Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease (ILD) of unknown origin characterized by epithelial cell dysfunctions, accumulation of fibroblasts and myofibroblasts and relentless deposition of extracellular matrix (ECM). Improved diagnostic accuracy and better trial design have provided important insights from recent clinical trials. Perhaps the most important insight was the realization that “standard therapy” was actually harmful! This review summarizes the current understanding of the cell types that are altered in IPF and the pathogenic mechanisms that have been identified. It also reviews recent clinical trial results and interpretations. Finally, we highlight attractive biologic targets and therapies in development with recommendations for future therapeutic avenues.
Introduction
Fibrosis in ILD is caused by the accumulation of ECM proteins within the interstitium and alveolar space of the lung. Some forms of ILD can be caused by environmental exposures (e.g. asbestos, inorganic dusts, organic particulates, drug toxicity or radiation). ILD can also occur in the context of systemic disease (e.g. collagen vascular disease) or due to genetic mutations (e.g. mutations in surfactant proteins or telomerase components)[1]. However, the majority of severe cases comprise a classification known as IPF for which the origin is unknown. The classification of IPF is based on radiographic patterns and/or the identification of the usual interstitial pneumonia (UIP) pattern on histology[2]. The disease is progressive, and often results in respiratory insufficiency within 3–5 years post-diagnosis[3]. The pathogenesis of the disease is poorly understood and most insights are based on inferences made from histology or various animal models which result in lung fibrosis. The variable natural history of IPF is another confounding factor. Some patients experience a relatively gradual decline in lung function, some experience stable disease over extended periods of time, and others have acute deteriorations with rapid worsening and disease progression[4]. This heterogeneity has complicated the design of clinical trials and the identification of potential therapies.
Pathogenesis of IPF
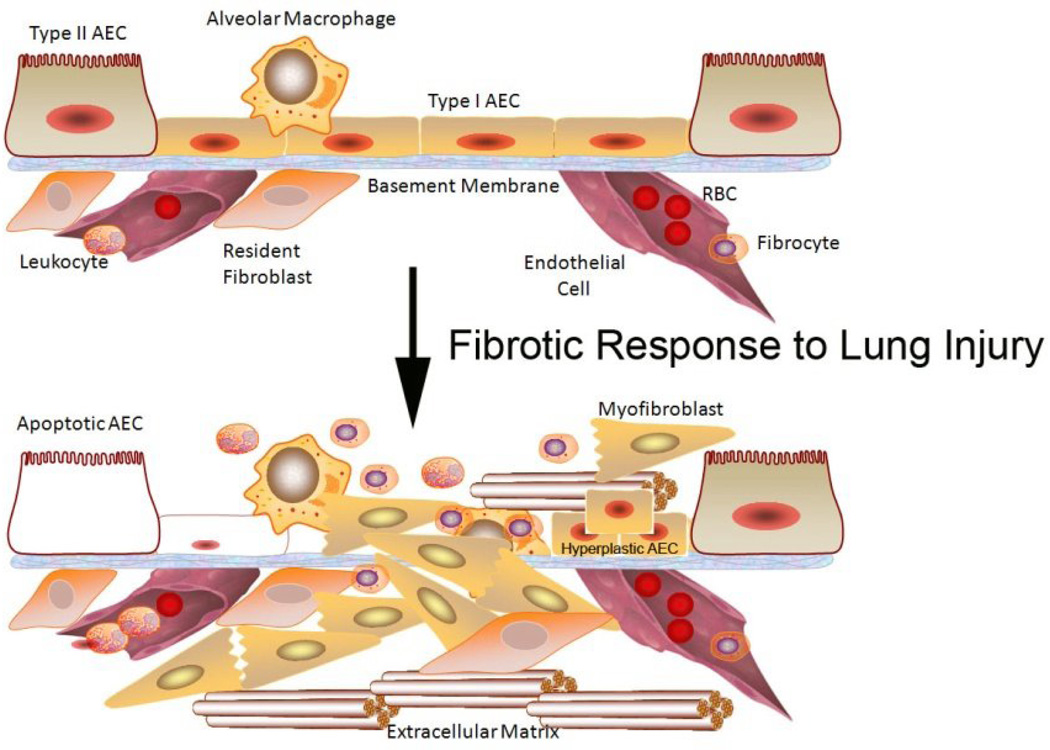
IPF is believed to be the result of an aberrant wound healing process. It is hypothesized that the initial/repetitive injury occurs to the lung epithelium, likely to type I alveolar epithelial cells (AECs) which line the majority of the alveolar surface [5]. Under homeostatic conditions, type I AECs are believed to keep pulmonary structural mesenchymal cells in check through the secretion of various mediators and cell-cell contact. When type I AECs are injured or lost, it is thought that type II AECs undergo hyperplastic proliferation to cover the exposed basement membranes. If this process is inefficient, alveoli can collapse and consolidate. In normal repair, the hyperplastic type II AECs will undergo regulated apoptosis. The remaining cells will spread and undergo a differentiation process to become type I AEC. Under pathologic conditions and in the presence of transforming growth factor (TGF)β, fibroblasts accumulate in these areas of damage and differentiate into α-smooth muscle actin (SMA)-expressing myofibroblasts that secrete collagen and other ECM proteins [2]. Pathologic examination of UIP tissues reveal the diagnostic lesions known as “fibroblastic foci” (dense collections of myofibroblasts and scar tissue). The AECs adjacent to these fibroblastic foci often remain hyperplastic and abnormal rather than undergoing appropriate repair [6](see Fig. 1).
Figure 1. Pathogenic alterations in IPF.
IPF is believed to be the result of injury to AECs which can result in damage or loss via apoptosis. Disruption of the homeostatic balance in the lung leads to the accumulation of fibroblasts and myofibroblast as well as chronic inflammation which may bring in monocytes/macrophages and fibrocytes. Vascular damage can also initiate provisional ECM deposition. Hyperplastic AECs are noted in areas of fibrotic injury. Mesenchymal cells in the lungs promote fibrosis via the secretion of ECM, including collagen 1. Stiffening of the ECM matrix may help perpetuate the profibrotic signals to lung mesenchymal cells.
Cellular processes that lead to the accumulation of the fibroblasts and myofibroblasts within IPF lungs may include expansion of resident mesenchymal cells, epithelial to mesenchymal transition (EMT), and differentiation of circulating precursors called fibrocytes [7,8]. While there is a general consensus that resident fibroblast accumulation plays an important role in IPF pathogenesis, the contribution of EMT and fibrocytes is less clear. EMT has been demonstrated to occur in animal models, and epithelial cells in IPF biopsies stain with mesenchymal markers as well as epithelial ones [7]. However, whether EMT can result in stable transformation to mesenchymal cells and myofibroblasts in vivo is unknown. Likewise, fibrocytes which are circulating cells which share both leukocyte (CD45) and mesenchymal markers (collagen 1) can stably differentiate into fibroblasts via loss of CD45 in vitro [8,9], but whether this occurs in vivo is not known. Adoptive transfer of fibrocytes can worsen fibrotic outcomes in murine models [10], but this result could be attributed to paracrine actions on resident epithelial cells and fibroblast rather than the result of in vivo differentiation of fibrocytes to myofibroblasts.
There are several aspects of IPF pathogenesis that have never been fully explained. These include the male predominance, the progressive nature of the disease and the association with aging. The male predominance has also been noted in murine models [11], but the reasons this occurs are unknown. Likewise, there are observations of age-related fibrosis in mice caused by murine gammaherpesvirus infection [12,13] or genetic ablation of the receptor for glycation end products (RAGE) [14]. Observations in the aged mice suggested that epithelial cells were more likely to undergo endoplasmic reticulum (ER) stress and apoptosis [13] and that fibroblasts from aged mice overexpressed the TGFβ receptors making them resistant to apoptosis [12]. An “apoptosis paradox” (enhanced apoptosis in epithelial cells, but resistance to apoptosis in mesenchymal cells) characterizes IPF [15]. The last feature that is unexplained is progression. No current animal models of lung fibrosis display a progressive disease course. In humans, it is believed that stiffening of the matrix may perpetuate the aberrant mesenchymal phenotype in IPF [16]. Matrix stiffening is caused by crosslinking of collagen monomers into fibrils and this is catalyzed by the actions of lysyl oxidase (LO) and lysl oxidase like 2 (LOXL2). The protease bone morphogenic protein (BMP)-1 cleaves LO to activate the enzyme. BMP-1 is localized to provisional ECM deposition via interactions with a matricellular protein known as periostin [17]. Two recent studies have demonstrated the elevation of periostin in lung tissue and circulation of IPF patients [18,19]. Interestingly, fibrocytes from IPF patients expressed elevated levels of periostin suggesting a paracrine mechanism via which fibrocytes that are recruited to sites of injury may promote fibrotic progression via paracrine actions [18]. It is also now appreciated that fibroblast interactions with stiffened ECM can promote survival and proliferation [16].
The role that inflammation plays in fibrosis is uncertain [20]. Many animal models of fibrosis (e.g. bleomycin administration) have exuberant early inflammatory responses that are followed by more chronic inflammation. It is presumed that human IPF may have originated from a lung injury associated with inflammation; however, that etiologic event may have occurred years before the patient presents with dyspnea. The initial inflammatory response may have dissipated by the time IPF is diagnosed. There is also a current belief that the nature of the chronic inflammatory infiltrates may influence IPF pathogenesis. In this regard, recent studies have suggested that macrophage polarization from an M1 to M2 phenotype may promote fibrogenesis [21].
Therapeutic Strategies
Despite extensive investigation into the pathogenesis of IPF, no therapy has been shown to definitively improve survival or quality of life. In 2011, the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society and the Latin American Thoracic Association published joint guidelines for evidence based management of IPF [4]. Provision of “best supportive therapy” is one of the principle approaches to management of IPF and is recommended despite variable strength in supporting evidence. Such therapies include supplemental oxygen for resting hypoxemia, pulmonary rehabilitation and lung transplantation in appropriate candidates. The second category of treatment includes the use of pharmacologic agents with therapeutic intent. Studied medications range from agents targeting co-morbid medical conditions to those aimed at halting fibroproliferation. A summary of such interventions, including rationale and results is provided below.
Treatment of Co-morbid Conditions
Gastroesophageal reflux (GER) is highly prevalent in IPF patients and GER and microaspiration may be inciting factors for repetitive lung injury in the development of IPF. A retrospective analysis of two cohorts of ILD patients found 47% reported current treatment with GER medications. Adjusted analysis demonstrated that the use of GER medications was an independent predictor for longer survival time and a lower high resolution computed tomography (HRCT) fibrosis score. This is intriguing retrospective data, but to date no randomized controlled trial has examined the effect of GER medications on morbidity and mortality in IPF [22].
Pulmonary arterial hypertension is another condition with increased prevalence in IPF patients. It is thought that the parenchymal distortion in severe IPF causes abnormalities in the pulmonary vasculature including impaired nitric oxide production, pulmonary vasoconstriction and impaired gas exchange. In the STEP-IPF trial, investigators treated patients with severe IPF and DLCO <35% with Sildenafil, a phosphodiesterase-5 inhibitor which enhances signaling through the nitric oxide pathway and promotes vasodilation. While Sildenafil did not meet the primary outcome measure of improved 6-minute walk distance compared to placebo, there were small but statistically significant improvements in secondary measures, including improved DLCO, arterial oxygenation, dyspnea symptoms and quality of life compared to placebo [23]. Information about clinical trials performed in the United States can be found at clinicaltrials.gov. The trial number for this trial was NCT00517933.
Patients with IPF are at increased risk of serious thromboembolism, and recently the ACE-IPF trial attempted to identify a therapeutic effect of warfarin in IPF. Primary outcome measures included time to death, hospitalizations and decline in forced vital capacity (FVC). This trial was terminated early after enrolling 145 of 256 patients secondary to increased mortality in the warfarin treated group and low likelihood of therapeutic benefit (NCT00957242).
Recent Anti-Fibrotic Clinical Trials
Several studies have examined immune-modulating therapies including corticosteroids, azathioprine and cyclophosphamide in the treatment of IPF. The 2000 ATS guidelines for the management of IPF recommended careful consideration of the risk and potential benefits of treatment with corticosteroids plus azathioprine or cyclophosphamide given the lack of evidence for other viable treatment options and the overall poor prognosis associated with the disease [24]. This recommendation was based on earlier studies suggesting variable impact on survival and pulmonary function with combinations of these agents. However, these studies were performed prior to the development of current IPF diagnostic criteria and the patient populations studied likely contained an overlap of IPF and non-specific interstitial pneumonia (NSIP) patients who do show some improvement with steroids [25,26].
The first large randomized trial of immuno-modulatory therapy was the IFIGENIA study [27]. In this study immuno-modulatory therapy with prednisone/azathioprine was evaluated alone or in combination with N-acetyl cysteine (NAC). NAC is a precursor to endogenous antioxidant glutathione and it has been postulated that treatment with high doses may repair an oxidant-antioxidant imbalance that propagates the progressive fibrotic reaction in IPF. This study demonstrated that patients with IPF treated with azathioprine/prednisone/N-acetyl cysteine had a slower decline in lung function compared to patients treated with azathioprine/prednisone/placebo [27]. This study led to the design of the IPFnet PANTHER-IPF study comparing placebo to N-acetyl cysteine alone or N-acetyl cysteine combined with azathioprine/prednisone. The triple therapy treatment arm of PANTHER-IPF was terminated early in 2011 secondary to increased risk of death and respiratory related hospitalization compared to placebo [28]. Based on the results of the PANTHER-IPF study immuno-modulatory therapy with azathioprine/prednisone is no longer recommended as therapy for patients with IPF. The PANTHER-IPF study continues with the NAC versus placebo arms and is estimated to be completed in 2013 (NCT00650091).
Interferon-γ is an endogenous cytokine with antifibrotic, antiinfective, antiproliferative and immuno-modulatory effects. In vitro and rodent models have demonstrated dose-dependent inhibition of fibroblast proliferation, collagen-matrix deposition, and collagen synthesis as well as alterations in Th1/Th2 balance in response to IFN-γ [29]. Early human studies demonstrated improved lung function in IPF patients treated with combined prednisolone and IFN-γ-1B [30]. Despite these promising results, a subsequent randomized-controlled trial found no benefit on duration of progression-free survival, pulmonary function or quality of life [31]. A follow-up mortality trial was stopped early due to lack of difference between INF- γ and placebo [32].
Endothelin-1 (ET-1) is a potent vasoconstrictor which has been demonstrated to promote smooth muscle proliferation and fibrosis in pulmonary hypertension. ET-1 has also been shown to have a pro-fibrotic effect in the lungs by promoting collagen production and inhibiting interstitial collagenase production [33]. Endothelin receptor antagonists are used in treatment of pulmonary hypertension and have been shown to reduce collagen production in rat models of bleomycin-induced pulmonary fibrosis [33]. Despite these results, two clinical trials evaluating for therapeutic benefit with bosentan compared to placebo have failed to show improvement in 6-minute walk distance [34] or time to IPF worsening or death [35]. A follow-up trial, ARTEMIS-IPF, failed to show therapeutic benefit on time to worsening of IPF or lung function with ambrisentan compared to placebo. In fact, ambrisentan treated patients had increased respiratory related hospitalizations (G Raghu, et al., abstract in Am J Respir Crit Care Med 2012, 185:A3632).
Etanercept gained interest as a potential therapy for IPF based on its TNF binding properties. TNF-α is an inflammatory and profibrotic cytokine which is expressed at increased levels in animal models of pulmonary fibrosis and humans with IPF [36]. The Etanercept treated group did not demonstrate any improvement in the primary outcomes of change in FVC, DLCO or arterial oxygenation [37].
In recent years, the therapeutic use of tyrosine kinase inhibitors has been explored given their inhibitory effect on platelet-derived growth factor receptors (PDGFR) which are known to have a proliferative effect. Initial in vitro studies demonstrated that imatinib has an inhibitory effect on PDGFR and TGF-β signaling with a resultant decrease in fibroblast-myofibroblast transformation, proliferation and extracellular matrix production [38]. Despite initial promise in vitro, a phase II, randomized-controlled clinical trial failed to show any benefit on survival or lung function in IPF patients compared to placebo [39].
The macrocyclic cell proliferation signal inhibitor, everolimus, gained interest as a potential therapy given its inhibitory effect on growth-factor dependent proliferation of hematopoietic cells as well as in vitro inhibition of human lung fibroblast proliferation when stimulated by platelet-derived growth factor [40]. Other studies have shown that everolimus or analogs inhibit human lung fibroblast proliferation after lung transplant and attenuates bleomycin-induced pulmonary fibrosis in rats [41]. However, in a randomized, placebo-controlled trial over 3 years, everolimus was associated with more rapid disease progression [42].
Finally, pirfenidone has shown promise as a therapeutic agent in the treatment of IPF. It has combined anti-inflammatory, antioxidant and antifibrotic effects in experimental models of fibrosis [43]. A phase II study of pirfenidone in IPF patients had to be terminated early after interim analysis demonstrated increased exacerbations in the placebo group compared to pirfenidone. Two follow-up phase III trials in Japan suggested that pirfenidone had an impact on secondary end-points including a decreased rate of decline in FVC, increased progression free survival, and decreased acute exacerbations of IPF [44,45]. There has been mixed opinion as to whether these two trials support further investigation of pirfenidone, as neither trial showed a positive effect on the primary endpoint.. The CAPACITY program attempted to clarify the role of pirfenidone in treatment of IPF by organizing two parallel, multi-center, international, randomized-controlled trials of pirfenidone versus placebo [46]. Results from this study were inconsistent with only a mild slowing in FVC decline in one of the trials and no benefit in the other. A third study is currently underway in an attempt to clarify the mixed results to date (NCT01366209).
Future Therapeutic Directions
A number of targeted therapies are currently in early testing or are being considered for development. These therapies are summarized in Table 1 and briefly outlined below.
Table 1.
Cell types and the target of action for current and suggested IPF therapeutics
| Cell Type | Profibrotic Actions | Target | Therapeutic |
|---|---|---|---|
| Fibroblast/Myofibroblast | Accumulation; Synthesis of Extracellular Matrix; Alveolar Contraction | PDGFR and FGFR kinase activity-stimulate fibroblast activation | BIBF1120 (Boehringer Ingelheim) NCT01335464 |
| Proliferation and TGFβmediated collagen synthesis | Perfinidone (Intermune) NCT01366209 | ||
| CTGF- mediates fibroblast activation | FG3019 (FibroGen) NCT01262001 | ||
| IL-13 receptor- signals fibroblast activation | QAX 576 (Novartis) NCT01266135 | ||
| Lysyl oxidase-like 2 (LOXL2)-enzyme that catalyzes collagen crosslinking in ECM | GS 6624 (Gilead Sciences) NCT01362231 | ||
| XIAP and Survivin- molecules that prevent fibroblast apoptosis | Suggested target | ||
| Periostin- activates fibroblasts and helps to stiffen the extracellular matrix | Suggested target | ||
| PDE4 inhibition- prevent cAMP degradation; estimated to limit fibroblast proliferation and ECM production |
Suggested target Roflumilast (Forest Pharmaceuticals) |
||
| Autotaxin- enzyme that generates LPA or the LPA1 receptor; can signal fibroblast activation | Suggested target | ||
| Fibrocyte | Migration to lung; Synthesis of extracellular matrix; Paracrine activation of resident cells | CCL2- mediates recruitment of fibrocytes and leukocytes to lung | CNTO 888 (Centecor) NCT00786201 |
| Provision of Serum Amyloid P/Pentraxin 2- endogenous compound; blocks fibrocyte to fibroblast differentiation | PRM-151 (Promedior) NCT01254409 | ||
| Epithelial Cells | TGFβ release can promote fibroblast activation | aαvβ6 integrin- critical for activation of TGFβ by epithelial cells | STX-100 (Stromedix) NCT01371305 |
| Apoptosis allows fibroproliferation; | Autotaxin- enzyme that generates LPA or the LPA1 receptor; can signal AEC apoptosis | Suggested Target | |
| Macrophages | M2 activation can promote tissue remodeling | Provision of Serum Amyloid P/Pentraxin 2, a naturally occurring compound that blocks M2 activation | PRM-151 (Promedior) NCT01254409 |
| Accumulation in tissue; Release of profibrotic mediators | CCL2- mediates recruitment of leukocytes to lung | CNTO888 (Centecor) NCT00786201 | |
| Anti-inflammatory/anti-profibrotic mediator secretion | Perfinidone (Intermune) NCT01366209 | ||
| Endothelial Cells | Angiogenesis to promote fibroproliferation | VEGF R kinase activity- stimulates angiogenesis | BIBF1120 (Boehringer Ingelheim) NCT01335464 |
| Increased vascular permeability promotes inflammation and provisional matrix deposition | Autotaxin- enzyme that generates LPA or the LPA1 receptor; can signal vascular permeability | Suggested target |
Fibroblast-Targeted Therapies
Several growth factors and cytokines are known to play a role in the proliferation, activation, differentiation or inappropriate survival of fibroblasts. Such molecules have become compelling drug targets to limit fibroblast activation. Pirfenidone limits TGFβ-stimulated collagen production in fibroblasts and is reported to decrease fibroblast proliferation [43]. Growth factor signaling via the platelet-derived growth factor (PDGF) receptor or the fibroblast growth factor (FGF) receptor can also trigger fibroblast proliferation via the activation of receptor associated tyrosine kinases [38]. This has promoted the development of a tyrosine kinase inhibitor (BIBF1120)[47] which has recently completed phase II trials. Two other growth factors known to stimulate fibroblast activation are connective tissue growth factor (CTGF) which is a potent downstream mediator of TGFβ actions and IL-13[48,49]. FG3019 is an anti-CTGF antibody-based therapy and QAX576 is an IL-13 receptor antagonist; both are hoped to limit fibroblast activation in vivo. Finally, a therapy directed against lysyl oxidase-like 2 (LOXL2) is in development and testing. LOXL2 crosslinks monomeric collagen fibers that are secreted by fibroblasts thus maturing and stiffening the ECM. The hope is that therapy directed against this molecule will prevent the maturation of the matrix and make it easier to digest and resorb the scar tissue.
Another potential target to reverse ECM stiffening is periostin. Periostin can bind to bone morphogenic protein-1, which is an activator of LO and sequester this enzyme within the ECM [17]. However, periostin can also directly influence fibroblast proliferation and migration. Monoclonal Abs that block the ability of periostin to signal through cell surface integrin receptors have been shown to limit fibrotic progression in animal models[18].
There are a number of fibroblast directed-targets that are well supported by basic science investigations, but are not yet in clinical trials for IPF. For example, therapies aimed at inducing myofibroblast apoptosis are likely to be beneficial. IPF fibroblasts have been demonstrated to upregulate survivin [50] and XIAP (J. Horowitz, personal communication). Both of the molecules are anti-apoptotic proteins that may contribute to inappropriate accumulation of fibroblasts in IPF. However, not all IPF patients showed a similar upregulation for each anti-apoptotic protein ([50] and unpublished observations). Fibroblasts from some patients had elevated XIAP while others had elevated survivin. This biologic heterogeneity is likely to correlate with clinical heterogeneity and highlights the importance of a personalized approach to therapy. Fibroblasts grown from patient biopsies could be screened to determine whether a small molecule directed against XIAP or survivin (or both) would be effective. It will be important to target anti-apoptotic therapies to targets that are preferentially expressed in fibroblasts, as opposed to epithelial cells to have the greatest likelihood of success in reversing the apoptosis paradox.
Other potential therapies anticipated to limit fibroblast activation are phosphodiesterase-4 (PDE4) inhibitors and antagonists of autotaxin or the lysophosphatidic acid (LPA)1 receptor. Elevations of cyclic adenosine monophosphate (cAMP) reduce fibroblast proliferation and matrix synthesis [51]. PDE4 inhibitors would prevent the breakdown of cAMP within the cell, thus potentiating the anti-fibrotic signaling. A PDE4 inhibitor known as roflumilast has been used in other lung diseases[52], and we speculate that it would be appropriate for consideration in IPF as well. Autotaxin is the enzyme that generates LPA and this molecule has potent profibrotic effects to induce epithelial cell apoptosis, endothelial cell leak and fibroblast accumulation. A pharmacologic inhibitor of autotaxin (GWJ-A-23) has been shown to ameliorate fibrosis in animal models[53] and strategies that limit the LPA1 receptor also limit fibrosis in other organs [54]. Thus, there is enthusiasm for testing such approaches in IPF.
Fibrocyte-directed therapies
Although the role of fibrocytes in human IPF pathogenesis is unconfirmed, several therapies that have been developed to limit fibroblast action are also anticipated to limit fibrocyte profibrotic actions. These include the CNTO888 which is an anti-CCL2 therapy. CCL2 is a critical ligand (at least in the mouse) for the recruitment of fibrocytes to the lung [9,10]. Additionally, serum amyloid P (PRM-151) has been shown to limit fibrocyte differentiation in vitro[55].
Macrophage-directed therapies
Targets which are anticipated to limit fibrocyte accumulation may also limit macrophage accumulation. CCL2 regulates monocyte and macrophage recruitment via CCR2 [56]. As such, CNTO888 is likely to limit macrophage recruitment to the lung as well. A recombinant form of Serum amyloid P which activates Fc gamma receptors, PRM-151, has been shown to prevent M2 polarization of macrophages [21]. When macrophages are polarized to an M2 phenotype, they overexpress arginase-1 which may promote collagen synthesis. Pirfenidone is also anticipated to block pro-fibrotic mediator release from macrophages [57].
Endothelial cell directed therapies
Endothelial cells are critical for the progression of fibrosis because angiogenesis is needed to support the cellular requirements for scar formation [58]. Barrier function of endothelial cells is also critical for limiting vascular leak and the spilling of plasma proteins into the alveolar space to begin the provisional ECM deposition. Targeting autotaxin or the LPA1 receptor should help limit vascular leak and improve fibrosis. Additionally, the BIBF1120 compound will block signaling via VEGF receptors, which in turn, should limit neoangiogenesis [59].
Epithelial-directed therapies
Epithelial repair is believed to be aberrant in IPF and it is likely that epithelial cell alterations promote fibrotic progression [7,8]. One therapy currently under development is aimed at blocking the ability of epithelial cells to activate latent TGFβ. This therapy (STX-100) is directed against the epithelial cell-selective αvβ6 integrin which is believed to physically interact with latent TGFβ complexes to promote activation of this potent profibrotic cytokine[60]. There is great enthusiasm for the success of this approach based on animal models.
Conclusions
In the last decade, numerous advances have been made in our basic science understanding of fibrotic pathogenesis and our accuracy in assigning a diagnosis of IPF. These discoveries have fueled a number of recent clinical trials with attractive targets and trial designs that include placebo arms. One of the most important recent advances was the demonstration that standard therapy (prednisone, azathioprine, and NAC) was actually harmful compared to placebo [28]; possibly due to the toxic actions of the immunosuppressive agents. The fact that no recent trials have identified a wide-ranging therapeutic is likely due to the clinical and biological heterogeneity of the disease. Most of the drug targets have been identified in genetically identical mouse strains. Approaches to target particular molecules in a uniform genetic background are more likely to be successful than when the drug is given to the human population which is genetically outbred. For this reason, our recommendation is that future clinical trials attempt to utilize biomarkers found in circulation or tissue samples to identify aberrant fibrotic pathways that are unique to that particular patient. Once this personalized approach to medicine is taken, it is likely that many of these currently available drugs or the ones in development will provide patient-specific benefit.
Therapy with prednisone/azathioprine/N-acetyl cysteine increases mortality in IPF.
Several molecular pathways contribute to the pathogenesis of IPF.
Heterogeneity in IPF emphasizes the need for patient specific treatments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hillary Loomis-King, Email: hloomis@med.umich.edu.
Kevin R. Flaherty, Email: flaherty@med.umich.edu.
Bethany B. Moore, Email: bmoore@umich.edu.
References
- 1.Steele MP, Schwartz DA. Molecular Mechanisms in Progressive Idiopathic Pulmonary Fibrosis. Annu Rev Med. 2012:27. in press. [Google Scholar]
- 2. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. Revised international, evidence-based comprehensive clinical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis.
- 3.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, Markart P. Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:152–160. doi: 10.1183/09059180.00001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kage H, Borok Z. EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med. 2012;18:517–523. doi: 10.1097/MCP.0b013e3283566721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coward WR, Saini G, Jenkins G, et al. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:367–388. doi: 10.1177/1753465810379801. [DOI] [PubMed] [Google Scholar]
- 9.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. Epub 2006 Mar 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redente EF, Jacobsen KM, Solomon JJ, Lara AR, Faubel S, Keith RC, Henson PM, Downey GP, Riches DW. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L510–L518. doi: 10.1152/ajplung.00122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik PN, Horowitz JC, Moore TA, Wilke CA, Toews GB, Moore BB. Pulmonary fibrosis induced by gamma-herpesvirus in aged mice is associated with increased fibroblast responsiveness to transforming growth factor-beta. J Gerontol A Biol Sci Med Sci. 2012;67:714–725. doi: 10.1093/gerona/glr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres-Gonzalez E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, et al. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol. 2012;46:748–756. doi: 10.1165/rcmb.2011-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Englert JM, Kliment CR, Ramsgaard L, Milutinovic PS, Crum L, Tobolewski JM, Oury TD. Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. Int J Clin Exp Pathol. 2011;4:241–254. [PMC free article] [PubMed] [Google Scholar]
- 15.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, et al. Periostin promotes fibrosis and predicts progression in patients with Idiopathic Pulmonary Fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;5:5. doi: 10.1152/ajplung.00139.2012. Elevated serum levels of periostin in IPF patients predicts progression of disease. Treatment with a periostin-directed antibody can prevent progression of pulmonary fibrosis in murine models of bleomycin-induced pulmonary fibrosis. Evidence for potential mediator of fibrocyte paracrine effects.
- 19. Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37:1119–1127. doi: 10.1183/09031936.00059810. Identification of a serum biomarker, periostin, which is elevated in IPF patients with the degree of elevation associated with level of impairment in lung function.
- 20.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray LA, Rosada R, Moreira AP, Joshi A, Kramer MS, Hesson DP, Argentieri RL, Mathai S, Gulati M, Herzog EL, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5:e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, King TE, Jr, Collard HR. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. Retrospective study supporting hypothesis that gastroesophageal reflex contributes to the pathogenesis of IPF and treatment may be a reasonable component of IPF treatment. No definitive conclusions can be drawn from the study given the retrospective design, but provides compelling evidence for future trials.
- 23. Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. Sildenafil did not improve the primary clinical outcome of improved 6-minute walk but there were small, non-significant improvements in secondary outcomes including dyspnea, gas exchange and quality of life which may provide hypotheses for further investigation.
- 24.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 25.Raghu G, Depaso WJ, Cain K, Hammar SP, Wetzel CE, Dreis DF, Hutchinson J, Pardee NE, Winterbauer RH. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective double-blind, randomized, placebo-controlled clinical trial. Am Rev Respir Dis. 1991;144:291–296. doi: 10.1164/ajrccm/144.2.291. [DOI] [PubMed] [Google Scholar]
- 26.Collard HR, Ryu JH, Douglas WW, Schwarz MI, Curran-Everett D, King TE, Jr, Brown KK. Combined corticosteroid and cyclophosphamide therapy does not alter survival in idiopathic pulmonary fibrosis. Chest. 2004;125:2169–2174. doi: 10.1378/chest.125.6.2169. [DOI] [PubMed] [Google Scholar]
- 27.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 28. Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. The previously recommended therapeutic standard-of-care actually increases mortality in IPF. This has dramatically altered the approach to treatment of the IPF patient and emphasizes the need for innovative therapeutic strategies.
- 29.Bouros D, Antoniou KM, Tzouvelekis A, Siafakas NM. Interferon-gamma 1b for the treatment of idiopathic pulmonary fibrosis. Expert Opin Biol Ther. 2006;6:1051–1060. doi: 10.1517/14712598.6.10.1051. [DOI] [PubMed] [Google Scholar]
- 30.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341:1264–1269. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]
- 31.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE., Jr A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 32.King TE, Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222–228. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 33.Fonseca C, Abraham D, Renzoni EA. Endothelin in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;44:1–10. doi: 10.1165/rcmb.2009-0388TR. [DOI] [PubMed] [Google Scholar]
- 34.King TE, Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stahler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 35.King TE, Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 36.Lasky JA, Brody AR. Interstitial fibrosis and growth factors. Environ Health Perspect. 2000;108:751–762. doi: 10.1289/ehp.00108s4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, Thomeer M, Utz JP, Khandker RK, McDermott L, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–955. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 38.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 40.Nair RV, Huang X, Shorthouse R, Adams B, Brazelton T, Braun-Dullaeus R, Morris RE. Antiproliferative effect of rapamycin on growth factor-stimulated human adult lung fibroblasts in vitro may explain its superior efficacy for prevention and treatment of allograft obliterative airway disease in vivo. Transplant Proc. 1997;29:614–615. doi: 10.1016/s0041-1345(96)00325-9. [DOI] [PubMed] [Google Scholar]
- 41.Simler NR, Howell DC, Marshall RP, Goldsack NR, Hasleton PS, Laurent GJ, Chambers RC, Egan JJ. The rapamycin analogue SDZ RAD attenuates bleomycin-induced pulmonary fibrosis in rats. Eur Respir J. 2002;19:1124–1127. doi: 10.1183/09031936.02.00281602. [DOI] [PubMed] [Google Scholar]
- 42.Malouf MA, Hopkins P, Snell G, Glanville AR. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology. 2011;16:776–783. doi: 10.1111/j.1440-1843.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- 43.Di Sario A, Bendia E, Svegliati Baroni G, Ridolfi F, Casini A, Ceni E, Saccomanno S, Marzioni M, Trozzi L, Sterpetti P, et al. Effect of pirfenidone on rat hepatic stellate cell proliferation and collagen production. J Hepatol. 2002;37:584–591. doi: 10.1016/s0168-8278(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 44.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 45.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 46. Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Jr, Lancaster L, Sahn SA, Szwarcberg J, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. Two parallel phase III randomized, placebo-controlled trials that provided further evidence supporting a small functional benefit in IPF patients treated with pirfenidone. This was an international study providing follow-up to two prior Japanese phase III trials. Results have led to a third trial to try to rectify discrepancies.
- 47.Antoniu SA. Nintedanib (BIBF 1120) for IPF: a tomorrow therapy? Multidiscip Respir Med. 2012;7:41. doi: 10.1186/2049-6958-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff HL, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40:2174–2182. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Sonnylal S, Shi-Wen X, Leoni P, Naff K, Van Pelt CS, Nakamura H, Leask A, Abraham D, Bou-Gharios G, de Crombrugghe B. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sisson T, Maher T, Ajayi I, King J, Higgins P, Booth A, Sagana R, Huang S, White E, Moore B, et al. Increased Survivin Expression Contributes to Apoptosis-resistance in IPF fibroblasts. Advances in Biosciences and Biotechnology. 2012;3:657–664. doi: 10.4236/abb.2012.326085. There is increased expression of survivin, an inhibitor of apoptosis, in the fibroblastic foci of IPF patients compared to the fibroblasts of normal subjects. Inhibition of surviving enhances fibroblast apoptosis, suggesting a novel target for treatment of IPF. This study also demonstrates the heterogeneity of expression within different IPF patient biopsy specimens.
- 51.Bozyk PD, Moore BB. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am. 45:445–452. doi: 10.1165/rcmb.2011-0025RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page CP, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol. 1016;12:275–286. doi: 10.1016/j.coph.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Jiang G, Madan D, Prestwich GD. Aromatic phosphonates inhibit the lysophospholipase D activity of autotaxin. Bioorg. 2011;21:5098–5101. doi: 10.1016/j.bmcl.2011.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 1405;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crawford JR, Pilling D, Gomer RH. FcgammaRI mediates serum amyloid P inhibition of fibrocyte differentiation. J Leukoc Biol. 1189;92:699–711. doi: 10.1189/jlb.0112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montovani A. The chemokine system: redundancy for robust outputs. Immunol. Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 57.Nakazato H, Oku H, Yamane S, Tsuruta Y, Suzuki R. A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level. Eur J Pharmacol. 2002;446:177–185. doi: 10.1016/s0014-2999(02)01758-2. [DOI] [PubMed] [Google Scholar]
- 58.Keane MP, Strieter RM, Lynch JP, 3rd, Belperio JA. Inflammation and angiogenesis in fibrotic lung disease. Semin Respir Crit Care Med. 2006;27:589–599. doi: 10.1055/s-2006-957331. [DOI] [PubMed] [Google Scholar]
- 59.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 1079;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 60.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]