Abstract
Rapid analysis of a cell's propensity to undergo apoptosis through the mitochondrial pathway is hindered by the complex network of interactions between more than fifteen known members of the BCL2 family that govern the decision to undergo mitochondrial apoptosis, and measurement of protein levels alone fails to account for critical interactions between the proteins. To address this issue, we have developed two functional assays for same-day analysis of cell lines or primary tissue samples. Using defined inputs in the form of peptides derived primarily from the BH3 domains of pro-apoptotic members of the BCL2 family, we invoke a response in the mitochondria in the form of mitochondrial outer membrane permeabilization measured indirectly using potential sensitive dyes. BH3 profiling can be applied to any viable single cell suspension and provides a response from the sum total of all known and unknown interactions within the BCL2 family for each stimulus, and the pattern of response can provide both a cell's propensity towards mitochondrial apoptosis, or ‘priming’, as well as indicate dependencies on specific anti-apoptotic proteins. Described here are optimized conditions for both plate-based and FACS-based BH3 profiling for homogeneous and heterogeneous samples.
Keywords: Mitochondria, Apoptosis, BCL-2, Flow Cytometry, Cancer, chemotherapy
1. Introduction
The mitochondrial pathway of apoptosis stands as a critical component in the development and maintenance of complex, multicellular organisms. [1] This pathway furthermore mediates the cell fate response to many toxic insults, including those therapeutically introduced into cancer patients by oncologists. The BCL-2 family of proteins controls the mitochondrial outer membrane permeabilization (MOMP) that is the key step in commitment to cell death by this pathway. There are many situations, both investigational and clinical, in which it would be useful to predict whether or not a given cell will undergo apoptosis in response to a particular insult. One might presume that comprehensive knowledge of the BCL-2 family proteins in a cell would provide the information required to provide such a prediction. However, the BCL-2 family proteins which regulate the mitochondrial apoptotic pathway are numerous and have a complex pattern of interaction[2]. Identifying all the interactions, expression levels, and post-translational modifications of the more than fifteen known members[3] of the family is by itself a feat beyond our accomplishing at present. Moreover, it is not clear that a model can be developed to receive these inputs that can be generalized among different cell types and insults.
Apoptosis may be thought of like a car,a very complex machine with a number of elements and interactions that any one of us could potentially isolate and examine. However, in practice most of us have a very incomplete understanding of it works in any detail. However, one does not need to measure the interaction of every gear to know that a fixed input from the accelerator will result in a proportional output from the tires. For most of us, this association of accelerator with motion is all we ever need to comprehend to operate an automobile. Similarly, while we may never understand the interactions of all of the BCL-2 family proteins simultaneously, the study of simple inputs and outputs of the mitochondrial apoptosis pathway we can nonetheless derive useful, actionable information.
The BCL2 family can be divided into three groups: multi-domain anti-apoptitic, multi-domain pro-apoptotic, and BH3-only pro-apoptotics. Multi-domain pro-apoptotics BAX and BAK are responsible forming the pores that cause MOMP following an activating conformational change and insertion into the mitochondrial outer membrane. The multi-domain antiapoptotic proteins all share a hydrophobic groove where the BH3-helix of the BH3 domain can bind and be inhibited or bind and displace another resident BCL2 protein[4]. It is from the BH3 helix of the BH3-only proteins that the peptides used here are derived and hence the name BH3 profiling. By using peptide mimics of the BH3 helices,
Peptides used in BH3 profiling (Table 1) are all 20-25mers containing the conserved LXXXXD motif, and can be divided into two categories: activators and sensitizers. The major difference in behavior between these two classes is the ability to directly induce the activating conformational change in BAX and BAK that leads to MOMP. BIM and BID peptides alone possess this ability while, regardless of their protein of origin, BAD, NOXAA, PUMA, BMF, and HRK derived peptides used in profiling cannot induce this change even at concentrations as high as 100 uM and are thus designated as sensitizer peptides. [5]
Table 1.
Peptide Sequences and Expected Behavior in BH3 Profiles
| Peptidea | Sequenceb | Classc | Expected Behaviord |
|---|---|---|---|
| BIM | MRPEIWIAQELRRIGDEFNA | A | Rapid depolarization when BAX and BAK are present/functional |
| BID-Y | EDIIRNIARHLAQVGDSMDRY | A | |
| PUMA | EQWAREIGAQLRRMADDLNA | S | Depolarizes primed cells. Pan-sensitizers. Generally depolarize next after BIM / BID |
| BMF-Y | HQAEVQIARKLQLIADQFHRY | S | |
| BAD | LWAAQRYGRELRRMSDEFEGSFKGL | S | Depolarization if dependent on BCL2, BCL-W, or BCL-xL |
| NOXAA | AELPPEFAAQLRKIGDKVYC | S | Depolarization only if dependent on MCL1 |
| W-HRK | WSSAAQLTAARLKALGDELHQ | S | Depolarization only if dependent on BCL-xL |
| PUMA2A | EQWAREIGAQARRMAADLNA | Control | Mutant control peptide. No depolarization |
–Y and W- denote amino acids added to the sequence for UV absorbance measurement of peptide concentration.
All have Acetyl N-termini and Amidated C-termini.
A=Activator, S=Sensitizer.
At 10-100 uM. Combining NOXAA+BAD peptides mimics PUMA
BH3 profiling is a functional assay that uses a defined input in the form of small molecules or peptides, most often derived from the BH3 domain of known Bcl-2 family members, to provoke a response from mitochondria. The responses measured are typically surrogates of MOMP, namely release of cytochrome c or the collapse of the transmembrane mitochondrial potential (ΔΨm).[2, 6-9] It is capable of taking the sum total of all modifications into account during its measurement without needing to directly probe for each modification or protein level. Furthermore, the assay is swift enough to be completed in a matter of hours from the time of sample arrival. BH3 profiling is a rapid and simple method of probing the functional competence of the intrinsic apoptotic pathway and a cell's propensity to undergo apoptosis.
Described here are the methods suitable for performing BH3 profiling on both homogeneous and heterogeneous cell populations from cell lines or primary tissues. In addition, a detailed explanation of the profiling system components has been included to aid in the understanding of its function, guide further optimization and elaboration, and provide information for troubleshooting.
2. Permeabilizing the Plasma Membrane
While mitochondrial potential dyes are capable of penetrating the plasma membrane, many BH3 profiling components are incapable of diffusing through this membrane. The most important of these are the BH3 peptides necessary to deliver the defined input used to trigger the apoptotic machinery, and the succinate that is needed to facilitate ΔΨm . In its earliest form, BH3 profiling surmounted this issue by isolating heavy membranes at the cost of losing all surface markers and most other intracellular structures, as well as requiring relatively large numbers of cells, 108 to 109. To improve the efficiency of the system, we turned to methods of selectively permeabilizing the plasma membrane without permeabilizing the mitochondrial membranes, to permit peptide diffusion into the cell to contact mitochondria.
A number of agents are capable of creating pores in the plasma membrane. Proteins including alphatoxin and streptolysin O (SLO) [10-12], peptides such as melittin [13], macrolides such as filipin [14], and glycosides including saponin and digitonin [15, 16]. While all of these compounds have been shown to create pores in the plasma membrane, their stability, ease of use, pore size, and specificity for the plasma membrane vary. This specificity is very important, for without it, mitochondria membranes may be permeabilized before peptides can be applied, rendering the assay useless.
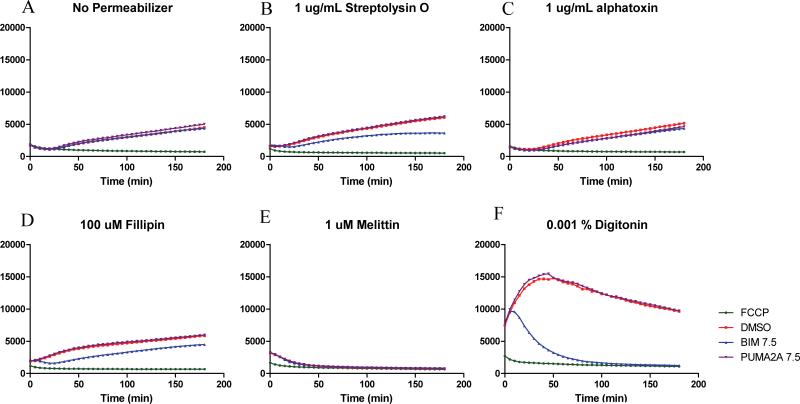
Both alphatoxin and SLO can create well controlled pores in the plasma membrane. However, the pores of alphatoxin are extremely small [10], too small for efficient peptide diffusion. SLO can be affected by both temperature and oxidation [10-12], and we found it incapable of inducing stable plasma membrane pores permissive to peptide diffusion. When used in the context of BH3 profiling, neither toxin was able to create a sufficient, stable degree of permeabilization that maintained a high degree of mitochondrial potential over time to allow for peptide-dependent depolarization. (Figure 1 B and C) Filipin, like alphatoxin and SLO, was unable to allow for peptide-dependent depolarization. (Figure 1D) Concentrations sufficient to permeabilize the plasma membrane permeabilized the mitochondrial membranes as well.
Figure 1. Digitonin provides superior mitochondrial membrane potential measurements in permeabilized cells.
Murine leukemia cells stained in the presence of JC-1 dye in DTEB under plate-based profiling conditions. Measurements are the average from triplicate wells of a 384 well plate. Doses displayed are the best results from a titration of each permeabilizer. Treatments included are BIM peptide as a depolarizing peptide control and PUMA2A as an inert peptide control. Peptide concentrations in uM are as indicated.
Melittin similarly formed plasma membrane pores, but not without disrupting mitochondrial membrane potential. (Figure 1E) As a cationic peptide, it is likely drawn towards the mitochondria and exerts its pore forming ability there when at high concentrations. At lower concentrations, near 0.1 uM, it appears to fail in forming pores even in the plasma membrane, eliminating access of BH3 peptides to mitochondria.
Both saponin and digitonin are glycosides that are capable of precipitating cholesterol and inducing pore formation in the plasma membrane in a dose dependent manner. [16] High concentrations of saponin in particular are used in the permeabilization of fixed cells for intracellular staining. At lower concentrations, digitonin in particular has been used to gently form pores in the plasma membrane to study intracellular processes including intracellular enzyme kinetics [17, 18], nuclear transport [19, 20], and calcium flux [21] without disruption of overall intracellular structure.[15]
Because they interact with cholesterol, they have a preference for the plasma membrane over other intracellular compartments including the nuclear envelope and mitochondrial membranes when used at the appropriate concentration. Both compounds can be used, but focused on optimizing digitonin for use in BH3 profiles.
Concentrations of digitonin were initially established at 50 ug/mL as suitable for profiling in that it produced a fluorescence difference between untreated and FCCP depolarized mitochondria of greater than 4:1 for up to three hours or more. (Figure 1F) Continued use has shown this to be close to the upper limit of its usefulness, and conditions ranging from 5-25 ug/mL (0.0005 – 0.0025% w/v) have proven to be more reliable with 10-25 ug/mL being the most typical range. Murine cells typically require slightly less digitonin than comparable cells of human origin.
Because the cells are permeabilized, it is possible for soluble proteins to escape after digitonin addition, and this may include members of the BCL2 family, such as uncleaved BID or PUMA. However, many of these BCL2 family proteins are either integrated into membranes or bound to cytoskeletal elements, such as BAX or BIM, (Figure S1) and the successful correlation of BH3 profiles to parameters such as drug sensitivity suggests that the proteins retained are sufficient to produce a meaningful profile.
3. Effects of Buffer Composition
It is the purpose of any buffer to provide an environment that remains sufficiently stable to eliminate it as a source of variance. While it is easy to take for granted, the alteration of only one of many components can result in different results. Buffers used for ΔΨm measurements usually contain a sugar, a pH buffer, salts, chelators, and a carbon source for the electron transport chain at a minimum. [22-25] Understanding the tolerance of ΔΨm measurements to variation of these parameters is not only important for producing the optimal conditions to perform measurements, but it is also crucial for troubleshooting as well as shortening later optimization cycles should a special application demand it. A buffer in this application ideally provides the greatest stability of ΔΨm in control mitochondria, while providing the greatest dynamic range of ΔΨm loss in response to BH3 peptide-induced MOMP.
Mitochondrial isolation is historically performed in the presence of a mannitol / sucrose buffer in the absence of electrolytes to obtain pure, functional mitochondria. [22-24, 26]However, BH3 profiling using whole cells does not need to protect the mitochondria through isolation and purification processes. Trehalose was found to be a superior non-ionic solute to maintain tonicity, capable of protecting rat liver mitochondria through freeze / thaw when compared to sucrose / mannitol isolation.[24]. The isolation and experimental buffers using trehalose formed the basis from which optimized BH3 profiling buffers were created. While the more standard 300 mM trehalose concentration used for isolated mitochondria provides sufficient dynamic range for ΔΨm measurements, ΔΨm measurements in 135 mM trehalose were as good as or of greater dynamic range than at higher concentrations.
While the presence of sugars aids in maintaining the stability of ΔΨm during profiling, without the proper amount of potassium chloride, treatment-dependent depolarization will be severely impaired. While it does not apply to all intermembrane space proteins, insufficient KCl can result in the retention of cytochrome c even after the outer mitochondrial membrane has been compromised while excessive levels can result in reduction or early collapse of the ΔΨm. While measurements can be conducted at KCl concentrations as high as 120 mM, the dynamic range and durability of the measurement suffers compared to measurements taken at 50-80 mM KCl. Lower concentrations, 25 mM and lower (Figure 2), result in incomplete depolarization as shown by a failure of the strong activator peptide BIM, which should result in full depolarization in this cell line, to reach the same level of depolarization as FCCP. While 80 mM is a suitable concentration, 50 mM provides a higher dynamic range and a more stable ΔΨm over time for data collection. A stable potential, defined by the difference between charged (DMSO) and depolarized (FCCP) controls, is important as we have previously demonstrated a robust correlation between ΔΨm collapse and cytochrome c release. [9]
Figure 2. KCl Concentration Modulates MMP and Peptide Response.
BCL-2 dependent murine leukemia cells were exposed to modified DTEB buffer with 10ug/mL digitonin containing the indicated concentrations of KCl. BIM, BAD, and PUMA peptides are included as depolarizing peptide controls. PUMA2A serves as an inert peptide control. Peptide concentrations in uM are as indicated.
Mitochondria do not exist in isolation within the cell. Since they are in intimate contact with the endoplasmic reticulum, the release of ER contents, particularly Ca2+, is a concern when isolating mitochondria or using permeabilized cells. Isolation buffers often use either EDTA or EGTA as chelators to remove such ions and prevent them from activating the mitochondrial permeability transition pore, mPTP, which is a BAX/BAK independent method of mitochondrial lysis. While isolation buffers often contain upwards of 1 mM chelator, this degree of ion sequestration exceeds what is necessary for whole cells and reduces the range of ΔΨm measurements. Reduction of chelators to 20 uM for both EDTA and EGTA makes ΔΨm measurement more robust. Complete removal of EDTA under these conditions, while not detrimental, reduces the quality of the measurement and suggests that a low level of buffering from EDTA is beneficial. These conditions work for many cell types, but tissues that contain high levels Ca2+ or Mg2+ levels may require high chelator concentrations to compensate.
The remaining components of the profiling buffer system are less flexible. The pH is normally held at 7.5, but this can range from 7.2 to 7.8 and still be successful. As with any system, it is best to maintain a standard condition to reduce variability. Succinate serves as a carbon source for the electron transport chain, and at 5 mM the electron transport chain appears to be saturated as the addition of more succinate does not provide an increase in potential. Finally, the presence of BSA, preferably IgG free, has remained a requirement for stable measurements in this buffer system. Sometimes listed as a membrane stabilizing agent, attempts to remove BSA resulted in low signal quality as determined by low dynamic range and early ΔΨm collapse. Because BSA will vary with lot, it is important to note the lot number and test new lots for equivalency before stocks of the previous lot are expended. Finally, 2-mercaptoethanol (2-ME) is included in the plate-based assay to dissociate JC-1 aggregates that are not associated with the mitochondria. The inclusion of 2-ME is required to reduce background in the plate-based profile, but reduced JC-1 concentration and methods of analysis makes 2-ME unneeded in FACS-based profiles.
4. Measurement of ΔΨm
The detection of mitochondrial outer membrane permeabilization (MOMP) is the endpoint for all forms of BH3 profiling. This can be accomplished by measuring the exit of intermembrane space proteins such as SMAC/DIABLO and cytochrome c, but detection of these proteins require the use of antibody staining, ELISA, or western blot which are not amenable to real time measurements or high throughput assays, and tend to be more expensive and time-consuming. While the loss of cytochrome c from the intermembrane space in an intact cell induces a loss of ΔΨm that may be reversible in some contexts[27], cytochrome c lost from permeabilized cells under profiling conditions causes a durable loss of ΔΨm that can only be restored by the addition of a large amount of exogenous cytochrome c. Therefore ΔΨm can serve as a measure of MOMP.
A number of commercially available small molecule probes exist for the measurement ΔΨm. A positive charge delocalized over a large conjugated system is the common theme among all ΔΨm probes. The positive charge leads to an accumulation of dye in the negatively charged matrix of the mitochondria as the dyes diffuse through the cell. Because the charge of the dye is highly delocalized, the dyes remain membrane permeable. In some cases, the plasma membrane potential is capable of causing dye accumulation, but permeabilization by digitonin removes this source of interference by completely depolarizing it.
While there is a variety of probes to choose from, some have unique properties that make them suitable for one form of profiling over another. Most ΔΨm dyes are either cyanine dyes or rhodamine derivatives. A few members of the cyanine dyes possess a shift in fluorescence to longer wavelengths based on their accumulation. The best known of these is JC-1. Monomers of JC-1 excited at 488 nm fluoresce with a green light near 525 nm. When the dye reaches high local concentrations, as in a normally polarized mitochondrial matrix, a J-aggregate forms and results in a second emission peak at 590 nm that is proportional to the ΔΨm. The 590 nm peak can be excited even more efficiently, and without monomer emission, if excited in the 540-560 nm range. The formation of J-aggregates is reversible.
Because JC-1 exists in two distinct and easily detected states, it does not require any washout steps when used in plate-based assays. Therefore, JC-1 is left in excess in wells with cells during plate-based profiles with only the red 590 nm fluorescence being read over time, and traces of ΔΨm of up to six hours have been obtained from cells without any further handling after being loaded into a plate reader. We have provided examples of what a typical profile should appear in several different cell types. (Figure 3) A large gap between DMSO and FCCP tracings, a lack of or only gradual loss of DMSO signal, and smooth curves are all signs of a good profile. Examples of three peptides also demonstrate typical peptide behavior in BAX/BAK replete cells. The direct activating peptide BIM shows a strong, fast depolarization typical of direct activation of BAX and/or BAK. Slower or sometimes inactive, the sensitizer peptide PUMA relies and pre-bound endogenous activators for its activity. This is common for sensitizer class peptides that cannot directly activate BAX or BAK. Finally, an inert control mutant peptide, PUMA2A serves as a second positive control. Additional information on the classification and sequences of these peptides can be found in Certoet al. [2]
Figure 3. Examples of Successful Plate-Based Profiles.
Cells lines are listed with their corresponding concentration of digitonin used in DTEB in a plate based BH3 profile. Depolarizing peptide controls BIM and PUMA and inert peptide control PUMA2A are included. Peptide concentrations in uM are as indicated.
While JC-1 is an exceptional dye, it has some shortfalls. It can be excited on 405 and 488 nm lasers of FACS machines, and the bright emissions covers nearly the whole spectrum from 500-600 nm. This produces very complicated compensation issue in FACS assays and limits the number of channels available for analysis of samples by FACS. However, the number of alternative fluorescent tags continues to increase, and compensation issues can be mitigated by intelligent selection of tags with non-overlapping spectra.
Excitation and emission characteristics of ΔΨm dyes vary widely. While a number of utilities exist to describe the excitation and emission spectra, it is sometimes more useful to have a practical example of each dye and how it appears on an instrument. In this case, MOLM13 cells stained with each dye at 50 nM, or 100 nM for JC-1, was examined on an LSR Fortessa with or without FCCP depolarization. For each dye, the FCCP control was set to 102 on a log scale and every channel for that dye was set to the same voltage without compensation. (Figure 4)
Figure 4. Spectral Overlap of MMP Dyes During Depolarization Measured by FACS.
MOLM13 cells stained with the indicated dye under FACS BH3 profiling conditions were treated with either DMSO (Blue) or FCCP (red.) Uncolored areas of curves indicate overlap between the FCCP and DMSO treated samples. All scales are log. Vertical colors on the far left indicate excitation laser wavelength. Colored blocks at left list the emission filter wavelength and bandwidth in nm.
A number of important properties can be observed from these data. First, JC-1 produces ΔΨm dependent shifts in fluorescence in many channels on all but the 633 nm emission. Second, the dynamic range of each dye varies greatly. No dye can compare to the 3-log shift in fluorescence of JC-1 with 561 nm excitation. Dyes like CMXRos only give large shifts in intensity when used with a 561 nm laser line. However, while these dyes have a smaller dynamic range, typically around one log between DMSO and FCCP controls, they occupy smaller emission ranges and allow for simplified compensation and additional colors for additional markers.
Any of these dyes can be used for BH3 profiling, but we have favored TMRE and JC-1 for their dynamics ranges and spectral properties. JC-1 is required for plate-based profiling, but FACS-based profiles also benefit from the large dynamic range with 561 nm emission. When more elaborate cell surface labeling, and a greater number of fluorescent labels, is needed, TMRE is a more tractable alternative if a 561 nm laser is available. Rhodamine 123 can be used in place of TMRE if such an excitation source is not available.
5. Cell Permeant Peptides
The use of a number of small peptide tags has allowed for whole proteins to be drawn into cells without the addition of other agents to facilitate their import. TAT, antennepedia, and polyarginine are the best known sequences, and all three are cationic peptides that associate with the cell membrane prior to import. In some cases, this import can be swift and very effective.
In the context of BH3 profiling, we have observed import to be slow and its effects do not correlate well with the behavior of peptides that are given direct access to mitochondria. It appears that the addition of these largely cationic peptide tags renders BH3 peptides capable of permeabilizing membranes in non-specific, BAX and BAK independent fashion. Introduction of these peptides also can require the removal of serum which may bind the peptides, and exposure typically resulted in non-specific, sequence-independent toxicity. Because alteration of tissue culture conditions over extended periods can alter profiling results, these peptide formats are not used for profiling.
6. Choice of Methods
6.1 Plate-based profiling
Current techniques for BH3 profiling are capable of handling both homogenous and heterogeneous cell samples. The choice of which techniques to use depends primarily on what sort of population is to be analyzed. In the case of homogeneous samples such as sorted cells, cells isolated from selection columns, or controlled cell lines, a plate-based approach (Figure 5A) provides the most information and greatest throughput for the lowest expense. In general, if there is only one population to analyze or there is no desire to discriminate between subpopulations, a plate-based approach should be used.
Figure 5. Overview of Plate and FACS Based BH3 Profiling Workflow.
A. Workflow for plate based BH3 profiling. B Workflow for FACS based BH3 profiling
6.1.1 Common Protocol materials
All BH3 profiles use a number of common components regardless of their analysis platform. Before beginning any profiling work, the following materials should be prepared:
DTEB (Derived from Trehalose Experimental Buffer) 135 mM trehalose, 50 mM KCl, 20 uM EDTA, 20 uM EGTA, 0.1% BSA, 5 mM succinate, 10 mM HEPES-KOH pH 7.5
5% digitonin in DMSO (w/v)
100 uM JC-1 in DMSO
1M 2-mercaptoethanol in water
20 mg/mL oligomycin in DMSO
1 mM FCCP or CCCP in DMSO
Treatments. Typical peptide stocks are 100-200X final working concentrations in DMSO. Peptide sequences are available in Certoet al[2]
6.1.1 Plate protocol
Prepare 8.5 uL per well of 4X Dig/Dye Mix composed of 4 mM JC-1, 40 ug/mL oligomycin, 20 mM 2-mercaptoethanol, and 0.01% digitonin (w/v) in DTEB. Store in the dark at RT. An initial bright pink color should fade in 10-15 min.
Prepare stocks of each treatment at 2X their final concentration in DTEB. It is recommended to prepare duplicate or triplicate wells for any given treatment-cell pair to provide redundancy against loading errors.
Load 15 uL of each 2X treatment into a well of a black, untreated 384 well plate.
Gently tap around the edges to knock droplets to the bottom of the wells. Never tap vertically as this favors droplets emerging from the wells and contaminated nearby wells.
- Prepare the fluorescence plate reader by setting the temperature control to 30°C. As close as possible, use an excitation of 545 nm and emission of 590 nm. Bandwidths up to 20 nm can be used for both. The reader should be programmed to take measurements every 5 minutes for 120-180 minutes with the latter being a common profile time.
- NOTE: Fluorescence will increase steadily in the first 30-45 minutes in a temperature dependent manner. Increased temperature can lead to faster charging but may cause earlier potential collapse. Pre-warming all materials to 30°C can increase initial separation of controls but is not necessary for profiling.
Prepare a single cell suspension and count the number of viable cells.
Prepare 8 uL/well of 4X Cell Solution by suspending cells in DTEB at a density of 1.33 × 106 / mL. This will result in 10000 cells/well final. Use 10000-20000 cells/well for adherent lines and 20000-50000 cells/well for suspension lines.
Add an equal volume of the 4X Dig/Dye Mix to the 4X Cell Solution to produce a 2X Dye/Cell Mix. Mix gently by pipetting and lets stand for 10 min at RT.
Add 15 uL 2X Dye/Dell Mix to each treatment well. Tap the edges of the plate lightly to knock droplets to the bottom of the wells as in step 4.
Load the plate into the plate reader and allow for automated fluorescence reading. If automated readings are not available, take measurements at 30, 60, 90, 120, and 180 min.
Proceed to data analysis.
6.1.2 Plate data analysis
One advantage of automated plate-based analysis is the integration of data over time. This provides less bias in the measurement as well as providing a more detailed examination of each sample's performance. Analysis of a BH3 profile curve generated from a plate begins by calculating the area under each curve. If a full curve is not available or only a single time point analysis is desired, the values of each treatment from the same time point may be used. For single time point analysis, it is recommended to use time points at least 90 minutes or greater.
While the absolute values of potential can be used, it is often useful to produce a normalized value for each profile to simplify comparison. To do these, the following equation is applied to each area or time point value:
This equation provides a background subtraction provided by the FCCP control value and a percent of the maximum range defined by the difference between DMSO and FCCP controls.
It is possible for a treatment to exceed 100% depolarization if it produces a potential loss that exceeds FCCP. This is common with irreversible and highly potent treatments such as the use of direct BAX/BAK activating peptides like BIM BH3. Likewise, treatments that cause a potential value in excess of the DMSO control will generate a negative depolarization value. If either of these are undesirable, the range can be redefined as a simple Min / Max value pair for background and range purposes.
As important as the analysis , understanding the curve shapes for troubleshooting is crucial. A failure of the positive, charged control (DMSO) to increase and separate from the negative, depolarized control (FCCP), will result in data that is difficult to analyze reliably. A large, stable separation of these controls is required for reproducible calculations of treatment-induced depolarization. This can result from cells that are in poor condition either from overgrowth, drug treatment, or any other condition that results in dying cells. To avoid this issue, cells used for profiling should not be over confluent and care should be taken to keep media conditions consistent between runs.
Additional factors that can lead to a failure of the controls to diverge may be from insufficient or excessive digitonin, or pH or other formulation errors in preparing DTEB. Curves that only slowly charge steadily over time may lack sufficient digitonin to allow for buffer and dye movement into the cell and typically happens when digitonin concentrations become less than 0.0005% w/v and resemble unpermeabilized cells (Figure 1A). Too much digitonin results in data that all look like FCCP and rarely rise from it. Excess or insufficient permeabilization can be verified by adding trypan blue to the cells. If none stain blue after 5 min at RT, there is not enough digitonin. For buffer formulation, it may be necessary to prepare a small, fresh batch and retest with a cell line.
Curves will generally start with low fluorescence, increase over the first 30-45 minutes, and then steadily decline for the remainder of the profile. This is due to the warming of the plate from RT to 30 degrees while the latter half is thought to result from damage to the mitochondria over time. Pre-heating the plate and solutions can reduce the charging time but may lead an earlier collapse of ΔΨm in controls. Once a sample reaches maximum fluorescence, it can be useful to note how long it takes the DMSO curve to reach half maximal fluorescence. Some healthy mitochondria will not reach this before the end of the profile. Samples that show collapse of DMSO controls to half maximum in less than 90-120 minutes should be treated with caution as they will be prone to higher background. This behavior typically arises from damaged cells or otherwise fragile mitochondria and may benefit from either a change in isolation procedures or cell culture.
Oligomycin has been included to provide a greater separation of charged and depolarized curves by blocking the ATP synthase and preventing it from allowing protons across the inner mitochondrial membrane. It also prevents the ATP synthase from hydrolyzing ATP to pump protons back across into the matrix to repolarize the inner mitochondrial membrane.However, it is not necessary for BH3 profiling but can provide a sharper depolarization curve when used.
6.2 Conditions favoring FACS based profiling
Fluorescence activated cell sorting, FACS, allows for the simultaneous analysis of populations within heterogeneous samples. Whether it is intractable to enrich for a single population suitable for plate-based profiling or because of a desire to observe many populations under identical conditions, FACS BH3 profiling (Figure 5B) provides mitochondrial response data for any viable population that can be gated.
Like any FACS experiment, care must be taken in the choice of fluorophores used. When few markers are needed and can be placed on 405 or 633 nm laser sources, then JC-1 is a suitable dye that can be used on machines with either a 488 or a 561 nm laser. When a 561 nm laser is available, TMRE is a more suitable dye. In either case, the depolarized FCCP control makes a more suitable compensation control than omitting the dye because cells almost always retain some amount of dye even when depolarized which may not always be localized in the mitochondria.
Except for the sample preparation, the data collection and interpretation of a FACS based profile is dissimilar to any other FACS experiment. Cells should be gated on forward (FSC) and side scatter (SSC) first with measurements of height and width in addition to area to allow for the discrimination of doublets or other higher order cell aggregates, so that these can be excluded from analysis. Using the desired markers, CD19 for B-cells as an example, gates are established to define the cell populations of interest. Once the populations have been identified, an assessment of their ΔΨm can be obtained.
The dynamic range of the ΔΨm measured will vary according to the choice of dye. In all cases, they will occupy a channel on the cytometer, and the data can be treated like any other stain used to label cells. Histograms are often sufficient to show the median of the selected population's charge, but the use of FSC or SSC vs. the dye can be useful to take the variable size of cells in G1 vs. S/G2 phase into account when making gates.
When preparing for FACS BH3 profiling, it is necessary to set the cytometer voltages where they will not exclude data. Dyes such as TMRE or rhodamine 123 are easily measured by adjusting the FCCP control to read approximately 103RFU. Fully charged cells should appear about one log higher in fluorescence, and the voltage can be reduced if cells exceed the range of a histogram.
When using JC-1 on a 488 nm laser in particular, ratios of red to green emission can be done, but a compensation to remove background fluorescence from the green emission peak is necessary to produce a useful dynamic range for the red emission. To use JC-1, the DMSO control should be used to set the green and red fluorescence to 104 initially. The FCCP control can then be used to make the necessary compensation to subtract the green from the red channel. The red channel should now have a range of more than two logs between FCCP and DMSO.
When using a 561 nm laser with JC-1, no compensation on the red color is needed if excited on the 561 nm laser. If the DMSO control is set to just under 105, the dye will often show a range of response over three logs.
In samples that contain large amounts of dead cells and debris, such as digests of primary tissue, the addition of a dye to discriminate between live and dead prior to peptide exposure can be useful. To date, the fixable LIVE/DEAD assay from Invitrogen has been successfully used with JC-1 and TMRE to exclude dead cells in primary samples. (unpublished data) By removing these cells with a gate, they cannot contribute to the measurement of the desired populations and they are used just before staining for cell surface markers.
6.2.1 FACS protocol
Prepare 100 uL 0.002% digitonin (w/v) and 20 ug/mL oligomycin in DTEB per tube.
Add 2 uL of a 100X stock of each treatment to its own tube.
Add 100 uL of the solution prepared in step 1 to each tube and vortex to mix.
Count cells and suspend 1-2 × 105 cells per treatment in 1% BSA in HBSS to a density of 1 × 107 / mL.
(optional) Add a 1:1000 dilution of fixable Live/Dead stain prepared according to manufacturer's instructions. Incubate for 30 min on ice.
Centrifuge cells at 300 xg for 5 min and remove the Live/Dead stain solution if used.
Suspend cells to a density of 1 × 107 cells / mL and add antibodies to stain for 30 min on ice in the dark. Antibodies should be titrated to find their optimal concentration.
Centrifuge cells at 300 xg for 5 min and remove the staining solution.
Suspend the cells in 100 uL DTEB per tube.
Add 100 uL stained cells in DTEB to each tube and gently mix.
Let tubes stand at RT protected from light for 60-180 min.
Prepare 25 uL / tube of either 900 nM JC-1 or 450 nM TMRE in DTEB
30 min before FACS, add 25 uL of the solution made in step 12 to each tube, mix gently.
Perform FACS acquisition.
6.2.2 FACS data analysis
There are two primary ways to analyze BH3 profiling generated by FACS: median fluorescence intensity and gating. The data for both of these methods can often be exported or obtained after acquisition with any number of FACS analysis packages including acquisition software such as Diva or analysis packages like FlowJo. Compensation can also be adjusted at this point if necessary.
Analysis using the median fluorescence intensity (MFI) is simple and uses the same calculation used for processing plate based profiles. As in plate based methods, DMSO and FCCP controls provide the bounds for normalization of the remaining data. This method is often sufficient as long as the sample does not contain a high background of depolarized or partially charged cells in the DMSO control. It also assumes Gaussian distributions, but the data rarely conforms to this.
Instead of relying on the MFI, it is also possible to use gating to determine the number of depolarized and fully charged cells. These gates are readily generated from the DMSO and FCCP control data. Using either a histogram or a plot of ΔΨm dye versus forward or side scatter, the FCCP control provides a simple means of gating on completely depolarized cells. As this gate is defined by the FCCP control, it is referred to as the FCCP or depolarized gate. (Figure 6C) This should contain at least 99% of the cells in the FCCP control. Likewise, a similar gate can be made for DMSO, but most samples will contain a number of partially depolarized cells even without treatment. Therefore, it is better to try to define a gate containing at least 90% of the cells with a lower bound as far away from the FCCP gate upper bound as possible. (Figure 6C)
Figure 6. Setting Gates for FACS-Based BH3 Profiling.
A: Intact singlet cells are first gated by FSC and SSC. B, Subpopulations are gated from the singlet population based on markers A and B. C MMP dye fluorescence from population gated in B is collected from DMSO and FCCP treated samples. Gates are made that contain these populations respectively. D: All other treatments are analyzed using the gates created in steps A-C.
The size of the gates can be altered to change their sensitivity. As defined above, the FCCP is the most stringent gate for depolarization. It can be considered to have a very low sensitivity but high specificity for depolarized cells. Raising the upper bound towards the DMSO gate will increase its sensitivity to perturbations. The DMSO gate is the most sensitive to changes in ΔΨm but does not require cells to be fully depolarized, and this gate is often preferred but is the more difficult of the two to create. The data from either gate can be reported directly as a percentage of the parent population, but it is often useful to report 1 –(DMSO Gate) so that a response is shown as an appearance, rather than a loss, of a signal analogous to the normalized area under curve and median fluorescence measurements.
7. Conclusion
BH3 profiling is a phenotypic assay that can provide information about how dependent a cell is on individual anti-apoptotic family proteins. We have used this application to identify cells that are dependent on MCL-1 or BCL-2. The latter finding is of particular interest, as it may have use in selecting patients best suited to receive innovative BCL-2 antagonists like ABT-263 and ABT-199.
More recently we have been focusing on using BH3 to measure how close a cell is to the threshold of apoptosis, a property we call “priming”. In the study of cancer, we have found that priming correlates with clinical response to chemotherapy in several cancers, including multiple myeloma, ovarian cancer, acute lymphoblastic leukemia, and acute myelogenous leukemia.[8] We are currently investigating whether these findings can be used to create a clinical useful predictive biomarker to help to better assign therapy to patients. Given the difficulty often experienced in linking genotype to phenotype, we anticipate that BH3 profiling may offer useful predictive information even in cases where whole genome sequencing cannot.
The addition of a FACS-based method has allowed us to investigate heterogeneous systems, not only in cancer, but also in normal physiology. For instance, we could discern differential priming among subsets of thymocytes.[9] More recently, we have shown that we can perform BH3 profiling even on very rare subsets such as hematopoietic stem cells.[28] These technical advances open the door to the more detailed study of heterogeneous populations, whether they be malignant or non-malignant. I would suggest that the knowledge of the mitochondrial preconditions that are provided by BH3 profiling will be essential to fully understand cell fate decisions induced by any number of agents, be they conventional chemotherapy, biological compounds, or targeted agents.
Supplementary Material
Highlights.
- Functional assay reports the response from the mitochondrial apoptotic pathway can be measured without prior knowledge of protein levels
- Plate reader assay is suitable for large sample numbers for homogenous samples
- FACS assay can determine priming in sub-populations from heterogeneous samples
- Suitable for any viable single cell suspension including primary samples
Acknowledgements
The authors gratefully acknowledge support from the following sources: NIH grants F31 CA150562, P01 CA139980, and R01CA129974. A.L. is a Leukemia and Lymphoma Society Scholar. A.L. was a cofounder and formerly served on the scientific advisory board of Eutropics Pharmaceuticals, which has a license for BH3 profiling.
Abbreviations
- MOMP
Mitochondrial outer membrane permeabilization
- ΔΨm
mitochondrial transmembrane potential
- MFI
Median fluorescence intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nature reviews Molecular cell biology. 2008;9:231–41. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Molecular cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer cell. 2002;2:183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 6.Brunelle JK, Ryan J, Yecies D, Opferman JT, Letai A. MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts. The Journal of cell biology. 2009;187:429–42. doi: 10.1083/jcb.200904049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer cell. 2007;12:171–85. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–33. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan JA, Brunelle JK, Letai A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12895–900. doi: 10.1073/pnas.0914878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhakdi S, Weller U, Walev I, Martin E, Jonas D, Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Medical microbiology and immunology. 1993;182:167–75. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- 11.Ogino S, Kubo S, Umemoto R, Huang S, Nishida N, Shimada I. Observation of NMR signals from proteins introduced into living mammalian cells by reversible membrane permeabilization using a pore-forming toxin, streptolysin O. Journal of the American Chemical Society. 2009;131:10834–5. doi: 10.1021/ja904407w. [DOI] [PubMed] [Google Scholar]
- 12.Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3185–90. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Bioscience reports. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 14.Lopes SC, Goormaghtigh E, Cabral BJ, Castanho MA. Filipin orientation revealed by linear dichroism. Implication for a model of action. Journal of the American Chemical Society. 2004;126:5396–402. doi: 10.1021/ja031782+. [DOI] [PubMed] [Google Scholar]
- 15.Fiskum G, Craig SW, Decker GL, Lehninger AL. The cytoskeleton of digitonin-treated rat hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:3430–4. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb RA, Granville DJ. Analyzing mitochondrial changes during apoptosis. Methods. 2002;26:341–7. doi: 10.1016/S1046-2023(02)00040-3. [DOI] [PubMed] [Google Scholar]
- 17.Aragon JJ, Feliu JE, Frenkel RA, Sols A. Permeabilization of animal cells for kinetic studies of intracellular enzymes: in situ behavior of the glycolytic enzymes of erythrocytes. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:6324–8. doi: 10.1073/pnas.77.11.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita K, Levine M, Pollard HB. Stimulatory effect of ascorbic acid on norepinephrine biosynthesis in digitonin-permeabilized adrenal medullary chromaffin cells. Journal of neurochemistry. 1986;46:939–45. doi: 10.1111/j.1471-4159.1986.tb13060.x. [DOI] [PubMed] [Google Scholar]
- 19.Cassany A, Gerace L. Reconstitution of nuclear import in permeabilized cells. Methods Mol Biol. 2009;464:181–205. doi: 10.1007/978-1-60327-461-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Xiao N, DeFranco DB. Use of digitonin-permeabilized cells in studies of steroid receptor subnuclear trafficking. Methods. 1999;19:403–9. doi: 10.1006/meth.1999.0876. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Meana M, Abellan A, Miro-Casas E, Agullo E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. American journal of physiology Heart and circulatory physiology. 2009;297:H1281–9. doi: 10.1152/ajpheart.00435.2009. [DOI] [PubMed] [Google Scholar]
- 22.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nature protocols. 2007;2:287–95. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 23.Hogeboom GH, Schneider WC, Pallade GE. Cytochemical studies of mammalian tissues; isolation of intact mitochondria from rat liver; some biochemical properties of mitochondria and submicroscopic particulate material. The Journal of biological chemistry. 1948;172:619–35. [PubMed] [Google Scholar]
- 24.Yamaguchi R, Andreyev A, Murphy AN, Perkins GA, Ellisman MH, Newmeyer DD. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell death and differentiation. 2007;14:616–24. doi: 10.1038/sj.cdd.4402035. [DOI] [PubMed] [Google Scholar]
- 25.Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. The Journal of biological chemistry. 2005;280:2266–74. doi: 10.1074/jbc.M411106200. [DOI] [PubMed] [Google Scholar]
- 26.Corcelli A, Saponetti MS, Zaccagnino P, Lopalco P, Mastrodonato M, Liquori GE, et al. Mitochondria isolated in nearly isotonic KCl buffer: focus on cardiolipin and organelle morphology. Biochimica et biophysica acta. 2010;1798:681–7. doi: 10.1016/j.bbamem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. The Journal of cell biology. 2001;153:319–28. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vo T-T, Ryan J, Carrasco R, Neuberg D, Rossi D, Stone RM, DeAngelo DJ, Frattini MG, Letai A. Relative Mitochondrial Priming of Myeloblasts and Normal HSCs Determines Chemotherapeutic Success in AML. Cell. 2012 doi: 10.1016/j.cell.2012.08.038. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.