Abstract
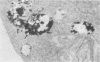
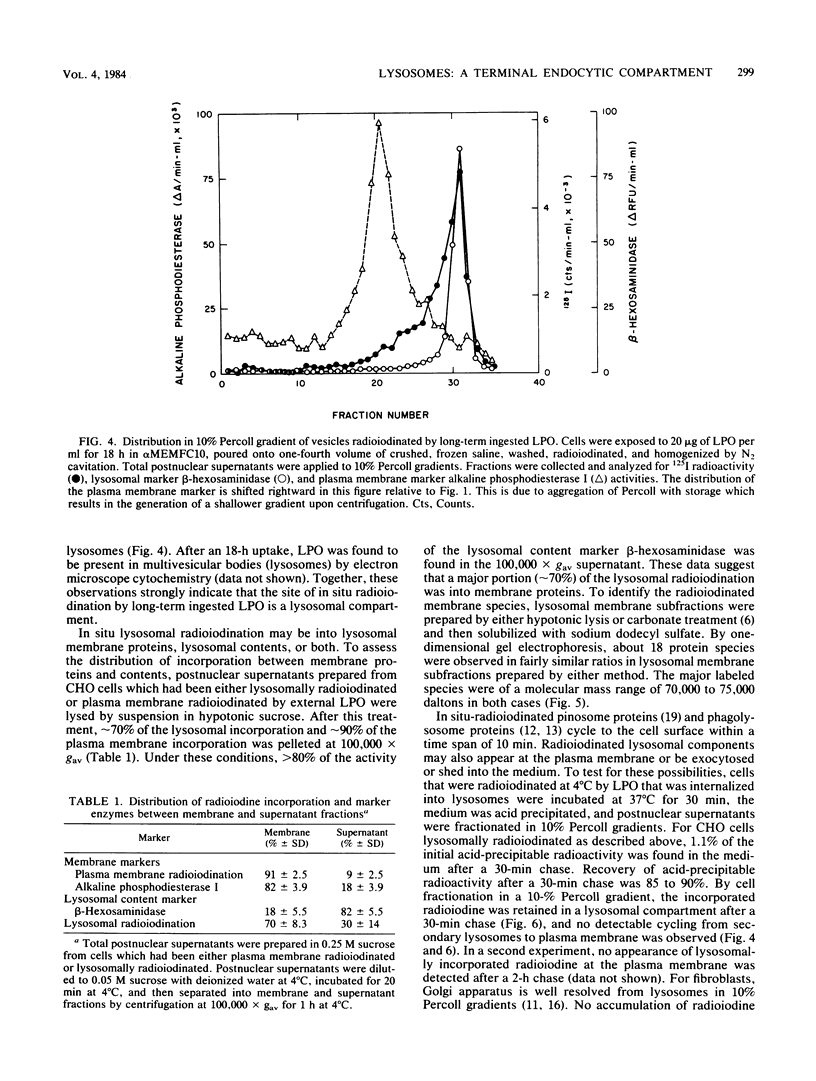
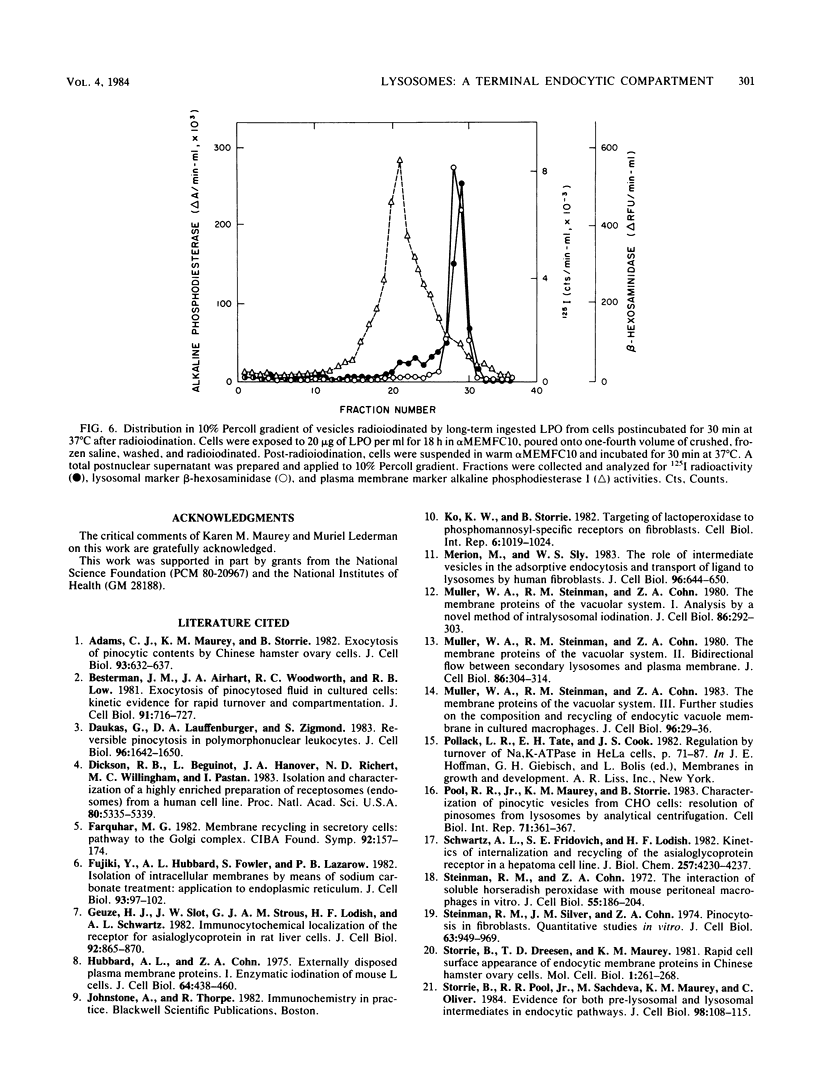
We used Chinese hamster ovary cells, a cell line of fibroblastic origin, to investigate whether lysosomes are an exocytic compartment. To label lysosomal contents, Chinese hamster ovary cells were incubated with the solute marker horseradish peroxidase. After an 18-h uptake period, horseradish peroxidase was found in lysosomes by cell fractionation in Percoll gradients and by electron microscope cytochemistry. Over a 24-h period, lysosomal horseradish peroxidase was quantitatively retained by Chinese hamster ovary cells and inactivated with a t 1/2 of 6 to 8 h. Lysosomes were radioiodinated in situ by soluble lactoperoxidase internalized over an 18-h uptake period. About 70% of the radioiodine incorporation was pelleted at 100,000 X g under conditions in which greater than 80% of the lysosomal marker enzyme beta-hexosaminidase was released into the supernatant. By one-dimensional electrophoresis, about 18 protein species were present in the lysosomal membrane fraction, with radioiodine incorporation being most pronounced into species of 70,000 to 75,000 daltons. After a 30-min or 2-h chase at 37 degrees C, radioiodine that was incorporated into lysosomal membranes and contents was retained in lysosomes. These observations indicate that lysosomes labeled by fluid-phase pinocytosis are a terminal component of endocytic pathways in fibroblasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. J., Maurey K. M., Storrie B. Exocytosis of pinocytic contents by Chinese hamster ovary cells. J Cell Biol. 1982 Jun;93(3):632–637. doi: 10.1083/jcb.93.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Airhart J. A., Woodworth R. C., Low R. B. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981 Dec;91(3 Pt 1):716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G., Lauffenburger D. A., Zigmond S. Reversible pinocytosis in polymorphonuclear leukocytes. J Cell Biol. 1983 Jun;96(6):1642–1650. doi: 10.1083/jcb.96.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Beguinot L., Hanover J. A., Richert N. D., Willingham M. C., Pastan I. Isolation and characterization of a highly enriched preparation of receptosomes (endosomes) from a human cell line. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5335–5339. doi: 10.1073/pnas.80.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Membrane recycling in secretory cells: pathway to the Golgi complex. Ciba Found Symp. 1982;(92):157–183. doi: 10.1002/9780470720745.ch9. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Immunocytochemical localization of the receptor for asialoglycoprotein in rat liver cells. J Cell Biol. 1982 Mar;92(3):865–870. doi: 10.1083/jcb.92.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K. W., Storrie B. Targeting of lactoperoxidase to phosphomannosyl-specific receptors on fibroblasts. Cell Biol Int Rep. 1982 Nov;6(11):1019–1024. doi: 10.1016/0309-1651(82)90017-0. [DOI] [PubMed] [Google Scholar]
- Merion M., Sly W. S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J Cell Biol. 1983 Mar;96(3):644–650. doi: 10.1083/jcb.96.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. Membrane proteins of the vacuolar system. III. Further studies on the composition and recycling of endocytic vacuole membrane in cultured macrophages. J Cell Biol. 1983 Jan;96(1):29–36. doi: 10.1083/jcb.96.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system I. Analysis of a novel method of intralysosomal iodination. J Cell Biol. 1980 Jul;86(1):292–303. doi: 10.1083/jcb.86.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system. II. Bidirectional flow between secondary lysosomes and plasma membrane. J Cell Biol. 1980 Jul;86(1):304–314. doi: 10.1083/jcb.86.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack L. R., Tate E. H., Cook J. S. Regulation by turnover of Na,K-ATPase in HeLa cells. Prog Clin Biol Res. 1982;91:71–87. [PubMed] [Google Scholar]
- Pool R. R., Jr, Maurey K. M., Storrie B. Characterization of pinocytic vesicles from CHO cells: resolution of pinosomes from lysosomes by analytical centrifugation. Cell Biol Int Rep. 1983 May;7(5):361–367. doi: 10.1016/0309-1651(83)90076-0. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Lodish H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982 Apr 25;257(8):4230–4237. [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Dreesen T. D., Maurey K. M. Rapid cell surface appearance of endocytic membrane proteins in Chinese hamster ovary cells. Mol Cell Biol. 1981 Mar;1(3):261–268. doi: 10.1128/mcb.1.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Pool R. R., Jr, Sachdeva M., Maurey K. M., Oliver C. Evidence for both prelysosomal and lysosomal intermediates in endocytic pathways. J Cell Biol. 1984 Jan;98(1):108–115. doi: 10.1083/jcb.98.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]