Abstract
Structure and dynamics of G proteins and their cognate receptors, both alone and in complex, are becoming increasingly accessible to experimental techniques. Understanding the conformational changes and timelines which govern these changes can lead to new insights into the processes of ligand binding and associated G protein activation. Experimental systems may involve the use of, or otherwise stabilize, non-native environments. This can complicate our understanding of structural and dynamical features of processes such as the ionic lock, Tryptophan toggle, and G protein flexibility. While elements in the receptor’s transmembrane helices and the C-terminal α5 helix of Gα undergo well defined structural changes, regions subject to conformational flexibility may be important in fine-tuning the interactions between activated receptors and G proteins. The pairing of computational and experimental approaches will continue to provide powerful tools to probe the conformation and dynamics of receptor-mediated G protein activation.
Introduction
Early structures of G protein coupled receptors (GPCRs) and G proteins reveal much of what we know about the conformations associated with distinct signaling states, but not the pathways that link these states or the dynamics associated with each of these states. Agonist binding to receptors and binding of cognate G proteins to activated receptors leads to the high-affinity state of the receptor, while catalyzing GDP release from the G protein. These events are accompanied by dynamic conformational changes in both receptors and G proteins on a time scale associated with receptor-mediated G protein activation. Each state is likely represented by an ensemble of conformations, however the experimental methods used to study these states may themselves perturb the system. While molecular dynamics (MD) simulations examine dynamics, there are challenges inherent with these approaches as well, such as convergence and under-sampling, especially as protein size increases. Conversion is generally thought to occur if system has sampled all possible states, and if the timescale is sufficiently long for a reliable prediction to be made1. While each approach has its own drawbacks, the combination of experimental data, molecular dynamics simulations, and crystallographic determinations together can be used in a complementary fashion to reveal protein dynamics and conformational flexibility associated with receptor-mediated G protein activation.
Conformational dynamics associated with GPCR activation
Dynamics of ligand binding
Rhodopsin, a prototypical class A GPCR, was the GPCR for which a structure was first determined2. Crystal structures of rhodopsin reveal distinctly different orientations for the retinal ligand2–4, resulting in some lack of certainty as to the orientation in vivo. Shedding light on this issue, Mertz et al.5 combined 2H NMR data with MD simulations to reveal that activation of rhodopsin (Rho) results in an ensemble of activated conformational states, which may help account for the divergent orientations of the ligand in crystal structures. Similarly, MD dynamics of dark Rho revealed that the beta-ionone ring of 11-cis-retinal is mobile in the binding pocket6. Results from experiments which examine protein structural dynamics, combined with molecular dynamics simulations and structural determinations together indicate that receptors are capable of adopting multiple conformations, depending on the nature of the bound ligand. Thus, conformational flexibility may combine with an induced fit mechanism to help stabilize a subset of conformations. Similarly, microsecond MD simulations of the A2A adenosine receptor demonstrate that a large degree of dynamics accompanies binding of adenosine, and reveal more than one binding orientation for ligand7. Only one of these orientations is reflected in the A2A receptor crystal structure7–9. On the other hand, binding to a synthetic agonist which is two to three orders of magnitude greater in efficacy than adenosine markedly reduces conformational variability in the receptor7; 10. This suggests that the difference in efficacy is due to the synthetic agonist’s ability to stabilize a smaller subset of active conformations, increasing the likelihood of G protein activation.
Ionic lock variability
The initial structure of dark rhodopsin2 lead to early hypotheses that an inactive-state ionic lock between residues in transmembrane (TM) helices 3 and 6, Arg 3.49 and Glu 6.30, respectively, would be broken in the process of GPCR activation. In the case of rhodopsin, breakage of this ionic lock exposes transducin binding elements11, and biochemical studies suggest that breakage of the lock accompanies agonist activation of β2AR12; 13. Somewhat surprisingly, the structures of activated β1AR14, β2AR15–17 and opsin18 were all seen with the ionic lock in the locked orientation, despite earlier predictions. Using microsecond MD simulations, Dror et al.19 demonstrate that the ionic lock forms and breaks spontaneously in the β2AR, suggesting that the lock is a dynamic process. Hints as to how this might occur in Rho was revealed by the NMR study cited above5, which suggests that destabilization of the ionic lock involves rotation of the C=NH+ group of the protonated Schiff base during retinal isomerization. Proton transfer from the protonated Schiff base during retinal isomerization results in a key rearrangement of E/DRY residues involved in the ionic lock. Taken together, these studies suggest that the ensemble of activated Rho conformations may be triggered by retinal isomerization5.
The ionic lock, its relation to the activation state of the receptor, and factors governing the equilibrium between the open and closed states may be receptor- and context-specific. However, since the simulations which observed the dynamic nature of the ionic lock were performed without the T4-lysozyme (T4L) used to stabilize the crystal structure of the β2AR19, it may be that the presence of T4L modulates the equilibrium between locked and unlocked states in the structural determination. A microsecond MD simulation of the β2AR performed by Romo et al. in 2010 in the absence of ligands or stabilizing proteins confirms the dynamic state of the ionic lock20. In addition to the open and locked conformation, this simulation reveals the presence of an intermediate, semi-open state containing a bridging water molecule. This is accompanied by changes in the orientation of TM helices, which remain hydrated throughout the simulation. However, these data are not meant to imply that the lock is unimportant for function. While the mutation of R in the E/DRY motif of rhodopsin type GPCRs abrogates G protein function21; 22, mutation of the conserved Glu in the ERY motif of the bradykinin B2 receptor to either R or A turns agonists into functional antagonists, decreasing phosphoinositol signaling and increasing constitutive internalization of receptors23. These types of studies help increase our understanding of processes such as biased agonism and functional selectivity that result in ligand-dependent differences in signaling pathways, through either arrestin binding or through differential signaling to G proteins24. These studies also point to a potential role for the E/DRY motif in signaling. It is interesting to note that in muscarinic as well as opioid receptor structures, the acidic residue in the DRY motif is linked thorugh a salt bridge to a conserved Arg in IC225. Ligands which alter the structural dynamics of this region may play a role in functional selectivity, given the ability of the agonists to act as antagonists in the bradykinin B2 system.
Energetics of ligand binding
MD simulations on the nanosecond timescale provide valuable information regarding structural dynamics of extracellular and intracellular loops26–28 and TM helices associated with ligand binding to GPCRs1. More recently, a long-timescale MD study in 2011 by Dror et al. was used to investigate the energetics of ligand binding to β2AR29. The authors observed that the ligand pauses in an entryway, or vestibule region before moving through a spatially restricted path to the site seen in crystallographic structures. Surprisingly, the highest energy barrier is associated with entry into the vestibule. This study suggests that the ligand is desolvated as it moves into the vestibule, and the remainder of its hydration shell is lost as it moves into the binding pocket seen in crystallographic studies. In contrast to small conformational changes seen on the ligand binding side, the intracellular side of the receptor exhibits changes in conformation of an even greater magnitude than that seen on the ligand binding side. Furthermore, a distinct intermediate state of the receptor was identified, and the authors propose that this state may facilitate G protein binding, offering new options to design therapies which stabilize or perturb specific receptor conformations.
Tryptophan conformation and receptor hydration
A combination of computational approaches can be used to address questions regarding receptor conformations associated with activation. Increasingly, normal mode analysis (NMA) is being paired with nanosecond and even microsecond MD simulations. With this approach, Louet and colleagues30 observed features of another Group A GPCR, ghrelin, which matches those of the activated β2AR and opsin structures. This includes a movement of TM 6 and 7 that opens a pocket for G protein binding. Furthermore, while early crystallographic studies of GPCRs suggested the presence of a Trp toggle switch, this too appeared to be questionable, in the light of later structures. Helping to reconcile these divergent observations, the combination of NMA and MD simulations by Louet et al.30 reveals that this highly conserved Trp in the CWLP motif of GPCRs is able to flip conformation. Furthermore, this flip is observed without applying any constraint to the simulation. An unbiased MD simulation by Hurst et al.31 demonstrates that the entrance of sn-2-arachidonylglycerol (2-AG) into the binding pocket of the cannabinoid receptor is sufficient to break the ionic lock, and full binding of 2-AG into the ligand binding site results in a reorientation of the conserved Trp in the CWLP motif of this class A GPCR. This reorientation is accompanied by influx of water upon receptor activation31, consistent with radiolytic footprinting of rhodopsin32, as well as in MD simulations of rhodopsin activation33.
A crystal structure of the A2A adenosine receptor bound to an antagonist contained three distinct water clusters which were visible at 1.8 Å34; on the extracellular face, in the TM core, and at the intracellular face, near the E/DRY motif. The waters in the central TM region are coordinated to a Na+ ion that may play a role in receptor activation. In the agonist bound A2A receptor, the ligand induced change in helix III prevents water binding9; 10. Thus, the presence of water and activation-induced changes in conformation which alter hydration of the receptor may be common features in GPCRs6; 31–33.
Conformational flexibility in the receptor core
Studies employing dynamic single-molecule force spectroscopy have also been used to investigate membrane-bound proteins35; 36. This approach allows the measurement of kinetic responses such that conformational variability during receptor activation can be quantified, along with other parameters such as unfolding free energy and mechanical flexibility35. Using this technique, Zocher and colleagues found that the basal activity of the β2AR is due to a high level of conformational variability in the core of the receptor, and that ligands alter the receptor’s energy landscape by modifying the receptor’s core36. Both agonists and inverse agonists increase the flexibility of the core, thus increasing the overall number of possible conformations, as well as enhancing the probability of the receptor adopting an activated conformation. However, this would not necessarily cause all receptor molecules to adopt an activated conformation. Binding of a G protein (or a molecule which mimics it) is predicted to further increase the number of receptor molecules in the active conformation. The ability to quantify the conformational variability of the receptor core may lead to a better understanding of how ligand binding stabilizes specific conformations through stabilization of structural segments within the core of the β2AR36.
Role of lipids in conformational flexibility and structural dynamics of receptors
However, we cannot consider the receptor in isolation. In addition to the myriad of membrane-bound and peripheral proteins in close proximity to receptors, receptors are surrounded by lipids in the membrane. To determine if lipids alter the dynamic state of receptors, Zocher et al. extended their 2012 study to include a lipid which mimics cholesterol37. Using dynamic single-molecule force spectroscopy, they found that cholesterol increases the kinetic stability of the β2AR, increasing the free energy barriers that stabilize each segment of the receptor against unfolding. These results suggest that the forces governing the structural dynamics of the receptor, and the energetics that stabilize receptor conformation, are influenced by lipids. This was not entirely unexpected, as early studies with rhodopsin demonstrated that cholesterol alters the metarhodopsin (Meta) I and Meta II equilibrium towards the inactive, Meta I state38. MD studies also suggest that more than one binding site exists for cholesterol in the A2A receptor39, and one of these sites was subsequently confirmed by structural determination of this receptor34. Since lipid rafts are thought to exhibit distinct lipid composition and subcellular localizations within the cell, rafts may play roles in the spatial regulation of signaling downstream of receptor activation37. However, the ability to isolate such membrane subdomains remains challenging, particularly because the methods used to isolate rafts may themselves influence a non-physiologic lipid composition.
Ligand binding alters dynamics on the intracellular face of the receptor
Since biased MD simulations can reveal trajectories that may or may not be relevant to biological signaling, despite well-defined endpoints40, the pairing of experimental evidence with simulation can enhance our understanding and increase confidence in the results of such studies. NMR has long been used as a tool for studying protein dynamics in solution. The propensity of ligands to alter the environment of both the extracellular and intracellular sides of the β2AR was demonstrated by a recent study combining NMR experiments with MD simulations by Nygaard and colleagues41. By examining the environment of a distinct set of residues in the receptor in the agonist-bound state, as well as bound to both an agonist and a G-protein mimicking nanobody, they found that ligand binding stabilizes the orientation of the extracellular side of the receptor, while increasing protein conformational variability at the intracellular side. Binding of both the agonist and G-protein mimic are required to reduce the dynamics at the intracellular side and fully stabilize the activated state of the receptor. Likewise, West and colleagues used hydrogen-deuterium exchange to identify changes in receptor conformation42. This study demonstrated that agonists increase conformational flexibility in the β2AR, while inverse agonists have a stabilizing effect. Activation of Rho also resulted in enhanced hydrogen-deuterium exchange, consistent with an activation-dependent increase in the conformational dynamics of the receptor43. The propensity for agonists to increase conformational variability in receptors may be responsible for the relatively fewer receptor structures determined in the activated state. However, as agonists which preferentially stabilize a specific active state are identified, such as in the structure of the agonist-bound A2A receptor10, more active state structures are likely to be determined.
Conformational variability in the nucleotide-free, receptor-bound G protein
Flexibility of the helical domain
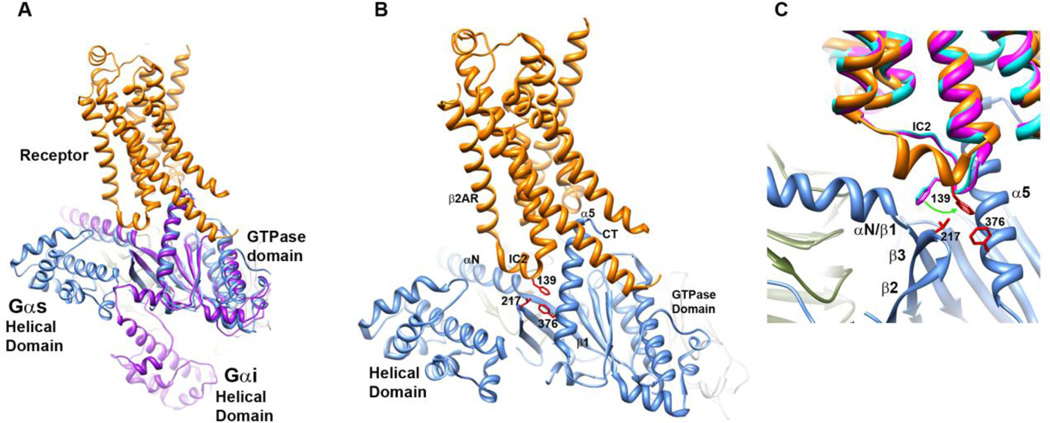
The receptor-bound Gs complex16 is the first structural determination of an activated receptor bound to a G protein. This study confirms numerous previous structural and biochemical studies which indicated that activation of a GPCR is accompanied by the outward movement of TM6 away from TM3, exposing a pocket for G protein binding. Not surprisingly, the structure confirms the interaction of the C terminus (CT) of the Gα protein with a pocket on the receptor opened by receptor activation. The structure also identifies a number of additional and less extensive interactions between the receptor and G protein, such as the interaction of intracellular loop 2 (IL2) of the receptor with αN/β1 hinge, β2/β3 loop, and TM5 of the receptor with α4 and β6 residues. Furthermore, this structure of the nucleotide-free receptor-G protein complex exhibits a loss of interdomain contacts, originally predicted in44 to accompany receptor-mediated G protein activation. Interestingly, an earlier computational study using MD simulations of isolated, nucleotide-bound Gαt proteins performed by Ceruso and colleagues45 hints at the interdomain reorientation that is now known to be a feature of receptor-bound G proteins. A more recent double electron electron resonance (DEER) study demonstrates that receptor activation is accompanied by a separation between the helical and GTPase domains in a rhodopsin-Gi model system46, an observation qualitatively confirmed shortly thereafter by the β2AR-Gs structural determination16. However, the exact placement of the helical domain in this crystal structure16 diverges from that in the DEER study (Fig. 1A), which may be due to the different conformations stabilized by the different techniques, or more likely due to an inherent flexibility of the helical domain upon GDP release.
Fig. 1.
The receptor-G protein complex. A. Comparison of the positions of the helical domains of Gα in β2AR-Gs (β2AR in orange, Gs in blue) vs. model derived from32, shown in purple. B. Hydrophobic triad of residues links IC2 of the β2AR to the β2/β3 loop and CT α5 helix of Gαs in the receptor-bound complex, side chains from hydrophobic triad shown in red. C. Overlay of β2AR receptor (teal, bound to antagonist, no G protein, PDB 3NYA; magenta, bound to inverse agonist, no G protein, PDB 3D4S) with that of activated complex, PDB 3SN6 (as in B).
The distribution of distances between pairs of residues spanning the helical and GTPase domains in this original DEER study46 indicated that there is a wide variability in the location of the helical domain in the receptor-bound Gα. Using a Rosetta-based approach to incorporating DEER distance distributions into a model of the receptor-bound G protein complex, we obtained an ensemble of structures which exhibited a highly flexible helical domain (in preparation). In this model, the helical domain was highly dynamic in the activated, receptor-bound, nucleotide-free state, in contrast to the GTPase domain, which remains in an orientation defined by the insertion of the CT of Gα into the receptor, as seen in the β2AR-Gs structure16; 47 and a previous model46. Importantly, the conformational variability associated with the nucleotide-free state is not simply due to the loss of nucleotide. Ridge and colleagues demonstrated in an NMR study in 200648 that receptor activation results in an increase in protein dynamics in the Gα subunit that are beyond the increases in dynamics observed in an isolated, nucleotide-free Gα protein49.
Communicating receptor activation to GDP release
Interaction of a G protein with an activated receptor results in a marked conformational change in the CT of Gα and a highly flexible helical domain16; 50. Using a combination of MD simulation and NMA, Louet et al.30 proposed that receptor-mediated nucleotide release occurs by a concerted mechanism that opens the GDP pocket as the receptor induces conformational changes in the C-terminal α5 helix, along with motions of α5, αG, α4, and the αN/β1 hinge. This study suggests that egress of the GDP may occur through either the base or phosphate side of the nucleotide. This study also predicts an important role for stabilization of the kink in the αA helix, necessary for a rigid body rotation of the helical domain away from the GTPase domain.
A hydrophobic triad links IC2 to αN/β1 hinge, β2/β3 loop and α5 helix of Gα
The CT of αG and residues in the α4 helix and α4/β6 loop have long been known from functional studies to be important for receptor-mediated G protein activation51–57. The CT of Gα plays well-established roles in receptor coupling, and both the crystal structure of the receptor-bound Gα complex and associated deuterium exchange studies demonstrate that this region is highly immobilized by interaction with activated receptors16; 32; 58. The β2AR-Gs structure also implicates regions other than the CT in receptor-G protein coupling, such as the α4 and α4/β6 loop, the β2/β3 loop and αN/β1 hinge of Gα16, as well as the IC2 of the receptor (Fig. 1B-C). Residues linked to the E/DRY motif in the IC2 loop of Rho also display reduced hydrogen-deuterium exchange in the activated Rho-Gt complex32, consistent with its role in coupling to Gα proteins. Loops and hinges are regions of high conformational variability that may enable fine-tuning of interactions between receptor and G protein. In Gα proteins, the β2/β3 loop is located in a critical region between Switches (Sw) I and II, and this loop contacts activated receptor in the β2AR-Gs complex16. In a recent study, site-specific labeling was used to demonstrate that receptor activation is communicated from the β2/β3 loop to Switches (Sw) I and II, resulting in enhanced packing of individual residues throughout Sw I and II of Gi proteins59.
In the β2AR-Gs complex, a hydrophobic triad of residues links receptor to G protein through a hydrophobic pocket59. This triad consists of F139 in IC2 of the β2AR, together with conserved residues in the β2/β3 loop (V217) and C-terminal α5 helix of Gαs (F376, Fig. 1B-C). In the deuterium exchange study by Palczewski and colleagues, the peptide that encompasses the residue homologous to V217 in Gαt displayed a low solvent accessibility when in complex with activated rhodopsin, roughly equivalent to the solvent accessibility of the CT, and the αN/β1 hinge also displayed a relatively low degree of solvent accessibility, in comparison to the remainder of the Gαt protein in the activated complex32. The αN/β1 hinge implicated in receptor coupling in the β2AR-Gs complex16 is allosterically linked to residues in the hydrophobic triad59 (Fig. 1C). In the cannabinoid receptor system, mutation of the homologous IC2 residue, L222 to either A or P, eliminates any coupling to Gs60, but does not perturb coupling to Gi, suggesting a role for the IC2 in G protein selectivity61. Furthermore, mutation of a nearby β2AR IC2 loop residue, Y141, eliminates potentiation of adenylyl cyclase activity by insulin. These results (and others) suggest a role for IC2 in modulating G protein signaling62–69, with some studies also implicating this region in the selectivity of receptor-G protein coupling70–72.
IC2 conformational flexibility
A study by Burstein et al.69 in the 1990’s implicates the IC2 in coupling of muscarinic receptors to Gαi proteins62–69. Based on mutational results alone, they predicted a helical conformation for the IC2 region, with one face containing residues important for receptor activation, and another other face involved in coupling to G proteins. Indeed, the crystal structure of the activated β2AR-Gs complex confirms not only the helical structure for IC2 when bound to the activated G protein, but also the linkage of residues on the intracellular side of IC2 to the DRY motif, with the opposing side of the helix in contact with G protein17. In the antagonist and inverse agonist bound β2AR, F139 in IC2 is angled away from the hydrophobic pocket formed by the juxtaposition with residues from the β2/β3 loop and the α5 helix (Fig. 1B-C)73; 74. Other receptor systems which exhibit a helical conformation for IL2 include β1AR, M2R and M3R, μ-OP and δ-OR, as well as the A2A adrenergic receptor25. This particular IC2 loop residue has been shown to play an important role in physiology, as a L to S mutation in the residue that is homologous to F139 in the GPCR, GPR54, causes idiopathic hypogonadotropic hypogonadism, a disorder associated with delayed puberty and infertility64.
Conformational flexibility of the hydrophobic triad and αN/β1 hinge
In Gαt, mutation of the Phe homologous to F376 in Gαs enhances receptor-mediated nucleotide exchange75, while mutation of the residue homologous to Gαs V217 in the β2/β3 loop of Gαi significantly reduces receptor-mediated nucleotide exchange59. Several studies have also implicated the αN and αN/β1 hinge in receptor activation, consistent with observations from the β2AR-Gs structure55; 76–78. An all-atom MD simulation of the rhodopsin-transducin complex also identified the β2/β3 loop, the αN/β1 hinge, and the α5 helix in the interactions of the Gα protein interactions with activated receptor79. This simulation indicates that the complex is dynamic, and samples many conformations during this microsecond simulation. These studies support a very dynamic receptor-G protein interface that includes contributions from regions far removed from the CT of Gα, in contrast to the low degree of solvent accessibility and dynamics in the CT of Gα itself. This is evident in deuterium exchange experiments of Gs and Gt with activated receptors32; 58, consistent with the well-established role of the CT in binding to activated receptors56; 57; 80; 81.
On the other hand, residues in the αN/β1 hinge region of Gαs, when incubated with activated receptors, exhibited increased exchange over the time course of the experiment, indicative of enhanced dynamics in this region in the receptor-G protein complex58. Interestingly, F139 in IC2, part of the hydrophobic triad linking receptor to the Gα protein, exhibits a distinctly altered conformation in the antagonist-bound and inverse-agonist bound β2AR structures (Fig. 1C), as compared to the G-protein bound structure. The helical conformation adopted by IC2 in the β2AR-Gs protein complex is absent without the bound G protein. Studies have shown that phosphorylation of Tyr 141 in the IC2 of β2AR shifts the receptor equilibrium towards the active conformation62, while mutation of Tyr 149 in the β1AR decreases stability of this receptor. In β2AR-Gs82, interaction of F139 of the receptor with residues 217 and 376 of Gαs would be expected to decrease packing surrounding the αN/β1 hinge region (Fig. 1C). In fact, deuterium exchange shows a time dependent increase in solvent exposure and the structural dynamics of αN/β1 hinge upon interaction with activated receptor58. More studies are needed to determine the functional importance of the increased structural dynamics in αN/β1 hinge in receptor-mediated G protein activation.
α5 α1 and αG Conformational variability in the receptor-bound complex
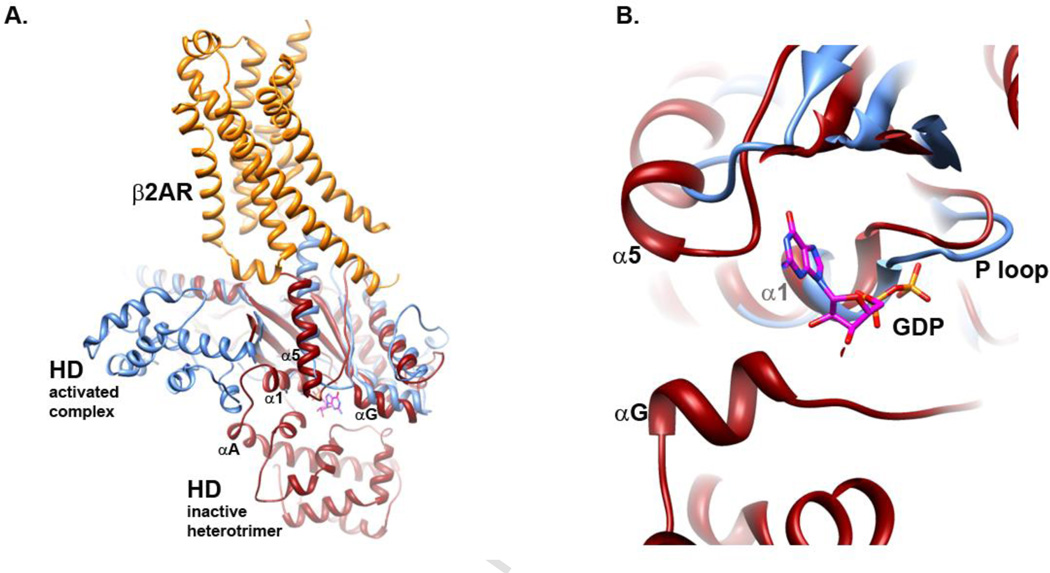
There is a marked increase in protein dynamics in αG of the Gα subunit when bound to β2AR, evidenced by the increase in the time-dependence of deuterium exchange in this region58. The activated Rho-Gt complex also exhibits enhanced deuterium exchange in the αG region of the Gα subunit32. Computational studies suggest that αG undergoes conformational changes upon receptor activation83, consistent with these deuterium exchange studies. The αG helix of Gα is in close proximity to bound GDP and the α5 helix, as well as proximity to residues in the helical domain (Fig. 2A), and thus may be a critical point linking the two domains. Another important allosteric linkage between the domains is likely mediated by interactions between the α1 and α5 helices of the G subunit. The α5 helix contacts the α1 helix (overview, Fig 2A), and α1 links the GTPase to the helical domain through the αA helix. At the bottom of the α1 helix is the P loop (Fig. 2B), so named due to its interaction with the phosphate of bound nucleotide (Fig. 2B, phosphates of GDP in orange and red). Thus, conformational changes at the CT of Gα may be communicated to the bound nucleotide, both directly and indirectly, leading to the observed increase in conformational flexibility of the helical domain (Fig. 3A-C)46; 47; 84. The receptor induces a large conformational change in the CT, which alters interaction with the guanine ring of the bound nucleotide51; 85; 86 through a rotation and translation of the C-terminal α5 helix50. Receptor-mediated changes in the CT may be communicated to the α1 helix and phosphate binding P-loop, as suggested by a study by Sakmar and colleagues86. In that study, mutations in the α5 and α1 helix result in perturbation of receptor-mediated nucleotide exchange. This is consistent with MD simulation by Weinstein and colleagues45 which reveals a role for the linkage between α5 and α1, as well as with the β2/β3 loop in interdomain flexibility associated with G protein activation.
Fig. 2.
Overlay of β2AR-Gs complex with GDP-bound heterotrimeric G protein Gαβγi (PDB files 3SN6 and 1GP2, respectively). Gαs shown in blue, β2AR in orange, and Gαi in red. Note that there is no high resolution of GαsGDP available for this comparison. A. Overview showing proximity of α5, αA and αG helices to bound GDP (sticks). B. Close up, rotated and slab view, showing proximity of P loop, α5, αG and αA to bound nucleotide.
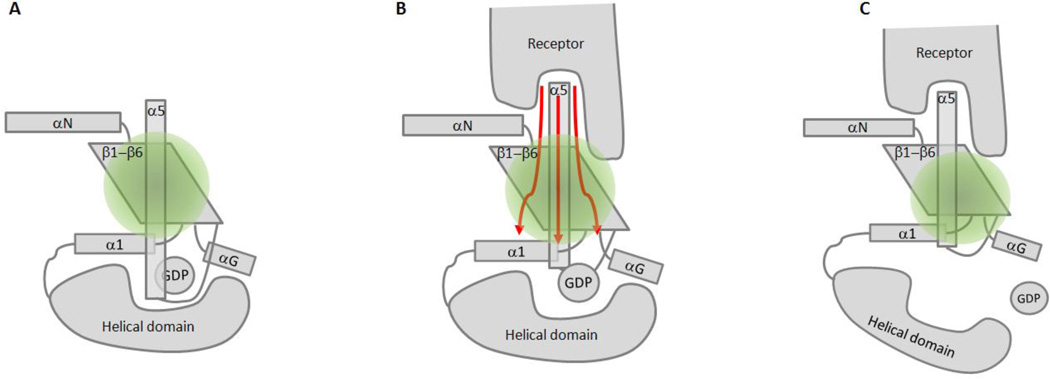
Fig. 3.
Receptor-mediated G protein activation schematic. A. Gα protein (Gβγ not shown), with specific elements in the GTPase domain labeled. GDP is held in the cleft between the GTPase and helical domains. B. Receptor activation impinges on the C-terminal α5 helix, and interactions of IC2 with secondary sites such as the αN/β1 hinge and α4/β6 loop dynamically alter interactions at the base of the α5 helix with surrounding regions. C. Receptor-mediated G protein activation results in the nucleotide-free, empty pocket state of the Gα protein, and a conformationally dynamic helical domain.
Nucleotide binding reduces G protein conformational flexibility
Nucleotide binding restores contacts between the domains, as seen in crystal structures of GTPγS-bound Gα proteins44; 87; 88. This is also seen in the reduction of line widths of spin-labeled Gα proteins upon GTPγS binding in EPR studies89. It is likely that nucleotide binding mediates decreased conformational flexibility, which stabilizes conformations that favor interaction with binding partners. Although the excess of GTP present within the cell overwhelmingly favors GTP binding to activated G proteins in the receptor-bound complex, a recent study indicates that the environment of individually labeled Sw I residues in the activated complex mimic that of the same residues in the GTPγS-bound state, suggesting that receptor activation may pre-organize these regions for subsequent GTP binding59. In the case of Gi proteins, N-terminal myristoylation (myr), a permanent co-translational modification of Gi family proteins, including Gt, reduces the already low degree of structural dynamics at the base of the α5 helix in the AlF4-activated protein90. This is consistent with a myr-dependent stabilization of bound nucleotide. Structural dynamics of the activated G protein are also influenced by myr in regions distal from the NT and in regions of Gα known to be involved nucleotide binding90. Thus, myristoylation may play a role in modulation G protein conformational flexibility in the GTP-bound protein.
Conclusion
The studies described here reveal potential pathways for activation and the activation dynamics implicated in receptor-mediated G protein activation. Taken together, these studies demonstrate that there is more than one conformation associated with activated receptors, as well as for activated, nucleotide-free Gα bound to these receptors. The inter-conversion between distinct activated states, and the timescale for inter-conversion between these states is still largely unknown. Furthermore, the ensemble of conformations that are associated with activation, and the relative energy of each state is still to be determined. In the receptor-G protein complex, these studies paint a picture of a highly dynamic Gα helical domain, with limited structural dynamics at the CT of Gα. In addition, receptor activation may alter dynamics in conformationally variable regions of the receptor and G protein that are known to participate in receptor G protein coupling, including the IC2 loop of the receptor, and the αN/β1 hinge and β2/β3 loop of Gα16. These structural dynamics may modulate effects of conformational changes that are mediated by the CT of Gα binding to activated receptors. These changes are likely propagated from the extreme Gα CT that binds to receptor to the base of the α5 helix of the G protein50; 85; 86 and throughout the GTPase domain, as well as across the nucleotide binding cleft to the helical domain. Together these result in a conformationally flexible helical domain in the receptor-bound, nucleotide-free state46; 47; 84. This may occur as a concerted mechanism, or step wise, and time-resolved experiments will be required in order to fully elucidate the order and pathway of the conformational changes that are induced by receptor activation to result in a fully activated Gα protein. Investigation of these questions will increase our understanding of conformation and dynamics that regulate G protein signaling in vivo.
Highlights.
Crystal structures represent lowest-energy conformations, while GPCRs and G proteins are dynamic.
Experimental and computational approaches can be used to conformation and dynamics.
The receptor-G protein complex exhibits a flexible and dynamic helical domain.
A hydrophobic triad of residues links IC2 of receptor to β2/β3 loop and C terminus of Gα
Flexible loops in receptor and G protein may fine tune interaction between receptor and G protein
ACKNOWLEDGEMENTS
H.E.H. supported by National Institutes of Health Grants EY006062 and GM095633. J.M supported by National Institutes of Health Grants GM080403, MH090192, GM099842 and NSF Career 0742762.
ABBREVIATIONS
- GPCR
G protein coupled receptor
- MD
molecular dynamics
- NMR
nuclear magnetic resonance
- DEER
double electron electron resonance
- DEER
double-electron electromagnetic resonance
- Rho
rhodopsin
- TM
transmembrane
- T4L
T4-lysozyme
- NMA
normal mode analysis
- 2-AG
sn-2-arachidonylglycerol
- Meta
metarhodopsin
- CT
C terminus
- IL2
intracellular loop 2
- P-loop
loop which binds phosphate in Gα
- myr
myristoylation
- EC
extracellular
- IL
intracellular
- OR
opioid receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ng HW, Laughton CA, Doughty S. Molecular Dynamics Simulations of the Adenosine A2a Receptor: Structural Stability, Sampling and Convergence. J Chem Inf Model. 2013 doi: 10.1021/ci300610w. In press. [DOI] [PubMed] [Google Scholar]
- 2.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal Structure of Rhodopsin: A G Protein-Coupled Receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 3.Nakamichi H, Okada T. Local peptide movement in the photoreaction intermediate of rhodopsin. Proc Natl Acad Sci U S A. 2006;103:12729–12734. doi: 10.1073/pnas.0601765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Standfuss J, Edwards PC, D'Antona A, Fransen M, Xie G, Oprian DD, Schertler GF. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature. 2011;471:656–660. doi: 10.1038/nature09795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertz B, Struts AV, Feller SE, Brown MF. Molecular simulations and solid-state NMR investigate dynamical structure in rhodopsin activation. Biochim Biophys Acta. 2012;1818:241–51. doi: 10.1016/j.bbamem.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau PW, Grossfield A, Feller SE, Pitman MC, Brown MF. Dynamic structure of retinylidene ligand of rhodopsin probed by molecular simulations. J Mol Biol. 2007;372:906–917. doi: 10.1016/j.jmb.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Lyman E. Agonist dynamics and conformational selection during microsecond simulations of the A(2A) adenosine receptor. Biophys J. 2012;102:2114–2120. doi: 10.1016/j.bpj.2012.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–525. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janz JM, Farrens DL. Rhodopsin Activation Exposes a Key Hydrophobic Binding Site for the Transducin -Subunit C Terminus. Journal of Biological Chemistry. 2004;279:29767–29773. doi: 10.1074/jbc.M402567200. [DOI] [PubMed] [Google Scholar]
- 12.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, Javitch JA. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- 14.Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a beta(1)- adrenergic receptor. Nature. 2011;469:241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, Chae PS, Gellman SH, Dror RO, Shaw DE, Weis WI, Caffrey M, Gmeiner P, Kobilka BK. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen SG, Devree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta(2) adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobodystabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 19.Dror RO, Arlow DH, Borhani DW, Jensen MO, Piana S, Shaw DE. Identification of two distinct inactive conformations of the beta2-adrenergic receptor reconciles structural and biochemical observations. Proc Natl Acad Sci U S A. 2009;106:4689–4694. doi: 10.1073/pnas.0811065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romo TD, Grossfield A, Pitman MC. Concerted interconversion between ionic lock substates of the beta(2) adrenergic receptor revealed by microsecond timescale molecular dynamics. Biophys J. 2010;98:76–84. doi: 10.1016/j.bpj.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rovati GE, Capra V, Neubig RR. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol. 2007;71:959–964. doi: 10.1124/mol.106.029470. [DOI] [PubMed] [Google Scholar]
- 22.Schneider EH, Schnell D, Strasser A, Dove S, Seifert R. Impact of the DRY motif and the missing "ionic lock" on constitutive activity and G-protein coupling of the human histamine H4 receptor. J Pharmacol Exp Ther. 2010;333:382–392. doi: 10.1124/jpet.109.163220. [DOI] [PubMed] [Google Scholar]
- 23.Leschner J, Wennerberg G, Feierler J, Bermudez M, Welte B, Kalatskaya I, Wolber G, Faussner A. Interruption of the ionic lock in the bradykinin B2 receptor results in constitutive internalization and turns several antagonists into strong agonists. J Pharmacol Exp Ther. 2013;344:85–95. doi: 10.1124/jpet.112.199190. [DOI] [PubMed] [Google Scholar]
- 24.Liu JJ, Horst R, Katritch V, Stevens RC, Wuthrich K. Biased signaling pathways in beta2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 26.Huber T, Menon S, Sakmar TP. Structural basis for ligand binding and specificity in adrenergic receptors: implications for GPCR-targeted drug discovery. Biochemistry. 2008;47:11013–11023. doi: 10.1021/bi800891r. [DOI] [PubMed] [Google Scholar]
- 27.Moro S, Hoffmann C, Jacobson KA. Role of the extracellular loops of G proteincoupled receptors in ligand recognition: a molecular modeling study of the human P2Y1 receptor. Biochemistry. 1999;38:3498–3507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avlani VA, Gregory KJ, Morton CJ, Parker MW, Sexton PM, Christopoulos A. Critical role for the second extracellular loop in the binding of both orthosteric and allosteric G protein-coupled receptor ligands. J Biol Chem. 2007;282:25677–25686. doi: 10.1074/jbc.M702311200. [DOI] [PubMed] [Google Scholar]
- 29.Dror RO, Arlow DH, Maragakis P, Mildorf TJ, Pan AC, Xu H, Borhani DW, Shaw DE. Activation mechanism of the beta2-adrenergic receptor. Proc Natl Acad Sci U S A. 2011;108:18684–18689. doi: 10.1073/pnas.1110499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louet M, Perahia D, Martinez J, Floquet N. A concerted mechanism for opening the GDP binding pocket and release of the nucleotide in hetero-trimeric G-proteins. J Mol Biol. 2011;411:298–312. doi: 10.1016/j.jmb.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Hurst DP, Grossfield A, Lynch DL, Feller S, Romo TD, Gawrisch K, Pitman MC, Reggio PH. A lipid pathway for ligand binding is necessary for a cannabinoid G proteincoupled receptor. J Biol Chem. 2010;285:17954–17964. doi: 10.1074/jbc.M109.041590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orban T, Jastrzebska B, Gupta S, Wang B, Miyagi M, Chance MR, Palczewski K. Conformational dynamics of activation for the pentameric complex of dimeric G protein-coupled receptor and heterotrimeric G protein. Structure. 2012;20:826–840. doi: 10.1016/j.str.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossfield A, Pitman MC, Feller SE, Soubias O, Gawrisch K. Internal hydration increases during activation of the G-protein-coupled receptor rhodopsin. J Mol Biol. 2008;381:478–486. doi: 10.1016/j.jmb.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, AP IJ, Cherezov V, Stevens RC. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janovjak H, Sapra KT, Kedrov A, Muller DJ. From valleys to ridges: exploring the dynamic energy landscape of single membrane proteins. Chemphyschem. 2008;9:954–966. doi: 10.1002/cphc.200700662. [DOI] [PubMed] [Google Scholar]
- 36.Zocher M, Fung JJ, Kobilka BK, Muller DJ. Ligand-specific interactions modulate kinetic, energetic, and mechanical properties of the human beta2 adrenergic receptor. Structure. 2012;20:1391–1402. doi: 10.1016/j.str.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zocher M, Zhang C, Rasmussen SG, Kobilka BK, Muller DJ. Cholesterol increases kinetic, energetic, and mechanical stability of the human beta2-adrenergic receptor. Proc Natl Acad Sci U S A. 2012;109:E3463–E3472. doi: 10.1073/pnas.1210373109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boesze-Battaglia K, Hennessey T, Albert AD. Cholesterol heterogeneity in bovine rod outer segment disk membranes. J Biol Chem. 1989;264:8151–8155. [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JY, Lyman E. Predictions for cholesterol interaction sites on the A2A adenosine receptor. J Am Chem Soc. 2012;134:16512–16515. doi: 10.1021/ja307532d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grossfield A. Recent progress in the study of G protein-coupled receptors with molecular dynamics computer simulations. Biochim Biophys Acta. 2011;1808:1868–1878. doi: 10.1016/j.bbamem.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Nygaard R, Zou Y, Dror RO, Mildorf TJ, Arlow DH, Manglik A, Pan AC, Liu CW, Fung JJ, Bokoch MP, Thian FS, Kobilka TS, Shaw DE, Mueller L, Prosser RS, Kobilka BK. The dynamic process of beta(2)-adrenergic receptor activation. Cell. 2013;152:532–542. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West GM, Chien EY, Katritch V, Gatchalian J, Chalmers MJ, Stevens RC, Griffin PR. Ligand-dependent perturbation of the conformational ensemble for the GPCR beta2 adrenergic receptor revealed by HDX. Structure. 2011;19:1424–1432. doi: 10.1016/j.str.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orban T, Jastrzebska B, Gupta S, Wang B, Miyagi M, Chance MR, Palczewski K. Conformational dynamics of activation for the pentameric complex of dimeric G protein-coupled receptor and heterotrimeric G protein. Structure. 2013;20:826–840. doi: 10.1016/j.str.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noel JP, Hamm HE, Sigler PB. The 2.2 Å crystal structure of transducincomplexed with GTPγS. Nature. 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 45.Ceruso MA, Periole X, Weinstein H. Molecular Dynamics Simulations of Transducin: Interdomain and Front to Back Communication in Activation and Nucleotide Exchange. Journal of Molecular Biology. 2004;338:469–481. doi: 10.1016/j.jmb.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 46.Van Eps N, Preininger AM, Alexander N, Kaya AI, Meier S, Meiler J, Hamm HE, Hubbell WL. Interaction of a G protein with an activated receptor opens the interdomain interface in the alpha subunit. Proc Natl Acad Sci U S A. 2011;108:9420–9424. doi: 10.1073/pnas.1105810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westfield GH, Rasmussen SG, Su M, Dutta S, DeVree BT, Chung KY, Calinski D, Velez-Ruiz G, Oleskie AN, Pardon E, Chae PS, Liu T, Li S, Woods VL, Jr, Steyaert J, Kobilka BK, Sunahara RK, Skiniotis G. Structural flexibility of the G alpha s alphahelical domain in the beta2-adrenoceptor Gs complex. Proc Natl Acad Sci U S A. 2011;108:16086–16091. doi: 10.1073/pnas.1113645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridge KD, Abdulaev NG, Zhang C, Ngo T, Brabazon DM, Marino JP. Conformational Changes Associated with Receptor Stimulated Guanine Nucleotide Exchange in a Heterotrimeric G-Protein a-subunit: NMR Analysis of GTPγS-Bound States. Journal of Biological Chemistry. 2006;281:7635–7648. doi: 10.1074/jbc.M509851200. [DOI] [PubMed] [Google Scholar]
- 49.Thomas CJ, Briknarova K, Hilmer JK, Movahed N, Bothner B, Sumida JP, Tall GG, Sprang SR. The nucleotide exchange factor Ric-8A is a chaperone for the conformationally dynamic nucleotide-free state of Galphai1. PLoS One. 2012;6:e23197. doi: 10.1371/journal.pone.0023197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nature Structural and Molecular Biology. 2006;13:772–777. doi: 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- 51.Marin EP, Krishna AG, Sakmar TP. Disruption of the 5 Helix of Transducin Impairs Rhodopsin-Catalyzed Nucleotide Exchange. Biochemistry. 2002;41:6988–6994. doi: 10.1021/bi025514k. [DOI] [PubMed] [Google Scholar]
- 52.Gilchrist A, Vanhauwe JF, Li A, Thomas TO, Voyno-Yasenetskaya T, Hamm HE. G alpha minigenes expressing C-terminal peptides serve as specific inhibitors of thrombinmediated endothelial activation. J Biol Chem. 2001;276:25672–25679. doi: 10.1074/jbc.M100914200. [DOI] [PubMed] [Google Scholar]
- 53.Bae H, Cabrera-Vera TM, Depree KM, Graber SG, Hamm HE. Two Amino Acids Within the α4 Helix of Gαi1 Mediate Coupling with 5-Hydroxytryptamine1B Receptors. Journal of Biological Chemistry. 1999;274:14963–14971. doi: 10.1074/jbc.274.21.14963. [DOI] [PubMed] [Google Scholar]
- 54.Natochin M, Granovsky AE, Muradov KG, Artemyev NO. Roles of the transducin alpha-subunit alpha4-helix/alpha4-beta6 loop in the receptor and effector interactions. Journal of Biological Chemistry. 1999;274:7865–7869. doi: 10.1074/jbc.274.12.7865. [DOI] [PubMed] [Google Scholar]
- 55.Blahos J, Fischer T, Brabet I, Stauffer D, Rovelli G, Bockaert J, Pin JP. A Novel Site on the Gα-protein That Recognizes Heptahelical Receptors. Journal of Biological Chemistry. 2001;276:3262–3269. doi: 10.1074/jbc.M004880200. [DOI] [PubMed] [Google Scholar]
- 56.Hamm HE, Deretic D, Arendt A, Hargrave PA, Koenig B, Hofmann KP. Site of G Protein Binding to Rhodopsin Mapped with Synthetic Peptides from the α Subunit. Science. 1988;241:832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- 57.Martin EL, Rens-Domiano S, Schatz PJ, Hamm HE. Potent Peptide Analogues of a G Protein Receptor-binding Region Obtained with a Combinatorial Library. Journal of Biological Chemistry. 1996;271:361–366. doi: 10.1074/jbc.271.1.361. [DOI] [PubMed] [Google Scholar]
- 58.Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL, Jr, Sunahara RK. Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature. 2011;477:611–615. doi: 10.1038/nature10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamm HE, Kaya AI, Gilbert JA, 3rd, Preininger AM. Linking receptor activation to changes in Sw I and II of Galpha proteins. J Struct Biol. 2013 doi: 10.1016/j.jsb.2013.02.016. in press March 4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters MF, Scott CW. Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. J Biomol Screen. 2009;14:246–255. doi: 10.1177/1087057108330115. [DOI] [PubMed] [Google Scholar]
- 61.Chen XP, Yang W, Fan Y, Luo JS, Hong K, Wang Z, Yan JF, Chen X, Lu JX, Benovic JL, Zhou NM. Structural determinants in the second intracellular loop of the human cannabinoid CB1 receptor mediate selective coupling to G(s) and G(i) Br J Pharmacol. 2010;161:1817–1834. doi: 10.1111/j.1476-5381.2010.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valiquette M, Parent S, Loisel TP, Bouvier M. Mutation of tyrosine-141 inhibits insulin-promoted tyrosine phosphorylation and increased responsiveness of the human beta 2- adrenergic receptor. Embo J. 1995;14:5542–5549. doi: 10.1002/j.1460-2075.1995.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moro O, Lameh J, Hogger P, Sadee W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J Biol Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- 64.Wacker JL, Feller DB, Tang XB, Defino MC, Namkung Y, Lyssand JS, Mhyre AJ, Tan X, Jensen JB, Hague C. Disease-causing mutation in GPR54 reveals the importance of the second intracellular loop for class A G-protein-coupled receptor function. J Biol Chem. 2008;283:31068–31078. doi: 10.1074/jbc.M805251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McClue SJ, Baron BM, Harris BA. Activation of Gi protein by peptide structures of the muscarinic M2 receptor second intracellular loop. Eur J Pharmacol. 1994;267:185–193. doi: 10.1016/0922-4106(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 66.Nussenzveig DR, Thaw CN, Gershengorn MC. Inhibition of inositol phosphate second messenger formation by intracellular loop one of a human calcitonin receptor. Expression and mutational analysis of synthetic receptor genes. J Biol Chem. 1994;269:28123–28129. [PubMed] [Google Scholar]
- 67.Pin JP, Gomeza J, Joly C, Bockaert J. The metabotropic glutamate receptors: their second intracellular loop plays a critical role in the G-protein coupling specificity. Biochem Soc Trans. 1995;23:91–96. doi: 10.1042/bst0230091. [DOI] [PubMed] [Google Scholar]
- 68.Zhou H, Yan F, Yamamoto S, Tai HH. Phenylalanine 138 in the second intracellular loop of human thromboxane receptor is critical for receptor-G-protein coupling. Biochem Biophys Res Commun. 1999;264:171–175. doi: 10.1006/bbrc.1999.1508. [DOI] [PubMed] [Google Scholar]
- 69.Burstein ES, Spalding TA, Brann MR. The second intracellular loop of the m5 muscarinic receptor is the switch which enables G-protein coupling. J Biol Chem. 1998;273:24322–24327. doi: 10.1074/jbc.273.38.24322. [DOI] [PubMed] [Google Scholar]
- 70.Erlenbach I, Kostenis E, Schmidt C, Serradeil-Le Gal C, Raufaste D, Dumont ME, Pausch MH, Wess J. Single amino acid substitutions and deletions that alter the G protein coupling properties of the V2 vasopressin receptor identified in yeast by receptor random mutagenesis. J Biol Chem. 2001;276:29382–29392. doi: 10.1074/jbc.M103203200. [DOI] [PubMed] [Google Scholar]
- 71.Schoneberg T, Kostenis E, Liu J, Gudermann T, Wess J. Molecular aspects of vasopressin receptor function. Adv Exp Med Biol. 1998;449:347–358. doi: 10.1007/978-1-4615-4871-3_44. [DOI] [PubMed] [Google Scholar]
- 72.Blin N, Yun J, Wess J. Mapping of single amino acid residues required for selective activation of Gq/11 by the m3 muscarinic acetylcholine receptor. J Biol Chem. 1995;270:17741–17748. doi: 10.1074/jbc.270.30.17741. [DOI] [PubMed] [Google Scholar]
- 73.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 74.Wacker D, Fenalti G, Brown MA, Katritch V, Abagyan R, Cherezov V, Stevens RC. Conserved binding mode of human beta2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J Am Chem Soc. 2010;132:11443–11445. doi: 10.1021/ja105108q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marin EP, Krishna AG, Sakmar TP. Rapid Activation of Transducin by Mutations Distant from the Nucleotide-Binding Site. Evidence for a Mechanistic Model of Receptor- Catalyzed Nucleotide Exchange by G Proteins. Journal of Biological Chemistry. 2001;276:27400–27405. doi: 10.1074/jbc.C100198200. [DOI] [PubMed] [Google Scholar]
- 76.Kostenis E, Degtyarev MY, Conklin BR, Wess J. The N-terminal Extension of Gαq Is Critical for Constraining the Selectivity of Receptor Coupling. Journal of Biological Chemistry. 1997;272:19107–19110. doi: 10.1074/jbc.272.31.19107. [DOI] [PubMed] [Google Scholar]
- 77.Slessareva JE, Graber SG. Reconstitution reveals additional roles for N- and Cterminal domains of g(alpha) in muscarinic receptor coupling. Biochemistry. 2003;42:7552–7560. doi: 10.1021/bi034133j. [DOI] [PubMed] [Google Scholar]
- 78.Preininger AM, Parello J, Meier SM, Liao G, Hamm HE. Receptor-mediated changes at the myristoylated amino terminus of Galpha(il) proteins. Biochemistry. 2008;47:10281–10293. doi: 10.1021/bi800741r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sgourakis NG, Garcia AE. The membrane complex between transducin and darkstate rhodopsin exhibits large-amplitude interface dynamics on the sub-microsecond timescale: insights from all-atom MD simulations. J Mol Biol. 2010;398:161–173. doi: 10.1016/j.jmb.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 80.Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. doi: 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- 81.Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 82.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-proteincoupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Louet M, Martinez J, Floquet N. GDP release preferentially occurs on the phosphate side in heterotrimeric G-proteins. PLoS Comput Biol. 2012;8:e1002595. doi: 10.1371/journal.pcbi.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdulaev NG, Ngo T, Ramon E, Brabazon DM, Marino JP, Ridge KD. The receptor-bound "empty pocket" state of the heterotrimeric G-protein alpha-subunit is conformationally dynamic. Biochemistry. 2006;45:12986–12997. doi: 10.1021/bi061088h. [DOI] [PubMed] [Google Scholar]
- 85.Preininger A, Funk M, Meier S, Oldham W, Johnston C, Adhikary S, Kimple A, Siderovski D, Hamm H, Iverson T. Helix dipole movement and conformational variability contribute to allosteric GDP release in Gi subunits. Biochemistry. 2009;48:2630–2642. doi: 10.1021/bi801853a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapoor N, Menon ST, Chauhan R, Sachdev P, Sakmar TP. Structural evidence for a sequential release mechanism for activation of heterotrimeric G proteins. J Mol Biol. 2009;393:882–897. doi: 10.1016/j.jmb.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 87.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal Structure of the Catalytic Domains of Adenylyl Cyclase in a Complex with Gsα GTPγS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 88.Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR. Structures of Active Conformations of Giα1 and the Mechanism of GTP Hydrolysis. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 89.Van Eps N, Oldham WM, Hamm HE, Hubbell WL. Structural and dynamical changes in an alpha-subunit of a heterotrimeric G protein along the activation pathway. Proc Natl Acad Sci U S A. 2006;103:16194–16199. doi: 10.1073/pnas.0607972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Preininger AM, Kaya AI, Gilbert JA, 3rd, Busenlehner LS, Armstrong RN, Hamm HE. Myristoylation exerts direct and allosteric effects on Galpha conformation and dynamics in solution. Biochemistry. 2012;51:1911–1924. doi: 10.1021/bi201472c. [DOI] [PMC free article] [PubMed] [Google Scholar]