Abstract
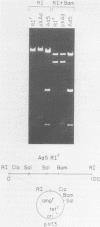
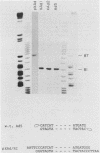
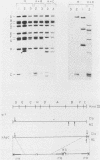
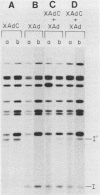
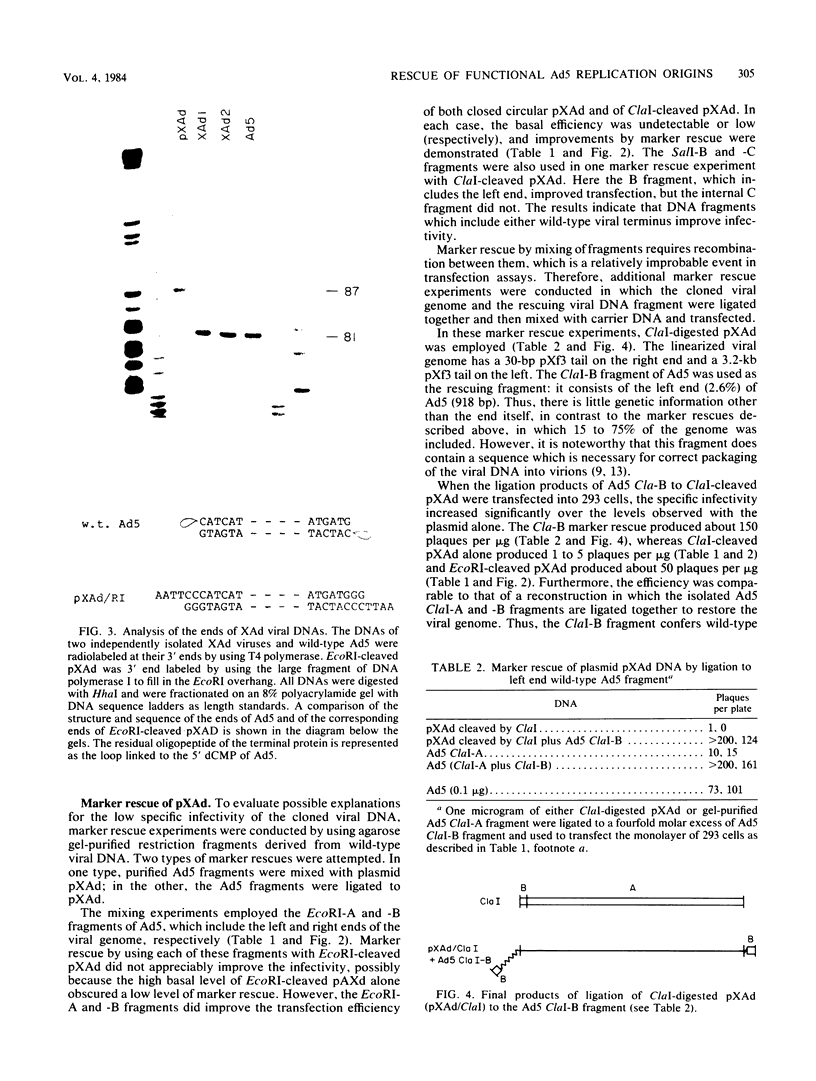
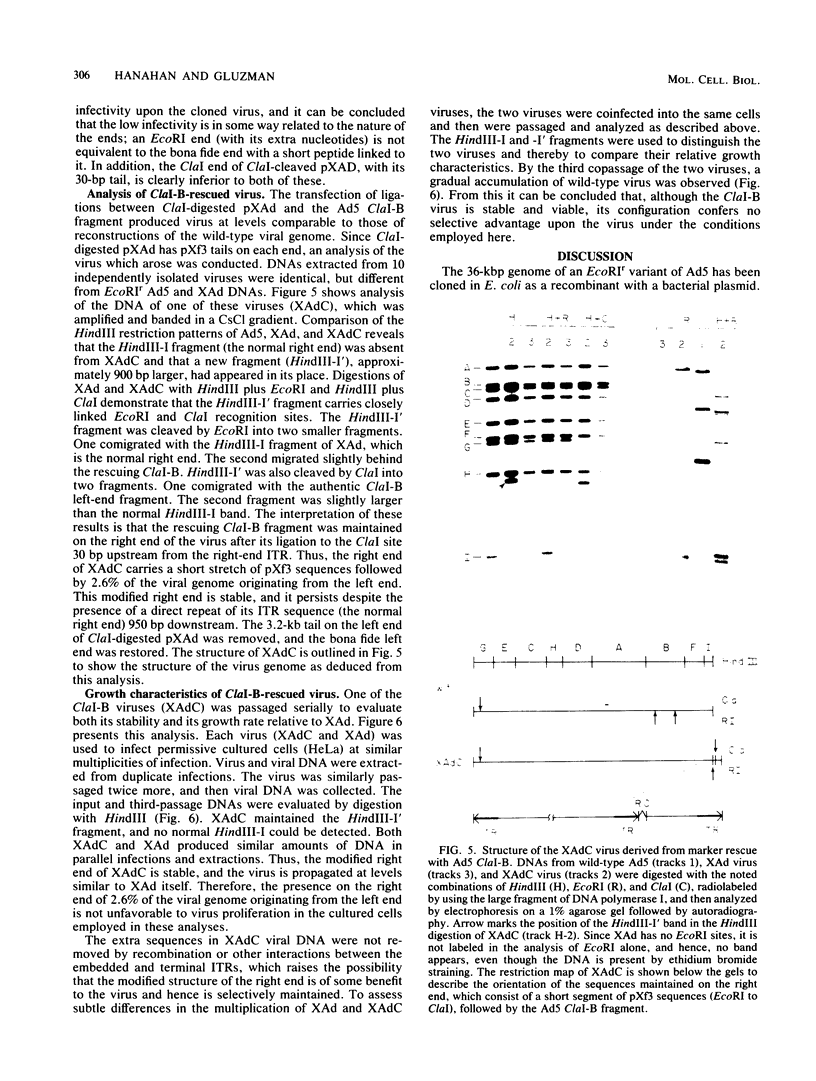
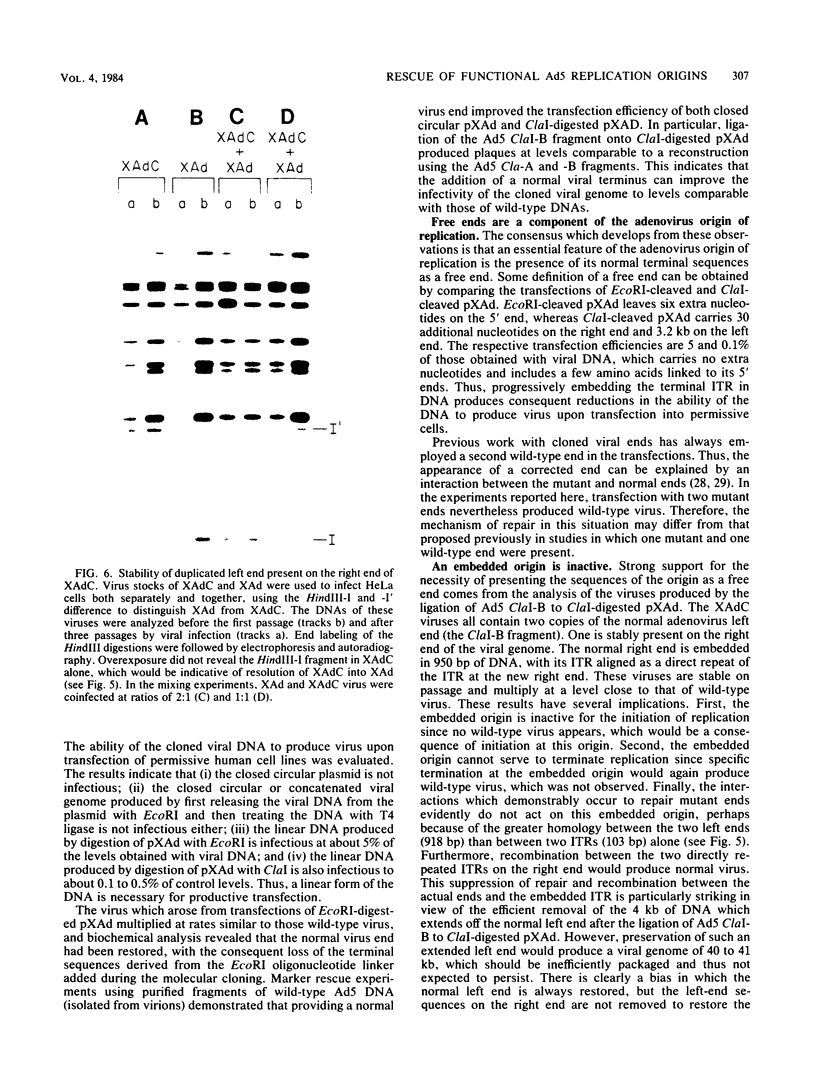
A variant of the adenovirus type 5 genome which lacks EcoRI sites has been cloned in a bacterial plasmid after the addition of EcoRI oligonucleotide linkers to its ends. Closed circular forms of the recombinant viral genome were not infectious upon their introduction into permissive eucaryotic cells. The linear genome released by digestion of the 39-kilobase recombinant plasmid (pXAd) with EcoRI produced infectious virus at about 5% of the level of wild-type controls. The viruses which arose were indistinguishable from the parental strain, and the normal termini of the viral genome had been restored. Marker rescue experiments demonstrate that provision of a DNA fragment with a normal viral end improves infectivity. When a small fragment carrying a wild-type left end (the 0 to 2.6% ClaI-B fragment) was ligated to ClaI-linearized pXAd, virus was produced with efficiencies comparable to a similar reconstitution of the two ClaI fragments of the wild-type genome. These viruses stably carry the left-end fragment at both ends, leaving the normal right end embedded in 950 base pairs of DNA. The embedded right origin is inactive. The consensus of the analyses reported here is that a free end is a necessary configuration for the sequences which make up the adenovirus origin of replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Eukaryotic DNA replication: viral and plasmid model systems. Annu Rev Biochem. 1982;51:901–934. doi: 10.1146/annurev.bi.51.070182.004345. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Processing of the adenovirus terminal protein. J Virol. 1981 Apr;38(1):272–277. doi: 10.1128/jvi.38.1.272-277.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- Deuring R., Klotz G., Doerfler W. An unusual symmetric recombinant between adenovirus type 12 DNA and human cell DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):3142–3146. doi: 10.1073/pnas.78.5.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Van Doren K. Palindromic adenovirus type 5-simian virus 40 hybrid. J Virol. 1983 Jan;45(1):91–103. doi: 10.1128/jvi.45.1.91-103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980 Jul;20(3):787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Chow K. C., Enns R. E., Ahern K. G., Corden J. L., Harpst J. A. In vitro replication directed by a cloned adenovirus origin. Gene. 1983 Sep;23(3):293–305. doi: 10.1016/0378-1119(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Srivastava A., Berns K. I., Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983 May;33(1):135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Sussenbach J. S. The nucleotide sequence of the right-hand terminus of adenovirus type 5 DNA: implications for the mechanism of DNA replication. Gene. 1979 Aug;6(4):307–318. doi: 10.1016/0378-1119(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Cloning of a DNA fragment from the left-hand terminus of the adenovirus type 2 genome and its use in site-directed mutagenesis. J Virol. 1981 Jan;37(1):171–180. doi: 10.1128/jvi.37.1.171-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D. The infectivity of adenovirus genomes lacking DNA sequences from their left-hand termini. Nucleic Acids Res. 1982 Sep 11;10(17):5105–5119. doi: 10.1093/nar/10.17.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Function of adenovirus terminal protein in the initiation of DNA replication. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2221–2225. doi: 10.1073/pnas.79.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Initiation of adenovirus DNA replication in vitro requires a specific DNA sequence. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6446–6450. doi: 10.1073/pnas.80.21.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ormondt H., Maat J., De Waard A., Van der Eb A. J. The nucleotide sequence of the transforming HpaI-E fragment of adenovirus type 5 DNA. Gene. 1978 Dec;4(4):309–328. doi: 10.1016/0378-1119(78)90048-3. [DOI] [PubMed] [Google Scholar]
- van Bergen B. G., van der Ley P. A., van Driel W., van Mansfeld A. D., van der Vliet P. C. Replication of origin containing adenovirus DNA fragments that do not carry the terminal protein. Nucleic Acids Res. 1983 Apr 11;11(7):1975–1989. doi: 10.1093/nar/11.7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]