Abstract
The mitochondrial pathway of apoptosis proceeds when the outer mitochondrial membrane (OMM) is compromised by the pro-apoptotic BCL-2 family members, BAK and BAX. Once activated, BAK and BAX form proteolipid pores in the OMM leading to mitochondrial outer membrane permeabilization (MOMP), and the release of inner membrane space proteins, such as cytochrome c, which promotes caspase activation. The use of isolated mitochondria has been instrumental to understanding the key interactions necessary to engage BAK and BAX activation, MOMP, and apoptosis. Furthermore, it is possible to biochemically define the relationships between BCL-2 family function and mitochondrial physiology using isolated systems. Our laboratory uses freshly isolated mitochondria from numerous sources to better understand BCL-2 family function and requirements for BAK and BAX activation. Here, we will discuss commonly used in vitro techniques to perform MOMP and cytochrome c release assays; and provide several key methodologies to implicate BAK and BAX activity in these processes.
Keywords: Apoptosis, BAK, BAX, BCL-2 family, Cytochrome c, Mitochondria, MOMP
1. Introduction
The role of mitochondria within the apoptotic pathways was first suggested when caspase activity resulted from Xenopus oocyte extract co-incubation with purified mitochondria [1]. This caspase activity was blocked by the addition of recombinant BCL-2 (B-cell CLL/lymphoma-2), suggesting that BCL-2 could prevent mitochondrial engagement of the cytosol [2]. Since then, the BCL-2 family has grown to include almost twenty members that are divided into two functional classes of proteins: anti-apoptotic and pro-apoptotic. Most cells express a variety of anti-apoptotic and pro-apoptotic BCL-2 proteins, and their interactions dictate survival or commitment to apoptosis [3].
Anti-apoptotic BCL-2 proteins are comprised of up to four BCL-2 homology domains (BH1-4) and are generally integrated within the outer mitochondrial membrane (OMM), but may be present in other membranes like the endoplasmic reticulum or in the cytosol. BCL-2, BCL-xL (BCL-2 related gene, long isoform), and MCL-1 (myeloid cell leukemia 1) are the major members of the anti-apoptotic BCL-2 repertoire that function to preserve OMM integrity by directly binding and inhibiting the pro-apoptotic BCL-2 proteins [3]. Most BCL-2 family interactions are conferred by the ‘BCL-2 core’ structural unit, which is comprised of several amphipathic alpha (α) helices creating a hydrophobic groove that binds pro-apoptotic BH3 domains [4].
The pro-apoptotic BCL-2 members are divided into effectors (which contain BH1-4) and the BH3-only proteins. The effector proteins BAK (BCL-2 antagonist killer 1) and BAX (BCL-2 associated × protein) homo-oligomerize into proteolipid pores within the OMM and are required to promote mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release [5, 6]. However, these effectors require an activation step, upon which they oligomerize and gain the capacity to permeabilize membranes [7–9]. It is thought that BAK and BAX activation results from at least two distinct scenarios: (1) interactions with cell stress-induced ‘direct activator’ BH3-only proteins, or (2) physico-chemical effects of heat, elevated pH, and hydrophobics (e.g., sphingolipids, the OMM) [9–12]. Here, we will primarily focus on BH3-only protein induced BAK and BAX activation and cytochrome c release.
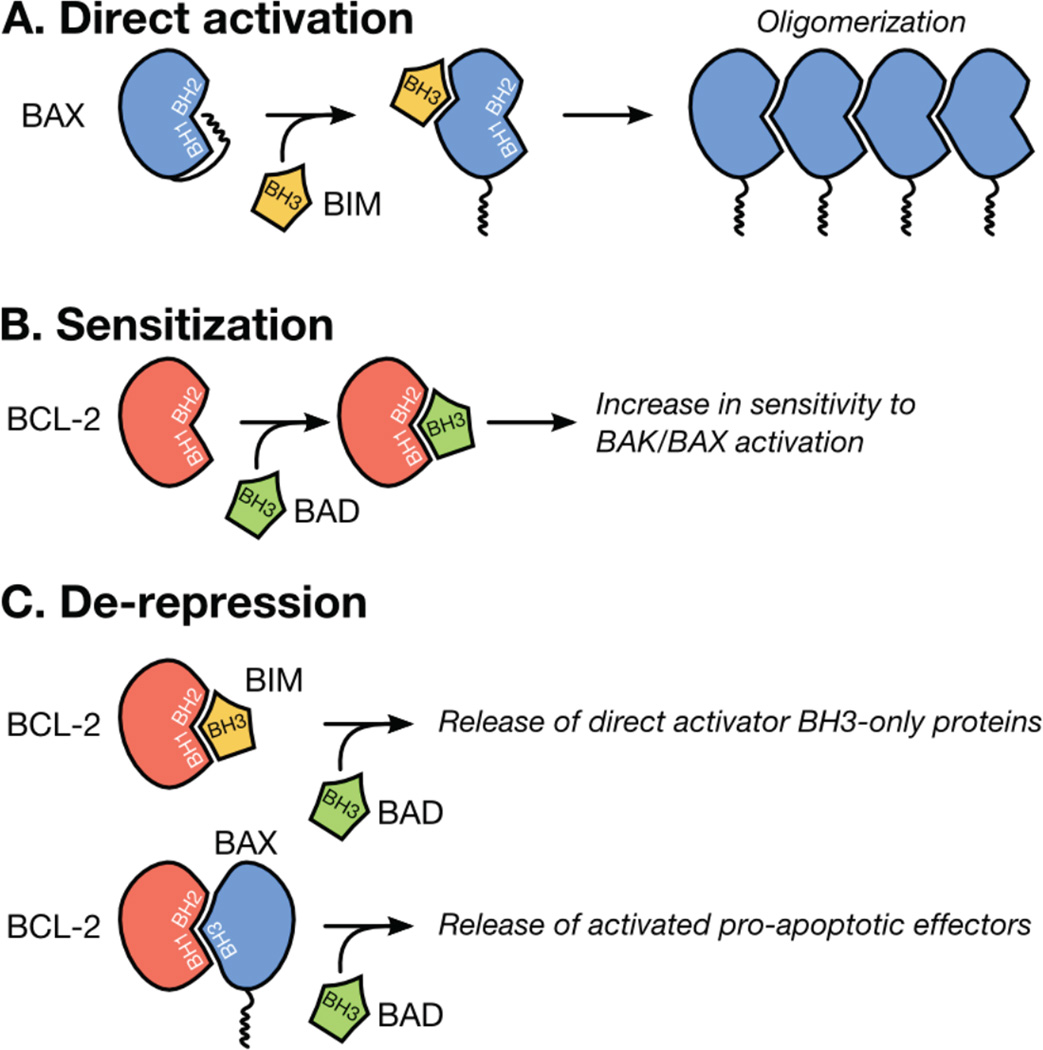
BID (BH3 interacting domain death agonist) and BIM (BCL-2 interacting mediator of cell death) are the best characterized direct activator BH3-only proteins and function via their BH3 domains to convert monomeric forms of BAK and BAX into potent killers (Figure 1A) [9, 13]. The remaining BH3-only proteins (e.g., BAD, BCL-2 antagonist of cell death) regulate MOMP by altering the availability of the anti-apoptotic BCL-2 repertoire to inhibit subsequent pro-apoptotic BCL-2 family members (Figures 1B–C) [9, 13, 14]; this function will be examined in the upcoming sensitization and de-repression experimental sections.
Figure 1.
BCL-2 family interactions that promote BAK/BAX activation and MOMP. (A) Pro-apoptotic effectors (e.g., BAX, in blue) are activated by direct activators BH3-only proteins (e.g., BIM, in yellow). After transient association with a direct activator BH3-only protein, BAX undergoes conformational changes, oligomerizes, and induces MOMP. (B) Sensitizer BH3-only proteins (e.g., BAD, in green) directly bind the anti-apoptotic BCL-2 proteins (e.g., BCL-2, in red); this inhibits their ability to neutralize subsequent pro-apoptotic proteins and lowers the cellular threshold leading to BAK/BAX activation and MOMP. (C) De-repression is possible when a direct activator BH3-only protein or activated BAK/BAX monomer is actively sequestered by an anti-apoptotic BCL-2 protein. De-repression occurs when an additional BH3-only protein competes for the binding to the anti-apoptotic protein and promotes the release and function of the sequestered direct activator BH3-only protein or pro-apoptotic effector.
Elegant biochemical studies have revealed the mechanisms by which direct activator BH3-only proteins promote BAK/BAX activation and oligomerization [15, 16]. In brief, BAK and BAX undergo amino-terminal re-arrangement, reorganize their BCL-2 core α helical structure, homo-dimerize, and then form high molecular weight, pore-forming units to disrupt the OMM. Each of these steps can be experimentally observed, and we will discuss appropriate assays later. Most structural insights into effector molecule activation have focused on BAX, but there are some data available on BAK [17–20]. Structural investigations suggest that BAX activation is initiated by BIM (and by extension, activated BID) binding to a ‘trigger site’ near BAX α1, which leads to amino terminal rearrangements, mobilization of α9, and exposure of the BAX BH3 domain [18]. Furthermore, BAX BH3 exposure is suggested to propagate auto-activation of downstream inactive BAX monomers, leading to dimerization and eventual pore forming units, however the interfaces that support BAX dimerization are debated [21, 22]. Moreover, the direct activation of BAK monomers also induces conformational changes that result in BAK BH3 exposure, and dimerization with another activated BAK monomer via BH3·groove interactions, resulting in symmetric dimers, and multimerization of dimers via α6 [23, 24]. The number of BAK and/or BAX dimers that are required to induce MOMP are not well established, and the structural details of how BAK or BAX position with the OMM are also not known.
Here, we will discuss the experimental details of examining the direct activation, conformational changes, and oligomerization of BAK and BAX that are associated with MOMP and apoptosis using isolated mitochondria and recombinant BCL-2 family proteins. These systems afford the opportunity to directly compare various sources of mitochondria (e.g., comparing wild-type and bak−/− bax−/− mitochondria), along with a myriad of recombinant BCL-2 family proteins, mutants, and peptides. In addition, these assays can determine the influence of non-BCL-2 family proteins on MOMP [25, 26], and can be extended to examine how cellular stress scenarios impact on mitochondrial responses to the BCL-2 family [11, 12].
2. Mitochondrial isolation techniques
There are numerous approaches to isolate functional, intact mitochondria. Our laboratory has had most success with utilizing detergent-free homogenization and differential centrifugation to routinely isolate high quality, high yield heavy membrane fractions. In the literature, one will read about purified heavy membrane fractions and mitochondria. In general, this nomenclature is interchangeable, as the heavy membrane (HM) fraction is normally comprised of a high percentage of mitochondria. Below, we will discuss how to isolate mitochondria from two common sources: (1) fresh murine liver, and (2) primary or cultured cells.
2.1 Liver heavy membrane fractions
Murine liver HM fractions are a robust model to examine BCL-2 family function, BAK/BAX activation, and MOMP for several reasons. Primarily, each liver normally yields ~ 20 mg of mitochondrial protein, which is sufficient for hundreds of MOMP reactions. In addition, these mitochondrial preparations are quick and inexpensive; in the event the donor mouse is precious, the heavy membrane fractions can be prepared using an alternative buffer, aliquoted into appropriate experimental volumes, and stored at −80°C for future use, as described [27]. A graphical summary of the isolation procedure is provided in figures 2A–C.
Figure 2.
An illustration of the procedure utilized to isolate heavy membrane fractions from fresh murine liver. (A) Processing the liver. The gall bladder is discarded and the liver excised, minced with a razor blade, and homogenized in a Potter-Elvehjem dounce in two batches. (B) Differential centrifugation. The homogenate undergoes a series of centrifugation steps described in Table 1. (C) Quantification. The heavy membrane pellet is resuspended in MIB and stored on ice. A 1:200 dilution of HMs in MIB is measured at OD520, and the resuspended HM fraction is adjusted to OD520 = 0.25.
HM fractions are purified from fresh murine liver, and we typically use three-month old female C57Bl/6 mice. After euthanizing the mouse according to your Institutional Animal Care and Use Committee guidelines, visualize the liver, and gently manipulate the bile duct and intact gall bladder away from the liver and discard. The four liver lobes are either dissected individually (which causes significant blanching of the lobes due to in situ bleeding), or the entire liver can be excised out of the abdomen (here, the liver loses very little blood, but the abdomen will quickly fill with blood). In either case, the freshly resected tissue should be immediately placed into ice-cold phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4; pH 7.4) and stored on ice until further processed. Pour off all the PBS, place the tissue onto a clean 10 cm2 Petri dish, and mince the liver into a fine paste using a razor blade. This step should be performed with the dish on ice, with no PBS on the dish. Mincing is complete when the paste contains no recognizable structures and is homogeneous in consistency.
Transfer half of the paste into a 15 ml Potter-Elvehjem dounce containing 10 ml of mitochondrial isolation buffer (MIB: 200 mM mannitol, 68 mM sucrose, 10 mM HEPES-KOH pH 7.4, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 0.1% BSA fraction V, add one Roche Complete EDTA-free protease inhibitor cocktail tablet per 50 ml MIB; n.b., the homogenizer and buffer should be waiting on ice during the liver resection and mincing), and begin to homogenize the paste. The first strokes will be the most difficult, and often these strokes will be abbreviated, meaning the pestle will not easily move from the top to the bottom of the dounce. To begin, it is best to insert the pestle into the dounce until it reaches the paste near the bottom of the dounce (do not crush the paste), then slowly move the pestle 3 – 5 centimeters up, and then return to the bottom. Repeat this abbreviated stroke until the all the liver paste has traveled past the Teflon pestle, indicating that the sample has been subjected to at least one complete homogenization stroke (do not twist the pestle, just move it smoothly up or down). The buffer above the Teflon pestle will become pink and cloudy during these abbreviated strokes and occasionally small bits of connective tissue or fat do not homogenize. Continue to homogenize the sample with five complete strokes, pour the homogenized sample into a chilled 17 × 100 mm tube, and store on ice while repeating the procedure with the second half of the liver paste.
The HM fraction is then isolated by a series of differential centrifugation steps that are outlined in table 1; all steps are performed in swinging bucket rotor (e.g., Sorvall HB-4) at 4°C, and the entire procedure is carried out without delays or stopping. The final HM pellet should be grayish brown, resuspended in 500 µl of MIB, and stored on ice for up to 2 hours. The HM preparation is quantified by measuring the OD520 with a 1:200 diluted sample of HMs in MIB. The OD520 reading is usually between 0.25 – 0.5, and the HM preparation is then diluted, if necessary, so the reading is 0.25. This is a rapid means of consistent quantification that eliminates additional protein assays.
Table 1.
Differential centrifugation steps required to isolate the liver HM fraction. All centrifugation steps are performed at 4°C in 17 × 100 mm tubes.
| Step | Centrifugal force | Time | Manipulation |
|---|---|---|---|
| 0 | - | - | Homogenize liver as described |
| 1 | 600 × g | 10 min | Pour supernatant into fresh tube, proceed to step 2, discard pellet |
| 2 | 3,500 × g | 15 min | Discard supernatant, resuspend pellet in 10 ml MIB, continue to step 3 |
| 3 | 1,500 × g | 5 min | Pour supernatant into fresh tube, proceed to step 4, discard pellet |
| 4 | 5,500 × g | 10 min | Discard supernatant, resuspend pellet in 10 ml MIB, repeat steps 3 & 4 |
| 5 | Discard supernatant, resuspend HM pellet in 500 µl MIB, store on ice |
BAK and BAX are both expressed in C57Bl/6 liver, but this HM isolation protocol does not co-purify cytosolic factors. Therefore, C57Bl/6 HM fractions only contain BAK as it is associated with the OMM, and no BAX since it is soluble. To ascertain BAX activity using isolated mitochondria, one must purify HM fractions from bak−/− or bak−/−baxf/− mice (available from The Jackson Laboratory, www.jax.org) and then add recombinant BAX [17, 28]. In the former genotype, the purified HM fraction is devoid of BAK, but low levels of activated and/or OMM-associated BAX could be present resulting in data misinterpretations. To avoid this, it is wiser to use bak−/−baxf/− animals where the final bax allele is genetically removed by a pharmacologically regulated, tissue-specific Cre recombinase (n.b., The bak−/−bax−/− phenotype is perinatal lethal, and one allele of bax rescues this lethality; therefore, the remaining bax allele is flanked by loxP sites and removed in a tissue-specific manner to generate a BAK/BAX-deficient liver) [28]. This ensures the purified HM fractions are free of both endogenous BAK and BAX.
2.2 Cell heavy membrane fractions
Primary or cultured cell lines are useful sources of mitochondria as numerous cell types and/or treatment conditions can be explored. For example, mitochondria isolated from staged lymphocytes can be analyzed for differential sensitivity to pro-apoptotic stimulation. Moreover, cultured cells can be pretreated with pro-apoptotic agents before HM isolation allowing for functional characterization of drug-induced BH3-only protein accumulation on the OMM. Two downsides to these systems are that limiting quantities of mitochondria are purified, and these mitochondria lose OMM integrity after minor experimental manipulation (i.e., cytochrome c release is observed in untreated samples); therefore, it is essential to organize experiments to maximize starting material, and minimize handling time/delays. As an aside, it is possible to perform cytochrome c release experiments on digitonin-permeabilized cells. Digitonin creates pores in the plasma membrane and allows for biochemical access to mitochondria in the absence of mitochondrial purification, but a thorough understanding of the total BCL-2 family expression and activation status is necessary to appropriately interpret the results [29].
We have had success using numerous cell culture lines for HM isolation, including: 293T, A375, HeLa, Jurkat, and mouse embryonic fibroblasts (primary and transformed) [14]. Cells are grown to approximately 100% confluency, and we typically culture enough cells to generate a 300 µl cell pellet for each isolation procedure. To begin, dissociate your cells from the culture vessels (or centrifuge if the cells are grown in suspension), pellet at a centrifugal force that is appropriate for your cells, and wash the cells with cold PBS twice to remove all residual cell culture media. Resuspend the cells in 1 ml of MIB, transfer to a 1.7 ml microcentrifuge tube, pellet, aspirate the supernatant, resuspend the cells in a volume of MIB that is equal to three times the cell pellet volume (e.g., resuspend a 300 µl cell pellet in 900 µl MIB), and incubate the cells on ice for 15 minutes.
Using a Pasteur pipette, transfer the cell suspension into a chilled 2 ml Potter-Elvehjem dounce. Support the dounce directly on the laboratory bench to improve stroke consistency, and only hold the top of the dounce to avoid heating the sample. Homogenize the cells with twenty consistent strokes (fast down, slow up; avoid completely removing the pestle from the suspension to prevent bubbles and foaming) and place the dounce on ice again. Place 5 µl of cell suspension on a glass slide, add trypan blue, mix, and observe for cell lysis using a light microscope. Lysed cells will not exhibit a halo by phase contrast, and there will be clusters of nuclei readily observable. Twenty stokes are usually sufficient, but some cells require more. Ideally, less than 50 strokes will lyse greater than 75% of the cells.
Once the cells are lysed, transfer the cellular suspension to a chilled 1.7 ml microcentrifuge tube, and centrifuge at 1,000 × g for 10 minutes to pellet unlysed cells and nuclei. Transfer the supernatant to another chilled 1.7 ml microcentrifuge tube, and repeat the spin, this will ensure no unlysed cells and nuclei are present in the HM fraction. Transfer the supernatant to another chilled 1.7 ml microcentrifuge tube, and centrifuge for at 8,000 × g for 10 minutes to pellet the HM fraction, which is typically white or pale yellow. The HM pellet is then resuspended in 100 µl of MIB, and quantified as above using OD520. All of these centrifugation steps are performed in a standard bench top centrifuge with a fixed angle rotor at 4°C.
It is important to note that we are presenting a general protocol for the isolation of HM fractions from cells. Before planning large experiments, we would recommend trying a few modifications of the above protocol. Considering the following items to find a condition that works best for your cells: (1) resuspending the cell pellet in a volume of MIB equal to 3 – 10 times the packed cell volume, cellular density often impacts on dounce homogenization success; (2) incubating the resuspended cells between 1 – 30 minutes on ice; and (3) occasionally, certain cell types appear to resist lysis using dounce homogenization, if this is the case after 50 strokes, we suggest using a 1 ml syringe fitted with a 26 gauge needle, try five passes and look for lysis.
3. BCL-2 family regulation of mitochondrial outer membrane permeabilization and cytochrome c release
In the upcoming sections, we will highlight the fundamental interactions within the BCL-2 family that can be experimentally analyzed using isolated mitochondria (Figure 3). Biochemical evaluation of the BCL-2 family requires several recombinant proteins and peptides (amino acid sequences for BH3 domain peptides are shown in table 2), most of which are commercially available through R&D Systems (www.rndsystems.com) and Anaspec (www.anaspec.com), respectively. The most difficult protein to purify is full-length BAX and we are not aware of any commercially available forms that are appropriate for these assays, but several papers describe methods to obtain functional protein [17, 30]. Finally, MOMP assays are set up the same for both cellular and liver HM fractions; however, the cellular HM fractions tend to be slightly less sensitive to BCL-2 family reagents, and we typically incubate cellular HM MOMP reactions at 37°C for less than 1 hour (e.g., 30 – 45 minutes) to minimize unwanted release in the negative control reactions. A graphical summary of a MOMP experimental protocol is provided in figure 3.
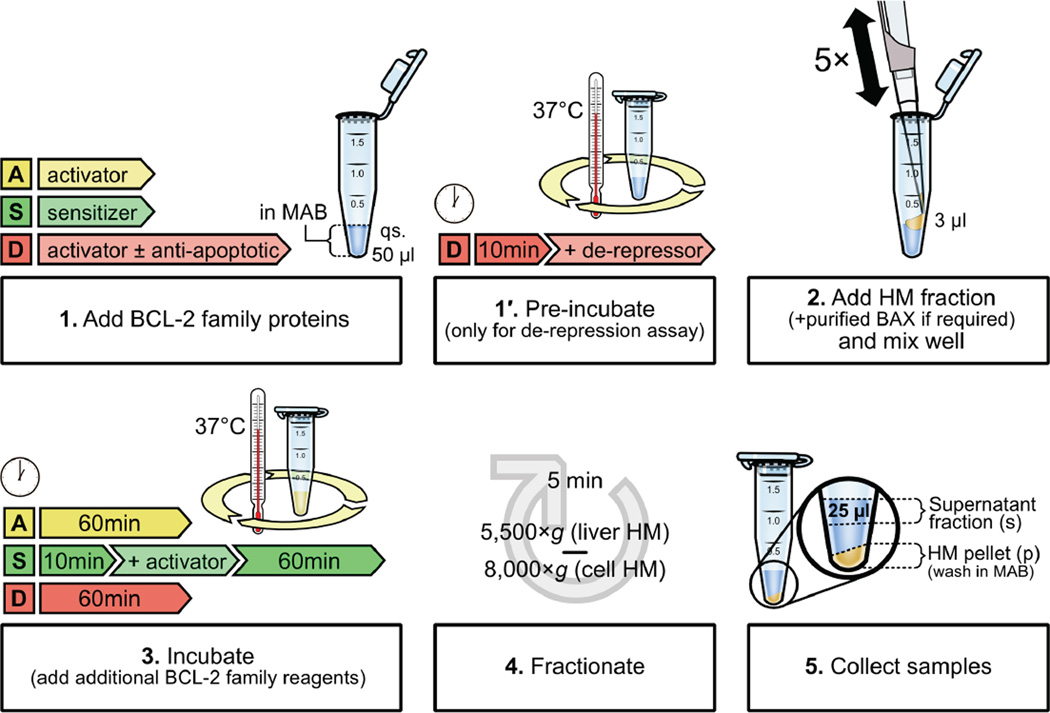
Figure 3.
Common BCL-2 family interactions to examine cytochrome c release with isolated mitochondria. BAK/BAX activation assays are performed in microcentrifuge tubes in a final volume of 50 µl MAB. Direct activation, sensitization and de-repression techniques are represented in yellow, green, and red, respectively. Reagents and incubation times before (1) and after (3) addition of the HM fraction (2) are represented for each assay. After incubation, the supernatant and HM pellet are separated by centrifugation (4), samples are collected (5), and analyzed by SDS-PAGE and western blot for cytochrome c.
Table 2.
BH3 domain-containing peptide sequences. Human and murine amino acid sequences for commonly used BH3 domain-containing peptides (amino to carboxy termini). Bold-faced leucines (L) and aspartic acids (D) are the conserved ‘L-x-x-x-x-D’ motif.
| Protein | Human peptide | Murine peptide |
|---|---|---|
| BAD | NLWAAQRYGRELRRMSDEFVDSFKK | NLWAAQRYGRELRRMSDEFEGSFKG |
| BAK | PSSTMGQVGRQLAIIGDDINRRYDS | PNSILGQVGRQLALIGDDINRRYDT |
| BAX | PQDASTKKSECLKRIGDELDSNMEL | QDASTKKLSECLRRIGDELDSNMEL |
| BID | QEDIIRNIARHLAQVGDSMDRSIPP | QEEIIHNIARHLAQIGDEMDHNIQP |
| BIK | CMEGSDALALRLACIGDEMDVSLRA | CVEGRNQVALRLACIGDEMDLCLRS |
| BIM | DMRPEIWIAQELRRIGDEFNAYYAR | DLRPEIRIAQELRRIGDEFNETYTR |
| BMF | QHQAEVQIARKLQCIADQFHRLHVQ | QHRAEVQIARKLQCIADQFHRLHTQ |
| HRK | RSSAAQLTAARLKALGDELHQRTMW | RWAAAQVTALRLQALGDELHRRAMR |
| Noxa (A) | PAELEVECATQLRRFGDKLNFRQKL | RAELPPEFAAQLRKIGDKVYCTWSA |
| Noxa (B) | Not in human protein. | VPADLKDECAQLRRIGDKVNLRQKL |
| PUMA | EEQWAREIGAQLRRMADDLNAQYER | EEEWAREIGAQLRRMADDLNAQYER |
3.1 Direct activation of BAK to promote cytochrome c release
As most cells and tissues express BAK, once mitochondria are purified using the above techniques, the methods to biochemically induce endogenous BAK activation are straightforward. In this section, we will discuss an approach to examine BAK-mediated cytochrome c release induced by a direct activator BH3-only protein or a BH3 domain peptide. We commonly refer to these experiments as “MOMP assays.”
Following death receptor ligation, BID is cleaved in cells by caspase-8 to generate a potent inducer of BAK activation (referred to as C8-BID). C8-BID is comprised of two polypeptides that remain associated until C8-BID interacts with the OMM, which causes release of the amino terminal fragment, thus revealing the carboxy terminal BID BH3 domain [31, 32]. Moreover, short synthetic peptides that encompass the BH3 domain of BH3-only proteins also function in these assays (Table 2; for a better understanding of core requirements within the BH3 domain, see Day et al., [33]). In this experiment, we will compare two direct activator proteins/peptides on C57Bl/6 liver and wild type MEF HM fractions: C8-BID (0.01, 0.1, 1, 10 nM), and the BID BH3 domain peptide (0.01, 0.1, 1, 10 µM). The purification of C8-BID generates a protein that maintains tertiary structure and therefore the protein functions at low nanomolar concentrations. In contrast, BH3 domain peptides lack secondary structure so a 2 – 3 log higher concentration is generally used. Structured peptides that work similarly to protein are described, but these are considerably more expensive [34].
C8-BID and BID BH3 domain peptide stock solutions are prepared (50X) for each dose by diluting in HE buffer (10 mM HEPES pH 7.4, 1 mM EDTA) or DMSO, respectively. Dilute and aliquot C8-BID after it arrives from the manufacturer and store at −80°C, we have not experienced any activity loss over years of storage. In contrast, BH3 domain peptides arrive as lyophilized peptides, and these should be reconstituted to 10 mM in DMSO, and stored in 1 – 2 µl aliquots at −80°C; these peptides rapidly lose activity, so we only dilute as necessary and do not freeze/thaw the 10 mM stock.
MOMP assays are performed in sterile 1.7 ml microcentrifuge tubes, using the following reagents assembled in this order at room temperature: (1) mitochondrial assay buffer (MAB, MIB supplemented with 3M KCl to a final concentration of 110 mM KCl), (2) proteins/peptides/vehicle buffers, and (3) 50 µg HM. For the assay described above, there will be ten MOMP reactions: two vehicle controls (HE buffer, DMSO), C8-BID (0.01, 0.1, 1, 10 nM), and BID BH3 domain peptide (0.01, 0.1, 1, 10 µM). Assemble the reactions so that MAB is pipetted into each tube first, then proteins/peptides/vehicles, then mitochondria; this will ensure that all the reactions initiate within the narrowest time frame. As example, each MOMP reaction will require 46 µl MAB, 1 µl of 50X protein/peptide/vehicle, and 3 µl HM (~ 50 µg); use the HM addition to mix the reaction by pipetting up and down five times. Incubate the MOMP reactions at 37°C for 60 minutes, centrifuge for 5 minutes at room temperature to pellet the HM fractions (liver HM fraction = 5,500 × g; cell HM fractions = 8,000 × g), and remove 25 µl of the supernatant to a fresh tube containing 8 µl of 4X SDS-PAGE loading buffer (0.25 mM Tris-HCl, 8% SDS, 40% v/v glycerol, 1% v/v β-mercaptoethanol, 0.04% bromophenol blue, pH 6.8), mix, and store at −20°C.
Remove and discard the remaining supernatant from each MOMP assay using a pipette, ensuring to not disrupt the pellet. Wash the pellets by adding 1 ml of room temperature MAB to each tube, do not mix, and spin again for 5 minutes. Aspirate the all the buffer. Solubilize the HM pellets in 10 µl of 1X SDS-PAGE loading buffer and store at −20°C. It is best to subject the pellets and supernatants to one round of freeze/thaw as cytochrome c is sensitive to precipitation, so equal treatment of all the samples is preferred.
Next, the samples will be analyzed by SDS-PAGE and western blot for the release of cytochrome c from the pellet to the supernatant. Denature the samples at 95°C for 5 minutes, centrifuge at 20,000 × g in a room temperature bench top centrifuge, and store at room temperature until loaded. The pellet and supernatant samples should be analyzed on separate gels and we typically load the entire supernatant sample (i.e., 32 µl) and half of the pellet sample (i.e., 5 µl), which is equivalent to 50% of both samples allowing for optimal comparison. Occasionally, a salt precipitate may form in the supernatant samples when the laboratory temperature is cold, but this does not influence the SDS-PAGE. As cytochrome c is quite small (~ 12 kDa), we use 12% bis-acrylamide gels for the SDS-PAGE. The preferred western blot antibody for cytochrome c is clone 7H8 diluted at 1:2000 in 5% milk/TBST (Tris-buffered saline + Tween-20: 100 mM Tris-HCl, 0.9% NaCl, 0.1% Tween-20). In general, the pellet western blot will develop quicker than the supernatant, and we typically expose the supernatant western blot until the positive controls appear robust and equivalent to the untreated pellet band. An example of a direct activation assay using primary liver HM fractions containing appropriate controls is presented (Figure 4A).
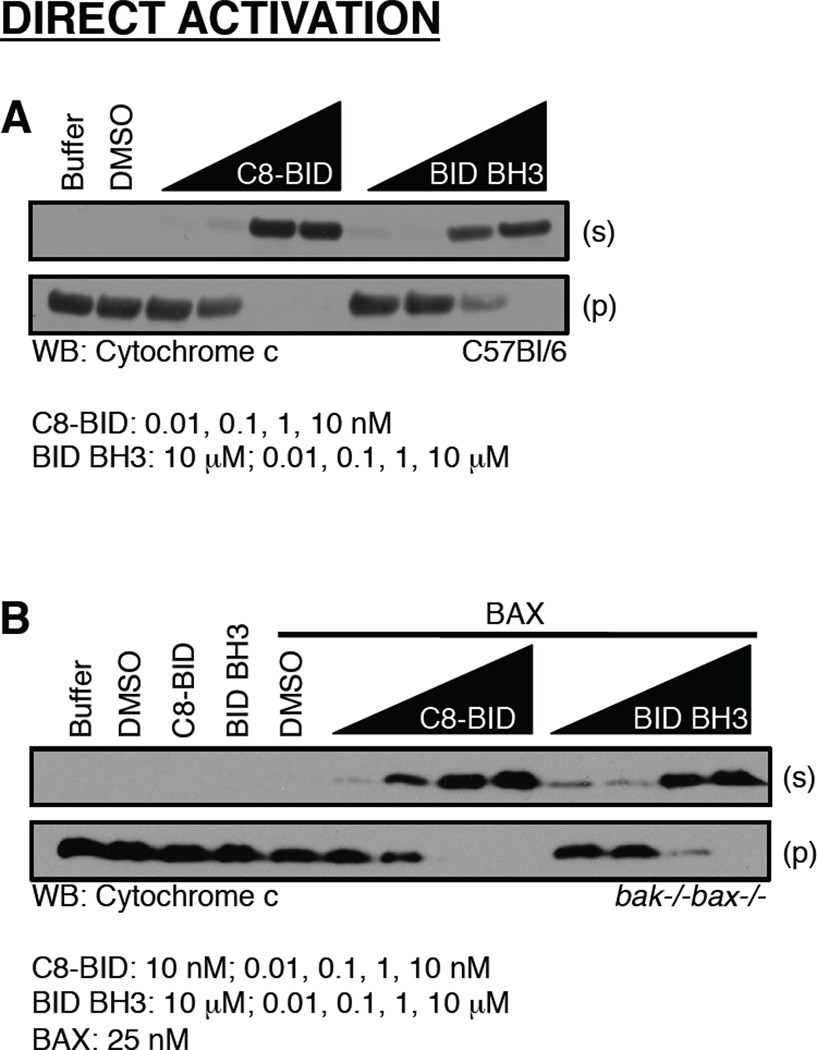
Figure 4.
The direct activation of BAK/BAX promotes cytochrome c release. (A) Mitochondria purified from C57Bl/6 liver were incubated with C8-BID or the BID BH3 domain peptide for 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c. (B) Mitochondria purified from bak−/−bax−/− liver were incubated with C8-BID or the BID BH3 domain peptide, in the presence of recombinant BAX, for 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c. C8-BID and BID BH3 treatments alone are at 10 nM and 10 µM, respectively.
3.2 Sensitization and de-repression leading to BAK activation
These experiments involve the use of several BCL-2 proteins and/or BH3 domain peptides. It is best to first establish a concentration range for direct activator stimulation required to induce 10 – 100% cytochrome c release using the conditions outlined in section 3.1. This information will minimize the amounts of proteins used in subsequent assays, and it is informative to gain a baseline for comparing subsequent BCL-2 family treatment combinations. Due to the time required to analyze for consistent direct activator stimulation, several HM isolations and days are required to move from direct activation to sensitization/de-repression assays.
Sensitization experiments are excellent to measure the impact of endogenous anti-apoptotic BCL-2 protein function on direct activator induced BAK activation. A concentration of 100 nM sensitizer BH3-only protein, or 10 µM BH3 domain peptide (Table 2), is generally sufficient to inhibit endogenous anti-apoptotic BCL-2 proteins leading to enhanced BAK activation. If using small molecules inhibitors to anti-apoptotic BCL-2 proteins, like ABT-737, 100 nM is also an appropriate starting concentration. The MOMP assays should be set-up as described in section 3.1, with a few adjustments: (1) MAB is added to all tubes, (2) followed by sensitizer BH3-only protein/peptide/small molecule/vehicle control, (3) HMs are added, mixed, and incubated for 10 minutes at 37°C, and then (4) direct activator stimulation is added and incubated for an additional 60 minutes at 37°C. The MOMP assay volumes should remain at 50 µl, and after step 4, proceed with the fractionation and SDS-PAGE/western blot analyses as described above. One caveat to interpreting data from these experiments is pre-existing endogenous direct activator function that may be present in the purified HM fraction. This may be due to previous cellular or tissue stress, and is often revealed by cytochrome c release that occurs following sensitizer treatment alone, in the absence of additional direct activator. This scenario suggests the sensitizer is actually ‘de-repressing’ a direct activator from the endogenous anti-apoptotic BCL-2 repertoire to induce BAK activation and cytochrome c release. Ideally, your choice of sensitizer should not induce substantial cytochrome c release unless additional direct activator stimulation is added. An example of a sensitization assay containing appropriate controls is presented (Figure 5A).
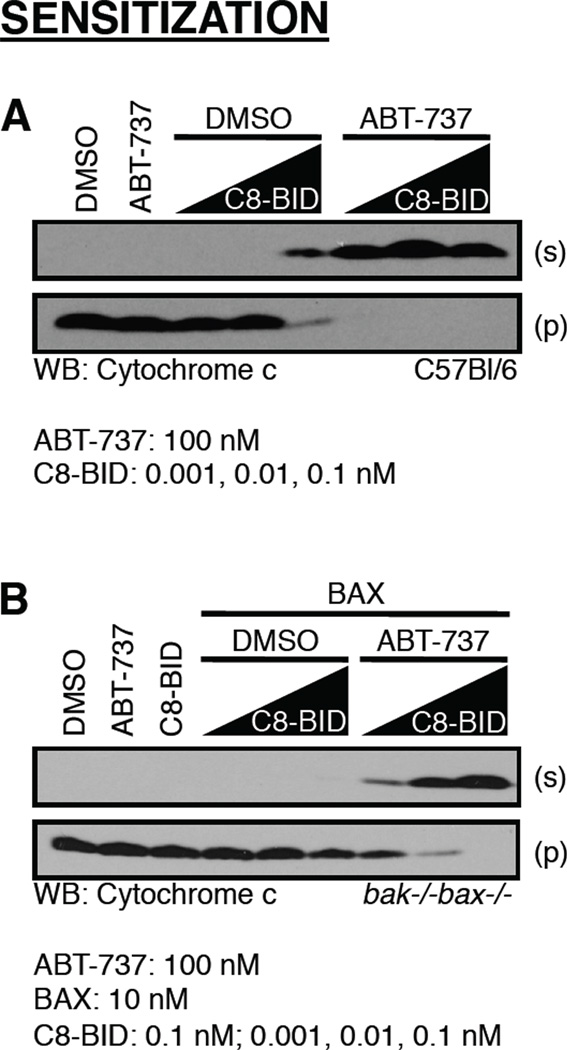
Figure 5.
Treatment of mitochondria with ABT-737 sensitizes to BAK/BAX activation and cytochrome c release. (A) Mitochondria purified from C57Bl/6 liver were pre-incubated with ABT-737 for 10 minutes at 37°C. C8-BID was added, and incubated for 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c. (B) Mitochondria purified from bak−/−bax−/− liver were pre-incubated with ABT-737 for 10 minutes at 37°C. Recombinant BAX along with C8-BID or the BID BH3 domain peptide was added, and incubated for 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c. The C8-BID treatment alone is 0.1 nM.
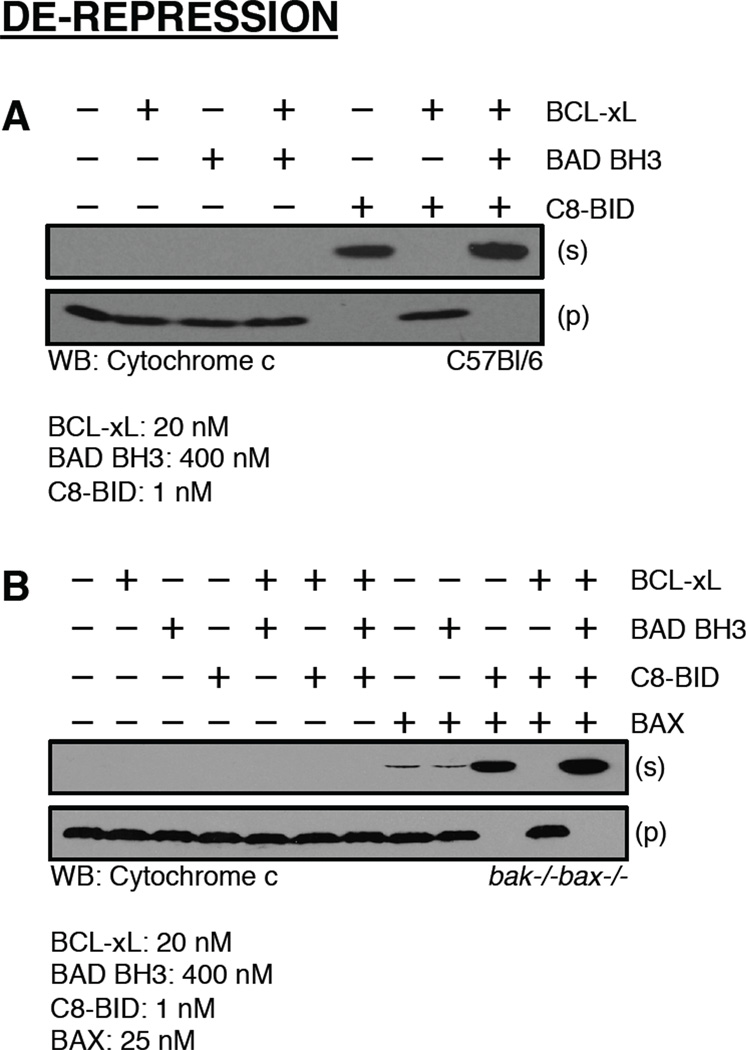
De-repression assays are set-up using three BCL-2 family reagents: a direct activator, an anti-apoptotic BCL-2 protein, and a de-repressor BH3-only that binds to the added anti-apoptotic BCL-2 protein. The direct activator should induce BAK-dependent cytochrome c release, which is then inhibited by the added anti-apoptotic BCL-2 protein, and this inhibition is reversed (referred to as ‘de-repressed’) by the addition of a de-repressor BH3-only. An experimental starting ratio for these proteins is: 25X molar excess (in relation to the direct activator concentration) of anti-apoptotic BCL-2 protein, and 20X molar excess of de-repressor BH3-only (in relation to the anti-apoptotic BCL-2 protein concentration). For example, C8-BID (1 nM) induces 100% cytochrome c release, this is blocked by co-treatment with recombinant BCL-xL (25 nM), which is de-repressed by recombinant BAD (500 nM).
These experiments are set-up as follows: (1) MAB is added to all tubes, (2) followed by direct activator ± anti-apoptotic protein and incubation for 10 minutes at 37°C, (3) followed by the addition of de-repressor, (4) HMs are added, the reaction is mixed, and incubated for 60 minutes at 37°C. The MOMP assay volumes should remain at 50 µl, and after step 4, proceed with the fractionation and SDS-PAGE/western blot analyses as described in section 3.1. Since there are several recombinant proteins added to these assays, numerous controls are key to ensure the effects are due to a de-repression scenario rather than sensitization itself. This assay allows for experimental examination of both anti-apoptotic and de-repressor activities, and proteins that may possess either anti-apoptotic or de-repressor like activities can easily be evaluated. Due to the significant amounts of recombinant anti-apoptotic and derepressor that are required for these assays to work, it is advisable to not use BH3 domain peptides as the source of direct activation since the scaled ratios will be in the millimolar range. An example of a de-repression assay containing appropriate controls is presented (Figure 6A).
Figure 6.
The BAD BH3 domain peptide de-represses BCL-xL to promote BAK/BAX activation and cytochrome c release. (A) C8-BID and BCL-xL were combined and incubated for 10 minutes at 37°C before the addition of BAD BH3 domain peptide and C57Bl/6 mitochondria. The reactions were incubated for an additional 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c. (B) C8-BID and BCL-xL were combined and incubated for 10 minutes at 37°C before the addition of BAX, BAD BH3 domain peptide, and bak−/−bax−/− mitochondria. The reactions were incubated for an additional 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c.
3.3 Direct activation of BAX to promote cytochrome c release
MOMP assays are set-up as described in section 3.1, but in this situation, bak−/− baxf/− or bak−/− mitochondria will be used and recombinant BAX needs to be added. The same amount of mitochondria is used as in the previous section (50 µg per reaction). In terms of BAX, each batch of recombinant protein displays slightly different background levels of cytochrome c releasing activity in the absence of direct activator stimulation, assumingly due to an unavoidable subpopulation of partially- or fully-activated BAX molecules. To address this, it is necessary to titrate BAX alone, and in the presence of C8-BID (10 nM). BAX titrations ranging between 5 – 50 nM should provide results that show BAX alone does not induce cytochrome c release, and synergy with C8-BID promotes complete release. After the reactions are set-up, the same incubation, fractionation, and analyses methods should be followed as described in section 3.1. In our experience, multiple freeze/thaws of recombinant BAX can increase the background release and reduce C8-BID synergy, so it is advisable to store BAX in smaller aliquots at −80°C. An example using a bak−/−baxf/− HM fraction reconstituted with BAX, and treated with either C8-BID or the BID BH3 domain peptide is provided in figure 4B.
3.4 Sensitization and de-repression leading to BAX activation
Once the above titrations are performed, and an optimal concentration of BAX is determined, the titrations need to be re-done in the presence of sensitizer protein/peptide/small molecule. The set-up is the same, but in step 4, BAX ± direct activator will be added. All the other steps remain unmodified. It is expected that the required concentrations for both BAX and the direct activator will decrease in the presence of sensitizers (Figure 5B). This is because less anti-apoptotic BCL-2 proteins will be available to inhibit the subpopulation of partially- or fully-activated BAX, and the same for the direct activator. One important control to consider is if the sensitizer directly impacts on BAX activity. As an example, many ‘sensitizer’ BH3 domain peptides can demonstrate weak direct activator function at high concentrations [29]. This can be determined by testing BAX in the presence of your sensitizer.
For de-repression leading to BAX activation, it is advisable to titrate your choice of anti-apoptotic protein into the direct activation assay in section 3.3. The lowest concentration of anti-apoptotic protein is optimal for the best de-repression results, and a starting point is 20X molar excess in relation to the BAX concentration. For example, if 25 nM BAX + 10 nM C8-BID gives 100% cytochrome c release, 500 nM BCL-xL is an expected concentration for inhibition (titrating 250 – 750 nM BCL-xL). After the anti-apoptotic concentration is known, a third titration of de-repressor is performed starting with 20X molar excess over the anti-apoptotic concentration (e.g., for the above scenario, 10 µM de-repressor). There are numerous controls to perform in a derepression assay (e.g., de-repressor + anti-apoptotic, de-repressor + direct activator, etc…), paying attention to the effect of the de-repressor on BAX in the absence of a direct activator. An example of a de-repression assay is provided in figure 6B.
4. Conformational changes correlated with BAK and BAX activation
Direct activators promote conformational changes in BAK and BAX that are correlated with their MOMP-inducing activities [21, 22, 35–38]. For instance, BID and BIM have been shown to cause BAK/BAX amino terminal rearrangements, BH3 domain exposure, membrane insertion (for BAX only, BAK is always at the OMM), and oligomerization. This activation sequence can be probed using mitochondria isolated from cells undergoing apoptosis, or by coupling the direct activation/sensitization/de-repression systems described above with the following assays.
4.1 Amino terminal rearrangements of BAK and BAX captured by conformation-specific antibodies
Immediately following a transient interaction with a direct activator BH3-only protein, BAK and BAX undergo amino terminal conformational changes that precede oligomerization and pore formation. This early step in the BAK/BAX activation process is biochemically examined with conformation-specific antibodies that recognize motifs that are buried in unactivated BAK and BAX molecules. The antibodies used in these assays are clones ‘AB-1’ and ‘6A7’, and these were developed against BAK (human amino acid residues 1 – 52) and BAX (human amino acid residues 12 – 24), respectively [10, 39].
MOMP assays are set-up as described in section 3, but instead of fractionating into pellet and supernatant, the reactions are lysed in BAK/BAX amino terminal capture buffer (BATCB: 10 mM HEPES, 135 NaCl, 5 mM MgCl2, 0.2 mM EDTA, 1% glycerol + 1% CHAPS, added fresh; pH 7.4), 1 µg of AB-1 (clone for human BAK IP, no conformation-specific antibody for mouse BAK is validated) or 6A7 (clone for human or mouse BAX IP) is added, and incubated for 3 hours at 4°C with end/end mixing, add an appropriate volume of protein A/G-agarose conjugate, and incubate for an additional 2 hours. The beads should be centrifuged according to manufacturer guidelines, and washed with each of the following buffers twice, 1 ml per wash, add buffer, centrifuge, aspirate, repeat until completed: (1) Wash A: 10 mM HEPES, 135 NaCl, 2% CHAPS; pH 7.4 (2) Wash B: 10 mM HEPES, 135 NaCl, 0.2% CHAPS; pH 7.4 and (3) Wash C: 100 mM Tris HCl, 100 mM NaCl, pH 8.0. Proteins are eluted by the addition of 1X SDS-PAGE loading buffer (50 µl), denatured for 10 minutes at 95°C, centrifuged for 1 minute at 15,000 × g, and the supernatant is subjected to SDS-PAGE and western blot analysis using G-23 or N-20 for BAK or BAX detection, respectively. If there are substantial issues with the light chain confounding BAK/BAX detection, TrueBlot® (www.ebioscience.com) reagents can be used to minimize light chain detection. In addition to coupling this technique with traditional MOMP assays (e.g., Figure 7A), it is possible to analyze apoptotic cells in place of isolated mitochondria using the same lysis (but starting with 0.25 – 1 mg cellular protein per condition), IP, and detection conditions.
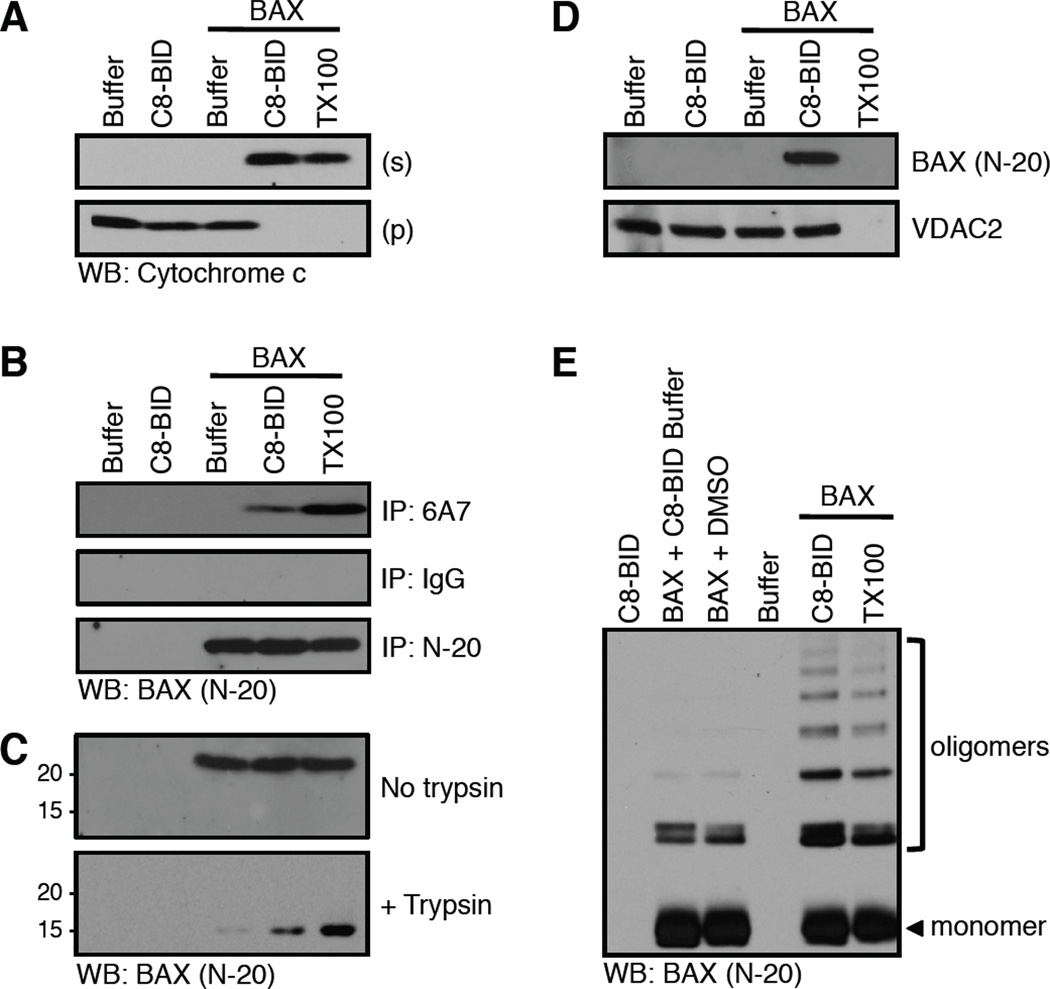
Figure 7.
Conformational changes correlated with BAX activation and cytochrome c release. (A) Mitochondria purified from bak−/−bax−/− liver were incubated with C8-BID (10 nM) and BAX (25 nM) for 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c. This MOMP reaction was set-up in quintuplicate, using 100 µl reaction volumes and 100 µg HM for analyses in A–E. 0.1% Triton X-100 (TX100) solubilizes mitochondria to release cytochrome c. (B) The amino terminal region of BAX is revealed by C8-BID, and captured following 6A7 immunoprecipitation (IP). IgG is an isotype control IP; N-20 is a non-conformation specific IP. TX100 artificially activates BAX and is a positive control. (C) BAX is trypsin (0.17 µg/µl) resistant when activated by C8-BID. TX100 artificially activates BAX and is a positive control. (D) C8-BID induced BAX integration into the OMM resists alkali extraction. VDAC2 is an integral OMM protein that is not carbonate sensitive. TX100 solubilizes mitochondria so both OMM integrated and associated proteins are not detectible as no pellet can be analyzed. (E) Cysteine cross-linking with BMH (1 µM) reveals BAX oligomerization induced by C8-BID. TX100 artificially activates BAX and is a positive control.
There are several controls that should be included in the assay. One is to create a sample that is detergent activated prior to the addition of BATCB. For example, the MOMP reaction should be supplemented with 0.1% Triton X-100, this will provide an indication of the total amount of BAK or BAX that is capable of undergoing activation (n.b., the mitochondria will solubilize, that is OK) [10]. Additionally, instead of conformation-specific antibodies, a pan-recognition IP antibody can be used as comparison to indicate the total amount of BAK (clones AB-2 or G-23) or BAX (clones N-20 or 2D2) within the assay. Keep in mind that the total amount of BAK or BAX present in the assay may differ than the total amount of protein capable of activation; this is particularly relevant when recombinant BAX is added. Finally, be aware of contaminating detergents within the assay, it is well known that non-ionic detergents alter the α helical conformation of BAK and BAX to promote their activation [10]. An example experiment is shown in figure 7B.
4.2 Structural rearrangements within BAK and BAX identified by protease sensitivity
Another method to observe activation-associated conformational changes in BAK/BAX measures increased protease sensitivity within the amino terminus of BAK/BAX, referred to as “BH4 cleavage” [35, 40]. MOMP assays are assembled and incubated as described above, but ensure the reactions are free of protease inhibitors: the use of protease inhibitors during the HM isolation procedure is fine, but leave them out when resuspending the final HM pellet and in the individual reactions. Proteolysis within the MOMP reactions is then triggered by the addition of trypsin (30 µg/ml final concentration, the tablets from Sigma-Aldrich are convenient, www.sigmaaldrich.com; alternatively, 20 nM recombinant Calpain-2 can also be used in the assay in the presence of 0.5 mM CaCl2 [41], available from EMD Biosciences, www.emdchemicals.com), and the samples are incubated at 4°C for 30 minutes. The proteolysis reaction is stopped by the addition of soybean trypsin inhibitor (100 µg/ml final concentration, for trypsin only; nothing added for Calpain inhibition). Samples are then supplemented with 4X SDS-PAGE loading buffer, denatured by boiling, and analyzed by SDS-PAGE and western blot for BAK or BAX cleavage.
The presence of BAK/BAX fragments of lower molecular weight indicates prior exposure of the amino terminus and subsequent cleavage. We suggest using clones G-23 and Δ21 for the detection of BAK and BAX, respectively. Please note that antibodies for the western blot analyses must not be specific to the amino terminus of BAK or BAX. For example, BAX clone N-20 will not work in this assay because the full-length protein band will only lose intensity and cleaved forms will not be detected. In addition to revealing amino terminal rearrangements, this assay can also provide evidence for exposure of the BAK BH3 domain, which is detected by an additional cleavage product, referred to as “BH3 cleavage” [41]. Detailed explanations of the cleavage sites and examples are provided elsewhere [40, 41].
In the previously described method, buffer conditions, protease concentration, and incubation time are critical to ensure controlled targeting of BAK or BAX leading to only BH4 and/or BH3 cleavage, and not degradation. Changing buffer conditions and increasing the trypsin concentration will degrade monomeric forms of BAX, while oligomerized BAX resist proteolysis [35, 42, 43]. Therefore, a modification of the above proteolysis technique can be applied to determine the oligomerization status of BAX. MOMP assays are setup as described earlier in sections 3 and 4.2, pellet the reactions at 5,500 × g in a room temperature bench top centrifuge, discard the supernatant, resuspend the mitochondrial pellets in KCl buffer (125 mM KCl, 2 mM MgCl2, 5 mM KH2PO4, 10 mM HEPES; pH 7.4) at a concentration of 4 µg mitochondrial protein/µl, and initiate proteolysis by the addition of trypsin (0.17 µg/µl final concentration). The samples are incubated at room temperature for 90 minutes, combined with 4X SDS-PAGE loading buffer, denatured by boiling, and analyzed with SDS-PAGE and western blot for BAX (clone N-20). The proteolysis will lead to either the complete degradation of monomeric BAX, or to the retention of a BAX 15 kDa fragment, suggestive of activation and oligomerization. As a positive control for BAX activation studies, add 0.1% Triton X-100 to an additional MOMP reaction, this will trigger maximal BAX oligomerization. An example experiment is shown in figure 7C.
4.3 BAX insertion into mitochondrial membrane identified by resistance to alkali extraction
Whereas BAK has constitutive mitochondrial localization, BAX has to re-localize and insert into the OMM to induce MOMP. Therefore, the determination of BAX insertion into the OMM is used as a marker of BAX activation and function. This is performed by examining the sensitivity of BAX to dissociate from the OMM in the presence of alkali sodium carbonate buffer. Associated, but not integrated BAX (e.g., BAX that is bound to BCL-2 or other OMM proteins), will readily dissociate from the OMM in alkali conditions, but membrane-integrated BAX resists extraction. A control for this assay is to compare a bone fide integral OMM protein (e.g., VDAC species, BCL-2, TOM22) for sodium carbonate buffer resistance.
Isolated mitochondria (from MOMP assays or treated cells, 100 µg) are pelleted, resuspended in 0.25 ml of 0.1 M sodium carbonate (pH 11.5), and incubated on ice for 20 minutes. The reaction is then centrifuged for one hour at 100,000 × g at 4°C. The supernatant is removed, and the pellet is resuspended in 1X SDS-PAGE loading buffer, denatured at 95°C for 10 minutes, and subjected to SDS-PAGE and western blot for BAX (clone N-20) and the bone fide integral OMM protein of your choice for comparison and loading control. A representative example of this assay is shown in figure 7D and [44].
4.4 Oligomerization of BAK and BAX detected by cysteine cross-linking
MOMP assays are set-up as described in section 3, but instead of fractionating into pellet and supernatant, the reactions are supplemented with bis-maleimido-hexane (BMH). BMH is homo-bifunctional cross-linker that is commonly used for conjugating sulfhydryl groups (−SH); it is very labile, and should be prepared as a 50X stock in DMSO just prior to use. For BAK-dependent MOMP and oligomerization, add BMH to a final concentration of 1 mM (a titration between 0.1 – 10 mM BMH may be necessary), mix, and incubate on ice for 30 minutes, and stop the reaction by quenching with DTT (final concentration 1 mM). Add 4X SDS-PAGE loading buffer, denature at 95°C, and analyze with SDS-PAGE and western blot for BAK (clone G-23). For BAX-dependent MOMP and oligomerization, use the above protocol, but it will be necessary to titrate the BMH using a concentration that is between 5 – 50 times higher than the recombinant BAX concentration. A crucial preliminary experiment is to also perform a BMH titration in the absence of direct activator stimulation, so a concentration of BMH that does not cross-link unstimulated BAK or BAX can be identified. A positive control for BAK and BAX homo-oligomerization is the addition of 0.1% Triton X-100, which will artificially activate both proteins to promote homo-oligomerization. An example experiment is shown in figure 7E. An alternative to BMH cross-linking of BAK is to incubate with the redox catalyst copper(II)(1,10-phenanthroline)3, as described [24].
5. Perspectives
The model systems and approaches outlined above provide substantial mechanistic insight regarding BAK/BAX activation, MOMP, and cytochrome c release. These assays require some practice to ensure mitochondria are functional and responsive to BCL-2 family stimulation. Furthermore, these techniques are sensitive to a range of contaminants, pH changes, and temperature, so preparing a controlled and consistent environment is key for experimental success.
Much of the BCL-2 family literature has been established using these approaches, but a plethora of questions remain within the field that will continue to utilize these highly informative techniques. As functional cooperation within the BCL-2 family can easily be examined using these systems, and numerous BCL-2 family partners and regulators continue to be described, we predict that these systems will further reveal, explore, and connect the complexities that govern the mitochondrial pathway of apoptosis.
Acknowledgments
We would like to thank everyone that has trained us to perform BCL-2 family studies with isolated mitochondria, in particular, Drs. Tomomi Kuwana and Jean-Ehrland Ricci. This work was supported by: NIH CA157740 (to J.E.C.), a pilot project from the NIH P20AA017067 (to J.E.C.), the William A. Spivak Fund (to J.E.C.), the Fridolin Charitable Trust (to J.E.C.), and the JJR Foundation (to J.E.C.). This work was also supported in part by a Research Grant 5-FY11-74 from the March of Dimes Foundation (to J.E.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newmeyer DD, Farschon DM, Reed JC. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 2.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 3.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 8.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 9.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 10.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 11.Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci U S A. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport, and F(0)F(1)-ATPase. J Biol Chem. 2001;276:6453–6462. doi: 10.1074/jbc.M006391200. [DOI] [PubMed] [Google Scholar]
- 13.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci U S A. 2008;105:20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Walensky LD, Gavathiotis E. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem Sci. 2011;36:642–652. doi: 10.1016/j.tibs.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 18.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, Huang DC, Kluck RM, Adams JM, Colman PM. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, Kluck RM. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM. Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol Cell. 2009;36:696–703. doi: 10.1016/j.molcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 26.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi R, Andreyev A, Murphy AN, Perkins GA, Ellisman MH, Newmeyer DD. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 2007;14:616–624. doi: 10.1038/sj.cdd.4402035. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. J Biol Chem. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem. 2003;278:48935–48941. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- 31.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational Nmyristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 32.Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 34.Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM. The Nterminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem. 2003;278:11633–11641. doi: 10.1074/jbc.M208955200. [DOI] [PubMed] [Google Scholar]
- 38.Peyerl FW, Dai S, Murphy GA, Crawford F, White J, Marrack P, Kappler JW. Elucidation of some Bax conformational changes through crystallization of an antibody-peptide complex. Cell Death Differ. 2007;14:447–452. doi: 10.1038/sj.cdd.4402025. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C, Hickman JA. Cell damage-induced conformational changes of the proapoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 41.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucken-Ardjomande S, Montessuit S, Martinou JC. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ. 2008;15:484–493. doi: 10.1038/sj.cdd.4402280. [DOI] [PubMed] [Google Scholar]
- 44.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]