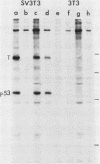
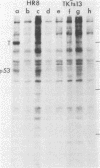
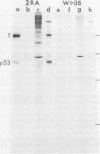
Abstract
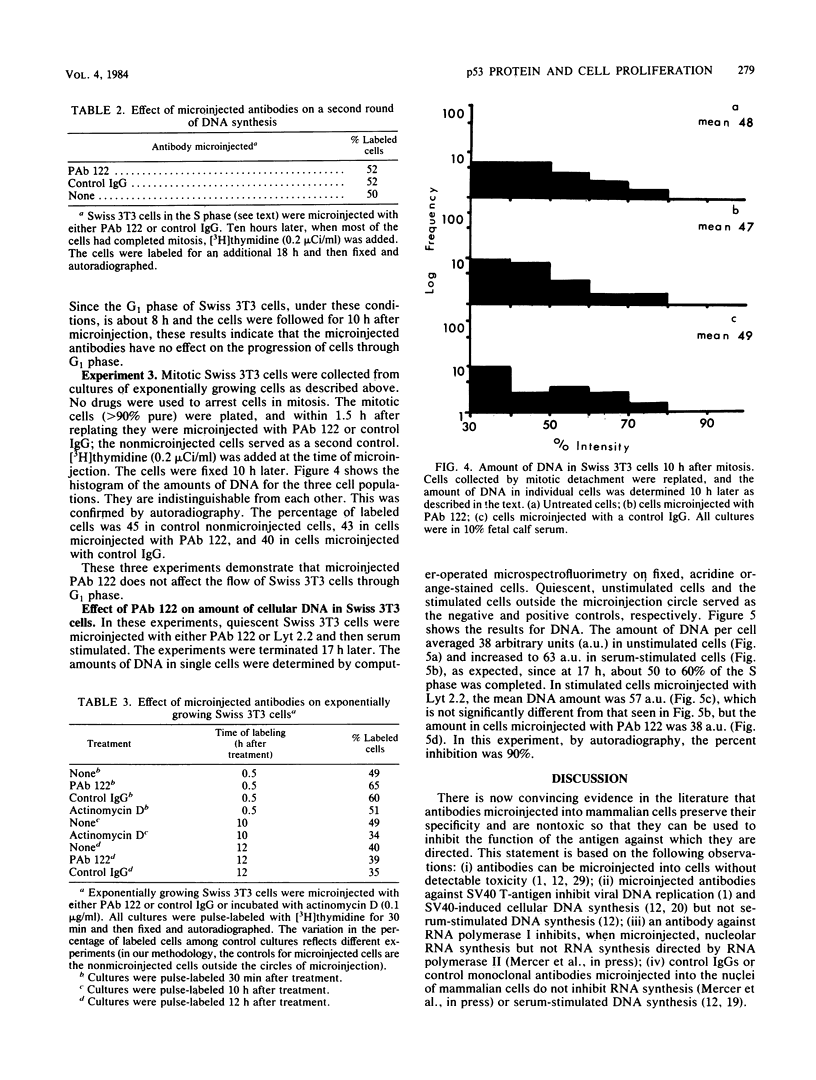
Two monoclonal antibodies against the p53 protein, PAb 122 and 200-47, were microinjected into mammalian cells as a probe to determine the role of the p53 protein in cell proliferation. PAb 122 recognizes the p53 proteins of mouse and human cells but not of hamster cells, whereas 200-47 recognizes the p53 proteins of mouse and hamster cells but not of human cells. The ability of these antibodies to inhibit serum-stimulated DNA synthesis of cells in culture correlates with their ability to recognize the species-specific antigenic determinants. More important, however, is the observation that microinjected PAb 122 inhibits the transition of Swiss 3T3 cells from G0 to S phase, but has no effect on the progression of these cells from mitosis to the S phase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antman K. H., Livingston D. M. Intracellular neutralization of SV40 tumor antigens following microinjection of specific antibody. Cell. 1980 Mar;19(3):627–635. doi: 10.1016/s0092-8674(80)80039-0. [DOI] [PubMed] [Google Scholar]
- Ashihara T., Traganos F., Baserga R., Darzynkiewicz Z. A comparison of cell cycle-related changes in postmitotic and quiescent AF8 cells as measured by cytofluorometry after acridine orange staining. Cancer Res. 1978 Aug;38(8):2514–2518. [PubMed] [Google Scholar]
- Baserga R., Estensen R. D., Petersen R. O. Inhibition of DNA synthesis in Ehrlich ascites cells by actinomycin D. II. The presynthetic block in the cell cycle. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1141–1148. doi: 10.1073/pnas.54.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R., Waechter D. E., Soprano K. J., Galanti N. Molecular biology of cell division. Ann N Y Acad Sci. 1982 Dec 10;397:110–120. doi: 10.1111/j.1749-6632.1982.tb43421.x. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Muello K., Melero J. A. Coordinate expression of the 48K host nuclear phosphoprotein and SV40 T ag upon primary infection of mouse cells. Virology. 1980 Apr 30;102(2):447–452. doi: 10.1016/0042-6822(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Gurney E. G., Goodfellow P., Taylor-Papadimitriou J. Detection of a common feature in several human tumor cell lines--a 53,000-dalton protein. Proc Natl Acad Sci U S A. 1981 Jan;78(1):41–45. doi: 10.1073/pnas.78.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D. P., Staiano-Coico L., Sharpless T. K., Melamed M. L. Correlation between cell cycle duration and RNA content. J Cell Physiol. 1979 Sep;100(3):425–438. doi: 10.1002/jcp.1041000306. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold W. G., Jay G., DeLeo A. B., Khoury G., Old L. J. p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1695–1699. doi: 10.1073/pnas.78.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floros J., Jonak G., Galanti N., Baserga R. Induction of cell DNA replication in G1-specific ts mutants by microinjection of SV40 DNA. Exp Cell Res. 1981 Mar;132(1):215–223. doi: 10.1016/0014-4827(81)90097-5. [DOI] [PubMed] [Google Scholar]
- Gaffney E. V., McElwain E. G. Selective detachment of mitotic cells by hypotonic salt solution. In Vitro. 1973 Jul-Aug;9(1):56–63. doi: 10.1007/BF02615990. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Maltzman W., Levine A. J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., Hyland J. K., Croce C. M., Baserga R. Inhibition of SV40-induced cellular DNA synthesis by microinjection of monoclonal antibodies. Virology. 1983 May;127(1):149–158. doi: 10.1016/0042-6822(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Milner J., McCormick F. Lymphocyte stimulation: concanavalin A induces the expression of a 53K protein. Cell Biol Int Rep. 1980 Jul;4(7):663–667. doi: 10.1016/0309-1651(80)90205-2. [DOI] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V., Boss M. A., Baltimore D. Increased concentration of an apparently identical cellular protein in cells transformed by either Abelson murine leukemia virus or other transforming agents. J Virol. 1981 Apr;38(1):336–346. doi: 10.1128/jvi.38.1.336-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V., Friedman H., Katz A., Zerivitz K., Wolf D. Variation in antigenic determinants of p53 transformation-related protein obtained from various species. J Immunol. 1983 Jul;131(1):329–333. [PubMed] [Google Scholar]
- Ruscetti S. K., Scolnick E. M. Expression of a transformation-related protein (p53) in the malignant stage of Friend virus-induced diseases. J Virol. 1983 Jun;46(3):1022–1026. doi: 10.1128/jvi.46.3.1022-1026.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. M., Hirschhorn R. R., Mercer W. E., Surmacz E., Tsutsui Y., Soprano K. J., Baserga R. Gene transfer: DNA microinjection compared with DNA transfection with a very high efficiency. Mol Cell Biol. 1982 Sep;2(9):1145–1154. doi: 10.1128/mcb.2.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavortink M., Thacher T., Rechsteiner M. Degradation of proteins microinjected into cultured mammalian cells. J Cell Physiol. 1979 Jul;100(1):175–185. doi: 10.1002/jcp.1041000118. [DOI] [PubMed] [Google Scholar]