Abstract
Background
Crizotinib is a tyrosine kinase inhibitor active against ALK, MET and ROS1. We previously reported that crizotinib decreases testosterone in male patients. The detailed etiology of the effect, its symptomatic significance, and the effectiveness of subsequent testosterone replacement have not been previously reported.
Methods
Male cancer patients treated with crizotinib had total testosterone levels measured, and results compared to non-crizotinib treated patients. Albumin, sex-hormone binding globulin (SHBG), follicle stimulating hormone (FSH) and/or luteinizing hormone (LH) were tracked longitudinally. A subset of patients had free testosterone levels measured and a hypogonadal screening questionnaire administered. Patients receiving subsequent testosterone supplementation were assessed for symptomatic improvement.
Results
Mean total testosterone levels were -25% below the lower limit of normal (LLN) in 32 crizotinib treated patients (27/32 patients below LLN, 84%) compared to +29% above LLN in 19 non-crizotinib treated patients (6/19 below LLN, 32%), p=0.0012. Levels of albumin and SHBG (which both bind testosterone) declined rapidly with crizotinib, but so did FSH, LH and free testosterone, suggesting a centrally mediated, true hypogonadal effect. Mean free testosterone levels were -17% below LLN (19/25 patients below LLN, 76%). 84% (16/19) with low free levels and 79% (19/24) with low total levels had symptoms of androgen deficiency. 5/9 (55%) patients with low testosterone given testosterone supplementation had an improvement in symptoms, coincident with increases in testosterone above LLN.
Conclusion
Symptoms of androgen deficiency and free or total/free testosterone levels should be tracked in male patients on crizotinib with consideration of testosterone replacement as appropriate.
Keywords: Crizotinib, NSCLC, Testosterone, ALK gene rearrangements, hypogonadism
Introduction
Crizotinib (Xalkori, Pfizer, La Jolla CA) is an inhibitor of several different tyrosine kinases including anaplastic lymphoma kinase (ALK), ROS1 and MET.1–3 Use of crizotinib in molecularly selected patients with ALK or ROS1 gene rearranged non-small cell lung cancer (NSCLC) leads to significant clinical benefit.1–5 Within the phase I-III studies of crizotinib reported to date, the range of side effects attributed to crizotinib include gastrointestinal disturbance, visual side effects, peripheral edema, and liver function test abnormalities.1, 4, 6, 7 Because of the demographics associated with ALK rearranged disease, patients treated with crizotinib are likely to be younger than most patients with NSCLC.8, 9 Recently, we reported that crizotinib reduced total testosterone over a period of weeks to below the lower limit of normal (LLN) in 19 out of 19 male patients treated with the drug, with free testosterone levels also being below the LLN in 9 out of 10 patients in whom this information was available.10 Levels of testosterone returned to normal within days following cessation of dosing with crizotinib. The mechanism of testosterone reduction appeared to include a central effect because longitudinal sampling in two patients showed that follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels decreased coincident with the drop in testosterone levels.10
However, many questions about crizotinib’s effects on testosterone remain.11 Fatigue, which can be associated with low testosterone, is a known side effect of crizotinib,1, 7 but fatigue could also have multiple other etiologies. Symptoms specifically associated with androgen deficiency may be missed in routine oncology practice,12 and are not routinely assessed in lung cancer studies including any reported crizotinib trials to date. Although the index case with low testosterone that prompted these observations presented with fatigue and loss of libido, the symptomatic consequences of these biochemical findings in most crizotinib-treated patients have not been documented.10 Similarly, the effectiveness of testosterone replacement in such cases has not been previously reported. Here we address in a larger series, across multiple different institutions, the incidence of low testosterone in advanced male cancer patients treated with crizotinib therapy, the impact of crizotinib on testosterone binding proteins (albumin and sex hormone binding globulin (SHBG)) which could differentially influence total and free levels of testosterone, the impact of crizotinib on gonadotrophin levels, the association of both total and free testosterone levels with symptoms of androgen deficiency, and whether treatment with testosterone replacement benefited these patients symptomatically.
Methods
Three groups of male patients with metastatic cancer were assessed within this study. The first group represented 32 crizotinib treated patients (crizotinib treated group 1, CTG1) assessed at the University of Colorado Cancer Center (10 of the 32) and six other institutions (Prince of Wales Hospital, Hong Kong; The Royal Marsden Hospital, London, United Kingdom; San Luigi Hospital, University of Turin, Italy; University of New Mexico Cancer Center, Albuquerque, NM; Billings Clinic, Billings, MT; and the University of California, Irvine, CA). Following the initial report of low testosterone secondary to crizotinib use from the University of Colorado, additional sites with experience treating patients with crizotinib were approached in April 2012.
The University of Colorado patients were tested for molecular abnormalities on-site within the CLIA-certified Colorado Molecular Correlates laboratory (CMOCO) and an IRB-approved protocol permits clinical correlates to be made on all in-house patients in whom molecular analyses have been conducted within the CMOCO laboratory. Information on patients treated with crizotinib at other institutions and assessed for testosterone levels and androgen deprivation symptoms following the initial publication of these data was captured and communicated for analysis within this study in accordance with each institution’s local practice guidelines.
Sites were asked to identify all patients with advanced cancer, regardless of their underlying histology or molecular status, who were currently on crizotinib and had received at least 21 days of continuous therapy. Total and/or free levels of testosterone using institutional standard assays were then checked at the next routine clinic visit. Pretreatment testosterone levels were not routinely collected. In addition, when possible, symptoms of androgen deficiency were documented at the same time as the initial testosterone assessment using the Androgen Deficiency in Aging Males (ADAM) questionnaire (Table 1), a validated screening assessment of hypogonadism in adult males.13 Serial albumin levels were assessable in most patients. Three patients from the University of Colorado within CTG1 also had serial total and free testosterone, SHBG and gonadotrophin assessments from the day of commencement of crizotinib. If patients had low total and/or free testosterone they were advised to be reviewed by an endocrinologist. Medical records from the University of Colorado patients referred to Endocrinology were subsequently reviewed for whether testosterone replacement was recommended, and the overall effectiveness of replacement therapy on subsequent symptoms of hypogonadism as assessed by the endocrinologist (improved/not improved).
Table 1.
Androgen Deficiency in Aging Males (ADAM questionnaire)13 (Yes/No answers)
| 1. Do you have a decrease in libido (sex drive)? |
| 2. Do you have a lack of energy? |
| 3. Do you have a decrease in strength and/or endurance? |
| 4. Have you lost height? |
| 5. Have you noticed a decreased “enjoyment of life” ? |
| 6. Are you sad and/or grumpy? |
| 7. Are your erections less strong? |
| 8. Have you noticed a recent deterioration in your ability to play sports? |
| 9. Are you falling asleep after dinner? |
| 10. Has there been a recent deterioration in your work performance? |
Answering Yes to number 1 or 7 or answering Yes to more than 3 questions, may indicate low testosterone.
The second group represented the 19 crizotinib treated patients (crizotinib treated group 2, CTG2) included in the original report from the University of Colorado who had a reanalysis of previously reported total and free testosterone levels to allow comparison across different institutional assays (see below).14 At the time of these assessments, all of these patients had received at least 21 days of continuous treatment with crizotinib. The ADAM questionnaire was also administered, where possible, to patients in CTG2 at the time of the testosterone assessment reported here. Patients with low total and/or free testosterone were similarly referred to Endocrinology and the impact of any replacement therapy assessed.
The third group represented a reference group of 19 patients with metastatic NSCLC (non-crizotinib treated group, NCTG), who had never received crizotinib, who were reported in the previous publication and similarly reanalyzed to allow comparison across different institutional assays. This group served as a control population to assess total testosterone measurements in advanced cancer patients in comparison to both CTG1 and CTG2.
Blood samples for hormonal and binding protein levels in all patients were obtained at routine clinic visits rather than at any consistent time of day, and when multiple assessments were made the first assay result after 21 days of continuous crizotinib therapy was used. At the University of Colorado Hospital, hormonal assessments were performed as previously described.10 Patients from the six other institutions had total and free testosterone measured using a range of different assays. Although previous research suggests that inter-assay variability makes only a small contribution to total variation in testosterone measurements,14 to facilitate comparisons between all patients across institutions each assay measurement was normalized by dividing individual measurements by the LLN of the specific assay. Using this approach each reading could then be expressed as an absolute percent relevant to the LLN.
To explore the relationship between total testosterone (protein bound plus unbound levels of testosterone) and free testosterone (unbound levels only), levels of albumin and/or SHBG were measured longitudinally in a subset of crizotinib treated patients in CTG1 and CTG2. For these patients, each of the serial readings was expressed as an absolute percent relative to the baseline measurement of the variable in the individual patient. Patient age, cancer type, molecular abnormality, and duration of therapy (days) with crizotinib were recorded on every patient.
Prism software (GraphPad, San Diego, CA) was used to calculate statistical significance in mean total testosterone between the CTG1/CTG2 and NCTG using a two-tailed unpaired t-test, and to compare levels of free testosterone with symptoms of androgen deficiency using Fisher’s exact test of assessment for contingency tables.
Results
All patients in CTG1 and CTG2 received crizotinib at 250mg twice daily orally, apart from 1 colorectal cancer patient in the CTG1 who received crizotinib at 650mg orally once daily as part of a dose escalation cohort within a crizotinib phase I trial. Two patients in CTG1 received crizotinib together with a pan-Her inhibitor (dacomitinib, Pfizer) within a phase I combination study.
Demographics of the CTG1, CTG2 and NCTG groups are listed in Table 2. Of the 32 patients within CTG1, 31 patients had NSCLC (27 with ALK gene rearrangements, 2 with ROS1 gene fusions, 1 with cMET amplification, 1 with a HER2 mutation and 1 with an EGFR mutation), and 1 patient had colorectal carcinoma with a KRAS mutation. The median duration of crizotinib therapy at the time of hormonal sampling was 6.3 months in CTG1 and 7.3 months in CTG2. The median age of the patients was 54 years in CTG1, 60 years in CTG2 and 63 years in the NCTG, consistent with the known younger demographics of ALK rearranged NSCLC comprising 82% and 90%, respectively, of the patients treated in CTG1 and CTG2.8, 9
Table 2.
| Crizotinib Treated Group 1 (CTG1) | Crizotinib Treated Group 2 (CTG2) | Non-Crizotinib Treated Group (NCTG) | |
|---|---|---|---|
|
|
|||
| No. patients | 32 | 19 | 19 |
|
| |||
| Age (Median) | 54 yrs | 60 yrs | 63 yrs |
| Range | 33 – 69 | 35 – 81 | 43 – 78 |
|
| |||
| Cancer | |||
| Non-small cell lung cancer | 31(97%) | 19 (100%) | 19 (100%) |
| Colorectal cancer | 1 (3%) | 0 (0%) | 1 (5%) |
|
| |||
| Molecular Status | |||
| ALK rearranged | 26 (82%) | 17 (90%) | 2 (11%) |
| ROS1 fusion gene | 2 (6%) | 1 (5%) | 0 (0%) |
| MET amplification | 1 (3%) | 1 (5%) | 0 (0%) |
| EGFR mutation | 1 (3%) | 0 (0%) | 5 (26%) |
| KRAS mutation | 1 (3%) | 0 (0%) | 3 (16%) |
| Undefined/Other | 1 (3%) | 0 (0%) | 9 (47%) |
|
| |||
| Current Therapy | |||
| Crizotinib | 32 (100%) | 19 (100%) | 0 (0%) |
| Median duration | 6.3 months | 7.3 months | - |
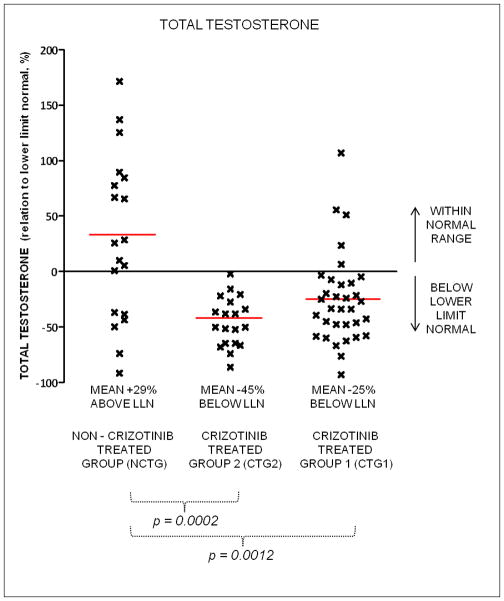
Mean total testosterone levels were lower in the CTG1 compared to the NCTG (mean -25% below the lower limit of normal (LLN) vs mean +29% above the LLN, p = 0.0012) (Figure 1). Mean total testosterone levels in the previously reported CTG2 were even lower (mean -45% below LLN, p = 0.0012). In the CTG1 group, 27/32 (84%) patients had levels of total testosterone below the LLN, compared to 19/19 (100%) in the CTG2, and 6/19 (32%) in the NCTG.
Figure 1.
Total testosterone levels in crizotinib treated and non-crizotinib treated patients in relation to lower limit of normal (LLN) of assay used. NCTG non-crizotinib treated group; CTG2 crizotinib treated group 2; CTG 1 crizotinib treated group 1.
Free testosterone levels were measured in 25 crizotinib treated patients (17 from CTG1, 8 from CTG2) and are shown in Figure 2. The free testosterone was below the LLN in 19/25 patients (76%), with a mean level −17% below the LLN. The relationship between the total testosterone measurement relative to LLN and free testosterone measurement relative to LLN in the 25 crizotinib treated patients is shown in Figure 3. The relationship was linear, with a r2=0.52.
Figure 2.
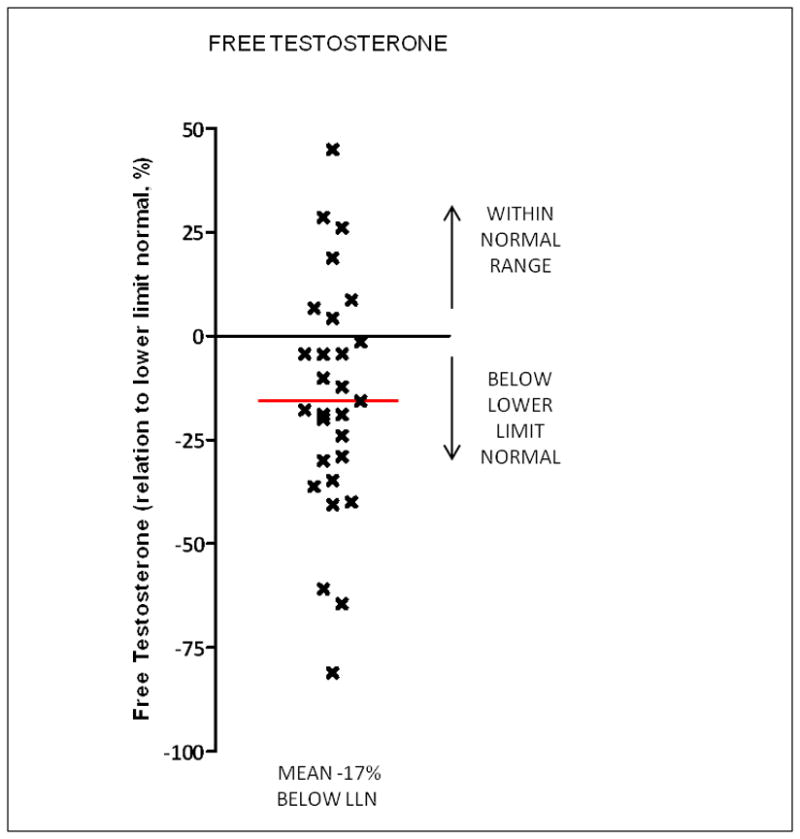
Free testosterone levels in crizotinib treated patients relative to lower limit of normal of assay used
Figure 3.
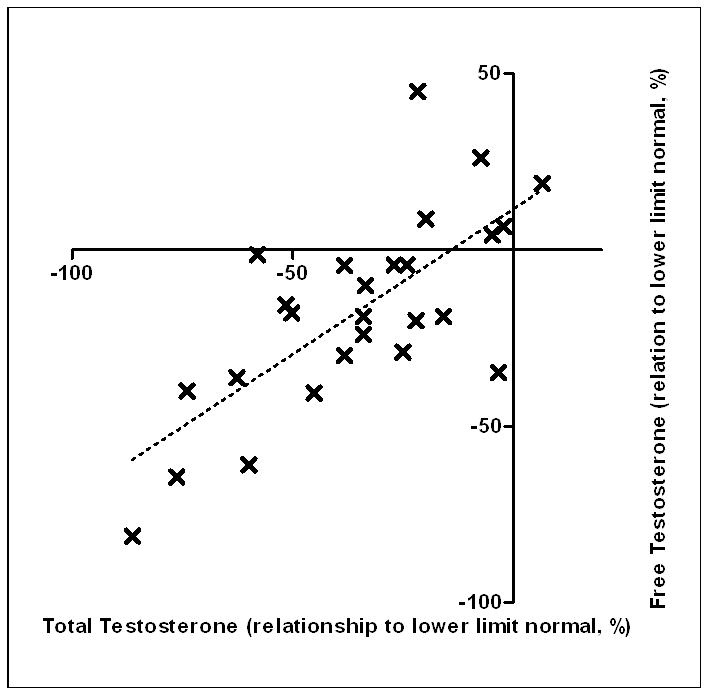
Correlation of each individual’s total testosterone relative to lower limit of normal of assay used to free testosterone relative to lower limit of normal of assay used. Total and free testosterone assessed on same occasion in each patient.
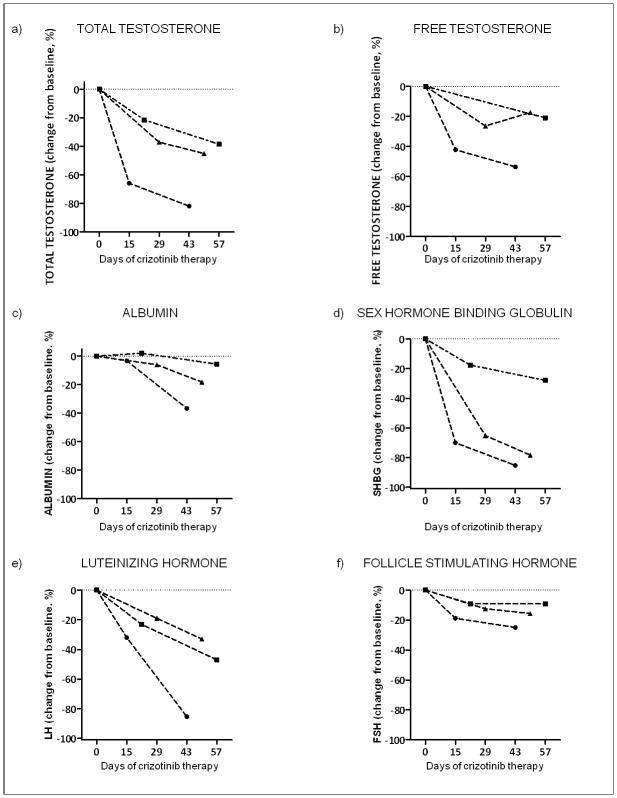
Albumin levels fell in crizotinib treated patients (Figure 4). In 20 patients from CTG1 and CTG2, after 6–8 weeks of treatment with crizotinib, mean albumin levels were −7% below baseline levels. SHBG levels were assessed in 3 patients in detail during the first 8 weeks of therapy with crizotinib, and declined precipitously in each patient, falling by up to 85% below baseline initial level over this time frame (Figure 4). Albumin levels checked over the same time frame also dropped, but more modestly. In each of the three patients, total and free testosterone, as well as FSH and LH levels were also assessed. Levels of total and free testosterone and both gonadotropins all declined following commencement of crizotinib, consistent with our previous report.10
Figure 4.
Dynamic assessment of sex hormones and testosterone binding proteins in three patients commenced on crizotinib showing decrease in total, free testosterone relative to baseline measurement, as well as rapid decreases in LH and FSH, albumin and sex hormone binding globulin.
Symptoms of low testosterone among those with both total and free testosterone data were assessed in 25 patients from CTG1 and CTG2 using the ADAM questionnaire (Table 1). Of these, 20/25 (80%) had responses to the questionnaire consistent with possible hypogonadism. The correlation of these responses to the assessment of total and free testosterone in these patients is shown in Table 3. 84% (16/19) of patients with low free testosterone and 79% (19/24) of patients with low total testosterone manifested symptoms of androgen deficiency.
Table 3.
Symptoms of hypogonadism from ADAM questionnaire and levels of free testosterone
| Symptoms | No Symptoms | Two tailed Fisher Test | |
|---|---|---|---|
| Free Testosterone below LLN | 16 | 3 | P=0.56 |
| Free Testosterone above LLN | 4 | 2 | |
| Total Testosterone below LLN | 19 | 5 | P=1.00 |
| Total Testosterone above LLN | 1 | 0 |
The benefits of testosterone replacement in crizotinib treated patients with low testosterone (total and/or free) were assessed in 9 patients treated at the University of Colorado from CTG1. Replacement testosterone in the form of a topical gel or injection was undertaken in 9 patients with low total testosterone under the supervision of an endocrinologist. At review between 2–3 months after commencing testosterone replacement, 5/9 (55%) reported improvement in symptoms of hypogonadism to the endocrinologist. 5/5 (100%) of patients who had testosterone replacement and increase in their testosterone levels above the LLN reported improvement in symptoms, whereas none of the 4 patients who took testosterone replacement but did not have total testosterone levels increase above the LLN reported a change in symptoms.
Discussion
Crizotinib use is associated with significant clinical benefit in ALK gene and ROS1 gene rearranged malignancies with additional early evidence of activity in some MET driven cancers.1–3 Previously we reported that crizotinib use was associated with rapid onset of low testosterone in male patients with metastatic non-small cell lung cancer, with 19 of 19 (100%) crizotinib treated patients manifesting low total testosterone levels and 9 of 10 (90%) manifesting low free testosterone levels.10 When levels were tracked longitudinally, testosterone declines were rapid, falling below the LLN within 3 weeks of commencing therapy in most cases. As crizotinib also led to decreases in both FSH and LH, activity of the drug within the central hypothalamic-pituitary axis was suggested as at least one possible mechanism underlying this effect.
However, from these initial findings it was unclear whether the effect of crizotinib on testosterone was functionally significant in the absence of both a larger dataset and information on the symptomatic consequences of these hormonal test results.11
Our initial findings are replicated here in a larger cohort of prospectively tested crizotinib treated patients, with 84% of patients manifesting total testosterone levels below the LLN. This effect was evident in patients with advanced cancer treated at multiple institutions, across different cancer histologies and molecular subtypes. Certainly, a proportion of all patients with advanced cancer would be expected to have low testosterone levels, as previously reported in the literature and shown by the 32% of patients in the NCTG with testosterone levels below the LLN.10, 15–18 However, despite the younger median age in the crizotinib treated groups, the levels of total testosterone were significantly lower in both CTG1 and CTG2 compared to the NCTG (Figure 1). Data was not collected in this study on the amount of prior chemotherapy, presence/absence of brain metastases, prior brain radiation or opiate/dexamethasone use in CTG1, all of which may influence testosterone levels. However, in our previous study we found no significant differences in these factors between our control and crizotinib-treated groups and therefore these factors alone could not explain the association between low testosterone levels and crizotinib treatment.10
Part of the rapid fall in total testosterone levels reflects a mild effect of crizotinib on albumin levels, and a much larger effect on SHBG levels, which represent the two major testosterone binding proteins (Figure 4). Anecdotally, among the University of Colorado patients, these drops occurred in the absence of proteinuria or significant hepatic dysfunction (as assessed by transaminase levels in the blood). The signaling pathways and feed-back loops controlling expression levels of these proteins are not well understood and the molecular basis for these effects remains unclear. Whether chronic use of crizotinib will drop albumin levels beyond those that we have noted after 6–8 weeks on therapy to contribute towards either the late-onset peripheral edema described with crizotinib, or to have meaningful effects on the activity of other drugs influenced by albumin levels, e.g. Coumadin, is unknown, but should be considered.7, 19 Importantly, in the subset in whom we have data, 76% of patients manifested free testosterone below the LLN suggesting that effects on testosterone physiology beyond binding protein levels are occurring and that these low levels are likely to be biologically significant. Consistent with this, 84% of patients (16 of 19) with low free testosterone levels on crizotinib and 79% (19/24) of patients with low total testosterone on crizotinib manifested symptoms of androgen deficiency (Table 3). In our small study, in which very few patients manifested normal testosterone levels on crizotinib, the presence/absence of symptoms was not statistically different between those with testosterone levels above and below the LLN. In addition, patents with testosterone levels above the LLN can certainly describe positive responses in the ADAM questionnaire, as, despite 88% sensitivity, the questionnaire only has a reported specificity of 60%.13 Specifically, in our study, the symptoms of androgen deficiency are likely to overlap with symptoms secondary to both advanced cancer and depression (Table 1). However consistent with the majority of symptoms in our patients being truly reflective of their low testosterone levels, 100% of patients who had testosterone replacement and increase in their testosterone levels above the LLN reported improvement in symptoms, whereas 0% of those who took testosterone replacement but did not have total testosterone levels increase above the LLN reported a change in symptoms.
Free and total testosterone levels are related (Figure 3), but the relationship is only moderately strong (r2=0.52). Although both low total and free testosterone levels are associated with symptoms of androgen deficiency, because reductions in albumin and SHBG also occur secondary to crizotinib exposure and the incidence of androgen deficiency symptoms was marginally higher among those with low free as opposed to low total testosterone levels, free testosterone alone or free and total levels together may be the most appropriate assessment methods to pursue in this indication in the future.
Building on our previous report of 2 patients tracked longitudinally, we have demonstrated in an additional three patients that the commencement of crizotinib is associated with a decrease in both FSH and LH (Figure 4), again suggesting that a central effect of crizotinib on the hypothalamic-pituitary axis may be occurring.10 Crizotinib is suspected to have very low blood-brain penetration, however the pituitary lies outside the blood-brain barrier.20, 21 Whether reductions in testosterone reflect an effect of crizotinib on ALK, on one of the other targets of crizotinib or some combination of the drug’s targets is unknown, but the presence or absence of this toxicity with some of the newer ALK inhibitors currently in development should elucidate this issue in the near future.22, 23
There are several limitations of this report that need to be accounted for. Firstly, the number of crizotinib treated patients described is still relatively low (32 in the CTG1, 19 in the CTG2). Secondly, testosterone concentrations were measured at variable times of day. Testosterone levels are known to peak in the early morning and to be lower in the evening, although there is some blunting of this diurnal variation with age. 24 However given the consistent and clear reduction of levels below the lower limit of normal in multiple individuals in two separate studies across seven different institutions, normal diurnal variation alone would not be able to account for the results seen in association with crizotinib. Thirdly, the patients who had more complete hormonal assessments (e.g. free testosterone levels or detailed longitudinal sampling of SHBG and gonadotrophin levels) and/or who completed the initial ADAM questionnaire represented subpopulations of the total and biases in the composition of each of these subgroups cannot be excluded. Finally, the number of patients assessed for the benefits of testosterone replacement was also small (n=9) and the formal ADAM questionnaire was not repeated, instead benefit was assessed more subjectively based on a retrospective review of the endocrinologist’s clinic notes. However, our results are strikingly consistent between both the initial and this new report and between institutions. In addition, the result of 55% of low testosterone cases deriving benefit from the replacement therapy is entirely consistent with the effectiveness of testosterone replacement in cancer patients reported in the literature. 25,26 Similarly, symptomatic benefit appeared entirely dependent on patients manifesting elevated testosterone levels from the replacement therapy.
Importantly, testosterone levels have not been formally assessed in any of the major studies of crizotinib to date. Overall, our data strongly suggest that crizotinib rapidly drops testosterone levels in men and that this effect is clinically significant in terms of both producing symptoms and in being amenable to appropriate therapy. The symptoms of androgen deficiency in such patients may be elicited in the clinic by using the ADAM questionnaire (Table 1). Oncologists typically avoid discussion of sexuality with patients, and without a clear awareness of these issues, symptoms of hypogonadism may be overlooked.12 Crizotinib is also currently being used to treat certain molecularly specific subtypes of cancer in pediatric populations, including in young male patients approaching puberty where maintenance of normal testosterone function may be essential. 27 We recommend tracking free or total/free testosterone levels in all male patients treated with crizotinib ideally measuring these levels in the early morning to adjust for diurnal variation 24 and referring those with low levels to an endocrinologist for discussion of the pros and cons of replacement therapy, but with no need to change crizotinib dosage.
Acknowledgments
Funding: In part with NCI grant P30CA046934
Footnotes
Financial Disclosures: Drs Weickhardt, Doebele, Popat, Ou, Camidge, have received honoraria for ad hoc consultancy/advisory meetings with Pfizer.
References
- 1.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010 Oct 28;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw AT, Camidge DR, Engelman JA, et al. Clinical activity of crizotinib in advanced non-small cell lung cancer (NSCLC) harboring ROS1 gene rearrangement. ASCO Meeting Abstracts; May 30, 2012; 2012. p. 7508. [Google Scholar]
- 3.Ou S-HI, Kwak EL, Siwak-Tapp C, et al. Activity of Crizotinib (PF02341066), a Dual Mesenchymal-Epithelial Transition (MET) and Anaplastic Lymphoma Kinase (ALK) Inhibitor, in a Non-small Cell Lung Cancer Patient with De Novo MET Amplification. Journal of Thoracic Oncology. 2011;6(5):942–946. doi: 10.1097/JTO.0b013e31821528d3. 910.1097/JTO.1090b1013e31821528d31821523. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Nagagawa K, Camidge DR, Solomon B. Phase III study of Crizotinib vs. Pemetrexed or Docetaxel Chemotherapy in Patients with Advanced ALK-Positive NSCLC (PROFILE 1007) ESMO. 2012;(2862) [Google Scholar]
- 5.Kim D-W, Ahn M-J, Shi Y, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts; May 30, 2012; 2012. p. 7533. [Google Scholar]
- 6.Camidge DR, Doebele RC. Treating ALK-positive lung cancer-early successes and future challenges. Nat Rev Clin Oncol. 2012 Apr 3; doi: 10.1038/nrclinonc.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012 Sep 3; doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weickhardt AJ, Camidge DR. The therapeutic potential of anaplastic lymphoma kinase inhibitors in lung cancer: rationale and clinical evidence. Clinical Investigation. 2011 Aug;1(8):1119–1126. [Google Scholar]
- 9.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009 Sep 10;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weickhardt AJ, Rothman MS, Salian-Mehta S, et al. Rapid-onset hypogonadism secondary to crizotinib use in men with metastatic nonsmall cell lung cancer. Cancer. 2012 Apr 4; doi: 10.1002/cncr.27450. [DOI] [PubMed] [Google Scholar]
- 11.Ramalingam SS, Shaw AT. Hypogonadism related to crizotinib therapy: Implications for patient care. Cancer. 2012 Apr 4; doi: 10.1002/cncr.27561. [DOI] [PubMed] [Google Scholar]
- 12.Lemieux L, Kaiser S, Pereira J, Meadows LM. Sexuality in palliative care: patient perspectives. Palliative Medicine. 2004 Oct 1;18(7):630–637. doi: 10.1191/0269216304pm941oa. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000 Sep;49(9):1239–1242. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- 14.Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clinical Endocrinology. 2007;67(6):853–862. doi: 10.1111/j.1365-2265.2007.02976.x. [DOI] [PubMed] [Google Scholar]
- 15.Howell SJ, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update. 2001 Jul-Aug;7(4):363–369. doi: 10.1093/humupd/7.4.363. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res. 1982 Jun;42(6):2495–2498. [PubMed] [Google Scholar]
- 17.Sperti C, Bonadimani B, Guolo P, et al. Androgen profile in patients with pancreatic carcinoma. Ital J Gastroenterol. 1992 Jul-Aug;24(6):328–331. [PubMed] [Google Scholar]
- 18.Burney B, Garcia J. Hypogonadism in male cancer patients. Journal of Cachexia, Sarcopenia and Muscle. 2012;3(3):149–155. doi: 10.1007/s13539-012-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petitpas I, Bhattacharya AA, Twine S, East M, Curry S. Crystal Structure Analysis of Warfarin Binding to Human Serum Albumin: ANATOMY OF DRUG SITE I. Journal of Biological Chemistry. 2001 Jun 22;276(25):22804–22809. doi: 10.1074/jbc.M100575200. [DOI] [PubMed] [Google Scholar]
- 20.Costa DB, Kobayashi S, Pandya SS, et al. CSF Concentration of the Anaplastic Lymphoma Kinase Inhibitor Crizotinib. Journal of Clinical Oncology. 2011 May 20;29(15):e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 21.Risau W, Wolburg H. Development of the blood-brain barrier. Trends in Neurosciences. 1990;13(5):174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- 22.Mehra R, Camidge DR, Sharma S, et al. First-in-human phase I study of the ALK inhibitor LDK378 in advanced solid tumors. ASCO Meeting Abstracts; May 30, 2012; 2012. p. 3007. [Google Scholar]
- 23.Gettinger S, Weiss GJ, Salgia R, Bazhenova L, Narasimhan NI, Dorer DJ, Rivera VM, Zhang J, Clackson T, Haluska F, Shaw AT, Camidge DR. A First-in-Human Dose-Finding Study of the ALK/EGFR Inhibitor AP26113 in Patients with Advanced Malignancies. ESMO 37th Annual Meeting; 2012. p. Abstract 4390. [Google Scholar]
- 24.Diver MJ Clinical Science Reviews Committee of the Association for Clinical Biochemistry. Analytical and physiological factors affecting the interpretation of serum testosterone concentration in men. Ann Clin Biochem. 2006 Jan;43(Pt 1):3–12. doi: 10.1258/000456306775141803. [DOI] [PubMed] [Google Scholar]
- 25.Chlebowski RT, Herrold J, Ali I, et al. Influence of nandrolone decanoate on weight loss in advanced non-small cell lung cancer. Cancer. 1986 Jul 1;58(1):183–186. doi: 10.1002/1097-0142(19860701)58:1<183::aid-cncr2820580131>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Howell SJ, Radford JA, Adams JE, Smets EM, Warburton R, Shalet SM. Randomized placebo-controlled trial of testosterone replacement in men with mild Leydig cell insufficiency following cytotoxic chemotherapy. Clin Endocrinol (Oxf) 2001 Sep;55(3):315–324. doi: 10.1046/j.1365-2265.2001.01297.x. [DOI] [PubMed] [Google Scholar]
- 27.Mosse YP, Balis FM, Lim MS, et al. Efficacy of crizotinib in children with relapsed/refractory ALK-driven tumors including anaplastic large cell lymphoma and neuroblastoma: A Children’s Oncology Group phase I consortium study. ASCO Meeting Abstracts; May 30, 2012; 2012. p. 9500. [Google Scholar]