Abstract
Background/ Objectives
The relationship between late-life blood pressure (BP) and cognitive function in the elderly is poorly understood. Inconsistent results have been reported from existing studies. We report the results from a prospective cohort study on the association between BP and cognitive function in elderly African Americans.
Design
Prospective cohort study conducted from 1997 to 2009.
Setting
Community-based study in Indianapolis.
Participants
3145 African Americans aged 65 years or older.
Measurements
At each assessment, participants’ cognitive function was measured by the Community Screening Interview for Dementia score. Other measurements included BP, height, weight, education level, antihypertensive medication use, alcohol use, smoking and histories of chronic medical conditions.
Results
5995 longitudinal assessments contributed by 2721 participants with complete independent variables were analyzed using a semiparametric mixed effects model. Systolic BP around 135 mmHg and diastolic BP around 80 mmHg were associated with optimal cognitive function after adjusting for other variables (P = 0.019). Weight loss with body mass index less than 30 kg/m2 was significantly related to poorer cognitive performance (P < 0.001). Older age at first assessment, lower education level, smoking, histories of depression, stroke and diabetes mellitus were related to worse cognitive function, while taking antihypertensive medication and drinking alcohol were associated with higher cognitive scores.
Conclusion
Both high and low BP levels were associated with poorer cognitive performance. A joint optimal region of systolic and diastolic BP for cognitive function has been identified, which may provide useful clinical information on optimal BP control in cognitive health and lead to improved quality of life for the elderly.
Keywords: blood pressure, cognitive function, elderly
INTRODUCTION
Most previous reports agree that maintenance of high cognitive function is a prerequisite for successful aging.1 There is extensive literature suggesting that hypertension in midlife is a risk factor for cognitive impairment and dementia in the elderly, and that treatment with antihypertensive medication reduces this risk.2–6 However, there is uncertainty about the relationship between late-life blood pressure (BP) and cognitive performance in the elderly.7, 8 Some studies report a positive association between elevated BP and cognitive impairment, but very low BP was also linked with poorer cognitive function.9–12 More recent reports found a U-shaped relationship between systolic BP and cognitive function, suggesting that both high and low systolic BP (e.g. ≥ 160 or < 130 mmHg) were related to poorer cognitive performance but this relationship did not hold for diastolic BP.13, 14 Because of these inconsistencies, the clinical determination of what might be the optimal BP to maintain in the elderly, in order to protect against the risk of cardiovascular and cerebrovascular diseases and yet maintain good cognitive function, is uncertain.7
Previous studies have examined the effects of systolic and diastolic BP on cognitive function in separate models, preventing the exploration of the joint relations of these two types of BP to cognitive function.15, 16 African Americans are known to have higher prevalence of hypertension than other ethnic groups.17, 18 To date the knowledge on the relationship between BP and cognitive function in African Americans has been limited. In this paper, we examine the joint relations of systolic and diastolic BP to cognitive performance in a cohort of elderly community-dwelling African Americans that were followed for up to 12 years.
METHODS
Study participants
Participants were from the Indianapolis cohort of the Indianapolis-Ibadan Dementia Project, a longitudinal comparative epidemiologic study examining risk factors for dementia and Alzheimer’s disease (AD). Recruitment to the study was conducted at two time points. In 1992, a cohort of African Americans aged 65 or older living in Indianapolis was enrolled in the study. Details on the assembling of this original cohort were described elsewhere.19 In 2001, the project enrolled additional community-dwelling subjects randomly selected from Medicare records, self-identified as African Americans, and who were at least 70 years of age.20 The original cohort was slightly older. The gender distribution was about the same for both cohorts, and included 65% women. The enrichment cohort had a higher level of education. Most importantly, prevalence rates for dementia or AD were similar between the two cohorts.21 All participants agreed to undergo follow-up cognitive assessments and clinical evaluations every two or three years. The study was approved by the Indiana University-Purdue University of Indianapolis Institutional Review Board. All subjects enrolled provided informed consent.
Cognitive assessment
Study participants underwent regularly scheduled cognitive assessments and clinical evaluations approximately every two or three years in 1992, 1995, 1997, 2001, 2004, 2007 and 2009. Cognitive function of study participants was measured by the Community Screening Interview for Dementia (CSID), which has been widely used as a screening tool for dementia that evaluates multiple cognitive domains including language, attention, memory, orientation, praxis, comprehension and motor response.22 For this analysis, we used a CSID score that incorporated all cognitive items from the screening exam, some of which had not been utilized previously.22 The CSID total score ranged from 0 to 80 with higher score indicating better cognitive function.
Blood pressure measure
BP was measured for all consenting participants starting from the second follow-up evaluation in 1997, i.e. year six after baseline for those enrolled in the original cohort, and at all evaluations for the enrichment cohort. Prior to measuring BP, participants were asked whether they had taken any medication for the treatment of hypertension within the previous 24 hours. Three measurements of systolic and diastolic BP were taken from the left arm by trained interviewers using the Omron digital units (Omron Health-Care Inc., Bannockburn, IL.) at approximately 15 minute intervals while the participant was seated. The average of the three BP measurements was used in all analyses.
Other assessments
The CSID screening process also included self and informant reports of medical histories of cancer, diabetes mellitus, heart attack, stroke, cardiovascular heart disease, depression and Parkinson’s disease. Participants’ weight and height were measured during each evaluation and were used to calculate their body mass index (BMI). Age, education level, smoking and alcohol use were also collected at each assessment.
Statistical analyses
The multiple cognitive scores collected throughout the study were examined as the outcome variable. Time-varying independent variables included systolic and diastolic BP, BMI, alcohol use, smoking, use of antihypertensive medication and history of medical conditions. Age at first assessment, sex and years of education were considered as time-fixed variables. All variables were included in a multiple regression model to examine the BP effects while adjusting for potential confounders. Instead of using the traditional linear models, we adopted a semiparametric mixed effects model which provides the flexibility to examine the nonlinear effects of BP as well as other variables on cognitive function.23 A subject-specific random intercept was used to account for the correlation between repeated measures from the same individual. For the fixed effects, nonparametric regression functions were adopted to accommodate nonlinear effects of the continuous variables, and regular parametric terms were used for the categorical variables.24 In particular, a bivariate thin plate regression spline was used to model the joint nonlinear influences of systolic and diastolic BP on concurrently measured cognitive function while accounting for the interaction between the two correlated variables.
In the semiparametric mixed effects model, we first included all medical conditions as independent variables in the preliminary analyses. Those that were statistically significant (with p-value less than 0·05) or marginally significant (p-value less than 0·10) were included in the final model. The analyses were conducted by the mgcv package in R 2.14.1.25
RESULTS
The original Indianapolis cohort had 2212 participants in the 1992 assessment and the enrichment cohort enrolled 1893 participants in 2001. BP measures were collected starting from the 1997 assessment for participants in the original cohort enrolled in 1992 and at all assessments for those in the enrichment cohort. Those without BP measures were excluded from the analysis. There were 3145 participants with at least one cognitive assessment, of which 1262 (40·1%) were from the original cohort and 1883 (59·9%) were from the enrichment cohort. There were 424 subjects with cognitive evaluations but were excluded from this analysis due to incomplete BP measures (n=264) or other variables (n=161). After excluding the records with missing values, 5995 longitudinal observations from 2721 subjects were used in this analysis. Among the 2721 subjects, 1814 (66·7%) were female. The median cognitive score was 68 with interquartile range = (62, 72) at participants’ first assessment. It is noteworthy that although the CSID total score ranges from 0 to 80, over 80% of the participants scored within a much narrower range of 61 and above. Table 1 compares the participant characteristics at the first evaluation when BP was measured with those not included in the analysis due to missing data. Participants with missing BP or other variables were significantly older, had less education, lower BMI, lower cognitive scores, and had more depression, stroke, diabetes, and Parkinson’s disease than those included in the analysis. However, the two groups did not differ on BP levels, or percentages of alcohol drinkers, smokers or those using antihypertensive medications.
Table 1.
Participants’ Characteristics Based upon Inclusion in Analysis at the First Evaluation When Blood Pressure Was Collected*
| Characteristic | Included in Analysis (n=2721) | Not Included due to Missing Values (n=424) | P-value |
|---|---|---|---|
| Cognitive score, median (IQR) | 68 (62, 72) | 62 (53, 68) | < 0·001 |
| Age, median (IQR) | 76·0 (72·6, 80·4) | 78·9 (74·6, 85·3) | < 0·001 |
| Female, N (%) | 1814 (66·7) | 281 (66·7) | 0·999 |
| Education, years, median (IQR) | 12 (9, 13) n=2704 |
10 (8, 12) n=397 |
< 0·001 |
| BMI, kg/m2, median (IQR) | 28·4 (24·9, 32·5) n=2662 |
27·3 (23·8, 32·2) n=240 |
0·020 |
| Systolic blood pressure, mmHg, median (IQR) | 145·7 (132·0, 160·0) n=2490 |
144·0 (130·0, 159·5) n=148 |
0·609 |
| Diastolic blood pressure, mmHg, median (IQR) | 80·0 (72·0, 88·0) n=2490 |
80·0 (70·0, 90·0) n=148 |
0·851 |
| Drink alcohol, N (%) | 1114 (42·5) n=2620 |
172 (45·9) n=375 |
0·221 |
| Smoker, N (%) | 1612 (59·7) n=2698 |
237 (56·6) n=419 |
0·219 |
| Antihypertensive medication, N (%) | 1470 (57·9) n=2538 |
129 (54·9) n=235 |
0·371 |
| Depression, N (%) | 321 (11·9) n=2693 |
68 (16·4) n=414 |
0·013 |
| Heart attack, N (%) | 401 (14·9) n=2688 |
69 (16·5) n=419 |
0·420 |
| Stroke, N (%) | 387 (14·3) n=2698 |
101 (24·2) n=417 |
< 0·001 |
| Cardiovascular heart disease, N (%) | 856 (31·7) n=2703 |
138 (32·8) n=421 |
0·653 |
| Diabetes mellitus, N (%) | 790 (29·3) n=2698 |
144 (34·4) n=419 |
0·039 |
| Cancer, N (%) | 403 (15·0) n=2689 |
39 (9·3) n=418 |
0·002 |
| Parkinson’s disease, N (%) | 18 (0·6) n=2700 |
8 (1·9) n=421 |
0·017 |
The data shown was collected at the 2nd follow-up (six years) after baseline for the original cohort and the baseline visit for the enrichment cohort. A few participants did not complete the full assessments at their first evaluations.
IQR = interquartile range
Table 2 presents results of the semiparametric mixed effects model on the repeated measures of cognitive scores. For the categorical variables, we report parameter estimates, standard error estimates and p-values. The parameter estimate of each variable indicates the association between the presence of the corresponding factor and the unit difference in CSID score adjusting for other variables in the model. Male participants scored on average 0·72 points higher than female subjects (P = 0·010). Participants who drank alcohol had significantly better cognitive function (P = 0·015) than abstainers. Participants with histories of depression, stroke or diabetes mellitus had lower cognitive scores compared to those without these conditions. Smoking was related to worse cognitive function (P = 0·039), while the use of antihypertensive medication was associated with better cognitive function (P < 0·001).
Table 2.
Semiparametric Mixed Effects Model on Cognitive Score
| Variable | Parameter Estimate (Standard Error) | P-value |
|---|---|---|
| Categorical | ||
| Male | 0·72 (0·28) | 0·010 |
| Antihypertensive Medication | 0·97 (0·20) | < 0·001 |
| History of Alcohol Drinking | 0·56 (0·23) | 0·015 |
| Depression | −1·37 (0·30) | < 0·001 |
| Stroke | −1·60 (0·29) | < 0·001 |
| Diabetes Mellitus | −0·61 (0·25) | 0·015 |
| Smoking | −0·46 (0·22) | 0·039 |
| Continuous | F Statistic | P-value |
| SBP, DBP | 2·4 | 0·019 |
| BMI | 19·6 | < 0·001 |
| Education | 216·6 | < 0·001 |
| Age at First Assessment | 212·3 | < 0·001 |
The effects of the continuous variables are summarized in the second half of Table 2. We included the F-values and p-values for testing each nonlinear smooth term with the null hypothesis that change in these variables has no effect on cognitive function. The nonlinear effects of BMI, years of education, age at first assessment, and the bivariate joint effects of systolic and diastolic BP on cognitive score were all statistically significant. The estimated associations of BMI, years of education and age at first assessment to cognitive score using nonparametric splines are presented in Figure 1. In Figure 1a, we can see that cognitive score rose with an increase in BMI until BMI reached approximately 30 kg/m2 and then stayed relatively flat after BMI increased beyond 30 kg/m2. Figure 1b shows that cognitive score was positively associated with education level, while Figure 1c shows that CSID scores were negatively associated with age.
Figure 1. Nonlinear effects of continuous variables.
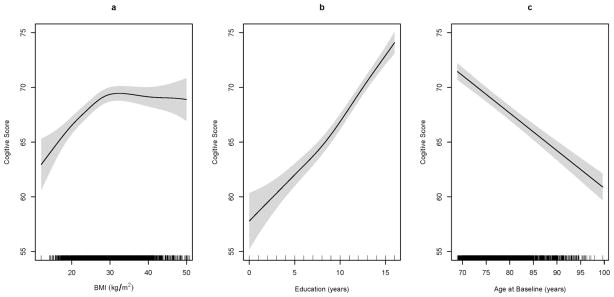
Cognitive score as a function of (a) Body mass index (BMI), (b) years of education and (c) age at baseline. Predicted cognitive scores are based on the semiparametric mixed effects regression model while adjusting for sex, blood pressure, antihypertensive medications, alcohol use, smoking status and history of medical conditions. The gray bands denote 95% pointwise confidence intervals.
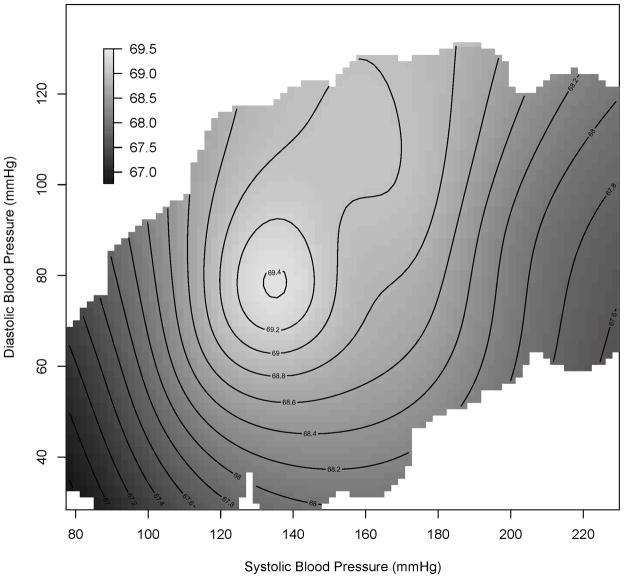
The joint nonlinear effects of systolic and diastolic BP on cognitive function while controlling for other variables are presented in Figure 2. Figure 2 is a bivariate contour plot with systolic and diastolic BP as the horizontal and vertical axes, respectively. Each contour line defines the ranges of systolic and diastolic values that correspond to CSID scores greater than or equal to the value marked on the contour line. In particular, the light grey circle in the middle of the contour plot depicts the systolic and diastolic BP values associated with CSID scores greater than or equal to 69·4. The color-coding represents relatively higher (light grey) or lower (dark) cognitive scores. The unimodal shape of the contour plot indicates that the association between the systolic and diastolic BP and cognitive score was nonlinear and that optimal cognitive function, represented by the peak area in light grey, occurred with a diastolic BP around 80 mmHg and a systolic BP around 135 mmHg. The dark areas shown on both ends of the systolic BP axis also demonstrate that both very low and very high systolic BP measures were associated with lower cognitive scores. In the regions with systolic BP less than 135 mmHg, it was positively associated with CSID scores, i.e. the higher systolic BP, the higher CSID scores. When systolic BP was greater than 135 mmHg, a negative association between BP and CSID score was observed, i.e. higher systolic BP was related to lower cognitive scores. Similar results hold for the relationship between diastolic BP and CSID scores.
Figure 2. Bivariate blood pressure effects.
Cognitive score as a bivariate function of systolic and diastolic blood pressure. Predicted cognitive scores are based on the semiparametric mixed effects regression model while adjusting for sex, age, body mass index, education level, antihypertensive medications, alcohol use, smoking status and history of medical conditions. Color-coding depicts relatively higher (light grey) and lower (dark) cognitive scores.
Based on the results of the bivariate contour plot, systolic BP seems to play a relatively more pronounced role in the BP-cognition relationship than diastolic BP. Although the joint optimal region of BP consists of both systolic and diastolic levels, when systolic BP was between 130 and 140 mmHg, cognitive scores remained relatively high even if diastolic BP was higher than the 76–82 mmHg optimal range. In contrast, even if diastolic BP was within the optimal range, systolic BP outside the 130–140 mmHg range was still associated with significantly lower cognitive scores.
DISCUSSION
In this study, we examined the joint nonlinear relationship of systolic and diastolic BP to cognitive function in a community-based elderly African American cohort. Our results suggest that optimal cognitive function is associated with BP around 135 and 80 mmHg for systolic and diastolic measures, respectively. Both elevated and low BP levels outside the optimal range were linked with poorer cognitive performance. These findings were adjusted for variables including age at first assessment, sex, BMI, years of education, alcohol use, smoking, use of antihypertensive medications and histories of depression, stroke, and diabetes mellitus. This study has a number of strengths. First, the use of flexible semiparametric models allowed us to explore the joint nonlinear effects of systolic and diastolic BP while taking into account the interaction between the two BP measures, rather than using separate models for systolic and diastolic BP, which is the standard in existing studies. Second, the relatively large study cohort with measurements of systolic and diastolic BP, cognitive function and other variables collected at each follow-up evaluation provided sufficient statistical power to examine factors associated with cognitive function.
Although the relationship between BP and cognitive function has been investigated in both cross-sectional and longitudinal population-based observational studies, the association between late-life BP and cognition in the elderly is poorly understood.5, 7 The notion that high BP is related to cognitive impairment is supported by some studies,9–12, 26 while other studies report no association between BP and cognitive function.27, 28 The inconsistent results may be partly due to the confounding effect of antihypertensive medication which is taken by a substantial proportion of elderly people.7 Also, the linearity assumption on the relationship between BP and cognitive function, which was adopted by most previous studies reporting null effects, may not be valid and could result in statistical non-significance if the true underlying association is indeed nonlinear. A U-shaped relationship between BP and cognition was reported by a few studies that explored the univariate effects of systolic and diastolic BP separately and did not take into consideration the correlation between the two measures.15, 16, 29 To the best of our knowledge, however, no investigation has explored a joint optimal region of systolic and diastolic BP on cognitive performance in elderly adults. Many previous studies were longitudinal in design and focused on examining the association between BP measured only at study baseline with future cognitive function during a follow-up evaluation. Our analyses reported here explored concurrent associations between BP measures and cognitive function collected during multiple assessments. Therefore, maximum statistical power was achieved by pooling the information collected from all follow-up evaluations in our study. It should be noted that the concurrent association between BP and cognitive function that we detected in this study does not provide information as to the direction of causality. This would require further clinical investigation.
Hypertension, especially untreated hypertension, was found to be related to poor cognitive function.30 A protective effect of antihypertensive medication against cognitive decline has been reported in observational studies.31 But evidence from large randomized trials on the association between antihypertensive therapy and incident dementia is less consistent.32 In this observational study, we have shown that taking antihypertensive medications was associated with better cognitive performance in the study cohort. The optimal region of BP we identified for cognitive function was ascertained after adjusting for antihypertensive medication use in the analysis. Using the method based upon the chi-square test in Liu and Tu (2012), no significant interaction between the BP-cognition association and use of antihypertensive treatment was found (P = 0.385).33
It is interesting to note that our finding of the optimal BP region near 135/80 mmHg falls outside the normal BP cut-off defined by the “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure” (JNC 7 report).17 In fact, the JNC 7 report considers individuals with a systolic BP of 120–139 mmHg or a diastolic BP of 80–89 mmHg as prehypertensive and subject to BP treatment with the intention of cardiovascular disease prevention. It is noteworthy that the JNC 7 report was based on studies in subjects aged 40 to 70. Current expert consensus on the management of BP in individuals 80 years or older recommends a BP goal of < 140/90 mmHg but it is not clear whether bringing BP lower than 140/90 is effective in the elderly population.34 Our results seem to suggest the target BP of 135/80 mmHg is sufficient in optimally maintaining cognitive function and BP lower than these optimal levels would not necessarily provide any cognitive benefit for older adults (e.g. 70 years old and above). However, it remains to be determined whether a BP target different than the 135/80 identified in this study would be beneficial for reducing cardiovascular risk. This outcome was not included in our analysis. Extremely low BP has been shown to have negative effect on cognitive function especially in very old adults, giving credence to the notion that an appropriate BP may be necessary to maintain adequate cerebral perfusion to preserve cognitive ability.11, 35 In our study, the poorest cognitive performance in this cohort was associated with extremely low levels of both systolic and diastolic BP, even after controlling for multiple cardiovascular diseases.
The model results presented in the contour plot demonstrate the trend of association between BP and cognitive function. Although the contour plot only covered CSID scores from 66·7 to 69·5 while adjusting for potential confounders, the predicted cognitive scores vary from 47·1 to 77·3, depending on the values of both BP and other variables. It is worth noting that in our cohort, the median baseline CSID for normal participants was 68 compared to that of 62 for demented subjects. Thus, the 2·8 units difference in CSID scores between the ideal BP and those associated with worst cognitive performance (lower left corner in Figure 2) accounted for approximately 50% of the differences between the median cognitive scores of the normal and demented participants.
Previous literature has reported conflicting findings on the relationship between obesity and cognitive impairment in the elderly, possibly due to the confounding effect of hypertension, commonly seen in overweight and obese adults.36, 37 Our study suggested that weight loss in late life was associated with poor cognitive performance, after adjusting for BP levels and other potential confounding factors. Specifically, BMI less than 30 kg/m2 was found to be related to reduced cognitive functioning, while excessive weight was not associated with significant cognitive change in this African-American cohort. Histories of diabetes mellitus, depression and stroke were related to poorer cognitive performance in the study, confirming the findings from existing investigations.38–40
There are several limitations of our study. First, this cohort study only involves African Americans. Whether the BP-cognition relationship we found could be extended to other ethnic groups is unclear. One study observed a significant U-shaped association between BP and cognitive function only in white participants but not in African Americans.41 It is likely that this nonlinear relationship would also exist in Caucasians, but this would require further investigation. Second, the outcome variable of this study is based upon cognitive scores. Although the CSID total score performs well in detecting dementia in this cohort as evidenced by the area under the receiving operating characteristic curve (AUC) equal to 0.918, whether or not a similar association between BP and a clinical diagnosis of either cognitive impairment or dementia exists remains to be confirmed. Also, these results are based upon using the CSID; thus results could vary if a different cognitive battery with different cognitive domains was adopted.
In conclusion, our study has identified a joint optimal region of systolic and diastolic BP for cognitive function in an elderly African-American cohort. BP levels further away from the optimal region represent increased risk for poor cognitive function. As the maintenance of good cognitive function plays such a major role in quality of life in the elderly, it is hoped that these results will stimulate similar studies in other populations to confirm these findings.
Acknowledgments
The research is supported by NIH grants R01 AG09956, P30 AG10133, R01 AG019181 and R24 MH080827.
Sponsor’s Role: The data used in this manuscript is from a NIH funded study entitled “Indianapolis-Ibadan Dementia Project”. The sponsor played no role in the design, method, analysis and preparation of this paper.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Concept and design: Liu, Gao, and Hendrie. Acquisition of data: Hall, Unverzagt, and Hendrie. Analysis and interpretation: Liu, Gao, and Hendrie. Statistical expertise: Liu, Gao, and Lane. Preparation of paper: Liu, Gao, Hall, Unverzagt, Lane, Callahan, and Hendrie.
References
- 1.Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee. Alzheimers Dement. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Elias MF, Wolf PA, Dagostino RB, et al. Untreated Blood-Pressure Level Is Inversely Related to Cognitive-Functioning - the Framingham-Study. Am J Epidemiol. 1993;138(6):353–64. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 3.Launer LJ, Masaki K, Petrovitch H, et al. The Association between Midlife Blood-Pressure Levels and Late-Life Cognitive Function - the Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 4.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: The Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 5.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: Common links. J Intern Med. 2006;260:211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 7.Qiu CX, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 8.Feldstein CA. Effects of blood pressure changes on Alzheimer’s disease. Neuroepidemiology. 2010;35:202–212. doi: 10.1159/000316872. [DOI] [PubMed] [Google Scholar]
- 9.Kilander L, Nyman H, Boberg M, et al. Hypertension is related to cognitive impairment -A 20-year follow-up of 999 men. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- 10.Budge MM, de Jager C, Hogervorst E, et al. Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. J Am Geriatr Soc. 2002;50:2014–2018. doi: 10.1046/j.1532-5415.2002.50614.x. [DOI] [PubMed] [Google Scholar]
- 11.Guo ZC, Fratiglioni L, Winblad B, et al. Blood pressure and performance on the Mini-Mental State Examination in the very old - Cross-sectional and longitudinal data from the Kungsholmen Project. Am J Epidemiol. 1997;145:1106–1113. doi: 10.1093/oxfordjournals.aje.a009073. [DOI] [PubMed] [Google Scholar]
- 12.Pandav R, Dodge HH, DeKosky ST, et al. Blood pressure and cognitive impairment in India and the United States - A cross-national epidemiological study. Arch Neurol-Chicago. 2003;60:1123–1128. doi: 10.1001/archneur.60.8.1123. [DOI] [PubMed] [Google Scholar]
- 13.Glynn RJ, Beckett LA, Hebert LE, et al. Current and remote blood pressure and cognitive decline. JAMA. 1999;281:438–445. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- 14.Molander L, Gustafson Y, Lovheim H. Low blood pressure is associated with cognitive impairment in very old people. Dement Geriatr Cogn. 2010;29:335–341. doi: 10.1159/000289821. [DOI] [PubMed] [Google Scholar]
- 15.Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology. 2002;21:123–130. doi: 10.1159/000054809. [DOI] [PubMed] [Google Scholar]
- 16.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function - The Baltimore Longitudinal Study of Aging. Hypertension. 2005;45(3):374–9. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure - The JNC 7 Report. Jama-J Am Med Assoc. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 18.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of Body Mass Index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 19.Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 20.Szwast SJ, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology. 2007;69:1873–1880. doi: 10.1212/01.wnl.0000279333.77404.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall KS, Gao S, Baiyewu O, et al. Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimers Dement. 2009;5:227–233. doi: 10.1016/j.jalz.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall KS, Ogunniyi AO, Hendrie HC, et al. A cross-cultural community based study of dementias: Methods and performance of the survey instrument Indianapolis, USA, and Ibadan, Nigeria. Int J Method Psych. 1996;6:129–142. [Google Scholar]
- 23.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. Cambridge ; New York: Cambridge University Press; 2003. [Google Scholar]
- 24.Wood SN. Generalized Additive Models: An Introduction with R. London: Chapman and Hall; 2006. [Google Scholar]
- 25.R Development Core Team. R. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 26.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77:1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farmer ME, White LR, Abbott RD, et al. Blood-Pressure and Cognitive performance – the Framingham-Study. Am J Epidemiol. 1987;126:1103–1114. doi: 10.1093/oxfordjournals.aje.a114749. [DOI] [PubMed] [Google Scholar]
- 28.Di Carlo A, Baldereschi M, Amaducci L, et al. Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian longitudinal study on aging. J Am Geriatr Soc. 2000;48:775–782. doi: 10.1111/j.1532-5415.2000.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 29.Gao SJ, Jin YL, Unverzagt FW, et al. Hypertension and cognitive decline in rural elderly Chinese. J Am Geriatr Soc. 2009;57:1051–1057. doi: 10.1111/j.1532-5415.2009.02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrington F, Saxby BK, McKeith IG, et al. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–1082. doi: 10.1161/01.hyp.36.6.1079. [DOI] [PubMed] [Google Scholar]
- 31.Murray MD, Lane KA, Gao S, et al. Preservation of cognitive function with antihypertensive medications. Arch Intern Med. 2002;162:2090–2096. doi: 10.1001/archinte.162.18.2090. [DOI] [PubMed] [Google Scholar]
- 32.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Tu W. A Semiparametric Regression Model for Paired Longitudinal Outcomes with Application in Childhood Blood Pressure Development. Ann Appl Stat. 2012;6:1861–1882. [Google Scholar]
- 34.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 Expert Consensus Document on Hypertension in the Elderly A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents Developed in Collaboration With the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens. 2011;5:259–352. doi: 10.1016/j.jash.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Verghese J, Lipton RB, Hall CB, et al. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61:667–1672. doi: 10.1212/01.wnl.0000098934.18300.be. [DOI] [PubMed] [Google Scholar]
- 36.Elias MF, Elias PK, Sullivan LM, et al. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obesity. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 37.Sakakura K, Hoshide S, Ishikawa J, et al. Association of body mass index with cognitive function in elderly hypertensive Japanese. Am J Hypertens. 2008;21:627–632. doi: 10.1038/ajh.2008.157. [DOI] [PubMed] [Google Scholar]
- 38.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol-Chicago. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 39.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type - A prospective cohort study. Arch Neurol-Chicago. 2004;61:290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 40.Zhu L, Fratiglioni L, Guo Z, et al. Incidence of stroke in relation to cognitive function and dementia in the Kungsholmen project. Neurology. 2000;54:2103–2107. doi: 10.1212/wnl.54.11.2103. [DOI] [PubMed] [Google Scholar]
- 41.Bohannon AD, Fillenbaum GG, Pieper CF, et al. Relationship of race/ethnicity and blood pressure to change in cognitive function. J Am Geriatr Soc. 2002;50:424–429. doi: 10.1046/j.1532-5415.2002.50104.x. [DOI] [PubMed] [Google Scholar]