Abstract
BACKGROUND
Cell-free hemoglobin (Hb) in the vasculature leads to vasoconstriction and injury. Proposed mechanisms have been based on nitric oxide (NO) scavenging by oxyhemoglobin (oxyHb) or processes mediated by oxidative reactions of methemoglobin (metHb). To clarify this, we tested the vascular effect and fate of oxyHb or metHb infusions.
STUDY DESIGN AND METHODS
Twenty beagles were challenged with 1 h similar infusions of (200uM) metHb (n=5), oxyHb (n=5), albumin (n=5), or saline (n=5). Measurements were taken over 3 h.
RESULTS
Infusions of the two pure Hb species resulted in increases in mean arterial blood pressure (MAP), systemic vascular resistance index, and NO consumption capacity of plasma (all p<0.05) with the effects of oxyHb being greater than that from metHb (MAP; increase 0 to 3h; 27±6 % vs.7±2 %, respectively) (all p<0.05). The significant vasoconstrictive response of metHb (vs. albumin and saline controls) was related to in vivo auto-reduction of metHb to oxyHb, and the vasoactive Hb species that significantly correlated with MAP was always oxyHb, either from direct infusion or after in vivo reduction from metHb. Clearance of total Hb from plasma was faster after metHb than oxyHb infusion (p<0.0001).
CONCLUSION
These findings indicate that greater NO consumption capacity makes oxyHb more vasoactive than metHb. Additionally, metHb is reduced to oxyHb post-infusion and cleared faster or is less stable than oxyHb. Although we found no direct evidence that metHb itself is involved in acute vascular effects, in aggregate, these studies suggest that metHb is not inert and its mechanism of vasoconstriction is due to its delayed conversion to oxyHb by plasma-reducing agents.
Keywords: methemoglobin, cell-free hemoglobin, nitric oxide, vasoconstriction, hemoglobin
INTRODUCTION
Cell-free hemoglobin (Hb) based blood substitutes (Hb-based oxygen carriers, HBOC) have been associated with hypertension,1–5 myocardial infarctions,6–10 and death.10–12 In hemolytic diseases, cell-free Hb has been postulated to be responsible for impaired endothelial function and pathogenic abnormalities of the vasculature; however, some controversies still persist.13–18 The exact mechanism(s) by which cell-free Hb causes endothelial dysfunction and vascular injury are unknown. Several hypotheses have been proposed: 1) Nitric oxide (NO) in the vasculature is scavenged by the ferrous (Fe2+) oxygen-carrying form of cell-free Hb (oxyHb) forming ferric (Fe3+) cell-free Hb (metHb). This fast and irreversible reaction results in NO oxidation to nitrate and a state of NO resistance producing systemic hypertension with potential vascular injury.19, 20 2) Cell-free oxyHb delivers oxygen prematurely to arterioles, resulting in systemic hypertension also with potential vascular injury.2 3) Cell-free oxyHb extravasates through the endothelial layer into various tissues where oxyHb either directly scavenges NO and/or oxyHb/metHb are catabolized to heme and globin, which may cause oxidative and inflammatory pathologies.21, 22 4) Circulating cell-free Hb is oxidized to ferryl Hb and reactive oxygen species which cause tissue injury.1, 23, 24
The effects of cell-free oxyHb have been extensively studied in animal models of sickle cell disease and experimental hemolysis.4, 17, 20, 25–27 The effects of formation of metHb in the intact red blood cell have also been well characterized,28 but the effects of cell-free metHb on vascular tone and redox reactions in animals have been less extensively studied. In two such preclinical studies, there were no significant increases in blood pressure associated with infusing cell-free metHb, but comparisons were not made to time-matched controls,4, 25 and one of these studies ended after only five minutes.25 At present, the in vivo effects of cell-free metHb have not been characterized.
The classic paradigm is that oxyHb released into plasma from RBC will undergo auto-oxidation and other oxidative reactions to form metHb and, eventually, other oxidative products (ferryl Hb, free heme, non-transferrin bound iron, etc.).23, 29, 30 However, in vitro studies in human plasma suggest that high concentrations of naturally-occurring plasma antioxidants (i.e., urate, ascorbate, and glutathione) will maintain Hb in the reduced ferrous redox state.31–33 The ascorbate levels in human plasma have been reported to be as high as ~50 µM.34 Indeed, in patients with hemolytic diseases, such as sickle cell and paroxysmal nocturnal hemoglobinuria, and in the plasma of stored red blood cell units, most Hb remains oxyHb, suggesting active reduction and stability of the heme center in the Hb tetramer. Even though metHb formation has been implicated in the pathogenesis of heme-oxidative injury, its hemodynamic effects have not been thoroughly studied. Importantly, metHb is formed by reaction of cell-free oxyHb with various agents being developed or considered for therapeutic uses to improve NO bioavailability, including various metabolites of NO such as nitrite, Angelis salt and compounds or nitrosyl Hb.35–37 Giving these NO donors therapeutically in animal models of hemolysis associated with high levels of cell-free oxyHb results in high plasma levels of cell-free metHb being formed (50 to 150 µM).35–38 The effects of high levels of circulating cell-free metHb are unknown and could be toxic and produce abnormal hemodynamic responses independent of any effect of cell-free oxyHb. To separate the effects of cell-free metHb and cell-free oxyHb, we gave pure infusions of each to different sets of animals and measured hemodynamics. We hypothesized the oxyHb would be more vasoconstrictive than metHb, as the former species would react with NO to convert it to nitrate (dioxygenation reaction) while metHb is known to react very slowly and to a minimal extent in vivo with NO. We found that high levels of circulating cell-free metHb has no measurable direct acute hemodynamic effects but surprisingly is indirectly vasoactive because it is reduced back to vasoconstrictive oxyHb in vivo.
MATERIALS AND METHODS
Experimental Design
All experiments were approved by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health. Twenty purpose-bred beagles (1–2 yr old, 10–14 kg) were studied over 3 h.
Four animals were randomized, each of 5 study days to one of four experimental groups: 1) cell-free metHb (n=5); 2) cell-free oxyHb (n=5); 3) albumin (n=5); and 4) 0.9% saline (n=5). We calculated cell-free Hb doses to produce 200 µM plasma levels at the end of a 60-min infusion. This plasma level in animals that previously received oxyHb is known to have marked hemodynamic effects.20 Each study day, a new stock solution of metHb and oxyHb was used. To calculate the infusion rate assuming a specific blood volume of 0.08 l/kg body weight, the volume (l) of fluid to be infused within 1 h was calculated as: [0.08 (l/kg) × body weight (kg) × target plasma concentration (0.2mM) × (1-Hct)/concentration in Hb stock solution (2.3 to 3.53 mM)], where Hct is a fraction. At end of the infusion, mean (+/− SE) peak plasma metHb and oxyHb levels were 189 +/− 27 (n=5) and 188 +/− 28 µM (n=5), respectively. The volumes infused ranged from 36 to 73 ml. For controls, each study day, doses of albumin (25% Human Albumin, Talecris Biotherapeutics, NC) equivalent to the concentration (µM) and volume of cell-free Hb were infused over 1 h to one animal, as was an equivalent volume of 0.9% saline to another. Two control groups were used to account for colloid osmotic effects of oxyHb or metHb (albumin vs. 0.9% saline). Measurements during the 3-h experiment were obtained before infusions (metHb, oxyHb, albumin, 0.9% saline) started at time 0 h, during the infusions from 0 to 1 h, and for 2 h after the infusion was completed.
On the day of the study, anesthesia was induced via mask inhalation using isoflurane (1–5%) and the animals were then intubated (6 mm, Rusch, Duluth, GA) and mechanically ventilated (Servo-I, Maquet, Wayne, NJ) (fractional inspired oxygen = 50%, positive end expiratory pressure = 5 cmH20, ventilation rate = 15 breaths/minute, tidal volume = 20 ml/kg) for the duration of the study. A high FiO2 was used to prevent hypoxemia from any cause during sedation and mechanical ventilation. Femoral arterial (20-gauge), external jugular venous (8-French) and radial venous (18-gauge) catheters (Maxxim Medical, Athens, TX) were placed percutaneously using aseptic techniques. Foley urinary catheters (Cook, Foley 8 Fr, 55 cm) were also placed in all animals using aseptic techniques to prevent bladder distention with hydration. After catheter placement, the anesthetic gas was discontinued and continuous infusions of midazolam (2.5–5 µg/kg/min infusion) and fentanyl (0.16 µg/kg/min infusion) were initiated and maintained for the duration of the study. Animals were continuously monitored for signs of distress and the infusions adjusted appropriately according to a protocol.39
Data Collection
MAP was obtained from the femoral artery catheter. A pulmonary artery thermodilution catheter (7-French, Abbott Critical Care, Chicago, IL) was introduced through the external jugular vein to measure central venous pressure (CVP) and to determine cardiac output (CO). The CO was measured by the thermodilution technique with 10 ml of sterile, room temperature 0.9% saline, injected through the proximal port of the triple lumen catheter, and the drop in temperature at the thermistor distal to the injection port was then recorded. CO was calculated using a CO module (Philips Medical Systems CO module, model M1012A). Three replicates were done at each time point and the average was used if there was less than 10% variance between measures. Systemic vascular resistance index was calculated (MAP − CVP) / (cardiac index) where cardiac index (CI) = CO / weight (kg). Hemodynamic measurements (MAP, CVP, and CO) and spectrophotometric-based quantification of cell-free Hb concentration, and chemiluminescence-based assays of NO consumption were obtained at 0-, 0.25-, 0.5-, 0.75-, 1.0-, 1.5-, 2.0-, 2.5-, and 3.0-h time points from arterial lines. Laboratory measurements (complete blood count, serum chemistries, and arterial blood gas analysis) were obtained from arterial lines at 0, 1, 2, 3 h. After the study was completed, while still sedated, all animals were euthanized (Beuthanol, 75 mg/kg IV).
Preparation of oxyHb and metHb solution from whole blood
Fresh blood was drawn into heparinized tubes from canines not enrolled in these studies and then centrifuged for 20 min at 3000g. Plasma was removed and red blood cells were washed 3 times using PBS and used immediately for preparation of oxyHb according to protocol developed by Rossi-Fanelli et al.40 The oxyHb was then dialyzed against PBS for at least 24 h in dialysis bags with cutoff of 10kDa, and the 100% purity of resulting oxyHb was confirmed spectroscopically and on a Sephadex G25 column. After dialysis, oxyHb aliquots were frozen in liquid nitrogen and stored at −80 °C or used to prepare metHb. Excess of potassium hexaferricyanide was used to oxidize ferrous heme of oxyHb to ferric heme of metHb. To remove potassium ferricyanide, the metHb solution was also dialyzed against PBS for at least 24 h in dialysis bags with cutoff of 10kDa, and the 100% purity of resulting metHb was confirmed spectroscopically and on a Sephadex G25 column.
Plasma Nitric Oxide Consumption and Plasma Nitrite Level Determination Assay
The ability of cell-free Hb in the supernatant to scavenge NO was measured with a previously published and validated NO consumption assay with a NO chemiluminescence analyzer (Sievers, Boulder, CO).41, 42 Additional experimental details are summarized in the online Supplemental Methods. Nitrite was measured using standard tri-iodide chemiluminescence assay.43
Cell-Free Plasma Hb Levels and Spectral Deconvolution of Species
Cell-free plasma Hb was measured only from plasma from arterial blood by conversion to cyanomet Hb with Drabkin’s reagent and then by spectrophotometric measurement of absorbance at 540 nm (Beckman Coulter DU 800 UV/visible spectrophotometer, Brea, CA).41, 42 Hb concentration and spectra were also measured on an Agilent 8453 UV-visible spectrophotometer (Agilent technologies, Santa Clara, CA) with 1-cm path-length cuvettes. Concentrations of oxyHb and metHb in plasma samples were analyzed by deconvolution of the spectrum into components from standard UV-visible spectra of human Hb composed of oxyHb, metHb, and deoxyHb in PBS buffer by a least-squares method, as described previously.44
STATISTICS
Unless noted otherwise, change from baseline values were used to account for baseline differences among animals. Linear mixed models (SAS PROC MIXED) were used to assess the effects of treatments over time. Random effects were included to account for the repeated measurements of each animal, and standard diagnostics were used to check model assumptions (e.g. normality, homoscedasticity). Log-transformation was used when necessary. Change-point linear regressions with changes in slopes fixed at the time of cessation of infusion (1 h) were used to estimate the rates of change in MAP, NO consumption, and Hb during and following infusion. Pearson’s correlations (SAS PROC CORR) were used to show the correlation of change in MAP with Hb measurements at each time point. Linear mixed models were used to assess the relationship between Hb and percent change in MAP, accounting for repeated measures. SAS version 9.2 (Cary, NC) was used. All p-values are two-sided and considered significant at the p ≤ 0.05 level.
RESULTS
Vasoconstrictive properties of cell-free Hb species vs. controls
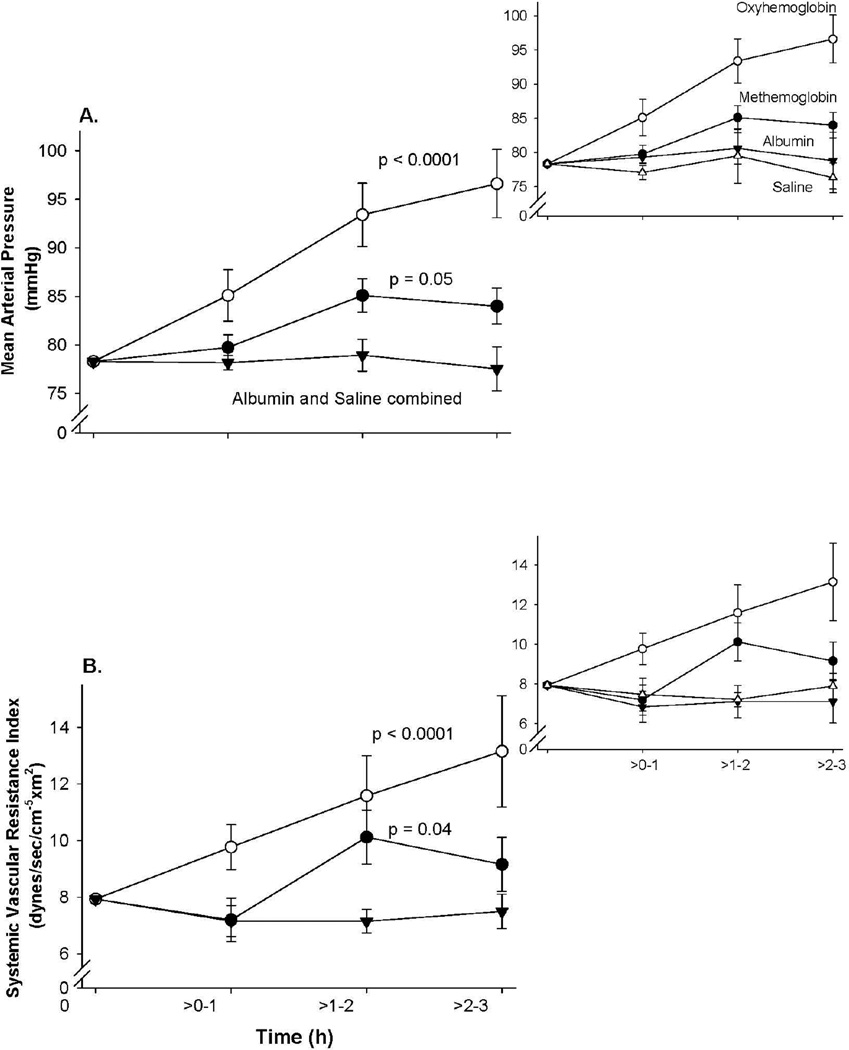
Figure 1 shows the time course of vascular pressure changes of the four study groups. The albumin and saline groups were combined since they are similar. After the cell-free oxyHb (Fe2+-O2) infusion was completed (0 to 1 h), there were until the end of the experiment (1 to 3 h) significant elevations in mean MAP (p <0.0001) and SVRI (p <0.0001) compared to controls (albumin and saline). Unexpectedly, after cell-free ferric metHb (Fe3+) infusions, there were also over this time period elevations in mean MAP (p = 0.05) and SVRI (p = 0.04) compared to control animals. However, despite infusing similar concentrations of Hb solutions over 1 h, the metHb infusions produced significantly less of an increase in MAP and SVRI compared to the oxyHb infusions (p = 0.006 and p = 0.04, respectively). For the rest of the Results section, we will focus only on changes in MAP, not SVRI, both to avoid redundancies in presentation, and because MAP is a direct measurement of vascular pressures whereas SVRI is calculated. The serial mean values for CI and HR which make up components of the calculation of the SVRI are shown by treatment group in the online Supplemental Figure 1 for completeness.
Figure 1. Changes in MAP and SVRI.
Serial mean (± SE) changes in (A) MAP, (B) SVRI in animals receiving oxyHb (n=5), metHb (n=5), albumin (n=5), or saline (n=5) are plotted. Hemodynamic values are plotted from a common origin representing the mean values for all animals at time 0. The inset above and to the right shows the individual serial changes for albumin and saline controls compared to the other two treatment groups. P-value represents changes over time compared to the combined controls.
Nitric oxide (NO) consumption potential of plasma after infusions of cell-free Hb species vs. controls
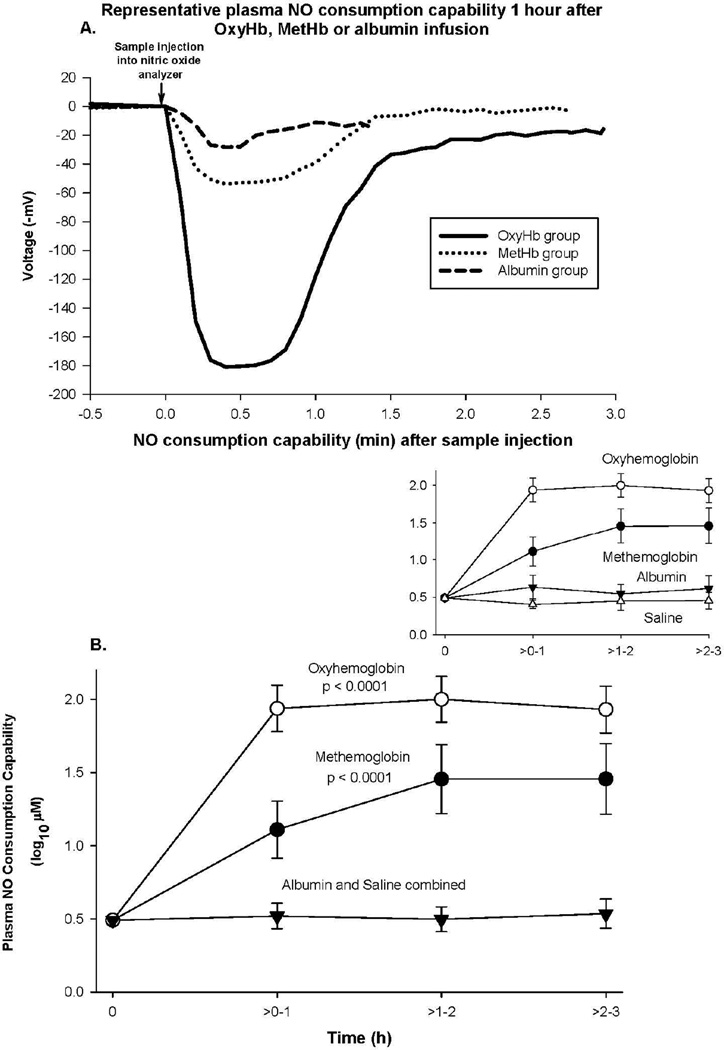
In order to better understand the weaker vasoconstrictive effect of metHb vs. oxyHb, we examined the NO consumption potential of plasma from these animals (Figure 2). This assay uses the fact that oxyHb is a very potent NO scavenger and that presence of any traces of oxyHb in plasma will result in loss of plasma NO. In practice, a chemiluminescence NO detector is used to measure changes in the steady-state NO in a bath with a NO donor present. If, with the addition of plasma, NO is scavenged, the steady state level of NO decreases which is observed as a drop in voltage in the detector of the nitric oxide analyzer (Figure 2A). This voltage drop indicates the presence of cell-free oxyHb (or potentially other NO-scavenging species such as ceruloplasmin) in plasma.41, 45 We measured elevated NO consumption ability of plasma in samples collected after oxyHb and metHb infusions compared to controls with infused albumin and saline (both p <0.0001) (Figure 2B). As expected, increase in NO consumption ability of plasma was highest with oxyHb. Unexpectedly, plasma from metHb infusions was also able to consume NO, albeit at a10-fold lower level than the infused oxyHb-containing plasma (p = 0.009) (Figure 2B), consistent with the decreased vasoconstrictive properties associated with metHb infusions in Figure 1. Since metHb is able to react only with very low affinity and very slowly with NO,46 we further pursued characterization of other Hb species present in plasma after metHb infusion.
Figure 2. NO consumption.
Panel A represents plasma NO consumption capability obtained from animal 1 h after infusion of various Hb species or albumin. Panel B represents the format similar to Figure 1, except now mean (± SE) log10 NO consumption capability of plasma is plotted.
Correlations between vascular pressure changes and levels of Hb species
Using a spectral deconvolution of absorption spectra, we quantified Hb species present in plasma. These results were used to determine the correlation between increased MAP and Hb species in plasma. We found that the “active” Hb species (more strongly correlated), was always plasma oxyHb levels, either infused directly or reduced in vivo (converted) in metHb-infused animals.
Infused cell-free oxyHb levels as well as the fraction in vivo oxidized to metHb and percent changes in MAP
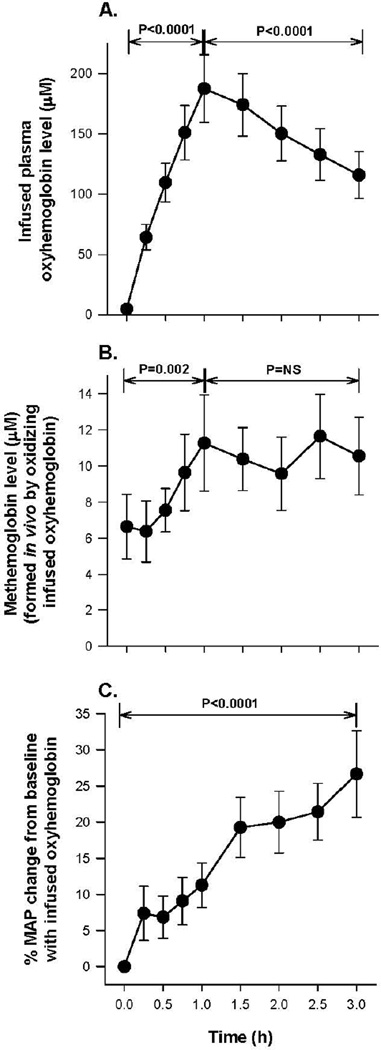
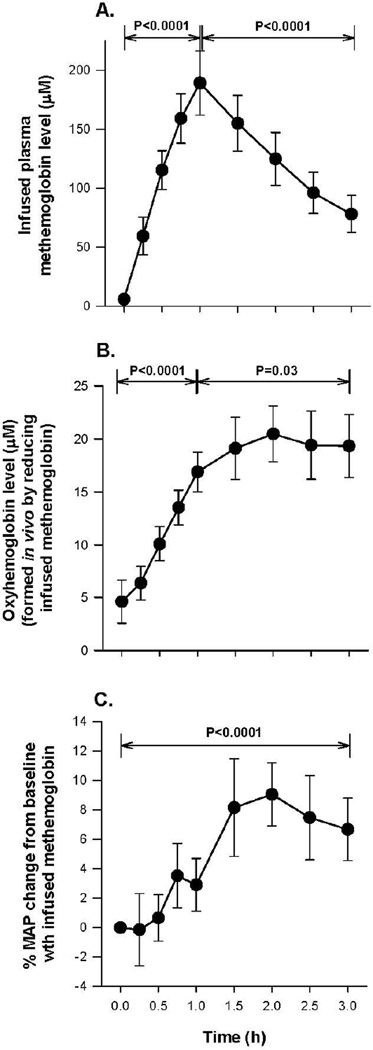
Figure 3A shows oxyHb levels in plasma as a function of time—levels increased progressively during the 1-h infusion (p <0.0001 for slope) and then monotonically decreased over the 2 h after the infusion stops (P < 0.0001 for slope). The concentration of cell-free oxyHb oxidized in plasma to metHb is plotted in Figure 3B and the levels of metHb progressively increased during the 1-h oxyHb infusion (p = 0.002 for slope) and remained elevated and unchanged during the last 2 h of the experiment. Figure 3C shows the MAP similarly increasing throughout the 3-h experiment (27% increase from 0 to 3 h, p<0.0001).
Figure 3. Hb species and percent changes in MAP during oxyHb infusions.
Panel A shows serial mean (± SE) values of oxyHb levels. Panel B shows serial mean (± SE) metHb levels formed by oxidizing a fraction of the oxyHb infusion in vivo. Panel C shows the mean (± SE) percent increase in MAP during the oxyHb infusion. All P values compare changes over the time period indicated by brackets.
Correlations of MAP with infused oxyHb levels and the fraction oxidized to metHb
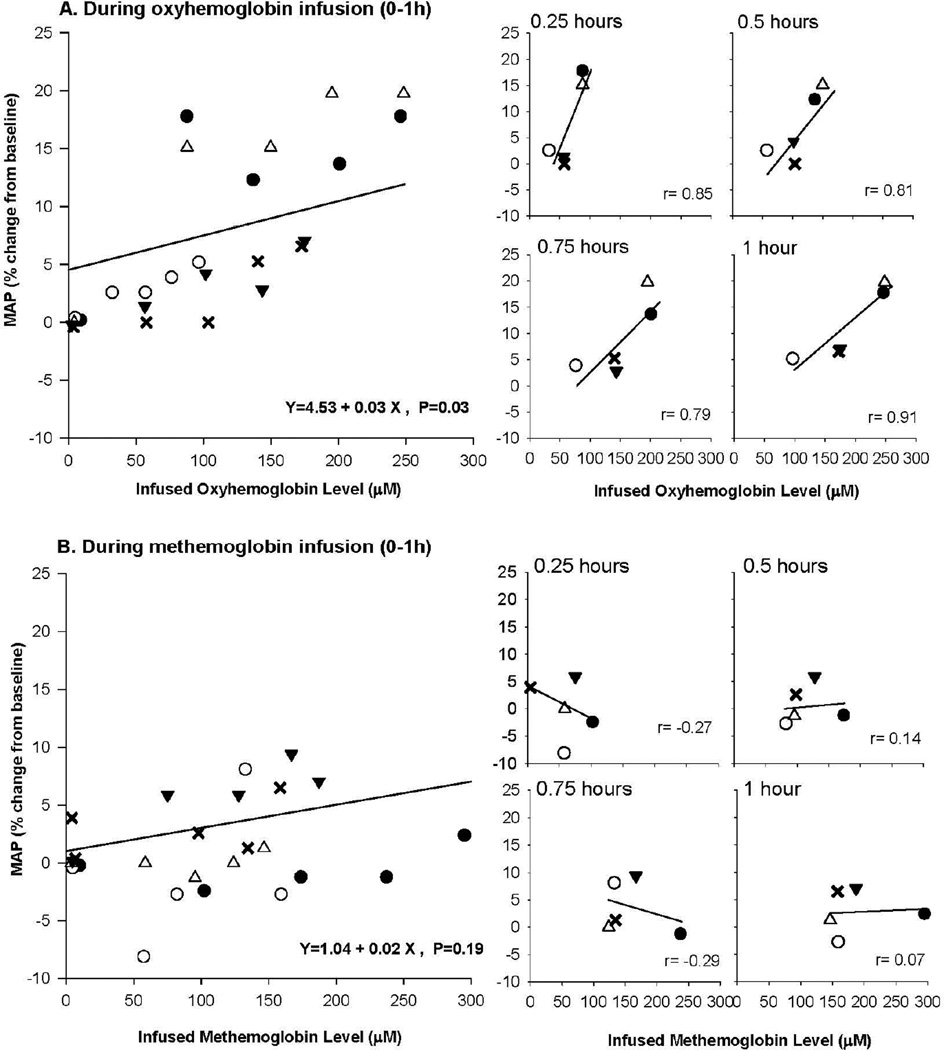
Next we examined if there was a correlation between oxyHb concentration in plasma and increases in MAP during infusion when levels of oxyHb were increasing (Figure 3). Using mixed models, we determined during infusion there was overall a significant positive relationship between increasing oxyHb levels and increases in MAP (p = 0.03 for slope) (Figure 4A, left side). Moreover, during infusion, there was at each time point studied a similar positive correlation between increases in MAP and oxyHb plasma levels (0.25, 0.50. 0.75, 1.0 h), (r = +0.79 to +0.91) (Figure 4A, right side). This strong positive correlation during the infusion occurred over a wide range of oxyHb values; near the start of the infusion (0.25 h), plasma concentrations in the five animals studied ranged from ~40 to 90 µM, and by the end of the infusion (1 h), they varied from ~90 to 250 µM. However, once the oxyHb infusion ended and oxyHb levels fell, the correlations between oxyHb plasma levels and increases in MAP at each time point measured became weaker (r = +0.80 to −0.06) (see online Supplemental Figure 2A right side) and overall non-significant (p = 0.62 for slope) (see online Supplemental Figure 2A left side). Finally, using similar analysis with mixed models, there was no significant relationship between the levels of oxyHb oxidized to metHb and percent increase in MAP throughout the experiment (p = 0.12 for slope, see online Supplemental Figure 3).
Figure 4. Comparison of percent change in MAP and infused oxyHb plasma levels.
The top four panels labeled A on the left shows the association of MAP and oxyHb levels during infusion 0 to 1 h. Each of the 5 animals is depicted by a different symbol (open circle, open triangle, closed circle, inverted closed triangle, and X), and the regression line was estimated using a mixed model. On the top panel to the right are shown at serial time points in animals during the oxyHb infusion, the correlation between MAP and plasma oxyHb levels. (The symbols are the same for each animal, as in the figure to the left.) The bottom panels labeled 5B are similar in format to the 5A top panels, except now infused metHb levels are shown from 0 to 1 h.
In summary, we found the relationship between oxyHb plasma levels and MAP was very strong during infusion of oxyHb over a wide range of plasma levels (Figure 4A, top panels). After the infusion ended and levels were decreasing, likely in part because the elimination of oxyHb from plasma became more prominent, the correlation progressively weakened and became overall non-significant (see online Supplemental Figure 2A). In contrast, metHb levels converted from infused oxyHb (product of oxyHb oxidation in plasma) were not correlated with changes in MAP throughout the experiment (see online Supplemental Figure 3).
Infused metHb levels and the fraction reduced to oxyHb and percent changes in MAP
Figure 5A shows cell-free metHb concentration in plasma as a function of time during the 3-h experiment. There is a progressive increase in plasma metHb levels during infusion (0 to 1 h) (p <0.0001 for slope) and the metHb concentration in plasma monotonically decreased after the infusion stopped (1 to 3 h; p <0.0001 for slope). Spectral deconvolution to determine the fraction in vivo reduced to oxyHb showed during the 1-h metHb infusion, oxyHb levels in the plasma progressively increased (p <0.0001 for slope) and after the metHb infusion stopped, oxyHb levels continued to rise (p=0.03 for slope) (Figure 5B). Figure 5C shows the time dependence of MAP changes throughout the 3-h experiment. There was an increase in MAP during and after the infusion of metHb (7% total increase; 0 to 3 h; p <0.0001 for slope).
Figure 5. Hb species and percent changes in MAP during metHb infusions.
The format is the same as Figure 3, except now Hb species and percent changes in MAP are shown during the metHb infusion. Of note, Panel B now shows the cell-free fraction of metHb that was reduced to cell-free oxyHb during infusion.
Correlations of MAP with infused metHb levels and the fraction of metHb reduced (converted) to oxyHb in vivo
Next, we looked for the presence of a relationship between infused Hb species and MAP during metHb infusions in a manner similar to that of infused oxyHb. Using mixed models, we found that there was no overall significant relationship between infused metHb plasma levels and MAP during the infusion (p = 0.19 for slope) (Figure 4B, left side). Moreover, there was actually a negative or very weak positive correlation between MAP and metHb plasma levels at each time point studied during infusion (0.25, 0.50, 0.75, 1.0) (r = −0.29 to r = 0.14) (Figure 4B, right side). Using mixed models, the relationship with MAP from 1 to 3 h was also non-significant when the metHb infusion was stopped and metHb levels were decreasing (p = 0.18 for slope, see online Supplemental Figure 2B).
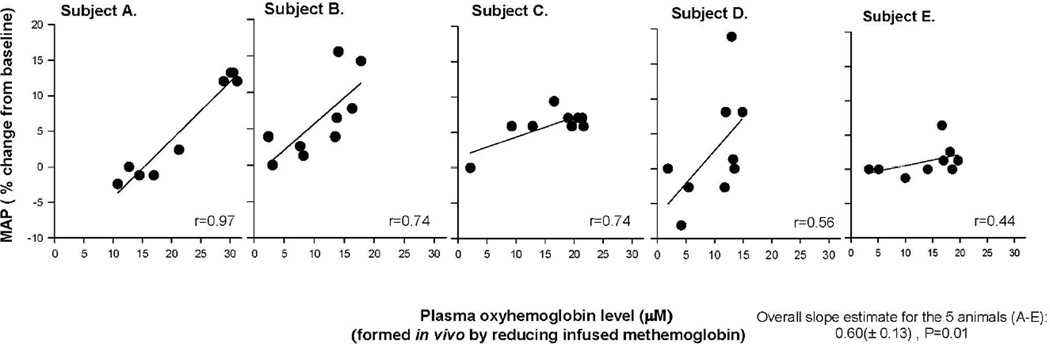
The in vivo reduced from metHb to oxyHb plasma levels were ~10-times lower than the infused plasma metHb levels. The converted oxyHb plasma levels followed a different time course pattern than infused metHb levels, peaking 1 h after the metHb infusion stopped (the 2 h time point) and remaining elevated and not decreasing until the end of the experiment (2 to 3 h) (Figure 5A and B). The pattern of changes over time in oxyHb (reduced from metHb) at these low plasma levels (6.3 to 20.5 µM) paralleled the changes in MAP over time (Figure 5B and C). To further test this, we examined the relationship between changes in MAP and changes in converted oxyHb levels in each animal and found there was a moderate to strong positive relationship in each animal (r = 0.97 to r = 0.44) (Figure 6) that was using mixed models overall statistically significant (slope: 0.60 ± 0.13, p = 0.01).
Figure 6. Comparison of the percent change in MAP and the fraction of metHb that was reduced (converted) to cell-free oxyHb during infusion.
Panels A to E show in each of these animals over the 3-h experiment the relationship between MAP and converted oxyHb levels. The overall slope of the lines is shown in the lower right-hand corner—analyzing this overall relationship between MAP, and converted oxyHb plasma levels using mixed models.
In summary, the following findings indicate that the increase in MAP after metHb infusions is mediated through converted oxyHb: 1) infused metHb levels do not correlate with MAP but infused oxyHb levels do correlate with MAP; 2) as reduction of infused metHb into oxyHb progresses over time, it correlates with observed increases of MAP over the 3-h experiment; and 3) the increases in converted oxyHb plasma levels across animals are significantly related to the increases in MAP, but the increases in converted to metHb levels across animals are not significantly related to MAP.
Plasma nitrite levels after cell-free Hb solution infusions vs. controls
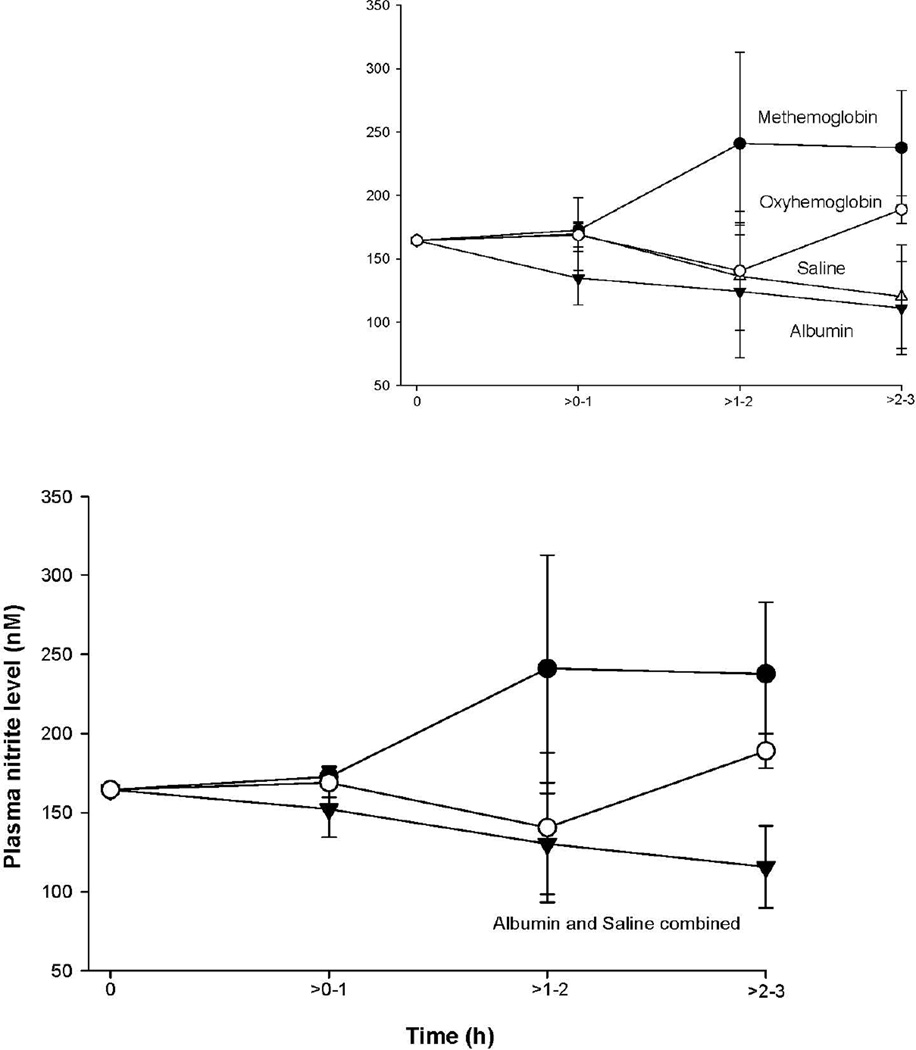
To determine if intravascular scavenging of NO by oxyHb (infused or converted) was responsible for the increases in MAP, we measured plasma nitrite levels in animals at several time points. Nitrite can be converted to NO and is also a biomarker for NO production by endothelial NOS.47 The mean nitrite levels were similar in animals receiving oxyHb and metHb infusions, compared to controls (p = 0.28 and 0.07, respectively) (Figure 7). Furthermore, in all four treatment groups, nitrite concentration did not significantly change throughout the experiment; the concentrations in plasma ranged on average from ~120 to 250 nM throughout (all, p >0.05).
Figure 7. Plasma nitrite level.
The format is similar to Figure 1 and 2, except now mean (± SE) nitrite level in plasma is plotted.
Plasma total Hb levels
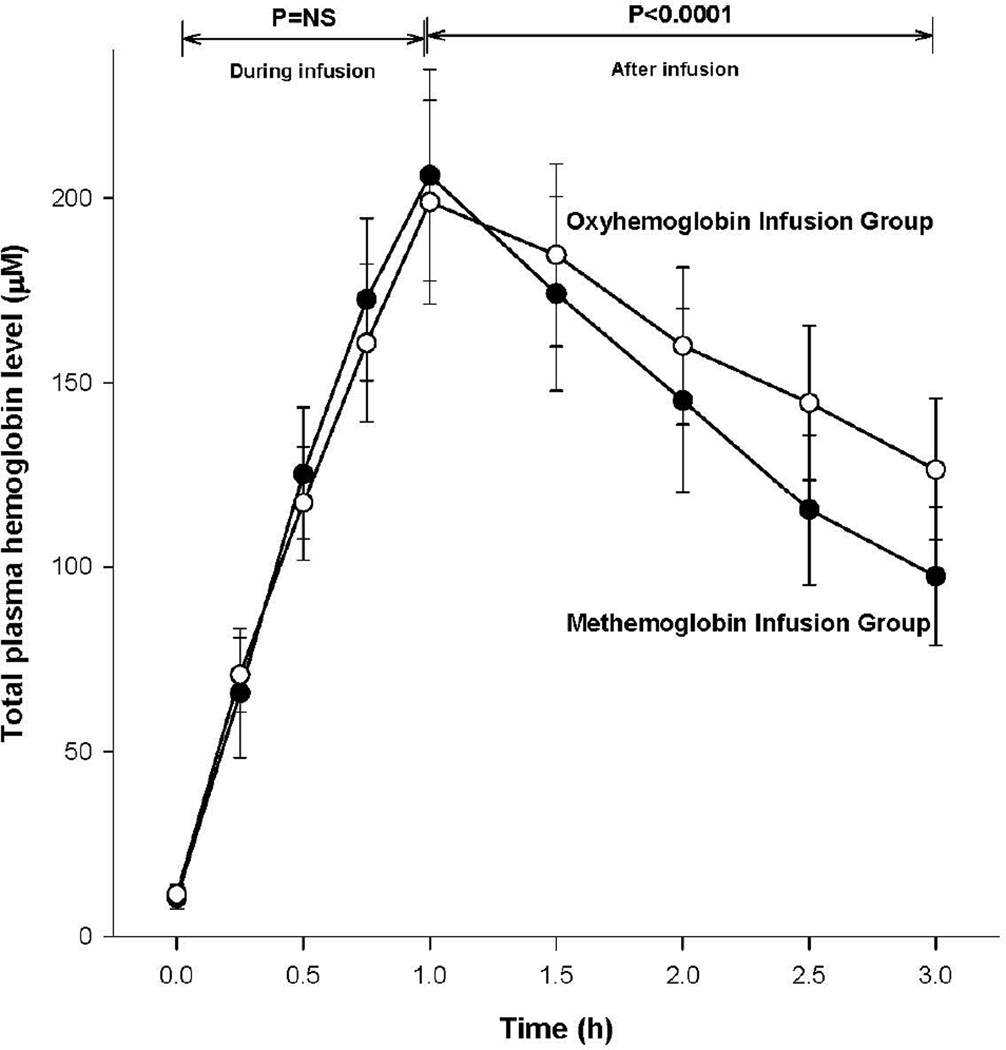
To confirm all animals actually received equivalent micromolar quantities of oxyHb or metHb during the 1-h infusion, we measured plasma total Hb concentrations serially. During infusion until the end of infusion, from 0 to 1 h, these two groups of animals had similar significant rate increases in plasma total Hb levels (Figure 8). However, total Hb concentration overall in plasma decreased post-infusion (from 1 to 3 h) at a faster rate in animals receiving metHb compared to those receiving oxyHb infusions (p <0.0001 for the difference in slopes), indicating that cell-free metHb is cleared faster or is less stable in plasma than cell-free oxyHb.
Figure 8. Serial mean (± SE) changes in total Hb levels in animals receiving oxyHb (open circles) and metHb (closed circles).
All p-values compare changes in these two treatment groups over the time period indicated by the brackets.
Other chemistries
For completeness, in an online Supplement Figure 4 (Panels A–K) it is shown that there were no significant abnormalities throughout the experiment in complete blood counts (Panels A–D), serum electrolytes (Panels E–H), and blood gases (Panels I–K) and no significant differences comparing treatment groups in each of these panels (Panels A–K) to explain these results (all, p=ns).
DISCUSSION
Cell-free metHb infusions in canines produced unexpected and, to the best of our knowledge, not previously described elevations in MAP and SVRI compared to control animals (Figure 1). At similar concentrations, cell-free oxyHb produced more marked increases in vascular pressures and tone than metHb. Cell-free metHb solution infusions were not only associated with progressive increases in plasma metHb levels, as expected (Figure 5A), but formation of the reduced species—oxyHb—in plasma was observed (Figure 5B). The infused metHb level was not positively correlated with MAP, but when plotted against the amount of oxyHb present (reduced from infused metHb), MAP and oxyHb plasma levels were correlated (Figure 6). These data indicate the increase in MAP after metHb infusion is mediated through reduction of infused metHb to oxyHb, resulting in NO scavenging and increases in vascular pressure. In human plasma, reducing agents such as urate, ascorbic acid, and glutathione were shown to be responsible for the conversion of cell-free metHb to oxyHb.48–50 In canines and humans, levels >20 µM of ascorbic acid have been reported in plasma and the processes we describe here could apply to both species.34, 51
We found that animals receiving cell-free metHb infusions were forming oxyHb in the plasma (Figure 5B) on a micromolar basis at a rate faster than animals that received cell-free oxyHb infusions were forming metHb (Figure 3B). This was surprising since the conversion of cell-free oxy- to metHb occurs through an auto-oxidation reaction and a dioxygenation reaction with NO (a reaction which is rate-limited by diffusion).52–54 This reaction with reducing agents has not been established as dominating over auto-oxidation or NO reactivity. Moreover, from the time the infusions stopped until the end of the experiment, converted plasma oxyHb levels remained higher (18 to 23 µM) in animals that received cell-free metHb (Figure 5B) than the metHb levels (8 to 12 µM) in the animals that received cell-free oxyHb (Figure 3B).
If a NO deficit in the luminal space of the vasculature is causing increases in MAP, then these two variables should be strongly correlated. As expected, there was a strong correlation between the level of oxyHb in plasma and MAP levels during the 1-h oxyHb infusions, measured every 15 min over a wide range of gradually increasing plasma levels from 90 to 250 µM (Figure 4, top panels). Unexpectedly, after the cell-free oxyHb infusion ended and the plasma oxyHb levels were decreasing over the ensuing 2 h (but still in the same range, 90 to 250 µM), the correlation with MAP became non-significant. Despite this loss of correlation after the infusion ended, the MAP continued to steadily rise over the next 2 h at the same rate as during the infusion (Figure 3C). The loss of correlation over time but continued rise in MAP could be explained in part by the fact that once the infusion stops, elimination of oxyHb from the systemic circulation becomes more prominent and the relationship between NO and oxyHb after this point becomes more complex. However, levels of oxyHb remain high enough vascularly or perivascularly to continue to increase MAP.
Based on in vitro experiments, the rate of oxyHb reaction with NO is limited only by diffusion, so any free NO in the plasma will be quickly scavenged in the presence of cell-free oxyHb.54 Recently, Hall and Garthwaite reported that there is immense variability of reported NO concentrations, depending on the method used and tissue/model studied, ranging from a few pM to ~1 µM.55 The authors based their estimate of the functional concentrations of NO on studies using soluble guanyl cyclase (sGC) as an innate NO sensor and concluded that 100 pM to 5 – 10 nM of NO could be considered as a functional NO range. However, there is one other source of NO present in the vasculature plasma-nitrite. The circulating nitrite levels varied from 100 to 250 nM levels in our animal experiments (Figure 8). Notably, even taking into account the highest estimate of 10 nM of cell-free NO in plasma from published sources55 and plasma nitrite levels measured in our experiments, there is still a large excess of oxyHb over nitrite and NO in both of our experimental setups: in the case of oxyHb infusions, 200 µM oxyHb compared to 100 to 250 nM available NO to be scavenged; and in the case of metHb infusions, 20 µM of oxyHb compared to 100 to 250 nM nitrite available to be scavenged. Without existence of some kind of “protective compartmentalization,” NO in plasma should be completely scavenged in a very short time after being released either from endothelial cells or red blood cells (RBCs)—probably on the order of milliseconds. Previous work has suggested that the extent of the effect of intravascular oxyHb on the concentration of NO at the smooth muscle decreases as the concentration of Hb increases to high enough levels.56 The fact that vascular pressures markedly differ even with very high plasma oxyHb levels in both oxyHb- and metHb-infused animals in excess of plasma NO available to be scavenged can only be explained if the effect of cell-free Hb is not limited to the luminal space and potentially occurs more perivascularly.
One possibility is that extravasation of cell-free Hb beyond the endothelium scavenges endothelial NO within the smooth muscle layer where NO levels may be higher, or there are other effects such as oxidative changes in the tissues. Hb has a molecular weight similar to albumin which has a wide extravascular circulation.57, 58 This extravasation of oxyHb into the extravascular circulation would explain why during the oxyHb infusion in these experiments intravascular plasma oxyHb levels and blood pressure were strongly correlated (i.e., intravascular and extravascular plasma levels should be correlated because they are similarly increasing during the infusion time). However, MAP and oxyHb levels gradually become less correlated after the infusion ends. After stopping the infusion, elimination of Hb from the intravascular space becomes a more significant factor. Because of this, post-infusion relationships between MAP and oxyHb in the extravascular and intravascular space may not be in step with one another and become more complex. This potentially explains the gradual loss of a significant correlation between MAP and plasma oxyHb levels, despite MAP still increasing.
The mechanism(s) of the multiple different toxic effects of cell-free Hb reported remain not fully understood. In terms of vasculopathy, multiple lines of clinical evidence support a role for NO scavenging by cell-free Hb in various disease processes.59 We have recently shown in a canine model of pneumonia that, after transfusion, older blood releases in vivo cell-free oxyHb over days in large quantities (50 to 150 µM on average), producing pulmonary hypertension, pulmonary vascular necrosis, and worsened gas exchange, associated with increased mortality.60 Further, giving NO donors can prevent the vaso-occlusive disease that occurs over 7–14 days following cerebral hemorrhage.61 This is the time when cerebral clots break up and cell-free Hb is potentially released near vascular tissue, causing vasospasm through NO scavenging. Notably, it is also possible this beneficial effect is not related to NO donors oxidizing these low levels of cell-free oxyHb but to the direct vasodilation effect of giving a NO donor. Also of note, in clinical trials, therapy with HBOCs associated with high circulating levels of modified cell-free Hb were associated with a 3-fold increased incidence of acute myocardial infarctions.10 If there is a loss of NO-related coronary vasodilatory tone and increased systemic pressures, patients are at risk for myocardial ischemic events.62–65 Both of these effects can potentially be precipitated by HBOCs scavenging NO. Other potentially important mechanisms, as discussed in the Introduction, mediated by oxidative reactions of metHb, may also be critical in producing these vascular toxicities.1, 2, 21–24, 50
In previous experiments, we showed inhaled NO, 80 parts per million in canines, which by itself has miniscule or no measureable effects on systemic blood pressure, completely eliminates by oxidizing oxyHb the hypertensive effects associated with intravascular free water-induced hemolysis and release of cell-free oxyHb.20 Yu et al. have done experiments in knockout mice without endothelial nitric oxide synthase (eNOS, enzyme that produces NO), which are hypertensive compared to wild type animals. Notably, HBOCs increase vascular pressure in wild type mice, but completely lose this ability in these eNOS knockout mice.25 The present study shows that cell-free metHb infusions, after being reduced to oxyHb, increase vascular pressures. Since the cell-free metHb is reduced in vivo in the plasma to oxyHb, and then increases vascular pressure, it is difficult to ascribe these hypertensive effects to red cell membranes or other impurities in the process of formation of ex vivo cell-free Hb (Figure 5B). Overall, the above data show that NO scavenging ability of the oxyHb molecule at a minimum are at least responsible for some of the hypertensive vascular effects and potentially the vasculopathies associated with cell-free Hb in various disease states.10, 60, 61
It should also be emphasized cell-free metHb is cleared faster and/or is less stable than cell-free oxyHb in plasma (Figure 8). This is an unexpected finding, as the classic mechanism of Hb clearance is through binding to haptoglobin and subsequent internalization through CD 163 receptor and clearance from plasma by either macrophages or liver hepatocytes.21 This clearance mechanism is not known to discriminate between oxyHb and metHb. We therefore speculate clearance is not increased but may be due to metHb dissociating faster favoring formation of dimers and heme dissociating faster from dimers.23, 66 With a higher percentage of dimers present, this might increase clearance by haptoglobin. Alternatively, since heme has a different absorption spectra than metHb, this could give the appearance of faster clearance given our use of spectroscopic methods to measure metHb in the plasma. The underlying reasons and exact mechanism for the faster clearance of metHb levels using spectroscopic assays from plasma requires further investigation.
We are aware of limitations to the interpretation of our findings. We are measuring short-term effects on hemodynamics by cell-free oxyHb. Although we speculate that over the long term this vasoconstrictive effect of cell-free Hb may produce permanent vascular injury, we may be observing only a short-term effect of oxyHb on a normal functioning endothelium with oxyHb scavenging endothelial NO in these experiments. Our control albumin is about the same molecular weight as Hb in its normal state, a tetramer, but some Hb could break down into dimers or monomers, making our albumin control slightly less comparable in terms of oncotic pressure to cell-free Hb.
This is the first systematic experimental study of metHb infusions that includes necessary time-matched controls. The major finding of this study is that vascular effects of cell-free metHb exist and they are related to reduction of cell-free metHb to cell-free oxyHb, likely by plasma-reducing agents. To the best of our knowledge, this finding has not been reported in any study in animals or humans. The results of this study also indicate metHb has no direct vascular effects during the time period studied and the results do not support the hypothesis that hypertension associated with cell-free Hb is directly and mainly mediated by oxidative reactions. However, in aggregate, metHb should not be considered inert and in the application of any therapies producing metHb in the plasma, this can result in potentially injurious vascular hypertensive effects by being reduced to oxyHb.
Supplementary Material
Acknowledgements
The authors of this paper would like to acknowledge Juli Maltagliati for typing and formatting the manuscript
This study was conducted using intramural NIH funds and NIH grants HL058091 (DK-S) and HL098032 (MTG and DK-S). Dr. Gladwin also receives research support from NIH grants RO1HL096973, and P01HL103455, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania. The work by the authors was done as part of U.S. government-funded research; however, the opinions expressed are not necessarily those of the National Institutes of Health.
Footnotes
Conflict of interest: All authors declare that they have no competing financial interests.
Authors / Contributors:
Dong Wang and Barbora Piknova contributed equally to writing the manuscript and should be considered co-first authors; Steven B. Solomon performed the experiments and helped write the manuscript; Irene Cortes-Puch made figures and helped with analysis; Steven J. Kern and Junfeng Sun did the statistical analysis; Christine Helms and Tamir Kanias did the laboratory analysis; Mark T. Gladwin, Daniel B. Kim-Shapiro, and Alan N. Schechter conceived of the experiments and helped write the manuscript; Charles Natanson designed the experiments and helped analyze the data and write the manuscript.
REFERENCES
- 1.Alayash AI, Cashon RE. Hemoglobin and free radicals: Implications for the development of a safe blood substitute. Mol Med Today. 1995;1:122–127. doi: 10.1016/s1357-4310(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 2.Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Jr, Vandegriff KD, Winslow RM. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273:12128–12134. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 3.Sprung J, Kindscher JD, Wahr JA, Levy JH, Monk TG, Moritz MW, O'Hara PJ. The use of bovine hemoglobin glutamer-250 (hemopure) in surgical patients: Results of a multicenter, randomized, single-blinded trial. Anesth Analg. 2002;94:799–808. doi: 10.1097/00000539-200204000-00006. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Smani Y, Fifre A, Labrude P, Vigneron C, Faivre B. Pharmacological and physicochemical factors in the pressor effects of conjugated haemoglobin-based oxygen carriers in vivo. J Hypertens. 2007;25:599–608. doi: 10.1097/HJH.0b013e3280119000. [DOI] [PubMed] [Google Scholar]
- 5.Buehler PW, Alayash AI. All hemoglobin-based oxygen carriers are not created equally. Biochim Biophys Acta. 2008;1784:1378–1381. doi: 10.1016/j.bbapap.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Lamy ML, Daily EK, Brichant JF, Larbuisson RP, Demeyere RH, Vandermeersch EA, Lehot JJ, Parsloe MR, Berridge JC, Sinclair CJ, Baron JF, Przybelski RJ. Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery. The dclhb cardiac surgery trial collaborative group. Anesthesiology. 2000;92:646–656. doi: 10.1097/00000542-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hill SE, Gottschalk LI, Grichnik K. Safety and preliminary efficacy of hemoglobin raffimer for patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2002;16:695–702. doi: 10.1053/jcan.2002.128416. [DOI] [PubMed] [Google Scholar]
- 8.Schubert A, Przybelski RJ, Eidt JF, Lasky LC, Marks KE, Karafa M, Novick AC, O'Hara JF, Jr, Saunders ME, Blue JW, Tetzlaff JE, Mascha E. Diaspirin-crosslinked hemoglobin reduces blood transfusion in noncardiac surgery: A multicenter, randomized, controlled, double-blinded trial. Anesth Analg. 2003;97:323–332. doi: 10.1213/01.ANE.0000068888.02977.DA. table of contents. [DOI] [PubMed] [Google Scholar]
- 9.Greenburg AG, Kim HW. Use of an oxygen therapeutic as an adjunct to intraoperative autologous donation to reduce transfusion requirements in patients undergoing coronary artery bypass graft surgery. J Am Coll Surg. 2004;198:373–383. doi: 10.1016/j.jamcollsurg.2003.11.020. discussion 384-375. [DOI] [PubMed] [Google Scholar]
- 10.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: A meta-analysis. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloan EP, Koenigsberg M, Gens D, Cipolle M, Runge J, Mallory MN, Rodman G., Jr Diaspirin cross-linked hemoglobin (dclhb) in the treatment of severe traumatic hemorrhagic shock: A randomized controlled efficacy trial. JAMA. 1999;282:1857–1864. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- 12.Kerner T, Ahlers O, Veit S, Riou B, Saunders M, Pison U. Dcl-hb for trauma patients with severe hemorrhagic shock: The european "on-scene" multicenter study. Intensive Care Med. 2003;29:378–385. doi: 10.1007/s00134-002-1622-x. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann RC, Jenkins DE, Jr, McKee LC, Heyssel RM. Paroxysmal nocturnal hemoglobinuria: Clinical and laboratory studies relating to iron metabolism and therapy with androgen and iron. Medicine (Baltimore) 1966;45:331–363. doi: 10.1097/00005792-196609000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Naumann HN, Diggs LW, Barreras L, Williams BJ. Plasma hemoglobin and hemoglobin fractions in sickle cell crisis. Am J Clin Pathol. 1971;56:137–147. doi: 10.1093/ajcp/56.2.137. [DOI] [PubMed] [Google Scholar]
- 15.Pepper JR, Mumby S, Gutteridge JM. Transient iron-overload with bleomycin-detectable iron present during cardiopulmonary bypass surgery. Free Radic Res. 1994;21:53–58. doi: 10.3109/10715769409056556. [DOI] [PubMed] [Google Scholar]
- 16.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 17.Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, Bronson RT, Halperin JA, Loscalzo J, Qin X. The critical roles of platelet activation and reduced no bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood. 2010;116:1613–1622. doi: 10.1182/blood-2010-01-267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebbel RP. Reconstructing sickle cell disease: A data-based analysis of the "hyperhemolysis paradigm" for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 2011;86:123–154. doi: 10.1002/ajh.21952. [DOI] [PubMed] [Google Scholar]
- 19.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 20.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated no inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graversen JH, Madsen M, Moestrup SK. Cd163: A signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol. 2002;34:309–314. doi: 10.1016/s1357-2725(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 22.Schaer DJ, Alayash AI. Clearance and control mechanisms of hemoglobin from cradle to grave. Antioxid Redox Signal. 2010;12:181–184. doi: 10.1089/ars.2009.2923. [DOI] [PubMed] [Google Scholar]
- 23.Everse J, Hsia N. The toxicities of native and modified hemoglobins. Free Radic Biol Med. 1997;22:1075–1099. doi: 10.1016/s0891-5849(96)00499-6. [DOI] [PubMed] [Google Scholar]
- 24.Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI. Blood aging, safety, and transfusion: Capturing the "radical" menace. Antioxid Redox Signal. 2011;14:1713–1728. doi: 10.1089/ars.2010.3447. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blood AB, Schroeder HJ, Terry MH, Merrill-Henry J, Bragg SL, Vrancken K, Liu T, Herring JL, Sowers LC, Wilson SM, Power GG. Inhaled nitrite reverses hemolysis-induced pulmonary vasoconstriction in newborn lambs without blood participation. Circulation. 2011;123:605–612. doi: 10.1161/CIRCULATIONAHA.110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashley Z, Jugg B, Brown RF, Kenward CE, Platt J, Rice P, Harban FM. Effects of inhaled nitric oxide on the anesthetized, mechanically ventilated, large white pig. Inhal Toxicol. 2002;14:1175–1185. doi: 10.1080/08958370290084854. [DOI] [PubMed] [Google Scholar]
- 28.Ashurst J, Wasson M. Methemoglobinemia: A systematic review of the pathophysiology, detection, and treatment. Del Med J. 2011;83:203–208. [PubMed] [Google Scholar]
- 29.Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc Natl Acad Sci U S A. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alayash AI, Patel RP, Cashon RE. Redox reactions of hemoglobin and myoglobin: Biological and toxicological implications. Antioxid Redox Signal. 2001;3:313–327. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- 31.Simoni J, Villanueva-Meyer J, Simoni G, Moeller JF, Wesson DE. Control of oxidative reactions of hemoglobin in the design of blood substitutes: Role of the ascorbate-glutathione antioxidant system. Artif Organs. 2009;33:115–126. doi: 10.1111/j.1525-1594.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 32.Chaves MA, Leonart MS, do Nascimento AJ. Oxidative process in erythrocytes of individuals with hemoglobin s. Hematology. 2008;13:187–192. doi: 10.1179/102453308X343356. [DOI] [PubMed] [Google Scholar]
- 33.Waugh WH. Inhibition of iron-catalyzed oxidations by attainable uric acid and ascorbic acid levels: Therapeutic implications for alzheimer's disease and late cognitive impairment. Gerontology. 2008;54:238–243. doi: 10.1159/000122618. [DOI] [PubMed] [Google Scholar]
- 34.Harrison FE, May JM. Vitamin c function in the brain: Vital role of the ascorbate transporter svct2. Free Radic Biol Med. 2009;46:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piknova B, Keszler A, Hogg N, Schechter AN. The reaction of cell-free oxyhemoglobin with nitrite under physiologically relevant conditions: Implications for nitrite-based therapies. Nitric Oxide. 2009;20:88–94. doi: 10.1016/j.niox.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: A mechanistic study. J Biol Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herold S, Rock G. Mechanistic studies of the oxygen-mediated oxidation of nitrosylhemoglobin. Biochemistry. 2005;44:6223–6231. doi: 10.1021/bi0475929. [DOI] [PubMed] [Google Scholar]
- 38.Solomon SB, Bellavia L, Sweeney D, Piknova B, Perlegas A, Helms CC, Ferreyra GA, Bruce King S, Raat NJ, Kern SJ, Sun J, McPhail LC, Schechter AN, Natanson C, Gladwin MT, Kim-Shapiro DB. Angeli's salt counteracts the vasoactive effects of elevated plasma hemoglobin. Free Radic Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.10.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minneci PC, Deans KJ, Hansen B, Parent C, Romines C, Gonzales DA, Ying SX, Munson P, Suffredini AF, Feng J, Solomon MA, Banks SM, Kern SJ, Danner RL, Eichacker PQ, Natanson C, Solomon SB. A canine model of septic shock: Balancing animal welfare and scientific relevance. Am J Physiol Heart Circ Physiol. 2007;293:H2487–H2500. doi: 10.1152/ajpheart.00589.2007. [DOI] [PubMed] [Google Scholar]
- 40.Rossi-Fanelli AAE, Caputo A. Studies on the relation between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J. Biol. Chem. 1961;236:391–396. [PubMed] [Google Scholar]
- 41.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci U S A. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and s-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Piknova B, Schechter AN. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol Biol. 2011;704:39–56. doi: 10.1007/978-1-61737-964-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of s-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res. 2003;37:1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
- 45.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a no oxidase and nitrite synthase that determines endocrine no homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 46.Tejero J, Basu S, Helms C, Hogg N, King SB, Kim-Shapiro DB, Gladwin MT. Low no concentration dependence of reductive nitrosylation reaction of hemoglobin. J Biol Chem. 2012;287:18262–18274. doi: 10.1074/jbc.M111.298927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 48.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348:1483–1485. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 49.Faivre B, Menu P, Labrude P, Grandgeorge M, Vigneron C. Methemoglobin formation after administration of hemoglobin conjugated to carboxylate dextran in guinea pigs. Attempts to prevent the oxidation of hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:551–558. doi: 10.3109/10731199409117883. [DOI] [PubMed] [Google Scholar]
- 50.den Boer PJ, Bleeker WK, Rigter G, Agterberg J, Stekkinger P, Kannegieter LM, de Nijs IM, Bakker JC. Intravascular reduction of methemoglobin in plasma of the rat in vivo. Biomater Artif Cells Immobilization Biotechnol. 1992;20:647–650. doi: 10.3109/10731199209119695. [DOI] [PubMed] [Google Scholar]
- 51.Viviano KR, Lavergne SN, Goodman L, Vanderwielen B, Grundahl L, Padilla M, Trepanier LA. Glutathione, cysteine, and ascorbate concentrations in clinically ill dogs and cats. J Vet Intern Med. 2009;23:250–257. doi: 10.1111/j.1939-1676.2008.0238.x. [DOI] [PubMed] [Google Scholar]
- 52.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 53.Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98:127–148. doi: 10.1016/s0301-4622(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 54.Gladwin MT, Lancaster JR, Jr, Freeman BA, Schechter AN. Nitric oxide's reactions with hemoglobin: A view through the sno-storm. Nat Med. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 55.Hall CN, Garthwaite J. What is the real physiological no concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- 58.Gattoni M, Boffi A, Sarti P, Chiancone E. Stability of the heme-globin linkage in alphabeta dimers and isolated chains of human hemoglobin. A study of the heme transfer reaction from the immobilized proteins to albumin. J Biol Chem. 1996;271:10130–10136. doi: 10.1074/jbc.271.17.10130. [DOI] [PubMed] [Google Scholar]
- 59.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112:586–594. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solomon SB, Wang D, Sun JF, Kanias T, Feng J, Helms CC, Solomon MA, Alimchandani M, Quezado M, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Mortality increases after massive older stored blood transfusion in canines with pneumonia. Blood. 2012 doi: 10.1182/blood-2012-10-462945. published ahead of print December 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 62.Kupatt C, Zahler S, Seligmann C, Massoudy P, Becker BF, Gerlach E. Nitric oxide mitigates leukocyte adhesion and vascular leak after myocardial ischemia. J Mol Cell Cardiol. 1996;28:643–654. doi: 10.1006/jmcc.1996.0060. [DOI] [PubMed] [Google Scholar]
- 63.Bai Y, Muqier, Murakami H, Iwasa M, Sumi S, Yamada Y, Ushikoshi H, Aoyama T, Nishigaki K, Takemura G, Uno B, Minatoguchi S. Cilostazol protects the heart against ischaemia reperfusion injury in a rabbit model of myocardial infarction: Focus on adenosine, nitric oxide and mitochondrial atp-sensitive potassium channels. Clin Exp Pharmacol Physiol. 2011;38:658–665. doi: 10.1111/j.1440-1681.2011.05550.x. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira JC, Mochly-Rosen D. Nitroglycerin use in myocardial infarction patients. Circ J. 2011;76:15–21. doi: 10.1253/circj.cj-11-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimokawa H, Tsutsui M. Nitric oxide synthases in the pathogenesis of cardiovascular disease: Lessons from genetically modified mice. Pflugers Arch. 2010;459:959–967. doi: 10.1007/s00424-010-0796-2. [DOI] [PubMed] [Google Scholar]
- 66.Benesch RE, Kwong S. The stability of the heme-globin linkage in some normal, mutant, and chemically modified hemoglobins. J Biol Chem. 1990;265:14881–14885. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.