Abstract
Complex communities of bacteria, fungi, and viruses thrive on our skin. The composition of these communities depends on skin characteristics, such as sebaceous gland concentration, moisture content, and temperature, as well as on host genetics and exogenous environmental factors. Recent metagenomic studies have uncovered a surprising diversity within these ecosystems and have fostered a new view of commensal organisms as playing a much larger role in immune modulation and epithelial health than previously expected. Understanding microbe-host interactions and discovering the factors that drive microbial colonization will help us understand the pathogenesis of skin diseases and develop new promicrobial and antimicrobial therapeutics.
Keywords: skin microbiome, metagenomics, Human Microbiome Project, atopic dermatitis, antibiotics, innate immunity, immunology, skin cancer
Introduction
Beginning with van Leeuwenhoek’s invention of the microscope in the 17th century, studies have linked microbes to human disease by uncovering direct, one-to-one relationships between pathogens and skin pathologies. Seminal discoveries include human papillomavirus (HPV) as a cause of squamous cell cancer and Treponema pallidum as the cause of syphilis. More recently, metagenomic advances have allowed us to examine not just one pathogen at a time but thousands of different microbes simultaneously. With these techniques, scientists have uncovered surprisingly diverse and complex microbial communities thriving on the epithelial surfaces of every individual. These communities influence human physiology, immunity, and disease in ways that we are now just beginning to appreciate.
An estimated 1 million bacteria, with hundreds of distinct species, inhabit each square centimeter of skin1. Many studies have suggested that microbes may contribute even to noninfectious pathologies, such as atopic dermatitis, psoriasis, rosacea, and acne though recent molecular studies are beginning to explain the complex relationship between host and microorganism2–6. These studies have established a new paradigm for how microbes cause disease, where not just pathogens but also imbalances in the commensal ecosystem cause skin pathology. Whether this imbalance is primary or secondarily caused by changes in host skin and immunity and how this imbalance potentiates epithelial dysfunction, immune dysregulation, or overgrowth of pathogenic microbes are new questions on the research frontier that will impact how we understand and treat skin diseases.
Recent reviews have comprehensively summarized the work to date on the skin microbiome7–10. This review will briefly describe representative studies of the skin microbiome but will focus primarily on the current gaps in research, relevant clinical questions, and potential methods for addressing these questions.
What is metagenomics?
Historically, characterizing cutaneous microbes involved culturing skin swabs or biopsies. However, less than 1% of bacterial species can be cultivated with standard lab conditions, and many that do grow are competed out by faster-growing organisms11. Consequently, easily cultivated bacteria or fungi, such as Staphylococcus or Malassezia species, were overrepresented in early microbial surveys. Recent advances in DNA amplification and sequencing technology can now bypass the culture steps and allow for more complete, unbiased views of skin microbiota and their genetic content, collectively called the “microbiome” (for glossary, see Table 1).
Table 1.
Glossary
| Term | Definition |
|---|---|
| metagenomics | culture-free, genomic analysis of microbes by direct extraction and cloning of DNA from a particular ecosystem, such as the skin1–3 |
| microbiome | original definition: the "ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space"4 |
| common usage: aggregate gene content within a microbial ecosystem5 | |
| 16S rRNA gene | ribosomal gene conserved across bacteria with conserved regions used for PCR amplification and variable regions used for taxonomic classification6 |
| MINE | maximal information-based nonparametric exploration |
| a group of statistical methods to find and characterize associations in large datasets with many variables7 | |
| metatranscriptomics | culture-free analysis of total RNA (both mRNA and rRNA) isolated from a microbial ecosystem8 |
References Handelsman J. Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev 2004;68:669-85.
Riesenfeld CS, Schloss PD , Handelsman J. Metagenomics: genomic analysis of microbial communities. Annu Rev Genet 2004;38:525-52.
Handelsman J, Rondon MR, Brady SF, Clardy J , Goodman RM. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem Biol 1998;5:R245-9.
Lederberg J MA. 'Ome Sweet 'Omics - a genealogical treasury of words. Scientist 2001;15.
A framework for human microbiome research. Nature 2012;486:215-21.
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R , Gordon JI. The human microbiome project. Nature 2007;449:804-10.
Reshef DN, Reshef YA, Finucane HK, Grossman SR, McVean G, Turnbaugh PJ et al. Detecting novel associations in large data sets. Science 2011;334:1518-24.
Urich T, Lanzen A, Qi J, Huson DH, Schleper C , Schuster SC. Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS One 2008;3:e2527.
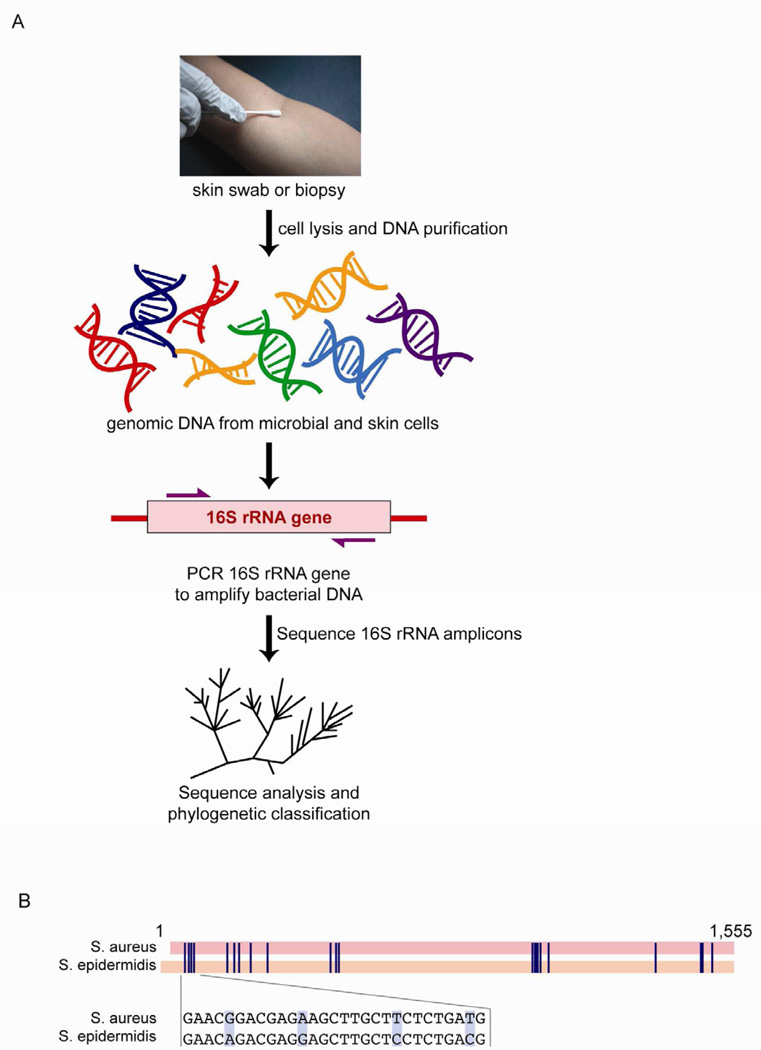
The culture-free, sequence-based method of analyzing any collection of microorganisms, such as skin microbiota, can be referred to as "metagenomics"12. In analyzing bacterial microbiomes, this method most often involves amplifying the 16S ribosomal RNA (16S rRNA) gene by PCR directly from skin samples (Fig. 1A)13, 14. The 16S rRNA gene exists in all bacteria and archaea but not in eukaryotes. It contains both conserved regions that serve as binding sites for PCR primers and variable regions for taxonomic classification after high-throughput sequencing of the PCR products (Fig. 1B)15, 16. Sequences that are more than 97% identical can often be classified within one species. Within one species, sequence variations are assumed to be due to intra-species strain variations. Also, the number of sequences counted within one species represents the relative abundance of that species in the original skin sample. Thus, this metagenomic approach gives a comprehensive picture of the bacterial community by providing both identification and relative abundances of all present species (Fig. 2).
Figure 1. Metagenomics is a culture-free method to assess skin microbiota.
(A) DNA is purified directly from a skin swab or biopsy. This DNA contains a mixture of genomic DNA from skin and microbial cells. PCR is used to amplify all bacterial DNA with primers that anneal to the conserved region of the 16S rRNA gene. Then, these PCR amplicons are sequenced. Finally, sequences can phylogenetically classified to give the species identities within the microbiome and sequences can be counted to give relative abundances of each species. (B) An alignment of the 16S rRNA gene between S. aureus and S. epidermidis downloaded from NCBI and aligned via Geneious (http://www.geneious.com/). Blue lines show nucleotides that differ between the two species. Inset shows an example of the specific sequence differences.
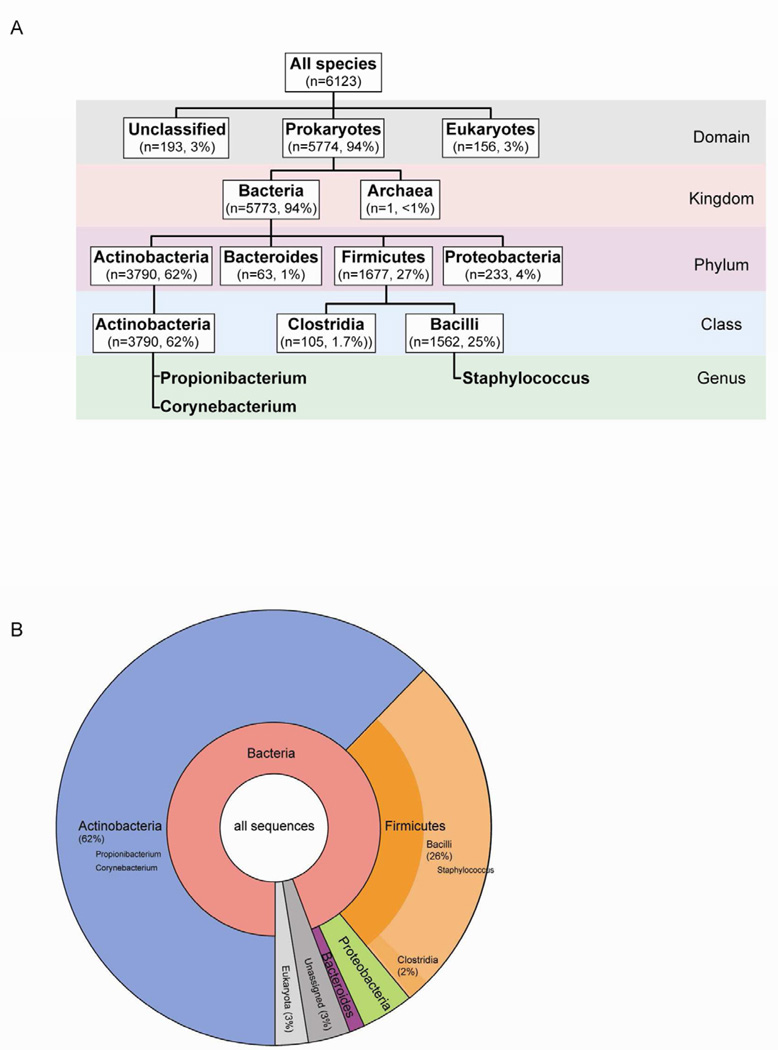
Figure 2. Composition of a single metagenome.
(A) Phylogenetic tree of an example metagenome downloaded from MG-RAST (data from Fierer et al26). The number of sequences in the metagenome that correspond to each phylogenetic category is listed. For example, 3790 sequences making up 62% of the metagenome's sequences were found to be Actinobacteria by similarity to references sequences. (B) Pie chart showing microbial composition within the same example metagenome. Chart was generated using Krona on the MG-RAST website (http://metagenomics.anl.gov/).
The normal microbiome on human skin
In 2007, the National Institutes of Health (NIH) launched the Human Microbiome Project to survey microbial content across 242 healthy adults, develop a reference catalog of microbial genome sequences, and understand how specific habitats in the gut, genitourinary system, and skin contribute to health and disease states14, 17–19. Recently, results from the Human Microbiome Project were published that describe their metagenomic methods and the publicly available databases of whole genome and 16S rRNA gene sequences18. This work and other studies in the past decade have characterized the skin microbiome of healthy volunteers and its variation across different spatial niches, individuals, and time (Table 2).
Table 2.
Summary of microbiome studies
| Author | Disease studied |
Skin site | Number patients | ||
|---|---|---|---|---|---|
| Method | Results | ||||
| Frank et al 20031 | normal | outer ear canal | 24 | RFLP; Sanger sequencing | 45 spp; microbial community complexity greater in males and older individuals; species composition correlated with consanguinity but not with household association |
| Dekio et al 20052 | normal | forehead | 5 | 16S rRNA gene sequencing | 32 OTUs; 62% sequences P. acnes; 9 species previously not known to live on skin |
| Gao et al 20073 | normal | volar forearm | 6 (4 resampled 8–10 mos later) | 16S rRNA gene sequencing | 182 OTUs; 19% sequences Proteobacteria, 51% Actinobacteria, 24% Firmicutes; 63% of sequences common to all subjects; 54% common to both time points; 50–77% identity between R and L arms |
| Grice et al 20084 | normal | antecubital fossa | 6 (4 resampled 8–10 mos later) | skin swab, scrape, or punch biopsy; 16S rRNA gene sequencing | 113 OTUs; swab, scrape, and punch gave similar results; >90% Proteobacteria (Pseudomonas spp predominant) |
| Fierer et al 20085 | normal | palm | 51 | 16S rRNA gene sequencing | 4,742 OTUs; 32% sequences Propionibacterium, 17% Streptococcus, 8% Staphylococcus; 17% identity between R and L hands; 13% identity between individuals; greater diversity in female hands |
| Grice et al 20096 | normal | 20 skin sites | 10 (5 resampled 4–6 mos later) | 16S rRNA gene sequencing | Similar habitats (moist, sebaceous, or dry) had similar microbial compositions; Outer ear and nares were most stable over time; Popliteal fossae, arms, buttocks least stable over time |
| Costello et al 20097 | normal | 18 skin sites | 9 (resampled 4 times in 3 mos) | 16S rRNA gene sequencing | Same habitats are similar between individuals; Inoculation of habitat with bacteria from another site did not significantly change microbiota over time |
| Dominguez-Bello et al 20108 | normal newborn | forearm, forehead | 10 newborns, 9 mothers | 16S rRNA gene sequencing | Newborns are homogenously colonized; Microbial composition dependent on delivery mode (C-section versus vaginal delivery) |
| Capone et al 20119 | normal infant | volar forearm, buttock, forehead | 31 infants age 3 to 52 wks; 5 mothers | 16S rRNA gene sequencing | Diversity "evenness" increases with age; Staph and Strep spp decrease in relative abundance with age. Bacilli, Clostridia, Actinobacteria are most frequent classes in infant skin. |
| Oh et al 201210 | normal child and adult | nares, volar forearm, antecubital fossa, popliteal fossa, | 28 individuals, age 2–40 yrs | 16S rRNA gene sequencing | Microbial diversity of nares increases with sexual maturity. Dominant phyla across all skin sites differed globally between children and adults. S. aureus was overrepresented in nares of younger children and correlated to presence at other skin sites. |
| Bek-Thomsen et al 200811 | acne | facial folicle, cheek | 5 pts (not on therapy), 3 normal | 16S rRNA gene sequencing | 30 OTUs; Healthy follicles contained only P. acnes; Acne follicles had 53–92% P. acnes but also had other bacteria |
| Price et al 200912 | chronic wounds | wound | 24 pts | 16S rRNA gene sequencing from wound cultures | Recent antibiotics changes wound microbiota, increases Pseudomonadaceae; Diabetic wounds have increased Streptococcaceae |
| Gontcharova et al 201013 | diabetic ulcers | wound and contralateral normal skin | 23 wound, 28 normal | 16S rRNA gene sequencing | Wounds less diverse than intact skin; Wounds have more Corynebacteriaceae, Streptococcaceae, and anaerobes; Intact skin and wound on same individual do not have more related microbiomes |
| Paulino et al 200614 | psoriasis | plaque, forearm | 3 pts, 5 normal | 18s rRNA gene sequencing | No significant difference in Malassezia populations in healthy versus psoriatic skin |
| Gao et al 200815 | psoriasis | plaque | 6 pts | 16S rRNA gene sequencing | Psoriatic plaques have more Firmicutes (39% vs 24% in normal skin) |
| Fahlen et al 201216 | psoriasis | plaque biopsy | 10 pts, 12 normal | 16S rRNA gene sequencing | 652 OTUs; Psoriatic plaques have more Streptococcus, similar levels of Firmicutes |
| Sugita et al 200117 | AD | scalp, back, nares | 32 pts (on topical steroids), 18 healthy | IGS gene sequencing | AD pts have different composition of Malasseiza species with more M. globosa, M. restricta, and M. furfur |
| Sugita et al 200418 | AD | 13 AD, 12 normal | IGS gene sequencing | Different strains of M. restricta in AD versus normal skin | |
| Dekio et al 200719 | AD | face | 13 AD, 10 normal | 16S rRNA RFLP analysis | 18 species; Stenotophomonas maltophilia most common colonizer of AD skin; S. aureus not seen in any samples |
| Kong et al 201220 | AD | antecubital fossa, popliteal fossa, forearm, nare | 12 AD, 11 healthy | 16S rRNA gene sequencing | AD flares characterized by 65% increase in Staphylococcus spp, predominantly S. aureus and S. epidermidis; AD flares while on intermittent treatment only 15% slight increase in Staphylococcus colonization; Complete treatment of flares re-diversified microbiome |
Abbreviations: AD = atopic dermatitis, OTU = operational taxonomic unit, pts = patients, mos = months, spp = species
References Frank DN, Spiegelman GB, Davis W, Wagner E, Lyons E, Pace NR. Culture-independent molecular analysis of microbial constituents of the healthy human outer ear. Journal of clinical microbiology 2003;41:295-303.
Dekio I, Hayashi H, Sakamoto M, Kitahara M, Nishikawa T, Suematsu M et al. Detection of potentially novel bacterial components of the human skin microbiota using culture-independent molecular profiling. Journal of medical microbiology 2005;54:1231-8.
Gao Z, Tseng CH, Pei Z , Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proceedings of the National Academy of Sciences of the United States of America 2007;104:2927-32.
Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW et al. A diversity profile of the human skin microbiota. Genome research 2008;18:1043-50.
Fierer N, Hamady M, Lauber CL , Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proceedings of the National Academy of Sciences of the United States of America 2008;105:17994-9.
Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC et al. Topographical and temporal diversity of the human skin microbiome. Science 2009;324:1190-2.
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI , Knight R. Bacterial community variation in human body habitats across space and time. Science 2009;326:1694-7.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences of the United States of America 2010;107:11971-5.
Capone KA, Dowd SE, Stamatas GN, Nikolovski J. Diversity of the human skin microbiome early in life. The Journal of investigative dermatology 2011;131:2026-32.
Oh J, Conlan S, Polley E, Segre JA, Kong HH. Shifts in human skin and nares microbiota of healthy children and adults. Genome medicine 2012;4:77.
Bek-Thomsen M, Lomholt HB, Kilian M. Acne is not associated with yet-uncultured bacteria. Journal of clinical microbiology 2008;46:3355-60.
Price LB, Liu CM, Melendez JH, Frankel YM, Engelthaler D, Aziz M et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PloS one 2009;4:e6462.
Gontcharova V, Youn E, Sun Y, Wolcott RD, Dowd SE. A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. The open microbiology journal 2010;4:8-19.
Paulino LC, Tseng CH, Strober BE, Blaser MJ. Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. Journal of clinical microbiology 2006;44:2933-41.
Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PloS one 2008;3:e2719.
Fahlen A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Archives of dermatological research 2012;304:15-22.
Sugita T, Suto H, Unno T, Tsuboi R, Ogawa H, Shinoda T et al. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. Journal of clinical microbiology 2001;39:3486-90.
Sugita T, Tajima M, Amaya M, Tsuboi R, Nishikawa A. Genotype analysis of Malassezia restricta as the major cutaneous flora in patients with atopic dermatitis and healthy subjects. Microbiology and immunology 2004;48:755-9.
Dekio I, Sakamoto M, Hayashi H, Amagai M, Suematsu M, Benno Y. Characterization of skin microbiota in patients with atopic dermatitis and in normal subjects using 16S rRNA gene-based comprehensive analysis. Journal of medical microbiology 2007;56:1675-83.
Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome research 2012;22:850-9.
In utero, fetal skin is sterile, but minutes after birth, colonization begins to occur20–22. Newborns are first homogenously colonized with a similar, low-diversity microbiome over all skin sites20, 22. As infants contact environmental microbiota and as different areas of the skin develop distinct moisture, temperature, and glandular characteristics, individual skin habitats arise with divergent, increasingly diverse microbiota22. These habitats then continue to transform with puberty, aging, and environmental exposures23–27. Metagenomic studies using 16S rRNA sequencing in adults show that the vast majority of skin bacteria as well as gut flora fall into four phyla: Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria, but within these phyla exist thousands of distinct species1, 26, 28–37. A survey of the palm microbiome, for instance, found 4,742 distinct species in 51 healthy subjects, with an average of 158 species coexisting on a single palm26.
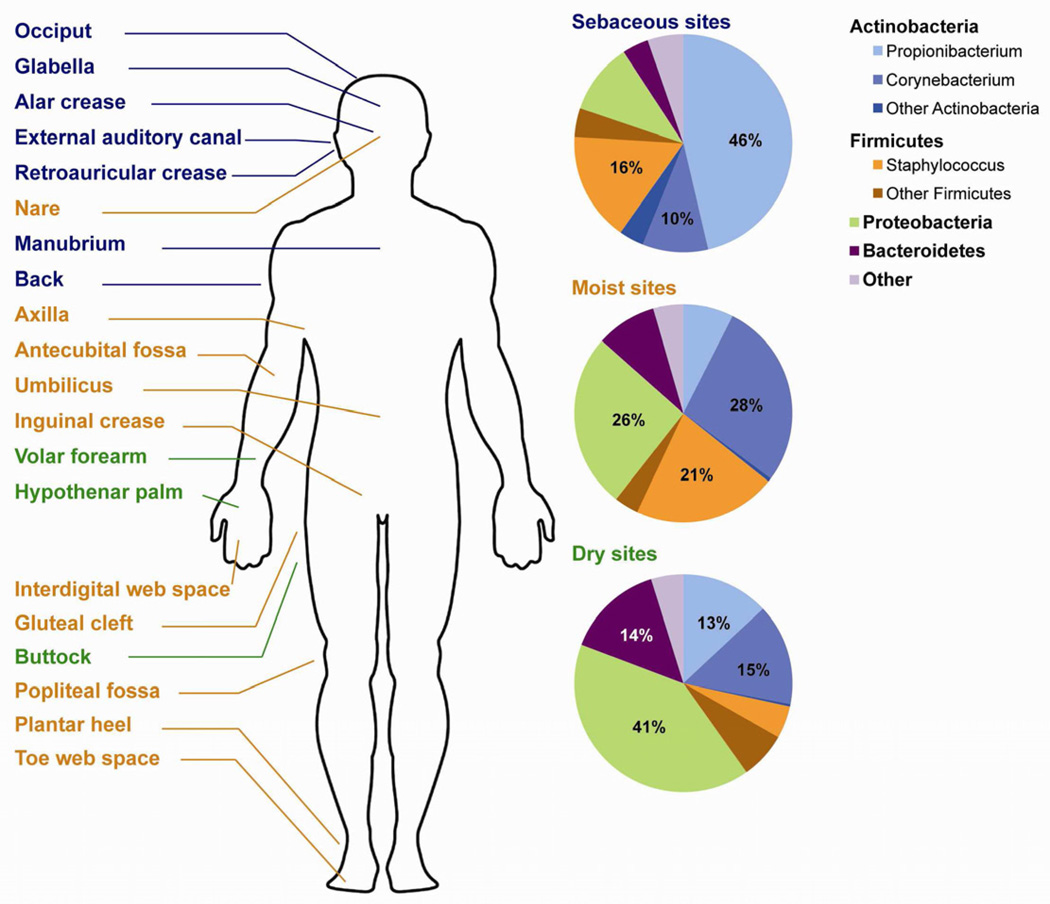
Surveys of microbiomes over 20 different skin sites show that similar habitats, such as the axillae and the popliteal fossae, have similar microbial compositions (Fig. 3)31, 38. For instance, in all individuals, Propionibacterium species dominate sebaceous areas like the forehead, retroauricular crease, and back, while Staphylococcus and Corynebacterium species dominate moist areas, such as the axillae (Fig. 3). Surprisingly, abundant Gram-negative organisms, previously thought to colonize the skin rarely as gastrointestinal contaminants, were found in the microbiomes of dry skin habitats, such as the forearm or leg.
Figure 3. Microbiome composition on normal human skin.
Sebaceous (blue text), moist (orange text), and dry (green text) habitats are labeled anatomically. Microbial composition differs among the habitats (pie charts at right). The four major phyla are shown: Actinobacteria, Firmicutes, Proteobacteri, and Bacteroidetes. Within these phyla, the three most abundant genera are also shown: Propionibacterium, Corynebacterium, and Staphylococcus. Figure is compiled with data pooled from many metagenomes, from Grice et al31. Figure is adapted from Figure 3 in Grice et al9 with permission from Nature Publishing Group.
In addition to differing species compositions, each habitat also has its own characteristic level of microbial diversity and temporal fluctuation. For example, antecubital fossae had the highest variance in species composition between subjects, called beta diversity, but each single antecubital fossa had less alpha diversity, or fewer unique species within one habitat when compared to other sites19. Different skin sites also have different levels of temporal variability. Partially occluded sites, such as the inguinal crease, had more stable bacterial communities over time31, while dryer and more exposed skin sites, such as the palm, had higher diversity and more temporal fluctuation38. Characterization of skin habitats by indices such as alpha diversity, beta diversity, and temporal volatility provides information about community structure and can be a quantitative method to follow changes in the skin microbiome after antibiotics, pathogen arrival, and other perturbations.
Consistent with the idea of ecological niches, transplanting microbes from one habitat to another, such as from the tongue to the forehead, caused only a transient presence of tongue microbiota on the forehead with eventual return to a forehead microbiome38. Individual genetics and environmental exposures also contribute to microbiome composition, as contralateral habitats within an individual are more similar than the same habitat across different individuals1, 31, 38. Additionally, within one species of bacteria, strain-level genotypic differences exist in subsets of the populations, potentially correlating to the genetic or immune characteristics of host individuals19.
Although metagenomics studies using 16S rRNA gene sequencing have revolutionized our understanding of the healthy skin microbiome, many questions need to be addressed. A recent study showed that the nares, antecubital fossa, volar forearm, and popliteal fossa of children differ globally from the same sites in adults in terms of bacterial composition39 (Table 2). For example, S. aureus was more abundant in the nares of children, and this was significantly correlated to S. aureus colonization at other skin sites39. Continued investigation of skin microbiome composition in a variety of age and ethnic groups may help elucidate why certain populations are more susceptible to certain pathologies and the host or environmental factors that determine the composition of skin ecosystems.
In addition to the abundant Propionibacterium, Staphyloccocus, and Corynebacterium species, most species in the skin microbiome each make up less than 1% of the total flora in any particular habitat. These minority species are not well studied and many were not previously known to colonize the skin, but low abundance species could nonetheless be linchpins of the skin ecosystem. Metagenomic studies of soil ecosystems have shown that several low abundance fungal species are actually highly active in essential decomposition processes40. Therefore, it is possible that low abundance skin microbes also exert large influences over abundant species, such as S. epidermidis, or pathogenic species, such as S. aureus. One way to detect relationships between pairs of species in a microbiome is to use maximal information-based nonparametric exploration (MINE) statistics. This statistical tool was recently developed and has been applied to a variety of large datasets, including the gut microbiome41. Application of MINE to skin microbiome data could hint at which pairs of bacterial species are functionally symbiotic or antagonistic and how disruptions in a few species could change the ecosystem as a whole.
Another method to study low abundance species in the skin microbiome is metatranscriptomics, which has been used to study soil microbiomes40, 42. All published studies surveying skin microbiomes have used a DNA-centered, genomic approach. By contrast, in metatranscriptomics, RNA, not DNA, is purified from a skin sample before sequencing. Since the cell itself has already amplified the RNA, this approach can better detect low abundance organisms. Additionally, transcriptome data capture metabolic activity and can reveal whether a low abundance species contributes proportionally more to the ecosystem. Furthermore, since RNA is much less stable than DNA, the meta-transcriptome would only identify microorganisms that are alive, providing a more accurate snapshot in time than metagenomics. However, one technical challenge to this approach is the limitation on skin biopsy size compared to a soil sample, which makes isolating enough RNA more difficult. Therefore, this metatranscriptomics approach may be more applicable when single-molecule DNA sequencing can be performed in a more inexpensive, high-throughput manner.
The microbiome in atopic dermatitis
One frequently studied disease using metagenomics is atopic dermatitis (AD). Although AD is noninfectious, flares may relate to changes in cutaneous microbes. AD is a chronic, relapsing disorder that affects approximately 15% of children in the United States. Many hypotheses have been invoked for the pathogenesis of AD, including a deficiency in the epithelial barrier protein filaggrin, colonization by S. aureus, and immune hypersensitivity43–47. Empirically effective treatments for AD include antibiotics, steroids, and dilute bleach baths48. These are thought to work by decreasing bacterial load and inhibiting a dysfunctional, exuberant immune response to skin flora.
Using culture methods, S. aureus colonization and infection have been commonly associated with AD49. Consistent with this, a metagenomic study showed that Staphylococcus species increased from 35% to 90% of the microbiome during flares, but surprisingly, both S. aureus and S. epidermidis increased50. Thus, microbiome data suggest that understanding how S. aureus affects AD will require understanding S. aureus fluctuations as part of a larger, complex ecosystem. S. epidermidis can produce molecules that selectively inhibit S. aureus51, arguing that S. epidermidis may be antagonistic to S. aureus. However, in the gut, pathogenic species can more easily colonize when closely related commensal species are also abundant52, suggesting that Staphylococcus species may be mutualistic. Given the differing data above, in the case of AD skin, it is still unclear whether S. aureus and S. epidermidis mutually enhance each other's colonization or whether S. epidermidis increases as an antagonistic response to an increasing S. aureus population. In addition to the obvious changes in S. aureus and S. epidermidis abundance, many unrelated, non-staphylococcal species also appear to change in abundance during an AD flare50. Future research should examine whether a change in host skin first triggers changes in species composition, thus allowing for Staphylococcus overgrowth, or if Staphylococcus overgrowth is a primary event that then forces other species to change in abundance.
These questions might be further investigated in mouse models of AD, such as the NC/Nga mouse, which develops disease that is clinically and histologically similar to AD after exposure to environmental aeroallergens53, 54. Importantly, understanding how S. aureus relates to microbiome fluctuations as a whole may reveal novel treatments of AD flares such as rebalancing and re-diversifying the skin microbiome rather than eliminating S. aureus or bacterial burden on the skin. Lessons learned from AD might also inform our understanding of other skin pathologies, such as psoriasis, acne, and chronic wounds, which may also be related to microbiome imbalances.
Microbiome studies similar to those in AD have been performed in patients with psoriasis5, 55, 56, chronic wounds57–59, or acne60 (Table 2). In chronic wounds, the microbiome was found to be less diverse than that of healthy skin but no consensus microbiome was found, even among wounds of the same etiology57–59, 61. In contrast, the follicular microbiome in acne was more diverse than that of healthy follicles, which are colonized almost exclusively by P. acnes60. And in psoriasis, there is a lack of consensus in how and if the microbiome of psoriatic plaques differs from that of normal skin5, 55, 56. Metagenomic studies with more detailed stratification based on patients’ clinical status and treatment regimens may help elucidate the clinical significance of these findings.
Antibiotics and the microbiome
A major gap in our current understanding is how current therapies affect the microbiome. Many dermatological treatments are bactericidal or immunosuppressive and may have unexpected effects on the microbiome. In the gut, antibiotics were found to cause not only a transient loss in bacterial diversity but also a long-term loss of microbiome members beyond the direct antibiotic targets62–64. Even though vancomycin targets only Gram-positive bacteria, Gram-negative populations were depleted after vancomycin treatment64. This effect on off-target microbes likely occurs due to indirect relationships between bacterial species that are forged through ecosystem-wide processes, such as metabolite exchange and waste product removal65.
Furthermore, after cessation of antibiotic treatment and even after restoration of bacterial density in the gut, the long-term changes in microbial community composition facilitate colonization by pathogens, such as vancomycin-resistant Enterococcus, which then potentiates bloodstream invasion66. Therefore, using bactericidal treatments like antibiotics in AD or UV light in psoriasis may have wide-reaching, unknown effects on the microbiome and disease recurrence. Currently, the data on probiotic treatments for skin diseases, such as atopic dermatitis remain controversial. A meta-analysis of seven Cochrane and non-Cochrane reviews showed no clear evidence that interventions such as probiotics, maternal antigen avoidance, and different antigen-avoidance diets reduced the incidence of atopic dermatitis67. Although pooled data showed a reduction in eczema incidence with exclusive breastfeeding for at least six months and with maternal probiotic supplementation, these data were based on small trials67. Additionally, these trials focused on the modulating the gut microbiome to affect skin health. Future investigation into treatments for microbe-related skin pathologies could be directed toward probiotic regimens that directly modulate the skin microbiome.
Metagenomics to investigate cutaneous infections
Metagenomic studies have provided insights into AD, psoriasis, acne, and chronic wounds. These diseases are noninfectious but can be influenced by shifts and imbalances in skin microbiota. Organisms that cause cutaneous infections can also be studied via metagenomics, which could be particularly useful in those infections associated with a wide range of clinical features and wide geographic and host variability. One such organism is Staphylococcus aureus, a major source of hospital- and community-acquired infections. Its manifestations range from asymptomatic nasal carriage to impetigo, enterotoxin-mediated desquamation, severe necrotizing pneumonia, and septicemia. In addition to a wide range of virulence and toxin-producing capabilities, S. aureus also exhibits variable antibiotic susceptibility, including methicillin and vancomycin resistance. Its widespread pathogenicity and increasing antibiotic resistance coupled with declining treatment options makes S. aureus an important pathogen to study from a patient safety and public health perspective68–71.
Thus far, 14 strains of S. aureus have been fully sequenced, with many more partially sequenced72–77. Additionally, DNA microarrays have been developed for genome comparisons between strains of S. aureus78–82. Studies using whole genome sequencing and DNA microarrays show that virulence and antibiotic resistance are associated with both host-specific and lineage-specific factors72, 80, 83 and are encoded in many different ways, including point mutations or small inserts in certain genes, large mobile genetic elements composed of many virulence genes that travel together78, 84, 85, and conjugative plasmids from unrelated species, such as vancomycin-resistant Enterococcus (VRE)86.
The study of vancomycin-resistant S. aureus (VRSA) provides an example of how genomic studies can characterize the emergence and epidemiology of antibiotic-resistant strains to identify future therapeutic targets. Since its emergence, all VRSA isolates have been found to be strains within the lineage CC5 and resistance seems to arise from acquisition of the plasmid Tn1546 from VRE during the course of each infection rather than spread of VRSA between individuals86–89. Recently, a comparative study of 12 whole VRSA genomes revealed that CC5 strains have several genetic features not present in other S. aureus lineages, which could promote acquisition of plasmids from other bacterial species while also impairing host immune function90.
In addition to techniques using whole genomes, methods such as multi-locus sequence typing (MLST)91 and spa typing92, 93 have been developed to analyze S. aureus epidemiology across hundreds of samples that differ in clinical, geographic, or host characteristics. Similar to the 16S rRNA metagenomic method described earlier, MLST and spa typing rely on culture-free sample collection, then sequencing of specific regions that exist in all S. aureus strains, and finally classification of strains based on strain-specific alleles. Studies using these approaches have shown that although a large number of S. aureus lineages are present worldwide, only ten predominate and among these, three lineages are rarely associated with methicillin resistance94. Further advances in and more widespread use of genomics to study pathogen epidemiology will continue to improve our understanding of how genetic information in pathogens encode pathology and host specificity. Similar studies outside of S. aureus have already shown strong associations between the genotypes of Helicobacter pylori strains and host ethnicity and migratory patterns95–97.
The microbiome in immune development
As a first line of defense against infection, the skin is both a physical and immunological barrier. Along with the gut, the skin is one of the most heavily immune-surveyed sites in the body. The immune system must not only distinguish between self and other but also perform the more difficult task of distinguishing between beneficial and pathogenic microbes. Since all microbes share similar molecular patterns of lipopolysaccharides and peptidoglycans, it has been a challenge to understand what exactly alerts the immune system to pathogenicity. Evidence now suggests that both skin and gut microbiota play a crucial role in educating and assisting the immune system.
Experiments in germ-free laboratory mice offered the first insights into how crucial microbiota are to immune development. These mice exhibit defective development of gut-associated lymphoid tissue and mesenteric lymph nodes, reduced epithelial expression of immune molecules, and improper T cell differentiation98–102. Studies have also shown that disturbances in gut microbiota contribute to diseases of immune dysregulation103–107. Similarly, a recent study has shown that germ-free mice without commensal skin microbes have abnormal cytokine production and cutaneous T cell populations108. These germ-free mice could not mount an appropriate immune response against intradermal Leishmania major infection; however, immunity could be rescued by allowing Staphyloccocus epidermidis colonization on the skin of germ-free mice108. These results offer tantalizing evidence that, like the gut, the skin has well-developed immune functions at both the epithelial and associated immune tissue levels. Thus, many of the same principles and lines of investigation in the gut microbiome can be applied to the skin microbiome.
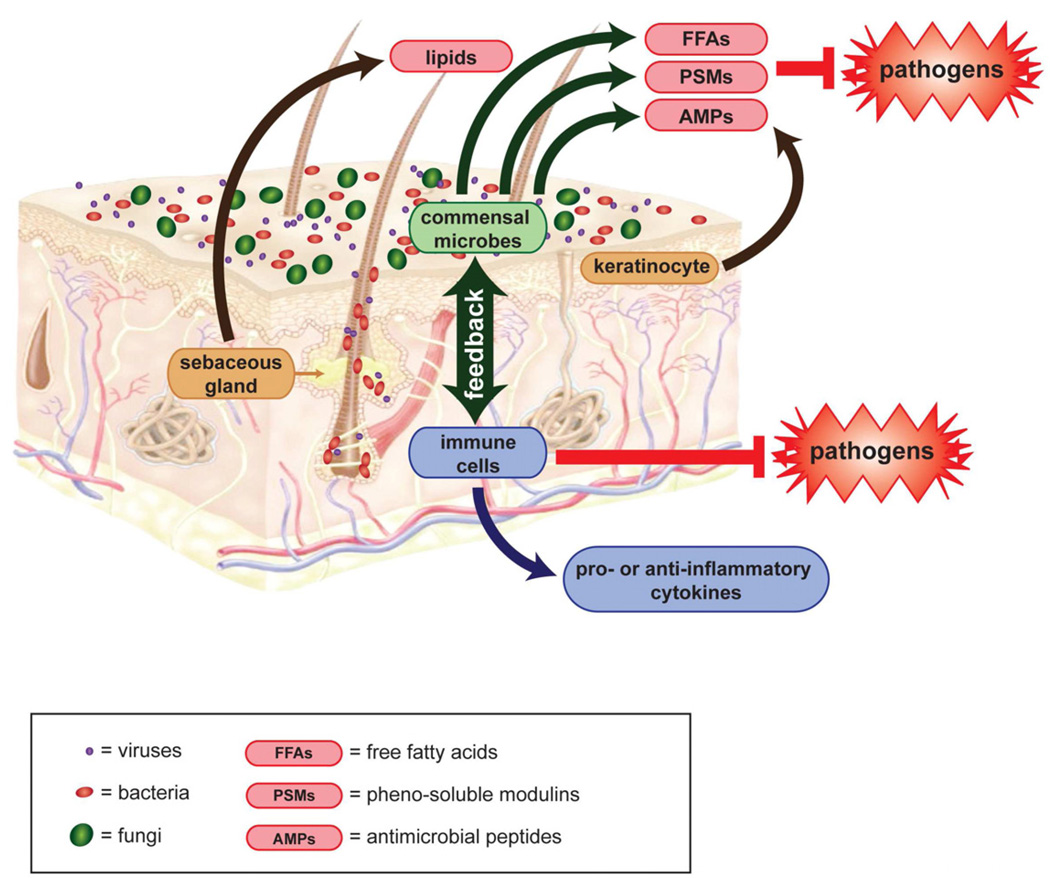
Healthy skin barrier consists of both immune surveillance and epidermal keratinocytes, which produce antimicrobial peptides (AMPs) that contribute to innate immunity (Fig. 4)109–111. Expression of these AMPs are upregulated by the presence of Propionibacterium species and other Gram-positive bacteria112, 113. In addition to AMPs, sebocytes can produce antimicrobial free fatty acids by hydrolyzing sebum triacylglycerides. This triacylglyceride hydrolysis is also performed by commensal bacterial flora such as P. acnes and S. epidermidis114, 115.
Figure 4. The microbiome and skin immunology.
Viruses, bacteria, and fungi (purple, red, or green dots) cover human skin and its appendages. Keratinocytes produce antimicrobial peptides (AMPs). Sebocytes produce free fatty acids (FFAs). Some commensal microbes also produce AMPs and FFAs, as well as pheno-soluble modulins (PSMs). These molecules all inhibit pathogen colonization. Commensal microbes may additionally inhibit pathogen growth by competition and crowding on the skin surface. The microbiota also interact with immune cells to activate them or modulate their production of pro- and anti-inflammatory cytokines. Backbone skin diagram downloaded from Docstoc (www.docstoc.com).
A large number of Gram-positive commensals, including Lactococcus, Streptococcus, and Staphylococcus species, also produce bactericidal factors de novo116. Peptides called pheno-soluble modulins (PSMs) are produced by S. epidermidis and have selective activity against S. aureus, Group A Streptocooccus, and E. coli but not other S. epidermidis117. Interestingly, S. aureus also produce PSMs, but these have minimal antimicrobial activity and instead induce lysis of neutrophils while S. epidermidis PSMs have bacteria-killing activity but no effect on neutrophils118, 119. Bacterially-produced AMPs do not just play a minor role in innate immunity but are abundant on skin and, in nanomolar amounts, can decrease the survival of pathogens on healthy human skin by 2–3 log fold119, 120.
Microbiota not only activate and assist innate immunity but also influence adaptive immunity, although these interactions are more complex and less well understood. Studies in the gut show that the commensal Bacterioides fragilis elicits anti-inflammatory cytokines, primarily IL-10, and regulatory T cells121. Other studies on how gut microbiota might modulate the immune system are reviewed elsewhere122, 123. How skin microbiota might influence the innate and adaptive immune system should now be an area of active investigation since so many autoimmune diseases—vitiligo, dermatomyositis, and lupus, to name a few—manifest on the skin even if they are also systemic.
Cancer immunology and the microbiome
Malignancy has been hypothesized to result from a breakdown in immune surveillance and from mutagenic and proliferative environments, such as chronic inflammation. Since the skin microbiome is important for developing a well-functioning immune system and for modulating inflammation, it may also protect against cancers. In support of this hypothesis, studies have shown that workers, such as farmers and waste incinerator workers, who were exposed heavily to environmental microbiota had lower cancer rates124–126.
Cancer and inflammation are linked in multiple ways. Studies have shown that chronic inflammation and tissue injury increases the risk for cancer, as in the relationship between Helicobacter pylori infection and gastric cancer108, 127 or between burns and squamous cell carcinoma128. On the other hand, acute inflammation can activate tumor necrosis factor and IL-12-induced antitumor activity, as in the case of Coley's toxin causing sarcoma regression129, 130. Commensal skin bacteria have been shown to both reduce inflammation during wound healing131 and activate innate immunity and inflammatory cytokines132. This begs the question, how do commensal bacteria affect skin inflammation and does this contribute to or protect against malignancy?
Evidence has now been provided that certain microbial components actually do have antitumor activity against bladder and colon cancers, at least in part by heightening immunosurveillance133–136. Thus far, there are no published studies on how the microbiome influences genesis and propagation of skin cancers. Global metagenomic assessments of microbiome differences between tumor sites and healthy skin may help explain the different propensities for cancer among individuals and skin habitats despite similar sun exposures and may open the door for new therapeutics.
Conclusions
Metagenomics has revolutionized our views about the skin microbiome and its interactions with the host epithelial and immune systems. Metagenomics has also yielded many new questions about what factors drive the composition and fluctuations in skin ecosystems, how changes in the microbiome contribute to disease, and how our medical interventions affect the microbiome. For a wide variety of diseases that relate to perturbations in the epidermis or the immune system, such as melanoma, graft-versus-host-disease, and autoimmune diseases, studying the microbiome may provide a new perspective to pathogenic factors and new therapeutic targets.
Capsule summary.
-
-
Recent metagenomic studies have revealed that diverse and complex microbial ecosystems inhabit the skin, collectively known as the skin microbiome.
-
-
This review summarizes recent studies characterizing the skin microbiome and highlights current gaps in research.
-
-
Understanding how the skin microbiome interacts with the host immune system and with pathogens could pave the way to new antimicrobial and promicrobial therapeutics for a wide array of diseases, including atopic dermatitis, psoriasis, chronic wounds, and cancer.
Acknowledgments
Funding source: None
Abbreviations
- 16S rRNA
16S ribosomal RNA
- OTU
operational taxonomic unit
- NIH
National Institutes of Health
- MINE
maximal information-based nonparametric exploration
- AD
dermatitis
- AMPs
antimicrobial peptides
- PSMs
pheno-soluble modulins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- 1.Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland KT, Cunliffe WJ, Roberts CD. Acne vulgaris: an investigation into the number of anaerobic diphtheroids and members of the Micrococcaceae in normal and acne skin. Br J Dermatol. 1977;96:623–626. doi: 10.1111/j.1365-2133.1977.tb05206.x. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen RJ, Stranieri A, Knutson D, Strauss JS. Topical clindamycin treatment of acne. Clinical, surface lipid composition, and quantitative surface microbiology response. Arch Dermatol. 1980;116:1031–1034. doi: 10.1001/archderm.116.9.1031. [DOI] [PubMed] [Google Scholar]
- 4.Till AE, Goulden V, Cunliffe WJ, Holland KT. The cutaneous microflora of adolescent, persistent and late-onset acne patients does not differ. Br J Dermatol. 2000;142:885–892. doi: 10.1046/j.1365-2133.2000.03467.x. [DOI] [PubMed] [Google Scholar]
- 5.Paulino LC, Tseng CH, Strober BE, Blaser MJ. Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. J Clin Microbiol. 2006;44:2933–2941. doi: 10.1128/JCM.00785-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth RR, James WD. Microbial ecology of the skin. Annu Rev Microbiol. 1988;42:441–464. doi: 10.1146/annurev.mi.42.100188.002301. [DOI] [PubMed] [Google Scholar]
- 7.Kong HH, Segre JA. Skin microbiome: looking back to move forward. J Invest Dermatol. 2012;132:933–939. doi: 10.1038/jid.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ursell LK, Clemente JC, Rideout JR, Gevers D, Caporaso JG, Knight R. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J Allergy Clin Immunol. 2012;129:1204–1208. doi: 10.1016/j.jaci.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong HH. Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trends Mol Med. 2011;17:320–328. doi: 10.1016/j.molmed.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staley JT, Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985;39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- 12.Handelsman J. Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev. 2004;68:669–685. doi: 10.1128/MMBR.68.4.669-685.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hugenholtz P, Pace NR. Identifying microbial diversity in the natural environment: a molecular phylogenetic approach. Trends Biotechnol. 1996;14:190–197. doi: 10.1016/0167-7799(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 16.Pace NR. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 17.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkany I, Gaylarde CC. Bacterial colonisation of the skin of the newborn. J Pathol Bacteriol. 1968;95:115–122. doi: 10.1002/path.1700950113. [DOI] [PubMed] [Google Scholar]
- 22.Capone KA, Dowd SE, Stamatas GN, Nikolovski J. Diversity of the human skin microbiome early in life. J Invest Dermatol. 2011;131:2026–2032. doi: 10.1038/jid.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somerville DA. The normal flora of the skin in different age groups. Br J Dermatol. 1969;81:248–258. doi: 10.1111/j.1365-2133.1969.tb13976.x. [DOI] [PubMed] [Google Scholar]
- 24.Somerville DA. The effect of age on the normal bacterial flora of the skin. Br J Dermatol. 1969;81(Suppl 1):14. [PubMed] [Google Scholar]
- 25.Marples DA. Sex, constancy, and skin bacteria. Arch Dermatol Res. 1982;272:317–320. doi: 10.1007/BF00509062. [DOI] [PubMed] [Google Scholar]
- 26.Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A. 2008;105:17994–17999. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55:144–149. doi: 10.1016/j.jdermsci.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A. 2007;104:2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank DN, Spiegelman GB, Davis W, Wagner E, Lyons E, Pace NR. Culture-independent molecular analysis of microbial constituents of the healthy human outer ear. J Clin Microbiol. 2003;41:295–303. doi: 10.1128/JCM.41.1.295-303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dekio I, Hayashi H, Sakamoto M, Kitahara M, Nishikawa T, Suematsu M, et al. Detection of potentially novel bacterial components of the human skin microbiota using culture-independent molecular profiling. J Med Microbiol. 2005;54:1231–1238. doi: 10.1099/jmm.0.46075-0. [DOI] [PubMed] [Google Scholar]
- 31.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy "core microbiome" of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bik EM, Long CD, Armitage GC, Loomer P, Emerson J, Mongodin EF, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010;4:962–974. doi: 10.1038/ismej.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A. 2004;101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh J, Conlan S, Polley E, Segre JA, Kong HH. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012;4:77. doi: 10.1186/gm378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldrian P, Kolarik M, Stursova M, Kopecky J, Valaskova V, Vetrovsky T, et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012;6:248–258. doi: 10.1038/ismej.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reshef DN, Reshef YA, Finucane HK, Grossman SR, McVean G, Turnbaugh PJ, et al. Detecting novel associations in large data sets. Science. 2011;334:1518–1524. doi: 10.1126/science.1205438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urich T, Lanzen A, Qi J, Huson DH, Schleper C, Schuster SC. Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS One. 2008;3:e2527. doi: 10.1371/journal.pone.0002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Mutius E. The environmental predictors of allergic disease. J Allergy Clin Immunol. 2000;105:9–19. doi: 10.1016/s0091-6749(00)90171-4. [DOI] [PubMed] [Google Scholar]
- 44.Cramer C, Link E, Horster M, Koletzko S, Bauer CP, Berdel D, et al. Elder siblings enhance the effect of filaggrin mutations on childhood eczema: results from the 2 birth cohort studies LISAplus and GINIplus. J Allergy Clin Immunol. 2010;125:1254 e5–1260 e5. doi: 10.1016/j.jaci.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Barker JN, Palmer CN, Zhao Y, Liao H, Hull PR, Lee SP, et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol. 2007;127:564–567. doi: 10.1038/sj.jid.5700587. [DOI] [PubMed] [Google Scholar]
- 46.Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–219. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 48.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:e808–e814. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 49.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 50.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 52.Stecher B, Chaffron S, Kappeli R, Hapfelmeier S, Freedrich S, Weber TC, et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6:e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka A, Amagai Y, Oida K, Matsuda H. Recent findings in mouse models for human atopic dermatitis. Exp Anim. 2012;61:77–84. doi: 10.1538/expanim.61.77. [DOI] [PubMed] [Google Scholar]
- 54.Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–466. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 55.Fahlen A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15–22. doi: 10.1007/s00403-011-1189-x. [DOI] [PubMed] [Google Scholar]
- 56.Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gontcharova V, Youn E, Sun Y, Wolcott RD, Dowd SE. A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. Open Microbiol J. 2010;4:8–19. doi: 10.2174/1874285801004010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price LB, Liu CM, Melendez JH, Frankel YM, Engelthaler D, Aziz M, et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One. 2009;4:e6462. doi: 10.1371/journal.pone.0006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolcott RD, Gontcharova V, Sun Y, Dowd SE. Evaluation of the bacterial diversity among and within individual venous leg ulcers using bacterial tag-encoded FLX and titanium amplicon pyrosequencing and metagenomic approaches. BMC Microbiol. 2009;9:226. doi: 10.1186/1471-2180-9-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bek-Thomsen M, Lomholt HB, Kilian M. Acne is not associated with yet-uncultured bacteria. J Clin Microbiol. 2008;46:3355–3360. doi: 10.1128/JCM.00799-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frank DN, Wysocki A, Specht-Glick DD, Rooney A, Feldman RA, St Amand AL, et al. Microbial diversity in chronic open wounds. Wound Repair Regen. 2009;17:163–172. doi: 10.1111/j.1524-475X.2009.00472.x. [DOI] [PubMed] [Google Scholar]
- 62.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 64.Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes. 2010;1:279–284. doi: 10.4161/gmic.1.4.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9:233–243. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 66.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. The Journal of clinical investigation. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. Overview of Reviews The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evid Based Child Health. 2011;6:1322–1339. doi: 10.1002/ebch.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goossens H. European status of resistance in nosocomial infections. Chemotherapy. 2005;51:177–181. doi: 10.1159/000086919. [DOI] [PubMed] [Google Scholar]
- 69.Lim SM, Webb SA. Nosocomial bacterial infections in Intensive Care Units. I: Organisms and mechanisms of antibiotic resistance. Anaesthesia. 2005;60:887–902. doi: 10.1111/j.1365-2044.2005.04220.x. [DOI] [PubMed] [Google Scholar]
- 70.Saiman L, O'Keefe M, Graham PL, 3rd, Wu F, Said-Salim B, Kreiswirth B, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis. 2003;37:1313–1319. doi: 10.1086/379022. [DOI] [PubMed] [Google Scholar]
- 71.Said-Salim B, Mathema B, Kreiswirth BN. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect Control Hosp Epidemiol. 2003;24:451–455. doi: 10.1086/502231. [DOI] [PubMed] [Google Scholar]
- 72.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 73.Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 75.Lindsay JA. Genomic variation and evolution of Staphylococcus aureus. Int J Med Microbiol. 2010;300:98–103. doi: 10.1016/j.ijmm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 76.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 78.Lindsay JA, Moore CE, Day NP, Peacock SJ, Witney AA, Stabler RA, et al. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol. 2006;188:669–676. doi: 10.1128/JB.188.2.669-676.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waldron DE, Lindsay JA. Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J Bacteriol. 2006;188:5578–5585. doi: 10.1128/JB.00418-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sung JM, Lindsay JA. Staphylococcus aureus strains that are hypersusceptible to resistance gene transfer from enterococci. Antimicrob Agents Chemother. 2007;51:2189–2191. doi: 10.1128/AAC.01442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarthy AJ, Witney AA, Gould KA, Moodley A, Guardabassi L, Voss A, et al. The distribution of mobile genetic elements (MGEs) in MRSA CC398 is associated with both host and country. Genome Biol Evol. 2011;3:1164–1174. doi: 10.1093/gbe/evr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Witney AA, Marsden GL, Holden MT, Stabler RA, Husain SE, Vass JK, et al. Design, validation, and application of a seven-strain Staphylococcus aureus PCR product microarray for comparative genomics. Appl Environ Microbiol. 2005;71:7504–7514. doi: 10.1128/AEM.71.11.7504-7514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCarthy AJ, Lindsay JA. The distribution of plasmids that carry virulence and resistance genes in Staphylococcus aureus is lineage associated. BMC Microbiol. 2012;12:104. doi: 10.1186/1471-2180-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist Updat. 2003;6:41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 85.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 87.Weigel LM, Donlan RM, Shin DH, Jensen B, Clark NC, McDougal LK, et al. High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob Agents Chemother. 2007;51:231–238. doi: 10.1128/AAC.00576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis. 2008;46:668–674. doi: 10.1086/527392. [DOI] [PubMed] [Google Scholar]
- 89.Flannagan SE, Chow JW, Donabedian SM, Brown WJ, Perri MB, Zervos MJ, et al. Plasmid content of a vancomycin-resistant Enterococcus faecalis isolate from a patient also colonized by Staphylococcus aureus with a VanA phenotype. Antimicrob Agents Chemother. 2003;47:3954–3959. doi: 10.1128/AAC.47.12.3954-3959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kos VN, Desjardins CA, Griggs A, Cerqueira G, Van Tonder A, Holden MT, et al. Comparative genomics of vancomycin-resistant Staphylococcus aureus strains and their positions within the clade most commonly associated with Methicillin-resistant S. aureus hospital-acquired infection in the United States. MBio. 2012;3 doi: 10.1128/mBio.00112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci U S A. 2002;99:7687–7692. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frenay HM, Bunschoten AE, Schouls LM, van Leeuwen WJ, Vandenbroucke-Grauls CM, Verhoef J, et al. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. Eur J Clin Microbiol Infect Dis. 1996;15:60–64. doi: 10.1007/BF01586186. [DOI] [PubMed] [Google Scholar]
- 93.Hallin M, Friedrich AW, Struelens MJ. spa typing for epidemiological surveillance of Staphylococcus aureus. Methods Mol Biol. 2009;551:189–202. doi: 10.1007/978-1-60327-999-4_15. [DOI] [PubMed] [Google Scholar]
- 94.Nubel U, Strommenger B, Layer F, Witte W. From types to trees: reconstructing the spatial spread of Staphylococcus aureus based on DNA variation. Int J Med Microbiol. 2011;301:614–618. doi: 10.1016/j.ijmm.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 95.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 96.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wirth T, Wang X, Linz B, Novick RP, Lum JK, Blaser M, et al. Distinguishing human ethnic groups by means of sequences from Helicobacter pylori: lessons from Ladakh. Proc Natl Acad Sci U S A. 2004;101:4746–4751. doi: 10.1073/pnas.0306629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lundin A, Bok CM, Aronsson L, Bjorkholm B, Gustafsson JA, Pott S, et al. Gut flora, Toll-like receptors and nuclear receptors: a tripartite communication that tunes innate immunity in large intestine. Cell Microbiol. 2008;10:1093–1103. doi: 10.1111/j.1462-5822.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 100.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 101.Matsumoto S, Setoyama H, Umesaki Y. Differential induction of major histocompatibility complex molecules on mouse intestine by bacterial colonization. Gastroenterology. 1992;103:1777–1782. doi: 10.1016/0016-5085(92)91434-6. [DOI] [PubMed] [Google Scholar]
- 102.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 103.Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–346. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 104.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 105.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 106.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 107.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 108.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 110.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 112.Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 113.Lee DY, Yamasaki K, Rudsil J, Zouboulis CC, Park GT, Yang JM, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill propionibacterium acnes. J Invest Dermatol. 2008;128:1863–1866. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marples RR, Downing DT, Kligman AM. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Invest Dermatol. 1971;56:127–131. doi: 10.1111/1523-1747.ep12260695. [DOI] [PubMed] [Google Scholar]
- 115.Gotz F, Verheij HM, Rosenstein R. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem Phys Lipids. 1998;93:15–25. doi: 10.1016/s0009-3084(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 116.Bastos MC, Ceotto H, Coelho ML, Nascimento JS. Staphylococcal antimicrobial peptides: relevant properties and potential biotechnological applications. Curr Pharm Biotechnol. 2009;10:38–61. doi: 10.2174/138920109787048580. [DOI] [PubMed] [Google Scholar]
- 117.Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 119.Cogen AL, Yamasaki K, Muto J, Sanchez KM, Crotty Alexander L, Tanios J, et al. Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One. 2010;5:e8557. doi: 10.1371/journal.pone.0008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974–1980. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Surana NK, Kasper DL. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev. 2012;245:13–26. doi: 10.1111/j.1600-065X.2011.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chervonsky AV. Microbial influences on immune function and more. Immunol Rev. 2012;245:7–12. doi: 10.1111/j.1600-065X.2011.01085.x. [DOI] [PubMed] [Google Scholar]
- 123.Chinen T, Rudensky AY. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol Rev. 2012;245:45–55. doi: 10.1111/j.1600-065X.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- 124.Enterline PE, Sykora JL, Keleti G, Lange JH. Endotoxins, cotton dust, and cancer. Lancet. 1985;2:934–935. doi: 10.1016/s0140-6736(85)90861-x. [DOI] [PubMed] [Google Scholar]
- 125.Rylander R. Environmental exposures with decreased risks for lung cancer? Int J Epidemiol. 1990;19(Suppl 1):S67–S72. doi: 10.1093/ije/19.supplement_1.s67. [DOI] [PubMed] [Google Scholar]
- 126.Rapiti E, Sperati A, Fano V, Dell'Orco V, Forastiere F. Mortality among workers at municipal waste incinerators in Rome: a retrospective cohort study. Am J Ind Med. 1997;31:659–661. doi: 10.1002/(sici)1097-0274(199705)31:5<659::aid-ajim23>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 127.Romero-Adrian TB, Leal-Montiel J, Monsalve-Castillo F, Mengual-Moreno E, McGregor EG, Perini L, et al. Helicobacter pylori: bacterial factors and the role of cytokines in the immune response. Curr Microbiol. 2010;60:143–155. doi: 10.1007/s00284-009-9518-4. [DOI] [PubMed] [Google Scholar]
- 128.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 129.Starnes CO. Coley's toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 130.Tsung K, Norton JA. Lessons from Coley's Toxin. Surg Oncol. 2006;15:25–28. doi: 10.1016/j.suronc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 131.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Akaza H, Iwasaki A, Ohtani M, Ikeda N, Niijima K, Toida I, et al. Expression of antitumor response. Role of attachment and viability of bacillus Calmette-Guerin to bladder cancer cells. Cancer. 1993;72:558–563. doi: 10.1002/1097-0142(19930715)72:2<558::aid-cncr2820720237>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 134.Juckett DA, Aylsworth CF, Quensen JM. Intestinal protozoa are hypothesized to stimulate immunosurveillance against colon cancer. Med Hypotheses. 2008;71:104–110. doi: 10.1016/j.mehy.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 135.Grange JM, Bottasso O, Stanford CA, Stanford JL. The use of mycobacterial adjuvant-based agents for immunotherapy of cancer. Vaccine. 2008;26:4984–4990. doi: 10.1016/j.vaccine.2008.06.092. [DOI] [PubMed] [Google Scholar]
- 136.Bojar RA, Eady EA, Jones CE, Cunliffe WJ, Holland KT. Inhibition of erythromycin-resistant propionibacteria on the skin of acne patients by topical erythromycin with and without zinc. Br J Dermatol. 1994;130:329–336. doi: 10.1111/j.1365-2133.1994.tb02929.x. [DOI] [PubMed] [Google Scholar]