Abstract
The histological spectrum of viral-associated lymphoid proliferations is quite broad, ranging from reactive lymphadenitis to atypical proliferations mimicking classical Hodgkin lymphoma or non-Hodgkin lymphoma. Virally associated reactive lesions can appear quite alarming on histological examination, because of direct (cytopathic) and indirect viral-induced changes eliciting a polymorphic cellular host response. In addition, the atypical lymphoid proliferation may show aberrant phenotypic features as well as restricted/clonal gene immunoglobulin or T-cell receptor rearrangements, further complicating the interpretation. In order to achieve an accurate diagnosis, it is important to be aware of the clinical history, including family history and ethnic background, clinical presentation, symptoms, and extent of the disease. Among the clinical data, particular emphasis should be placed on serology and viral load studies, and the use of immunosuppressive drugs. The clinical course and outcome vary greatly, from an indolent, self-limited to aggressive clinical course, blurring at times the distinction between neoplastic and reactive proliferations. It is now recognized that immunosenescence also plays a significant role in the development of these viral-associated lymphoid proliferations, and new entities have been described in recent years. In this review we discuss mostly Epstein–Barr virus-associated viral proliferations that may be confused with lymphomas, which the practicing pathologist may encounter.
Keywords: Lymphoid proliferation, EBV, Chronic active EBV, HHV6
Introduction
In general, during the acute phase of a viral infection, there is an active viral replication (the lytic phase) and the host immune response is able to handle the infection, control replication, and eliminate the virus. Several forms of persistent, chronic, or latent infection have been recognized. We refer to a chronic infection when there is a continuous viral replication and high systemic viral load (Human Immunodeficiency Virus) and when the infections are not readily controlled by the host immune system (Hepatitis B and C Virus). Evidence of continued infection beyond 6 months is arbitrarily required for the process to be considered chronic. In cases of persistent or latent infection, no viral progeny is usually produced, since only limited transcription and translation of the viral genome occur, such as in Herpes viruses, Epstein–Barr Virus (EBV), Herpes Simplex Virus (HSV) and Varicella–Zoster Virus (VZV) infections; in reactivation usually a larger set of genes is transcribed, but it is often non-productive and clinically asymptomatic, and only occasionally can it lead to a full replicative phase with the expression of more than 80 viral genes and release of a new viral progeny.
EBV
EBV is a gamma herpes virus (HHV4) and linear double-stranded DNA virus, with worldwide distribution. It is orally transmitted and most people become infected in the course of their life. When the primary infection occurs in children (>90% positive by age 5) it is usually asymptomatic or a self-limited brief viral illness like many others. In the US and other developed countries, the primary acute infection often occurs later in life (adolescence or early adult) and causes acute infectious mononucleosis (AIM) in 35–50% of cases.1 The virus is transmitted through saliva. EBV is highly immunogenic and at the first contact (acute primary infection) elicits a strong immune response against lytic antigens-mediated predominantly by CD8 and CD4 positive T cells. This strong immune response then forces the virus into a lifelong immunologically silent, latent state.2 How the virus infects naïve B cells and ends up surviving in memory B cells has been the focus of extensive research since its discovery in 1964 by Epstein.2 Briefly, by combining in vivo and in vitro observations, it has been postulated that EBV infects through the tonsillar epithelium naïve B cells.3 In acute primary infection, the pre-dominant T-cell population, composed of naive T cells, needs time to mount a targeted response to the new pathogen and indirectly allows the virus to promote cellular proliferation of the infected naïve B cells into naïve blasts by transitioning from G0 to G1 and by switching viral promoters (Wp to Cp), leading to the expression of EBV nuclear antigen-2 (EBNA-2) with subsequent differentiation through the germinal center. The subsequent expression of all 6 EBNAs and LMP-1 and LMP-2 mimics the signaling required by the B cells to proliferate, differentiate, and establish their fate within the germinal centers (mimicking CD40 and BCR signaling). By shutting off BCL-6 (mediated through LMP-1), the B cells will exit the germinal center as latently infected memory B cells.4 The sequential expression of viral proteins, also mediated by micro-RNAs (miRNA), exploits the cellular genes involved in mature B-cell proliferation and differentiation and may explain the ability of the virus to transform a variety of B cells at different stages of differentiation.5
It is important also to underline the fact that as a result of the primary infection, the T-cell repertoire is modified with the induction of virus specific cytotoxic T cells (CTLs). Indeed, it has been shown that the diversity of the T-cell repertoire is more limited and less effective in individuals who have difficulties in clearing the primary acute infection (persistent/chronic infection), while it is broader in healthy sero-positive individuals.6 The limited repertoire also accounts for the detection of oligoclonal/clonal T-cell populations in AIM patients, since these are antigen driven and comprising few dominant clones.7,8 These clonal bursts rapidly decay upon resolution of the AIM, and the TCR repertoire returns to normal.8
The main compartment of latently infected cells is the memory B cell. At this stage of latency, the viral gene expression is limited to two small non-coding, non-polyadenylated RNAs (EBER1 and 2) EBV and a set of transcripts from BamA rightward transcript (BART) RNAs with no expression of protein coding transcript, also known as latency 0, which allows the virus to evade the immune system and become immunologically silent.2 EBERs are the only transcripts expressed in latently infected cells in great abundance (greater than 106 copies), which allow us to use them as target for in situ hybridization on tissue sections. EBERs functions are still largely unknown, but it has been shown that they can induce interleukin-10 and interact with cellular proteins and may play a role in antiviral innate immunity.9
The full range of latent gene expression, so-called latency type III, may be observed in the setting of immunosuppression, such as in post-transplant lymphoproliferative disorders (PTLDs) as well as in EBV immortalized cell lines (so-called lymphoblastoid cell lines). Other forms of latency in between latency types 0 and III, namely type I and type II, are usually associated with EBV-associated malignancies, both lymphoid and epithelial, and show a limited expression pattern (EBNA-1 and LMPs).2
Acute primary EBV infection, consistent with acute infectious mononucleosis
The histological features of tonsils or lymph nodes in patients with AIM vary greatly, ranging from follicular and paracortical hyperplasia to marked paracortical expansion, and occasionally to a worrisome proliferation of large immunoblasts with Reed–Sternberg-like (RS-like) cells. This range of changes may be observed even in the same biopsy (often tonsil) and may be helpful in reaching a diagnosis. Inmost cases, areas of necrosis are present and mitoses may be easily seen. Foci of monocytoid B cells may also be present. The majority of the histological changes are thought to be due to the immune response elicited by the virus to control the infection and often, because of the tissue damage, EBV may infect other cell types besides B cells. RS-like cells may be easily identified and occasionally cluster near the areas of necrosis. Usually, in contrast to classical Hodgkin lymphoma (CHL), the RS-like cells show a greater range in cell size and are often associated with typical immunoblasts (Fig. 1). Immunohistochemical stains may be helpful, since these cells tend to be CD30 positive and usually lack expression of CD15 (Fig. 1D). They are positive for B-cell markers, such as CD20, Pax-5, and CD79a (weak) (Fig. 1F). Typically, the background T cells are numerous and predominantly CD8 positive with an inversed CD4:CD8 ratio. Usually one of the most helpful findings is the marked and diffuse positivity for EBV by in situ hybridization with only a subset of LMP-1 positive cells (Fig. 1E). On tonsillar sections, the infected cells (EBER positive) are mostly interfollicular with only few intrafollicular positive cells.10 In situ hybridization marks more dramatically the variation in cell size among the positive cells, which is very uncharacteristic for CHL (Fig. 1E). From a practical standpoint, in cases of suspected AIM, it is better to perform in situ hybridization for EBV over immunohistochemistry; however, if the quality of the fixation of the tissue is sub-optimal or the necrosis is a prominent feature, immunohistochemistry for LMP may be informative.
Fig. 1.
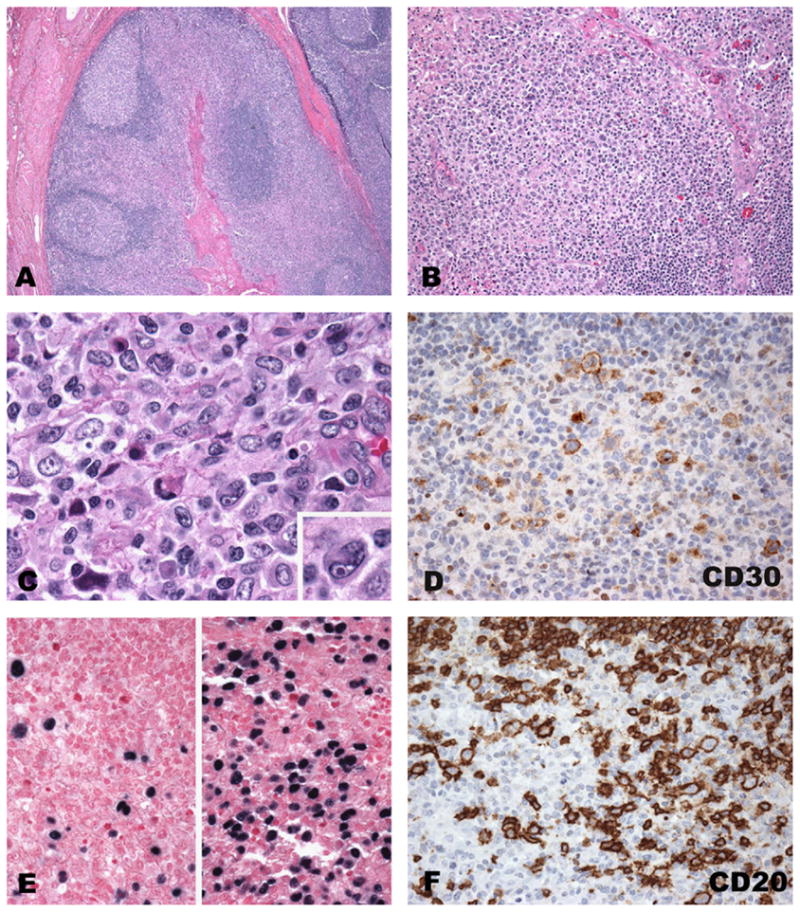
Acute infectious mononucleosis involving tonsils. (A) Low power revealing architectural preservation with reactive secondary B follicles and expanded interfollicular areas with a polymorphic cellular composition and focal necrosis (B and C). Large atypical cells with Reed–Sternberg-like features (inset C) are positive for CD30, EBV and CD30 (D–F). In situ hybridization for EBV (E) reveals a great variation in the number of positive cells and their size (separate fields, same tonsil).
In cases with marked immunoblastic proliferation, if molecular studies for IGH and TCR gene rearrangement are performed, they usually show a polyclonal pattern and often a restricted/oligoclonal pattern, respectively.
Because of the diagnostic difficulties that a case of AIM may pose, leading to different therapeutic implications, it is important to obtain a detailed clinical history, including serologic studies and viral load. A negative Paul–Bunnell heterophile antibody test (monospot) does not preclude the diagnosis of acute primary EBV infection, since it can be negative in 10–15% of cases, particularly in children younger than 10 years of age. Other serologic tests are needed to confirm the diagnosis of AIM and rule out the possibility of other viral illnesses with similar clinical presentation. For a primary infection, serologic tests are the method of choice.1
In routine reactive tonsils or lymph nodes, in which AIM is not suspected, the number of EBV-positive cells varies greatly, but they are usually small lymphocytes, present in small numbers and predominantly found in the interfollicular areas (60%), followed by tonsillar crypt epithelium (27%) and germinal centers (15%).11 An accumulation of EBV-positive cells within germinal centers has been described in immunodeficiency states, either primary or secondary, including HIV associated.12
In vivo EBV is also capable of infecting other hematopoietic cells, such as T- and NK cells, as well as epithelial and mesenchymal cells; however, in vitro systems are much less well characterized mainly due to the inability to maintain long term culture of EBV virally infected T- or NK cells. Most neoplastic conditions associated with these cell types show type II latency.
Chronic active EBV (CAEBV)
CAEBV was originally described by Dr. Stephen Straus13 as a disease related to chronic or persistent EBV infection. It was described as a severe illness lasting over 6months subsequent to acute EBV infection, with persistent elevated titers of EBV and evidence of organ damage in patients without evidence of an underlying immunodeficiency. Based on the Western experience, it was initially viewed as a progressive EBV infection targeting B cells; however, over the years, the term CAEBV has been used, especially in Japan and Korea, to identify a clinical syndrome primarily associated with EBV infection of T cells or NK cells.14 The current definition of CAEBV includes the following criteria: (1) follows an acute EBV infection with chronic EBV infection of B-, T-, or NK cells; (2) clinically presents with fever, lymphadenopathy, and hepatosplenomegaly; (3) demonstrates increased EBV DNA in the peripheral blood (104–107 EBV genomes/106 cell); and (4) demonstrates EBV-EBER positive cells in tissues.15
In our experience, CAEBV of B-cell type is very rare, in comparison with T-cell type, and tends to occur in a slightly older population (mean age 23 y versus 7 y). Patients show persistent lymphadenopathy, often lasting several years and, less frequently, show evidence of hemophagocytic syndrome; however, a preceding acute EBV infection is not documented in all patients. Many of these patients have a progressive loss of B cells and develop hypogammaglobulinemia.16 Histologically, the lymph nodes show features often resembling a polymorphic post-transplant lymphoproliferative disorder (PTLD) with paracortical expansion, numerous immunoblasts admixed with cells with plasmacytoid differentiation, plasma cells, and occasional RS-like cells. EBV by in situ hybridization shows numerous EBV-positive B cells mainly in the expanded paracortex ranging in cell size from small to large. In some of the cases with multiple biopsies, histological progression towards a monomorphic PTLD-type lesion may be observed (Fig. 2A–F). Immunoglobulin gene rearrangement may be polyclonal/oligoclonal with a restricted T-cell pattern for TCR.
Fig. 2.
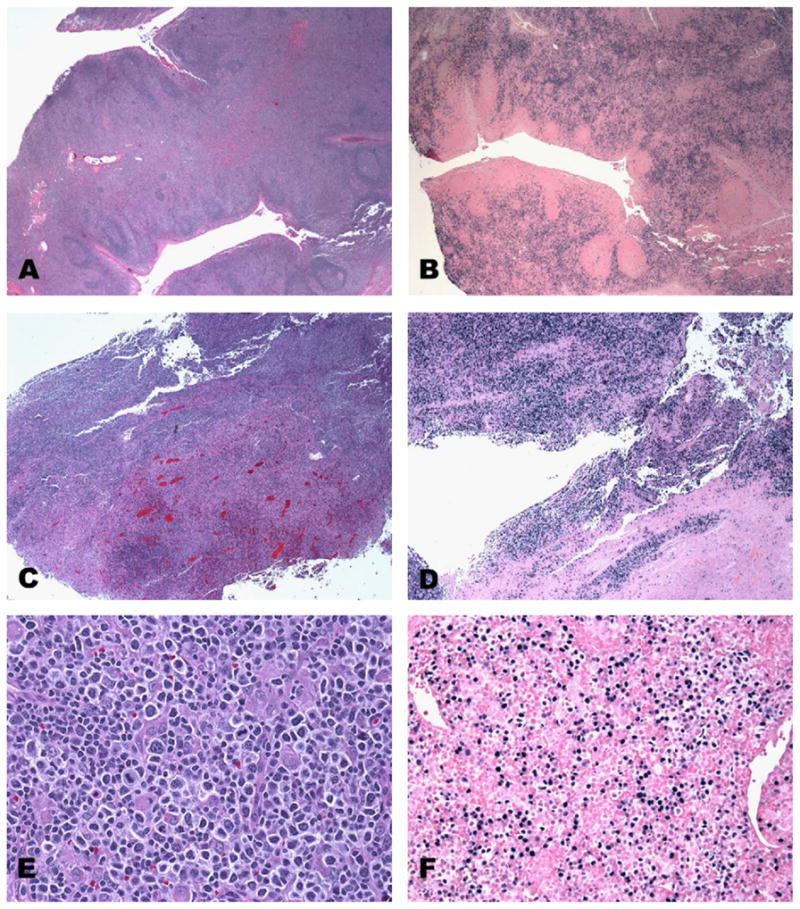
CAEBV of B-cell type. (A–F) The three panels represent 3 separate biopsies (tonsil and two lymph nodes) from the same young woman with persistent EBV infection. (A, C and E) H&E stained sections, (B, D and F) EBV in situ hybridization using EBER probe, showing the diffuse and marked positivity through time and the progressive increase in necrosis and architectural effacement.
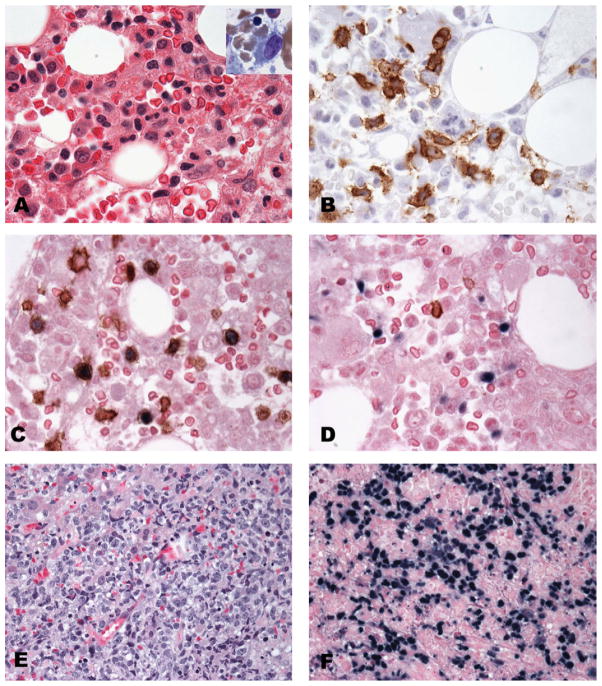
CAEBV of T-cell or NK-cell type is most common, but not limited, to the pediatric age group in Asians, and Native American populations from Mexico, Peru, and Central America. It is rare in Caucasians and African-Americans. The term T/NK/CAEBV has been used to include a range of lymphoproliferative disorders (LPDs) with a broad spectrum including polyclonal, oligoclonal and monoclonal proliferation of cytotoxic T and/or NK cells. All patients have elevated EBV viral loads at presentation. The majority of patients have evidence of systemic disease presenting with high fevers, hepatomegaly, splenomegaly, and lymphadenopathy with subsequent pancytopenia related to hemophagocytic syndrome.17,18 Ohshima et al.19 proposed to classify CAEBV T/NK into 4 categories, based on cytological atypia and clonality as follows: A1, polymorphic LPD, polyclonal; A2, polymorphic LPD, clonal; A3, monomorphic LPD, clonal; and category B monomorphic LPD clonal with fulminant clinical course. The latter categories (A3 and B) are equivalent to systemic EBV-positive T-cell LPD of childhood (also known as infantile fulminant EBV-associated T-LPD, fatal hemophagocytic syndrome or severe CAEBV).20 For a systemic and clonal process, the terminology of the WHO classification is preferred, i.e. systemic EBV-positive T-cell lymphoproliferative disease of childhood,21 and although the disease may arise in a background of CAEBV, it usually follows shortly after primary acute EBV infection and has a rapidly fatal clinical course with hemophagocytic syndrome. Common sites of involvement are liver and spleen followed by lymph nodes and bone marrow. Histologically, the infiltrating T cells show minimal cytological atypia, although cases with marked atypia and pleomorphism have also been described (Fig. 3A–F). The atypical infiltrate is usually sinusoidal in liver and spleen and often associated with prominent hemophagocytosis. EBV-EBER is uniformly positive in the cytotoxic T cells (typically CD3+, CD2+, CD56−, and TIA-1+; expression of CD4 and CD8 was not consistent) with clonal TCR rearrangement (Fig. 3C and D).
Fig. 3.
CAEBV of T-cell type. (A–D) Bone marrow involvement by a slightly atypical lymphoid infiltrate. Hemophagocytosis best appreciated on the bone marrow aspirate (inset A). The scattered T cells are positive for CD8 (panel B), with EBV infecting CD3 positive T cells as shown by double in situ hybridization and immunohistochemistry for CD3 (C), CD20 positive B cells are negative for EBV (D). Massive liver involvement showing a greater degree of cytologic atypia (different patient) (E) with strong and diffuse positivity for EBV by in situ hybridization (F).
Cases with minimal cytologic atypia may be easily missed if EBV in situ studies are not performed. The severity of the clinical presentation and laboratory findings may contrast with the lack of significant histological changes, and this discrepancy should prompt EBV investigation. Another important sign is the presence of hemophagocytosis that can be appreciated more easily in the liver biopsy and bone marrow aspirates (Fig. 3A and B).
Hydroa vacciniforme-like (HV-like) lymphoma is also considered a CAEBV infection, predominantly involving T cells with a similar epidemiology to systemic EBV-positive T-cell lymphoproliferative disorder of childhood.21,22 It is considered a pediatric EBV-positive cutaneous T-cell lymphoma, most commonly involving sun-exposed areas and often with a chronic clinical course with worsening of cutaneous symptoms and eventual systemic dissemination.21 Most, but not all, cases show clonal T-cell gene rearrangement and it is not clear whether the T-cell clonality is always predictive of a more aggressive clinical behavior.15
Also severe mosquito bite allergy is considered part of the spectrum of CAEBV with a similar ethnic distribution as HV and HV-like T-cell lymphoma. It has been more frequently, but not uniquely, associated with NK-cell disease characterized by rash with severe skin reaction, fever, liver dysfunction, high IgE levels, and circulating large granular lymphocytes.21,23 Despite a broad clinical spectrum, about half of the patients die of HPS or lymphoproliferative disorders.24
Age-related EBV lymphoid proliferations
EBV reactivation also plays a central role in the development of lymphoid proliferative processes in the context of primary and iatrogenic immunodeficiencies. With aging, a reduced ability to handle infectious diseases also occurs and it is considered as part of the physiological aging process. However, the phenomenon of immunosenescence is multifactorial, involving both innate and adaptive arms of the immune system, and is still poorly understood. Numerous factors and complex mechanisms are involved in the remodeling of the immune system during the aging process, such as alteration of T-cell homeostasis due to thymic involution with dramatically decreased output of naive T cells and accumulation of certain specific lifelong memory CD8+ T cells, which together have a dramatic effect in reducing the diversity of the T-cell pool. Other events include telomere shortening, T-cell transduction changes and alterations, impaired DNA repair, and antioxidant mechanisms.25
Oyama et al.26 recently described an EBV-positive LPD in elderly Japanese patients with striking similarities to polymorphic and monomorphic forms of post-transplant LPD. The overall clinical behavior was aggressive with frequent extranodal presentation, but the polymorphic group seemed to have a better prognosis (p=0.003), at least in their initial report. The EBV-positive cells were of B-cell lineage expressing CD20 and or CD79a in all cases with variable expression of CD30 in the large pleomorphic cells, which were negative for CD15. The type of latency was either II (the majority of cases) or III as seen in PTLD (based on EBNA-2 stain in addition to uniform expression of LMP in all cases). In the subsequent larger series,27 the overall survival was poor in both polymorphic and monomorphic groups and inferior to EBV negative diffuse large cell lymphoma. This led to inclusion in the WHO classification (2008)21 as a provisional entity. In reviewing our cases of age-related EBV LPDs, we identified a subset of patients with localized extranodal disease, manifesting as mucocutaneous ulcer28 with an indolent clinical course, and a high rate of spontaneous remission. Although a subset of patients had received immunosuppressive therapy, the majority did not, suggesting a common underlying pathogenetic mechanism of reduced immunosurveillance at specific anatomic sites. Typical features include well-delineated, shallow ulcers with atypical cells at the base of the ulcers often with RS-like cells with variable expression of CD20/CD79a, often CD30 positive, and CD15 positive (about 50% of cases). The infiltrate tends to be superficial with an underlying rim of reactive T cells.
We also identified another subset of patients within the age-related LPDs with a good prognosis and relatively low risk to develop lymphoma.29 These patients tended to be younger and presented with localized nodal disease with a high rate of spontaneous resolution and excellent overall survival. The lymph nodes show a variable degree of follicular and paracortical hyperplasia. Monocytoid B-cell reactions and epithelioid granulomas were also variably present. CD20 and CD30 marked the frequent immunoblasts seen in the paracortical areas, but the immunoblasts lacked CD15 expression. EBV-EBER was either restricted to germinal centers or sparing them and dispersed through the paracortex. Molecular studies revealed a polyclonal pattern for IGH and evidence of a restricted T-cell repertoire in a quarter of the cases.29
In our experience, the pathological spectrum of age-related LPD is broader than that previously reported by Oyama et al.27 In order to recognize these cases it is important to perform in situ hybridization for EBV, since some of the early lesions may be easily missed. Most cases show expanded paracortex with a polymorphic infiltrate with plasmacytoid differentiation and immunoblasts with features resembling RS cells. In general, by immunohistochemistry, CD20 expression is variable, while CD79a is more diffusely positive with strong expression of Pax-5 and Oct-2. Also CD30 is usually positive, while co-expression of CD15 is more variable. This phenotype raises the possibility of CHL. Besides the lack of the appropriate inflammatory infiltrate usually present in CHL, the EBV-positive cells are usually more numerous with a great range in cell size and they are not limited to the large RS-like cells. In our experience, CD15 expression may occur in age-related LPD and it is not synonymous with CHL-EBV-positive, as suggested by Oyama et al.27 and Asano et al.30
HHV6
Human herpes virus 6 (HHV6) is a beta herpes virus, nearly ubiquitous with a broad distribution worldwide with a sero-prevalence approaching 100%. Two variants are recognized HHV6A and B, which are closely related. No disease is associated with type A, while type B is associated with exanthem roseola infantum (exanthem subitum) of infancy. Both are also opportunistic pathogens in immunocompromised hosts. The majority of infections in healthy infants are caused by HHV6B, which preferentially infects CD4 positive cells through the surface marker CD46, which acts as co-receptor. 31,32 CD46 (human membrane cofactor protein, MCP) is a central component of the innate immune system, thus explaining the broad tissue tropism of the virus. The immunosuppression is enhanced by the CD4 T-cell depletion, which may occur at early developmental stages in the thymus. The virus persists in a latent state, infecting macrophages/monocytes in kidneys, brain, and salivary glands.
The primary infection is usually asymptomatic and it is widespread among infants between 6 months and 2 years of age; however only 17% develop roseola. Febrile seizures may also occur during primary infection with HHV6. Rare complications include hepatitis, arthritis, encephalopathy, and hemophagocytic syndrome.31 Immunosuppression may lead to reactivation and cause severe limbic encephalitis. HHV6 has been implicated also as possible etiologic agent for multiple sclerosis, myocarditis, encephalitis, and acute liver failure. Detection of the virus by PCR in peripheral blood mononuclear cells or CSF is often positive in asymptomatic individuals, while the detection by immunohistochemistry of infected cells has been elusive. HHV6 remains in the host for life after primary infection, and the actual site or the cell type of latency has yet to be identified. Possible candidates include monocytes, macrophages, and early bone marrow progenitor cells.
We have previously described two cases of viral HHV6 lymphadenitis occurring in immunocompetent hosts and presenting as an acute viral illness.33 Histologically the lymph nodes show marked paracortical expansion due to a proliferation of CD4 positive T cells with nuclear and cytoplasmic inclusions, as shown by the strong positivity with an antibody against the envelope glycoprotein gp60/110 kDa. The presence of the virus was also confirmed by electron microscopy and by molecular studies.
Summary
The histological spectrum of viral-associated (EBV, HHV6) lymphoid proliferations is quite broad, ranging from reactive lymphadenitis to extremely atypical proliferations mimicking malignant lymphoma. However, by being aware of the histological, serologic, and molecular results that may occur in these viral-associated lymphoid proliferations and correlating this information with the specific clinical history, including family history and ethnic background, clinical presentation, symptoms, and extent of disease, the appropriate diagnosis and clinical management should be reached.
Footnotes
Supported in part by the Intramural Research Program, National Cancer Institute, NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen JI. Epstein–Barr virus infection. N Engl J Med. 2000;343(7):481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 2.Rickinson AB, Kieff E. Epstein–Barr Virus. 5. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 3.Thorley-Lawson DA. Epstein–Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1(1):75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 4.Roughan JE, Torgbor C, Thorley-Lawson DA. Germinal center B cells latently infected with Epstein–Barr virus proliferate extensively but do not increase in number. J Virol. 2010;84(2):1158–1168. doi: 10.1128/JVI.01780-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalla M, Hammerschmidt W. Human B cells on their route to latent infection—early but transient expression of lytic genes of Epstein–Barr virus. Eur J Cell Biol. 2011 doi: 10.1016/j.ejcb.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Bharadwaj M, Burrows SR, Burrows JM, Moss DJ, Catalina M, Khanna R. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following primary Epstein–Barr virus infection. Blood. 2001;98(8):2588–2589. doi: 10.1182/blood.v98.8.2588. [DOI] [PubMed] [Google Scholar]
- 7.Callan MF, Steven N, Krausa P, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2(8):906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 8.Callan MF. The evolution of antigen-specific CD8+ T cell responses after natural primary infection of humans with Epstein–Barr virus. Viral Immunol. 2003;16(1):3–16. doi: 10.1089/088282403763635401. [DOI] [PubMed] [Google Scholar]
- 9.Iwakiri D, Takada K. Role of EBERs in the pathogenesis of EBV infection. Adv Cancer Res. 2010;107:119–136. doi: 10.1016/S0065-230X(10)07004-1. [DOI] [PubMed] [Google Scholar]
- 10.Niedobitek G, Hamilton-Dutoit S, Herbst H, et al. Identification of Epstein–Barr virus-infected cells in tonsils of acute infectious mononucleosis by in situ hybridization. Hum Pathol. 1989;20(8):796–799. doi: 10.1016/0046-8177(89)90075-0. [DOI] [PubMed] [Google Scholar]
- 11.Hudnall SD, Ge Y, Wei L, Yang NP, Wang HQ, Chen T. Distribution and phenotype of Epstein–Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol. 2005;18:519–527. doi: 10.1038/modpathol.3800369. [DOI] [PubMed] [Google Scholar]
- 12.Niedobitek G, Herbst H, Young LS, et al. Patterns of Epstein–Barr virus infection in non-neoplastic lymphoid tissue. Blood. 1992;79(10):2520–2526. [PubMed] [Google Scholar]
- 13.Straus SE. The chronic mononucleosis syndrome. J Infect Dis. 1988;157(3):405–412. doi: 10.1093/infdis/157.3.405. [DOI] [PubMed] [Google Scholar]
- 14.Kimura H, Hoshino Y, Kanegane H, et al. Clinical and virologic characteristics of chronic active Epstein–Barr virus infection. Blood. 2001;98(2):280–286. doi: 10.1182/blood.v98.2.280. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein–Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. 2009;20(9):1472–1482. doi: 10.1093/annonc/mdp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein–Barr virus disease: a 28-year experience in the United States. Blood. 2011;117(22):5835–5849. doi: 10.1182/blood-2010-11-316745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura H. Pathogenesis of chronic active Epstein–Barr virus infection: is this an infectious disease, lymphoprolifertaive disorder, or immunodeficiency? Rev Med Virol. 2006;16:251–261. doi: 10.1002/rmv.505. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H, Morishima T, Kanegane H, et al. Prognostic factors for chronic active Epstein–Barr virus infection. J Infect Dis. 2003;187(4):527–533. doi: 10.1086/367988. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima K, Kimura H, Yoshino T, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Inter. 2008;58(4):209–217. doi: 10.1111/j.1440-1827.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 20.Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96(2):443–451. [PubMed] [Google Scholar]
- 21.WHO. Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: IARC; 2008. [Google Scholar]
- 22.Barrionuevo C, Anderson VM, Zevallos-Giampietri E, et al. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. App Immunohistochem Mol Morphol. 2002;10(1):7–14. doi: 10.1097/00129039-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Satoh M, Oyama N, Akiba H, Ohtsuka M, Iwatsuki K, Kaneko F. Hypersensitivity to mosquito bites with natural-killer cell lymphocytosis: the possible implication of Epstein–Barr virus reactivation. Eur J Dermatol. 2002;12(4):381–384. [PubMed] [Google Scholar]
- 24.Rodriguez-Pinilla SM, Barrionuevo C, Garcia J, et al. EBV-associated cutaneous NK/T-cell lymphoma: review of a series of 14 cases from Peru in children and young adults. Am J Surg Pathol. 2010;34(12):1773–1782. doi: 10.1097/PAS.0b013e3181fbb4fd. [DOI] [PubMed] [Google Scholar]
- 25.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck- Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2010 doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27(1):16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13(17):5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- 28.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer—a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34(3):405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117(18):4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano N, Yamamoto K, Tamaru J, et al. Age-related Epstein–Barr virus (EBV)-associated B-cell lymphoproliferative disorders: comparison with EBV-positive classic Hodgkin lymphoma in elderly patients. Blood. 2009;113(12):2629–2636. doi: 10.1182/blood-2008-06-164806. [DOI] [PubMed] [Google Scholar]
- 31.Dockrell DH. Human herpesvirus 6: molecular biology and clinical features. J Med Microbiol. 2003;52(Pt 1):5–18. doi: 10.1099/jmm.0.05074-0. [DOI] [PubMed] [Google Scholar]
- 32.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99(7):817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 33.Maric I, Bryant R, Abu-Asab M, et al. Human herpesvirus-6-associated acute lymphadenitis in immunocompetent adults. Mod Pathol. 2004;17(11):1427–1433. doi: 10.1038/modpathol.3800179. [DOI] [PMC free article] [PubMed] [Google Scholar]