14, 35). In our study, the number of subjects with TSH ≤ 0.1 mIU/L was low ( i.e., only 16 subjects, out of N. 83 SHyper subjects), and this could have affected our data, by a reduction, at least theoretically, in the number of cardiovascular deaths. Small sample size could also have determined the lack of a significant association between this subgroup of SHyper subjects and all-cause mortality which was close to, but did not reach, the statistical significance. It is of interest to note, however, that the increase in all-cause mortality was confirmed in the subgroup of SHyper subjects with TSH >0.1 mIU/L, when compared to euthyroid subjects. This is an important observation which may be clinically relevant in the management of SHyper subjects with mildly decreased TSH levels.
Therefore, in spite of the lack of a clear-cut association between SHyper and cardiovascular mortality our data indicate that all-cause mortality is increased in SHyper older individuals. This may be explained by the numerous reports demonstrating that SHyper may exert negative influences other than those on the cardiovascular system. For example, a SHyper-associated reduction in cognitive function and physical performance has been demonstrated in the same aging population which is being considered in this study (5, 36, 37). All these effects of SHyper may lead to increased frailty and eventually increase the risk of death.
Although randomized controlled trials on the effect of treatment of SHyper on clinical outcomes are still needed, the fact that this thyroid dysfunction may be generally responsible for negative clinical outcomes has been underlined by the guidelines on the management and treatment of thyrotoxicosis, which have been recently issued (38) and which recommend the treatment of SHyper in subjects aged more than 65years, especially in the presence of comorbidities.
The relationship between SHypo and mortality in elderly subjects is controversial. Some studies have demonstrated that SHypo is associated with an increased risk of all-cause mortality and a modest increase in cardiovascular mortality especially in subjects with TSH concentration of 10 mIU/L or greater (9, 39–41). However, in many of these studies, old subjects were only poorly represented. We did not find any correlation between SHypo and all-cause mortality. It has to be pointed out, however, that the sample size of our subclinically hypothyroid subjects was low with a relatively low power of our analysis.
Interestingly, our results also demonstrated that even in euthyroid elderly subjects plasma TSH is independently associated with a reduced risk for all-cause mortality. Plasma FT4 levels were not significantly associated with all-cause mortality in euthyroid subjects; however, the point estimate suggests a tendency toward an association between higher FT4 and all-cause mortality in these group of subjects. This could be in line with our findings of a higher all-cause mortality in subjects with high FT4 in the overall analysis of the whole our cohort. Also, in euthyroid subjects we found an inverse association between FT3 and all-cause mortality which was close to the statistical significance. This could suggest that lower FT3 values in euthyroid subjects may reflect clinical conditions which elevate the risk of mortality, such as systemic illnesses which may determine a reduced FT3-associated sick euthyroid syndrome. In this perspective, low circulating FT3 levels in our euthyroid subjects could be interpreted as negative markers for the overall clinical outcome. These data represent an important information about the overall relationship between thyroid function and mortality and are in line with the concept of a general protective effect of mild hypothyroidism in elderly people (6, 11, 20, 42). All these findings are also supported by data from van den Beld and co-workers who demonstrated a better 4-year survival in elderly men who had low serum thyroxine concentrations (43).
A particular strength of this study is represented by the fact that thyroid function test were not provided to participants. Therefore, the possibility of the beginning of a thyroid treatment as a potential confounder in the results is unlikely to have occurred. One major limitation of this study is represented by the fact that only baseline thyroid function tests are available, so we do not know whether the diseases were stable, progressive, or reversible after diagnosis. However, this is a characteristic shared by many epidemiological studies on these topic.
In conclusion, the results of this study demonstrate that endogenous SHyper is associated with an increase in all-cause mortality in aging subjects. Intervention study, especially in late-adulthood or in middle-aged subjects are needed in order to define the effects of treatment of this thyroid disorder on clinical outcomes
ACKNOWLEDGMENTS
Funding Sources: This work was supported as a “target project” (ICS 110.1|RS97.71) by the Italian Ministry of Health and, in part, by the U.S. National Institute on Aging ( contracts 263_MD_9164_13 and 263_MD_821336), and by grant n. FIL0774249 from MURST, Rome (to G.C.)
Sponsor’s Role : none
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author’s Contributions:
All authors meet the following conditions: 1) Substantial contributions on conception and design, acquisition of data, analysis and interpretation of data; 2) drafting the article and revising it critically for important intellectual content; 3) final approval of the version to be published.
REFERENCES
- 1.Mariotti S, Franceschi C, Cossarizza A, et al. The Aging Thyroid. Endocr Rev. 1995;16:686–715. doi: 10.1210/edrv-16-6-686. [DOI] [PubMed] [Google Scholar]
- 2.Roberts LM, Pattison H, Roalfe A, et al. Is sub clinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573–581. doi: 10.7326/0003-4819-145-8-200610170-00006. [DOI] [PubMed] [Google Scholar]
- 3.Vitale G, Fatti LM, Prolo S, et al. Screening for hypothyroidism in older hospitalized patients with anemia: A new insight into an old disease. J Am Geriatr Soc. 2010;58:1825–1827. doi: 10.1111/j.1532-5415.2010.03040.x. [DOI] [PubMed] [Google Scholar]
- 4.Aghini-Lombardi F, Antonangeli L, Martino, et al. The spectrum of thyroid disorders in an iodine-deficient community: The Pescopagano survey. J Clin Endocrinol Metab. 1999;84:561–566. doi: 10.1210/jcem.84.2.5508. [DOI] [PubMed] [Google Scholar]
- 5.Ceresini G, Lauretani F, Maggio, et al. Thyroid function abnormalities and cognitive impairment in elderly people: Results of the Invecchiare in Chianti Study. J Am Geriatr Soc. 2009;57:89–93. doi: 10.1111/j.1532-5415.2008.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biondi B, Cooper DS. The Clinical Significance of Subclinical Thyroid Dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 7.Parle JV, Maisonneuve P, Sheppard MC, et al. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: A 10-year cohort study. Lancet. 2001;358:861–865. doi: 10.1016/S0140-6736(01)06067-6. [DOI] [PubMed] [Google Scholar]
- 8.Haentjens P, Van Meerhaeghe A, Poppe K, et al. Subclinical thyroid dysfunction and mortality: An estimate of relative and absolute excess all-cause mortality based on time-to-event data from cohort studies. Eur J Endocrinol. 2008;159:329–341. doi: 10.1530/EJE-08-0110. [DOI] [PubMed] [Google Scholar]
- 9.Sgarbi JA, Matsumura LK, Kasamatsu TS, et al. Subclinical thyroid dysfunctions are independent risk factors for mortality in a 7.5-year follow-up: The Japanese Brazilian thyroid study. Eur J Endocrinol. 2010;162:569–577. doi: 10.1530/EJE-09-0845. [DOI] [PubMed] [Google Scholar]
- 10.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jongh RT, Lips P, van Schoor NM, et al. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol. 2011;165:545–554. doi: 10.1530/EJE-11-0430. [DOI] [PubMed] [Google Scholar]
- 12.Waring AC, Harrison S, Samuels MH, et al. Osteoporotic Fractures in Men (MrOS) Study. Osteoporotic Fractures in Men (MrOS) Study. Thyroid function and mortality in older men: a prospective study. J Clin Endocrinol Metab. 2012;97:862–870. doi: 10.1210/jc.2011-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochs N, Auer R, Bauer DC, et al. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148:832–845. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 14.Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172:799–809. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biondi B. Should we treat all subjects with subclinical thyroid disease the same way? Eur J Endocrinol. 2008;159:343–345. doi: 10.1530/EJE-08-0527. [DOI] [PubMed] [Google Scholar]
- 16.Biondi B. Invited Commentary: Cardiovascular mortality in subclinical hyperthyroidism: an ongoing dilemma. Eur J Endocrinol. 2010;162:587–589. doi: 10.1530/EJE-09-1095. [DOI] [PubMed] [Google Scholar]
- 17.Biondi B. How could we improve the increased cardiovascular mortality in patients with overt and subclinical hyperthyroidism? Eur J Endocrinol. 2012;167:295–299. doi: 10.1530/EJE-12-0585. [DOI] [PubMed] [Google Scholar]
- 18.Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for Ischemic Heart Disease and All-Cause Mortality in Subclinical Hypothyroidism. J Clin Endocrinol Metab. 2004;89:3365–3370. doi: 10.1210/jc.2003-031089. [DOI] [PubMed] [Google Scholar]
- 19.McQuade C, Skugor M, Brennan DM, et al. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: A PreCIS Database Study. Thyroid. 2011;21:1–7. doi: 10.1089/thy.2010.0298. [DOI] [PubMed] [Google Scholar]
- 20.Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 21.Mariotti S. Editorial: Thyroid function and aging: do serum 3,5,3’-triiodothyronine and thyroid-stimulating hormone concentrations give the Janus response? J Clin Endocrinol Metab. 2005;90:6735–6737. doi: 10.1210/jc.2005-2214. [DOI] [PubMed] [Google Scholar]
- 22.Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 23.Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med. 2005;165:2467–2472. doi: 10.1001/archinte.165.21.2467. [DOI] [PubMed] [Google Scholar]
- 24.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 25.Hak AE, Pols HA, Visser TJ, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: The Rotterdam Study. Ann Intern Med. 2000;132:270–278. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Aghini Lombardi FA, Pinchera A, Antonangeli L, et al. Mild iodine deficiency during fetal/neonatal life and neuropsychological impairment in Tuscany. J Endocrinol Invest. 1995;18:57–62. doi: 10.1007/BF03349700. [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the In CHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 28.Deshpande N, Metter EJ, Lauretani F, et al. Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J Am Geriatr Soc. 2008;56:615–20. doi: 10.1111/j.1532-5415.2007.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pisani P, Faggiano F, Krogh V, et al. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centers. Int J Epidemiol. 1997;26:S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Berghout A, Wiersinga WM, Smits NJ, et al. Interrelationships between age, thyroid volume, thyroid nodularity, and thyroid function in patients with sporadic nodular goiter. Am J Med. 1990;89:602–608. doi: 10.1016/0002-9343(90)90178-g. [DOI] [PubMed] [Google Scholar]
- 34.Radácsi A, Kovács G, Bernard W, et al. Mortality rate of chronically ill geriatric patients with subnormal serum thyrotropin concentration: A 2-yr follow-up study. Endocrine. 2003;21:133–136. doi: 10.1385/endo:21:2:133. [DOI] [PubMed] [Google Scholar]
- 35.Sttewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year followup of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 36.Kalmijn S, Mehta KM, Pols HA, et al. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam Study. Clin Endocrinol. 2000;53:733–737. doi: 10.1046/j.1365-2265.2000.01146.x. [DOI] [PubMed] [Google Scholar]
- 37.Ceresini G, Ceda GP, Lauretani F, et al. Mild thyroid hormone excess is associated with a decreased physical function in elderly men. Aging Male. 2011;14:213–219. doi: 10.3109/13685538.2011.606514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahn RS, Burch HB, Cooper DS, et al. ATA/AACE Taskforce on Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2011;6:593–647. [Google Scholar]
- 39.Razvi S, Weaver JU, Vanderpump MP, et al. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: Reanalysis of the Wickham Survey cohort. J Clin Endocrinol Metab. 2010;95:1734–1740. doi: 10.1210/jc.2009-1749. [DOI] [PubMed] [Google Scholar]
- 40.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce EN. In people with subclinical hypothyroidism, TSH levels >10 mIU/l may predict increased risk of coronary heart disease and related mortality. Evid Based Med. 2011;16:31–32. doi: 10.1136/ebm1166. [DOI] [PubMed] [Google Scholar]
- 42.Waring AC, Arnold AM, Newman AB, et al. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97:3944–3950. doi: 10.1210/jc.2012-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Beld A, Visser TJ, Feelders RA, et al. Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90:6403–6409. doi: 10.1210/jc.2005-0872. [DOI] [PubMed] [Google Scholar]