Abstract
Multiple myeloma, a clonal plasma cell malignancy, has long provided a prototypic model to study regulatory interactions between malignant cells and their microenvironment. Myeloma-associated macrophages have historically received limited scrutiny but recent work points to central and non-redundant roles in myeloma niche homeostasis. The evidence supports a paradigm of complex, dynamic and often mutable interactions between macrophages and other cellular constituents of the niche. We and others have shown that macrophages support myeloma cell growth, viability and drug resistance through both contact-mediated and non-contact-mediated mechanisms. These tumor-beneficial roles have evolved in opposition to, or in parallel with, intrinsic pro-inflammatory and tumoricidal properties. Thus, simple blockade of protective ‘don't eat me’ signals on the surface of myeloma cells leads to macrophage-mediated myeloma cell killing. Macrophages also enhance the tumor-supportive role of mesenchymal stem/stromal cells (MSCs) in the niche: importantly, this interaction is bidirectional, producing a distinct state of macrophage polarization that we termed “MSC-educated macrophages”. The intriguing pattern of cross-talk between macrophages, MSCs and tumor cells highlights the myeloma niche as a dynamic multicellular structure. Targeted reprogramming of these interactions harbors significant untapped therapeutic potential, particularly in the setting of minimal residual disease, the main obstacle towards a cure.
Multiple myeloma and macrophages: a long-neglected link
Multiple myeloma, a malignant disorder of plasma cells, is the second most common hematological malignancy with approximately 20,000 new diagnoses per year in the United States [1,2]. Its premalignant phase, monoclonal gammopathy of undetermined significance (MGUS), is common in the general population, affecting 4% of Caucasians over the age of 50 [3]. Dramatic changes in the therapeutic landscape in last 10-15 years have prolonged the median survival from 3 years to 6 years or more [4], but the disease remains largely incurable.
Myeloma cells are dependent on microenvironmental interactions for their homeostasis under steady-state conditions, as well as to evade stress, such as pharmacological agents administered for therapy [5-7]. We and others have hypothesized that relapse following effective antiproliferative therapy may reflect the persistence of residual tumor cells within tumor-protective, drug-resistant niches in the bone marrow [8-13]. Whether minimal residual disease consists of a distinct tumor cell subpopulation with enhanced self-renewal, and whether this subpopulation is fully committed to the plasma cell lineage, are topics of active investigation and intense debate at present [14,15]. Regardless of the precise identity of the clonal component of minimal residual disease, macrophages are necessary for proper niche orchestration and homeostasis (Figure 1). In this review article, we delineate regulatory interactions between macrophages and other cellular constituents of the myeloma niche and suggest potential therapeutic approaches to redirect these interactions against myeloma tumor cells, particularly in the setting of minimal residual disease [16,17].
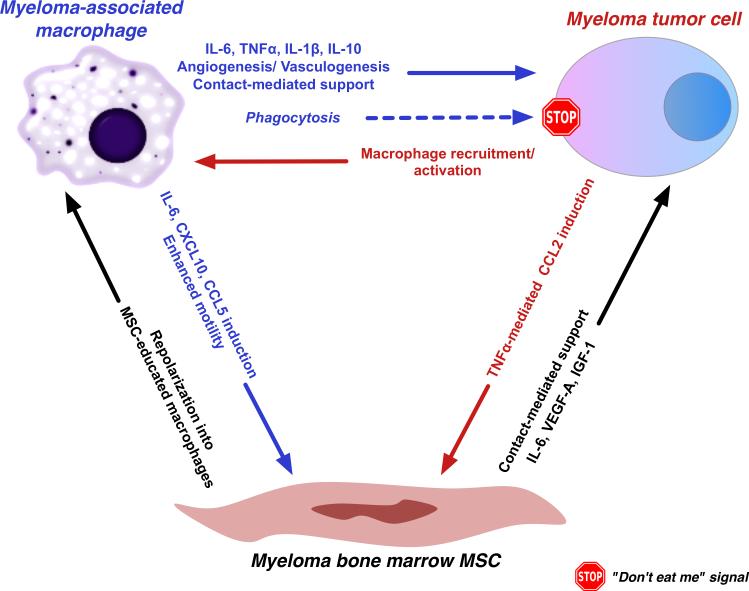
Figure 1. Regulatory interactions between macrophages, mesenchymal stem/stromal cells (MSCs) and malignant plasma cells in the myeloma niche.
Macrophages directly support malignant plasma cells through contact-mediated interactions, cytokine secretion and indirectly, through orchestration of the “angiogenic switch” and an immunosuppressive environment conducive for tumor cell propagation. These tumor-beneficial roles are balanced by inherent tumoricidal and phagocytic properties of activated macrophages. Myeloma-associated macrophages also engage in bidirectional interactions with mesenchymal stem/stromal cells (MSCs) and the latter, in turn, modulate the polarization state of macrophages (“MSC-educated macrophages”, see text) as well as provide direct support to tumor cells.
Macrophages in hematological malignancies: the more you look, the more you find
Macrophages have emerged as important regulators of cancer-associated inflammation, the seventh hallmark of cancer [18,19]. Although the mechanisms of tumor promotion by tumor-associated macrophages (TAM) have been mostly established from study of solid tumors [20], investigation into the role of tumor-associated macrophages in the evolution of hematological malignancies has recently gained momentum. In lymphoma, increased macrophage infiltration is associated with adverse prognosis, albeit with exceptions. This association appears strongest in the case of Hodgkin's lymphoma [21-23] and more tenuous in non-Hodgkin's lymphomas. Among lymphoma subtypes in the latter category, the presence of large numbers of CD68+ macrophages has been associated with poor prognosis in follicular lymphoma [24,25] but results have been variable in diffuse large cell lymphoma (DLBCL) [26,27]. However, when appropriate markers were used to differentiate between “classically-activated” (or M1-polarized macrophages) and “alternatively-activated” (or M2-polarized macrophages) on DLBCL biopsies, a correlation between macrophage infiltration and adverse outcome was again seen [28] (see below for definition of macrophage polarization states).
In circulating (“liquid”) hematological malignancies, there is some evidence to suggest that macrophages constitute important components of the tumor niche, or site of propagation of clonogenic progenitors. “Proliferation centers” in chronic lymphocytic leukemia (CLL) contain abundant numbers of macrophages and non-macrophage stromal elements [29]. While the significance of the presence of macrophages in these structures needs further study, it is likely that these macrophages also contribute to the survival of clonogenic malignant cells. It is interesting that macrophages in CLL proliferation centers are STAT1-positive, resembling “classically-activated” macrophages. Recent evidence presented at the 2012 American Society of Hematology Meeting suggested that selective depletion of macrophages from an animal model of polycythemia vera could ameliorate clinical manifestations of disease such as spleen size and importantly, the hematocrit, a surrogate of total red cell mass [30]. Therefore, even in “liquid” hematological malignancies, macrophages are likely to have important roles in supporting clonogenic progenitors in the tumor niche, whether located in the bone marrow or peripheral lymphoid organs.
Macrophage polarization in myeloma: nuances in concepts and phenotypes
Macrophages are key components of the myeloid infiltrate of most tumors [20,31]. Tumor-associated macrophages (TAMs) arise from in situ maturation of recruited circulating monocytes [32]. Tumors, including myeloma, secrete monocyte-attractant chemokines, such as CCL2 and MIP-1α, abundantly [33,34]. The notion that myeloma-associated macrophages derive from recruited monocytes and not from bone marrow-resident monocytic precursors is further supported by two facts: Firstly, extramedullary plasma cell tumors (plasmacytomas) are rich in tumor-associated macrophages [35]. Secondly, hematopoietic activity in myelomatous bone marrow is suppressed, partly due to the cytostatic effects of cytokines such as TGFβ [36]. Once recruited to the microenvironment of the nascent tumor, monocytes acquire a pro-inflammatory profile (“classically-activated” or “M1-polarized”)[37]. In the case of myeloma, macrophage activation within the early lesion may be multifactorial. Myeloma cells secrete inflammatory mediators that promote macrophage activation [38-42]. Mere local tissue disruption from tumoral expansion may elicit or augment innate and adaptive inflammatory responses. Consistently with the notion that the myeloma microenvironment fosters “smoldering” inflammation, myeloma tumor cells express, and respond to signaling from, a broad range of Toll-like receptors [39,43]. Toll-like receptors in the myeloma microenvironment may bind to exogenous ligands carrying pathogen-associated molecular patterns (PAMPs) (components of bacteria and viral pathogens) and/or endogenous ligands carrying danger-associated molecular patterns (DAMPs), such as fibronectin, soluble hyaluronan, heat shock proteins, or endogenous RNA, released in the context of tissue damage, extracellular matrix breakdown, cellular stress, or cell death [43]. Activated oncogenes (particularly RAS homologues) or novel oncogene products (e.g., fusion proteins) may directly elicit potent pro-inflammatory responses [44,45]. Whatever the relevant signals, macrophage activation promotes the synthesis and secretion of pro-inflammatory mediators such as TNFα, IL-1β, IL-6, IL-8 and others [46]. These factors enhance malignant cell growth, protect from stress-inducing stimuli and promote genetic instability and malignant clonal evolution [47]. As tumors expand and progress, selective pressures incite macrophages to acquire characteristics of “alternative activation” or “M2 polarization” [33,48]. M2 macrophages are better suited to carry out tissue–remodeling, to aid local invasion, to promote angiogenesis and to orchestrate a locally immunosuppressive microenvironment.
Although the recognition of macrophage plasticity has been of enormous value in understanding macrophage-mediated modulation of the tumor microenvironment in line with the evolving requirements of the growing tumor, the very concept of “polarization” has led to oversimplification. It is important for M1 and M2 states to be understood as extremes of a dynamic continuum rather than as mutually exclusive cell fates. Importantly, “intermediate” states of macrophage activation may be better suited to the physiology of specific tumor types compared to either of the extremes. Myeloma and activated-type diffuse large B cell lymphoma are prime examples illustrating this principle. Both tumor types are characterized by constitutive NFκB signaling that, in most cases, is non-cell autonomous [49-51]. Macrophages in the immediate tumor microenvironment must be capable of elaborating pro-inflammatory cytokines that elicit constitutive NFκB activity in the tumor cell, particularly when this activity is not conferred by cell-autonomous mutations [52,53]. This “intermediate” state of macrophage polarization may be distinct from “tolerization” [54], because of the continued robust expression of inflammatory mediators [55]. However, the pro-tumoral role of macrophage activation comes at the price of enhanced cytolytic and tumoricidal activity that must be curbed to allow tumor progression (Figure 1). Moreover, distinct states of macrophage polarization may not only be tumor stage-specific but also tumor site-specific. Macrophages in the expanding invasive rim of the tumor secrete pro-inflammatory cytokines and tissue-remodeling enzymes to allow local invasion as well as recruitment of subsequent waves of inflammatory cells [56]. By contrast, the necrotic center of the tumor is likely to require the presence of M2 macrophages to promote tissue remodeling and angiogenesis [57]. Thus, macrophages at different stages of polarization may co-exist within the same myeloma lesion. Lastly, macrophage polarization is not fixed but may be modulated therapeutically. Several investigators have used therapeutic approaches to reprogram macrophage polarization to elicit strong tumoristatic or tumoricidal effects. Work from the Sondel laboratory has shown that therapeutic activation of macrophages results in effective anti-tumor activity, including in B cell malignancies, that is particularly beneficial in cases where defects in adaptive immunity have arisen as the result of therapy or the tumor pathophysiology per se [58-69] (see section on “therapeutic implications”, below). Other investigators have also shown the feasibility and value of this approach in hard-to-treat cancers, such as pancreatic carcinoma [70].
Macrophage activation in the myeloma niche: the central role of TPL2 kinase
In an early landmark study, Brian Durie highlighted the importance of macrophages as a source of paracrine IL-6 in myeloma [71]. Depletion of macrophages from co-cultures with myeloma cells dramatically reduced the growth rate of the latter, an effect simulated by IL-6 depletion. However, it was not clear why macrophages co-cultured with myeloma cells were able to produce excess IL-6. The authors proposed a feedback loop between macrophages and tumor cells in the myeloma niche. According to this model, stimulation of macrophages either by myeloma cells or other components of the microenvironment led to production of excess IL-6 by macrophages which, in turn, promoted myeloma growth and iterative waves of macrophage activation.
These early findings were corroborated and expanded in recent work by our groups [8,55]. We showed that macrophages promoted growth and decreased apoptosis of myeloma cell lines in combined cultures [55]. These tumor-beneficial effects of macrophages were observed in co-cultures with myeloma cells of various genotypes. The growth-promoting effect of macrophages was partially abrogated following treatment with an IL-6-neutralizing antibody. Furthermore, we showed that CD14+ monocytic cells freshly explanted from myeloma bone marrow expressed much higher amounts of IL-6 transcript compared to fresh peripheral blood monocytes from normal donors or macrophages derived from normal peripheral blood monocytes [55]. Mesenchymal stem/stromal cell (MSC)-educated macrophages (MEM, see relevant section below) also expressed high amounts of IL-6 mRNA. Indeed, the inhibitory effect of neutralizing anti-IL-6 antibody was most pronounced in three-way co-cultures of myeloma cells with macrophages and MSCs.
To begin to understand the mechanisms underlying these findings, we focused on TPL2 (Cot, MAP3K8), a serine/threonine kinase with central and non-redundant roles in regulating innate immune responses and cytokine secretion in macrophages [72-75]. TPL2 modulates multiple signal transduction pathways and regulates several pro-inflammatory (TNFα, IL-1β, IL-6, IL-12) and anti-inflammatory cytokines (IL-10, IL-1RA) [73,76-80]. The balance of pro- and anti-inflammatory activities of TPL2 is tissue- and context specific. Our recently published data demonstrated constitutive activation of pro- and anti-inflammatory, TPL2-dependent pathways in myeloma-associated monocytes/macrophages [8]. Furthermore, we established a cell-autonomous effect of TPL2 activity on myeloma cell growth that was attenuated in myeloma cells carrying activating MAPK pathway mutations. Lastly, we observed that TPL2 was activated in myeloma tumor cells undergoing mitosis, raising the possibility that TPL2 activity may be particularly important for regulation of dividing clonogenic progenitors. Our findings support a model in which TPL2 promotes homeostasis of the myeloma niche by both cell-autonomous and non-autonomous mechanisms (Figure 2). Interference with TPL2 activity may disrupt crucial cross-talk between macrophages and tumor cells in the myeloma niche (see section on “therapeutic implications” below).
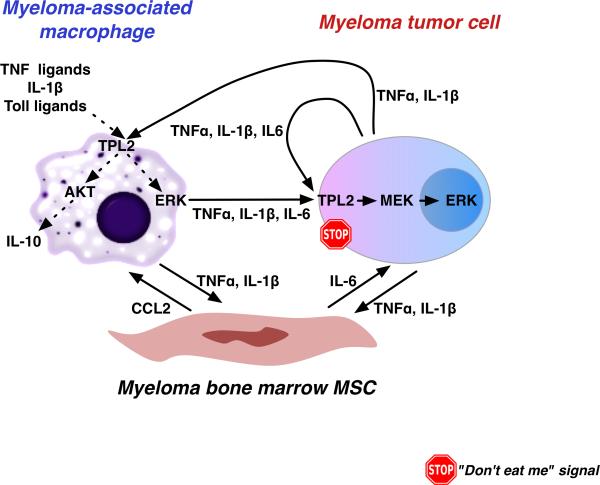
Figure 2. TPL2 kinase regulates myeloma growth through tumor cell-autonomous and non-autonomous mechanisms, the latter involving myeloma-associated macrophages.
TPL2 is a key regulator of cytokine secretion by myeloma-associated macrophages. In malignant plasma cells, TPL2 activates downstream MAP kinases in response to growth and inflammatory signals. Targeted inhibition of TPL2 activity may disrupt crucial regulatory cross-talk between macrophages and malignant cells in the myeloma niche.
Macrophages promote survival of myeloma cells through both contact-mediated and non-contact mediated mechanisms
Work from the Yi laboratory established macrophages as an important component of the myeloma microenvironment by showing that physical interactions between macrophages and tumor cells activate signaling pathways that protect myeloma cells from apoptosis induced by drug treatment [81]. These investigators generated macrophages by treating monocytes with M-CSF followed by treatment with supernatant from cultured myeloma cells to induce the tumor-associated macrophage phenotype. These macrophages were able to prevent drug-induced apoptosis by inhibiting the caspase pathway, especially caspase 3 and PARP. Macrophage-mediated protection from apoptosis was observed with both myeloma cell lines and primary myeloma cells and required direct cell-to-cell contact. Interestingly, IL-6 did not appear to contribute to protection of myeloma cells against drug-induced apoptosis. A subsequent study from the same laboratory established a mechanism behind these observations [82]. They found that the interactions between PSGL-1 and ICAM-1 on myeloma cells and E/P selectins and CD18 on macrophages, respectively, allowed macrophages to protect myeloma cells from drug-induced apoptosis. The interactions stimulated SRC, ERK1/2 kinases and c-MYC and suppressed drug-induced caspase activation.
Contact-mediated support of myeloma cells by macrophages likely acts in concert with non-contact mediated mechanisms. Indeed in our previous study [55], we showed that macrophages protected myeloma cells from apoptosis even when physically separated in transwell plates. Together with findings by the Yi group, our results suggest that macrophages protect myeloma cells from apoptosis through both contact-mediated and non-contact mediated mechanisms. The former may be more important in the setting of drug-induced apoptosis.
Macrophages and osteoclasts belong to the same hematopoietic cell lineage and may support myeloma cell survival and growth, as well as protect myeloma cells from drugs, through common molecular mechanisms (e.g. production of pro-inflammatory cytokines and growth factors [83]). This may suggest that while myeloma cells localized in focal lesions and close to bone surface are protected by osteoclasts, myeloma cells that are localized within the diffuse marrow may be similarly supported by macrophages. Osteoclasts and macrophages may also use common pathways in the induction of angiogenesis in myeloma lesions (see next section) [84].
A bone-resident subpopulation of macrophages has been recently described (osteal macrophages, “OsteoMacs”) [85,86]. Osteal macrophages appear to be involved in bone remodeling and local immunosurveillance. Their role in myeloma niche orchestration and bone pathology merits further investigation and may be significant because they have been shown to be capable to respond to inflammatory stimuli [87] and elaborate pro- and anti-osteoclastogenic cytokines such as TNFα, IL-6, IL-1 and interferon-β. Moreover, OsteoMacs may serve as a pool of osteoclast precursors within myeloma bone lytic lesions.
Macrophages in myeloma angiogenesis and vasculogenesis
Myelomas are highly vascularized tumors and increased vascular density imparts a poor prognosis [88]. Microvascular density in myeloma bone marrows was recently reported to correlate with the prevalence of CD163+ macrophages [89]. Therefore, macrophages are likely to be central orchestrators of the “angiogenic switch” in myeloma, similar to other tumors [90]. Macrophages are likely to promote neoangiogenesis through both cytokine secretion and physical contribution to the generation of a vascular network.
A major mechanism behind angiogenic induction by macrophages is through secretion of vascular endothelial growth factor- A (VEGF-A) in poorly vascularized areas of tumors [91]. A paracrine loop between myeloma cells and stroma ensures robust induction of angiogenesis as myeloma lesions expand [92]: macrophages secrete VEGFs to promote myeloma cell growth and angiogenesis, leading to further waves of myeloma cell-derived secretion of VEGF-A and basic fibroblast growth factor (bFGF) that directly contributes to angiogenesis but also induces stromal cells to secrete VEGF-C and –D that stimulate myeloma cell growth through VEGFR-3 in a self-perpetuating loop [93]. Additionally, activated macrophages synthesize nitric oxide, leading to vasodilation and enhanced angiogenesis [94]. Lastly, the angiogenic factors secreted by macrophages stimulate mast cell migration [95,96], and mast cells contribute to angiogenesis [97,98].
Intriguing observations from the Ribatti and Vacca groups have highlighted direct, structural contributions of macrophages to the myeloma blood vessel network by “vasculogenic mimicry” [99]. In a study by Scavelli et al. [100], they reported that bone marrow macrophages from myeloma patients assumed a vascular endothelial cell-like phenotype when activated with VEGF and basic fibroblast growth factor (bFGF). In contrast, macrophages from healthy donors, non-active myeloma and monoclonal gammopathy of unknown significance (MGUS) did not exhibit similar behavior. Importantly, the angiogenic and vasculogenic properties of bone marrow macrophages in myeloma were inhibited following treatment with the proteasome inhibitor, bortezomib, as well the bisphosphonate, zolendronic acid [101].
Macrophages in the myeloma niche: friend or foe?
The literature presented above supports the hypothesis that macrophages are integral components of the myeloma niche and support myeloma cell growth and viability under both steady-state and stress conditions as well as promote niche angiogenesis and vasculogenesis. However, recent work from the Weissman lab has shown that macrophages also possess inherent tumoricidal potential that would be detrimental for malignant plasma cells if the latter did not express protective “don't eat me” signals [102]. Indeed, myeloma cells, both primary cells and lines established in culture, universally upregulate CD47, an integrin-associated receptor protein. CD47 interacts with SIRPa on the surface of myeloma-associated macrophages to deliver a potent anti-phagocytosis (“don't eat me”) signal [103]. Simple inhibition of this interaction by a CD47-blocking antibody elicits frank tumoricidal responses by macrophages, resulting in tumor regression in xenotransplantation models, including models utilizing human primary myeloma cell grafts into human fetal bone implants [102]. Importantly, anti-CD47 antibody bound on myeloma cells did not induce complement-mediated lysis or antibody-dependent cell-mediated cytotoxicity (ADCC). These results demonstrate that macrophages in the myeloma niche display inherent anti-tumor potential and simple perturbation of the balance between macrophage activation and defenses put up by myeloma cells suffices to elicit macrophage-mediated tumor regression.
MSC-educated macrophages: a novel subtype of the alternatively-activated macrophage?
Bone marrow mesenchymal stem/stromal cells (MSCs) are thought to play major regulatory roles in the myeloma microenvironment [5,7,13,104]. Myeloma cells receive key supportive signals from MSCs [105-107], including MSC-derived cytokines that are important for growth and survival of myeloma cells [108-111]. Moreover, cell adhesion is thought to be another mechanism by which bone marrow MSCs support myeloma cell survival [112,113].
Macrophages induce MSCs to express IL-6, CCL5, and interferon gamma-induced protein-10 (CXCL10) and to exhibit increased mobility in response to multiple soluble factors produced by macrophages including IL-8, CCL2, and CCL5 [114]. Macrophage-MSC cross-talk is bidirectional: the interaction results in a distinct state of macrophage polarization that we have termed “MSC-activated macrophages” (MEM) [115]. MEMs bear many phenotypic characteristics of M2 polarization, such as expression of the surface marker CD206. However, the pattern of cytokine secretion by MEMs is unique: high IL-10, low IL-12, low TNFα and high IL-6. Whereas high IL-10 and low IL-12 levels are characteristic of the “alternatively-activated” M2 phenotype, the continued expression of high levels of IL-6 sets MEMs apart from classical M2 macrophages, although there is some recent evidence to suggest that IL-6 may have a role in M2 polarization [116]. With regard to TNFα, it should be noted that low-level tonic stimulation of myeloma cells may be more optimal for tumor propagation compared to acute or steep surges in availability of this pleiotropic cytokine in the tumor microenvironment [117]. Moreover, TNFα signaling may become pro-tumoral in the presence of RAS mutations, a frequent genetic alteration in myeloma [118-120]. This MEM-specific cytokine profile was corroborated by the findings of Zhang et al., who showed that MSC-conditioned medium induced higher expression of IL-6 and IL-10 but lower level of TNFα in macrophages [121]. MSCs also modulated cytokine release by macrophages in a study by Maggini et al. [122]. The pattern of cytokine expression characteristic of MEMs renders this cell type particularly suitable for the support of tumor cells in the myeloma niche.
MSCs modulate macrophage polarization in the niche but also they orchestrate monocyte recruitment to sites of active tumor cell propagation. Thus, in follicular lymphoma, MSCs in the niche upregulate CCL2 to recruit inflammatory monocytes [123]. Follicular lymphoma-derived MSCs cooperate with macrophages to sustain malignant B cell growth, at the same time as skewing macrophage polarization towards a pro-angiogenic and LPS-unresponsive phenotype [123]. Similar results were recently confirmed in mouse models of lymphoma [124,125]: interestingly, the ability of lymphoma-derived MSCs to promote lymphoma growth was abolished in CCR2-null mice, demonstrating that MSC support of tumor cells required the recruitment and presence of monocytes/macrophages. Control marrow-derived MSCs acquired the tumor-promoting properties of lymphoma-derived MSCs when pre-treated with TNFα [124]. Thus, bidirectional crosstalk between MSCs and macrophages orchestrates a tumor-protective niche.
Macrophages in myeloma therapy: repolarization versus depletion
The last decade has witnessed the advent of several new therapies for multiple myeloma [2,126]. In addition to cytotoxic chemotherapy, including high-dose chemotherapy followed by autologous stem cell rescue (autologous transplant), proteasome inhibitors [127] and thalidomide analogues (thalidomide, lenalidomide) are used [128,129]. These approaches have prolonged survival for many myeloma patients. Myeloma therapies mainly act by direct antiproliferative effect on tumor cells. “Immunomodulatory” activities have been ascribed to thalidomide analogues based on in vitro observations [128], however the significance of the immunomodulatory effect in vivo is unclear, particularly as they are often co-administered with potent immunosuppressive agents, primarily steroids [130,131]. It is important to note however, that an inhibitory effect of thalidomide and its analogues on TNFα production by LPS-stimulated monocytes was recognized early [132,133]. Compared with thalidomide, inhibition of TNF〈 was 2000-fold more potent with lenalidomide and 20000-fold more potent with pomalidomide [134]. These observations suggest that thalidomide analogues may directly modulate the activation/polarization status of myeloma-associated monocytes/macrophages.
Despite the success of traditional and novel agents, myeloma remains virtually incurable. Current therapies, including autologous transplantation, cannot eradicate the disease in most patients and even after allogeneic transplantation, relapses are frequently seen [135]. We hypothesize that the persistence of residual tumor cells nested within tumor-protective niches constitutes a major mechanism for relapse and that macrophage-tumor cell interactions are crucial determinants of the homeostasis of the myeloma niche. Therefore, targeted approaches to redirect these interactions may overcome the limits of current therapies and potentially lead to a cure.
Four possible approaches to therapeutically exploit macrophage activation and function in the myeloma niche can be envisaged. First, reprogramming of macrophages to an overtly activated phenotype through M1-polarizing signals (eg. CD40-agonistic antibody). Second, interference with signaling pathways that promote shift to an “alternatively activated” M2 phenotype or activate anti-inflammatory responses, e.g., through IL-10 inhibition or TPL2 blockade. Third, inhibition of “don't eat me signals” on myeloma tumor cells (anti-CD47 antibody). Fourth, interference with monocyte recruitment to the niche (eg. through CCL2-CCR2 axis inhibition) or selective depletion of tumor-associated macrophages. Combinations of any of these approaches might have additive or synergistic effects.
Therapeutic repolarization of macrophages to an “unopposed” M1 phenotype has been achieved following the administration of signals that directly and potently activate intracellular pro-inflammatory pathways. Pioneering work from the Sondel group has demonstrated that it is possible to repolarize macrophages through administration of a first, or “priming” signal (CD40 ligation through an agonistic CD40 antibody) followed by a second, or “triggering” signal that has typically consisted of a Toll-like receptor (TLR) ligand [58-62,64,66-69]. Although in vitro assays have utilized lipopolysaccharide (LPS) for TLR stimulation, concerns about the systemic toxicity of endotoxin-type agents have led to exploration of CpG, a TLR9 ligand, as “triggering” signal. Indeed, the combination of CD40 ligation and CpG-mediated macrophage activation is cytostatic and cytocidal in vitro and leads to tumor regression in vivo, through elaboration of factors such as NO, TNFα and TRAIL [58]. More recently, the Vonderheide group has shown that administration of an agonistic CD40 antibody together with chemotherapy led to meaningful clinical responses in a particularly recalcitrant tumor, pancreatic carcinoma [70]. Although initially the investigators hypothesized that the effect was lymphocyte-driven, dissection of the relevant mechanisms in a genetically-engineered animal model demonstrated that the anti-tumor effects were mediated entirely by activated macrophages. In B cell malignancies and other cases where the malignant cells express CD40, there is a potential concern that CD40 stimulation may lead to (at least transient) tumor stimulation and growth. However, in a model of CLL (a CD40-expressing malignancy), a modest effect on tumor cell proliferation was overcome by macrophage-mediated tumoricidal activity following anti-CD40 antibody administration [62]. Moreover, binding of CD40 antibody on the surface of CD40+ tumor cells may kindle a primary tumoricidal effect through antibody-mediated cell cytotoxicity (ADCC), acting in parallel with macrophage activation [58].
Direct interference with immunosuppressive pathways may offer an alternative or complementary approach to reprogram macrophages to an anti-tumor role. Disruption of IL-10-dependent immunosuppressive pathways have been explored in cancer immunotherapy [136]. Targeted inhibition of the IL-10 axis may be achieved through small molecules targeting TPL2 kinase activity. Indeed, in a genetically-engineered model of colonic carcinogenesis, genetic ablation of Tpl2 led to enhanced inflammation and tumor promotion, predominantly through inhibition of IL-10 production coupled with defects in regulatory T cell (Treg) generation [137]. Pharmacological TPL2 inhibitors have been under continuous development for over a decade [138-144]. TPL2 has low homology to other kinases and unique structural features in its ATP-binding loop that will likely allow the design of highly specific inhibitors [75]. Moreover, Tpl2 activity is not inhibited by staurosporine, a non-specific kinase inhibitor [145]. However, the lack of crystal structure has hampered the pace of TPL2 inhibitor development. Several classes of compounds have been shown to have good activity in kinase inhibition assays as well as in vivo activity, by blocking TNFα responses to systemic LPS administration [146]. A natural compound, luteolin, has recently been shown to inhibit TPL2 activity, albeit with a high IC50 [147]. Because Tpl2 nullizygosity is compatible with normal hematopoietic development and function [148], therapeutic TPL2 blockade is likely to be well tolerated in patients with hematological malignancies.
A third approach involves blocking of “don't eat me’ signals on the surface of tumor cells. Anti-CD47 antibody-based approaches in particular have found applicability in several tumor models and are fast moving to the clinic [103]. It is unlikely that anti-CD47-based approaches will suffice as monotherapy, particularly in the setting of bulky disease. However, CD47 blockade may be particularly attractive in the setting of minimal residual disease, alone or in combination with antiproliferative therapies. Interestingly, cancer stem cells appear to upregulate CD47 strongly, making anti-CD47 therapy particularly attractive in those tumors where minimal residual disease may have stem-cell characteristics [149].
Lastly, therapeutic interference with monocyte recruitment or selective depletion of tumor-infiltrating macrophages may delay tumor growth, e.g. through inhibition of angiogenesis, as well as disrupt essential cross-talk with other constituents of the niche. Monocytes are recruited to nascent tumors through their expression of CCR2, the receptor for the potent monocyte chemo-attractant cytokine CCL2, produced by the tumor microenvironment [150]. Indeed, inflammatory monocytes in both mice and humans are CCR2+ and monocyte migration through the CCR2-CCL2 axis is important for the generation of the metastatic niche [151]. There are several CCR2 inhibitors in development (reviewed in [152]). Although the main applications have so far focused on therapy of autoimmunity, atherosclerosis and metabolic disease, CCR2-CCL2 axis inhibition is beginning to be explored in the context of cancer. Clinical trials using anti-CCL2 antibodies, alone or in combination with chemotherapy, have been conducted in solid tumors aiming at inhibition of angiogenesis (www.clinicaltrials.gov). Robust expression of CCL2 by the rich vascular network of myeloma bone marrow [153,154] underscores CCL2-CCR2 axis inhibition as a particularly attractive therapeutic strategy in myeloma. Lastly, selective depletion of tumor-infiltrating macrophages may be envisaged. Powerful proof-of-principle in favor of therapeutic macrophage targeting was recently provided by the demonstration that trabectedin, a novel marine-derived compound, exerted powerful anti-tumor effects through depletion of monocytes/macrophages and associated collapse of tumor vascular networks [155]. Trabectedin, or similar approaches, may hold great promise in macrophage-rich, vascular tumors, such as myeloma.
Conclusions
Recent and expanding investigations suggest that macrophages play a major, and hitherto poorly appreciated, role in the development and propagation of hematological malignancies, including multiple myeloma. Work from our laboratories and others, has provided insight into the bidirectional interactions between macrophages and malignant plasma cells and between macrophages and MSCs in the myeloma microenvironment. Taken together, these mechanistic studies support the hypothesis that macrophages are central to the homeostasis of the myeloma niche and therefore, therapeutic approaches that exploit these interactions may hold the key for improved control and ultimately cure in patients with myeloma.
ACKNOWLEDGMENTS
Work in our laboratories is supported by a Leukemia Research Foundation New Investigator Award (FA), a Kirschstein National Research Service Award (T32 HL007899, Sheehan) (CH), a CTSA TL1 award by the National Center for Advancing Translational Sciences (9U54TR000021, Drezner) (JLJ), an American Society of Hematology Trainee Research Award (RAD), the UWCCC Trillium Fund for Multiple Myeloma Research and the UW Carbone Cancer Center Core Grant (P30 CA014520).
Footnotes
CONFLICTS OF INTEREST
The authors have no financial conflicts-of-interest to declare.
REFERENCES
- 1.Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012;122:3456–63. doi: 10.1172/JCI61188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ., 3rd Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8:479–91. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaire M, Deleu S, De Bruyne E, Van Valckenborgh E, Menu E, Vanderkerken K. The microenvironment and molecular biology of the multiple myeloma tumor. Adv Cancer Res. 2011;110:19–42. doi: 10.1016/B978-0-12-386469-7.00002-5. [DOI] [PubMed] [Google Scholar]
- 6.Roodman GD. Targeting the bone microenvironment in multiple myeloma. J Bone Miner Metab. 2010;28:244–50. doi: 10.1007/s00774-009-0154-7. [DOI] [PubMed] [Google Scholar]
- 7.Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, Munshi NC, Anderson KC. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007;21:1007–34, vii-viii. doi: 10.1016/j.hoc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Hebron E, Hope C, Kim J, Jensen JL, Flanagan C, Bhatia N, Maroulakou I, Mitsiades C, Miyamoto S, Callander N. MAP3K8 kinase regulates myeloma growth by cell-autonomous and non-autonomous mechanisms involving myeloma-associated monocytes/macrophages. Br J Haematol. 2012 doi: 10.1111/bjh.12175. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groen RW, Noort WA, Raymakers RA, Prins HJ, Aalders L, Hofhuis FM, Moerer P, van Velzen JF, Bloem AC, van Kessel B. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood. 2012;120:e9–e16. doi: 10.1182/blood-2012-03-414920. others. [DOI] [PubMed] [Google Scholar]
- 10.Basak GW, Srivastava AS, Malhotra R, Carrier E. Multiple myeloma bone marrow niche. Curr Pharm Biotechnol. 2009;10:345–6. doi: 10.2174/138920109787847493. [DOI] [PubMed] [Google Scholar]
- 11.Nair JR, Rozanski CH, Lee KP. Under one roof: The bone marrow survival niche for multiple myeloma and normal plasma cells. Oncoimmunology. 2012;1:388–389. doi: 10.4161/onci.18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipori D. The hemopoietic stem cell niche versus the microenvironment of the multiple myeloma-tumor initiating cell. Cancer Microenviron. 2010;3:15–28. doi: 10.1007/s12307-009-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe M. Targeting the interplay between myeloma cells and the bone marrow microenvironment in myeloma. Int J Hematol. 2011;94:334–43. doi: 10.1007/s12185-011-0949-x. [DOI] [PubMed] [Google Scholar]
- 14.Matsui W, Borrello I, Mitsiades C. Autologous stem cell transplantation and multiple myeloma cancer stem cells. Biol Blood Marrow Transplant. 2012;18:S27–32. doi: 10.1016/j.bbmt.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Park CY, Medeiros BC, Weissman IL. CD19(-)CD45(low/-)CD38(high)/CD138(+) plasma cells enrich for human tumorigenic myeloma cells. Leukemia. 2012;26:2530–7. doi: 10.1038/leu.2012.140. [DOI] [PubMed] [Google Scholar]
- 16.Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28:2612–24. doi: 10.1200/JCO.2009.25.4250. [DOI] [PubMed] [Google Scholar]
- 17.Hart AJ, Jagasia MH, Kim AS, Mosse CA, Savani BN, Kassim A. Minimal residual disease in myeloma: are we there yet? Biol Blood Marrow Transplant. 2012;18:1790–9. doi: 10.1016/j.bbmt.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 20.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steidl C, Farinha P, Gascoyne RD. Macrophages predict treatment outcome in Hodgkin's lymphoma. Haematologica. 2011;96:186–9. doi: 10.3324/haematol.2010.033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, d'Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin's lymphoma. Haematologica. 2011;96:269–76. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005;106:2169–74. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 25.Byers RJ, Sakhinia E, Joseph P, Glennie C, Hoyland JA, Menasce LP, Radford JA, Illidge T. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR-based gene expression profiling. Blood. 2008;111:4764–70. doi: 10.1182/blood-2007-10-115915. [DOI] [PubMed] [Google Scholar]
- 26.Hasselblom S, Hansson U, Sigurdardottir M, Nilsson-Ehle H, Ridell B, Andersson PO. Expression of CD68+ tumor-associated macrophages in patients with diffuse large B-cell lymphoma and its relation to prognosis. Pathol Int. 2008;58:529–32. doi: 10.1111/j.1440-1827.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- 27.Cai QC, Liao H, Lin SX, Xia Y, Wang XX, Gao Y, Lin ZX, Lu JB, Huang HQ. High expression of tumor-infiltrating macrophages correlates with poor prognosis in patients with diffuse large B-cell lymphoma. Med Oncol. 2012;29:2317–22. doi: 10.1007/s12032-011-0123-6. [DOI] [PubMed] [Google Scholar]
- 28.Wada N, Zaki MA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y, Ishikawa J, Tominaga N, Sakoda H, Take H. Tumour-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology. 2012;60:313–9. doi: 10.1111/j.1365-2559.2011.04096.x. others. [DOI] [PubMed] [Google Scholar]
- 29.Herreros B, Rodriguez-Pinilla SM, Pajares R, Martinez-Gonzalez MA, Ramos R, Munoz I, Montes-Moreno S, Lozano M, Sanchez-Verde L, Roncador G. Proliferation centers in chronic lymphocytic leukemia: the niche where NF-kappaB activation takes place. Leukemia. 2010;24:872–6. doi: 10.1038/leu.2009.285. others. [DOI] [PubMed] [Google Scholar]
- 30.Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Gupta R, Guy E, Mariongiu MF, Levine RL, Abdel-Wahab O. Removal of Macrophages From the Erythroid Niche Impairs Stress Erythropoiesis but Improves Pathophysiology of Polycythemia Vera and Beta-Thalassemia. ASH Annual Meeting Abstracts. 2012;120:81. others. [Google Scholar]
- 31.Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2012 doi: 10.1002/jcp.24260. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–5. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 33.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roussou M, Tasidou A, Dimopoulos MA, Kastritis E, Migkou M, Christoulas D, Gavriatopoulou M, Zagouri F, Matsouka C, Anagnostou D. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia. 2009;23:2177–81. doi: 10.1038/leu.2009.130. others. [DOI] [PubMed] [Google Scholar]
- 35.Peng KW, Dogan A, Vrana J, Liu C, Ong HT, Kumar S, Dispenzieri A, Dietz AB, Russell SJ. Tumor-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma. Am J Hematol. 2009;84:401–7. doi: 10.1002/ajh.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruns I, Cadeddu RP, Brueckmann I, Frobel J, Geyh S, Bust S, Fischer JC, Roels F, Wilk CM, Schildberg FA. Multiple myeloma-related deregulation of bone marrow-derived CD34+ hematopoietic stem and progenitor cells. Blood. 2012 doi: 10.1182/blood-2011-04-347484. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 38.Gerlo S, Haegeman G, Vanden Berghe W. Transcriptional regulation of autocrine IL-6 expression in multiple myeloma cells. Cell Signal. 2008;20:1489–96. doi: 10.1016/j.cellsig.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Abdi J, Engels F, Garssen J, Redegeld F. The role of toll-like receptor mediated signalling in the pathogenesis of multiple myeloma. Crit Rev Oncol Hematol. 2011;80:225–40. doi: 10.1016/j.critrevonc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Jourdan M, Tarte K, Legouffe E, Brochier J, Rossi JF, Klein B. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw. 1999;10:65–70. [PMC free article] [PubMed] [Google Scholar]
- 41.Kline M, Donovan K, Wellik L, Lust C, Jin W, Moon-Tasson L, Xiong Y, Witzig TE, Kumar S, Rajkumar SV. Cytokine and chemokine profiles in multiple myeloma; significance of stromal interaction and correlation of IL-8 production with disease progression. Leuk Res. 2007;31:591–8. doi: 10.1016/j.leukres.2006.06.012. others. [DOI] [PubMed] [Google Scholar]
- 42.Donovan KA, Lacy MQ, Gertz MA, Lust JA. IL-1beta expression in IgM monoclonal gammopathy and its relationship to multiple myeloma. Leukemia. 2002;16:382–5. doi: 10.1038/sj.leu.2402374. [DOI] [PubMed] [Google Scholar]
- 43.Chiron D, Jego G, Pellat-Deuceunynck C. Toll-like receptors: expression and involvement in multiple myeloma. Leuk Res. 2010;34:1545–50. doi: 10.1016/j.leukres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 44.de Launay D, Vreijling J, Hartkamp LM, Karpus ON, Abreu JR, van Maanen MA, Sanders ME, Grabiec AM, Hamann J, Orum H. Silencing the expression of Ras family GTPase homologues decreases inflammation and joint destruction in experimental arthritis. Am J Pathol. 2010;177:3010–24. doi: 10.2353/ajpath.2010.091053. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okumura T, Ericksen RE, Takaishi S, Wang SS, Dubeykovskiy Z, Shibata W, Betz KS, Muthupalani S, Rogers AB, Fox JG. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010;70:8435–45. doi: 10.1158/0008-5472.CAN-10-1506. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 47.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42:161–70. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 48.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 49.Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rui L, Schmitz R, Ceribelli M, Staudt LM. Malignant pirates of the immune system. Nat Immunol. 2011;12:933–40. doi: 10.1038/ni.2094. [DOI] [PubMed] [Google Scholar]
- 51.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–44. doi: 10.1016/j.ccr.2007.07.003. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahindra A, Hideshima T, Anderson KC. Multiple myeloma: biology of the disease. Blood Rev. 2010;24(Suppl 1):S5–11. doi: 10.1016/S0268-960X(10)70003-5. [DOI] [PubMed] [Google Scholar]
- 53.Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget's disease and multiple myeloma. Immunol Rev. 2005;208:252–66. doi: 10.1111/j.0105-2896.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 54.Ivashkiv LB. Inflammatory signaling in macrophages: transitions from acute to tolerant and alternative activation states. Eur J Immunol. 2011;41:2477–81. doi: 10.1002/eji.201141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Denu RA, Dollar BA, Escalante LE, Kuether JP, Callander NS, Asimakopoulos F, Hematti P. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br J Haematol. 2012;158:336–46. doi: 10.1111/j.1365-2141.2012.09154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–4. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 57.Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 58.Rakhmilevich AL, Alderson KL, Sondel PM. T-cell-independent Antitumor Effects of CD40 Ligation. Int Rev Immunol. 2012;31:267–78. doi: 10.3109/08830185.2012.698337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rakhmilevich AL, Baldeshwiler MJ, Van De Voort TJ, Felder MA, Yang RK, Kalogriopoulos NA, Koslov DS, Van Rooijen N, Sondel PM. Tumor-associated myeloid cells can be activated in vitro and in vivo to mediate antitumor effects. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson EE, Buhtoiarov IN, Baldeshwiler MJ, Felder MA, Van Rooijen N, Sondel PM, Rakhmilevich AL. Enhanced T-cell-independent antitumor effect of cyclophosphamide combined with anti-CD40 mAb and CpG in mice. J Immunother. 2011;34:76–84. doi: 10.1097/CJI.0b013e318200b28a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, Rakhmilevich AL. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology. 2011;132:226–39. doi: 10.1111/j.1365-2567.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu QL, Buhtoiarov IN, Sondel PM, Rakhmilevich AL, Ranheim EA. Tumoricidal effects of activated macrophages in a mouse model of chronic lymphocytic leukemia. J Immunol. 2009;182:6771–8. doi: 10.4049/jimmunol.0801847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buhtoiarov IN, Rakhmilevich AL, Lanier LL, Ranheim EA, Sondel PM. Naive mouse macrophages become activated following recognition of L5178Y lymphoma cells via concurrent ligation of CD40, NKG2D, and CD18 molecules. J Immunol. 2009;182:1940–53. doi: 10.4049/jimmunol.0800443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rakhmilevich AL, Buhtoiarov IN, Malkovsky M, Sondel PM. CD40 ligation in vivo can induce T cell independent antitumor effects even against immunogenic tumors. Cancer Immunol Immunother. 2008;57:1151–60. doi: 10.1007/s00262-007-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buhtoiarov IN, Sondel PM, Eickhoff JC, Rakhmilevich AL. Macrophages are essential for antitumour effects against weakly immunogenic murine tumours induced by class B CpG-oligodeoxynucleotides. Immunology. 2007;120:412–23. doi: 10.1111/j.1365-2567.2006.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. Tumoristatic effects of anti-CD40 mAb-activated macrophages involve nitric oxide and tumour necrosis factor-alpha. Immunology. 2006;118:261–70. doi: 10.1111/j.1365-2567.2006.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J Leukoc Biol. 2006;79:1181–92. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 68.Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–18. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 69.Buhtoiarov IN, Lum H, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 2005;174:6013–22. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 70.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durie BG, Vela EE, Frutiger Y. Macrophages as an important source of paracrine IL6 in myeloma bone marrow. Curr Top Microbiol Immunol. 1990;166:33–6. doi: 10.1007/978-3-642-75889-8_4. [DOI] [PubMed] [Google Scholar]
- 72.Babu GR, Jin W, Norman L, Waterfield M, Chang M, Wu X, Zhang M, Sun SC. Phosphorylation of NF-kappaB1/p105 by oncoprotein kinase Tpl2: implications for a novel mechanism of Tpl2 regulation. Biochim Biophys Acta. 2006;1763:174–81. doi: 10.1016/j.bbamcr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Vougioukalaki M, Kanellis DC, Gkouskou K, Eliopoulos AG. Tpl2 kinase signal transduction in inflammation and cancer. Cancer Lett. 2011;304:80–9. doi: 10.1016/j.canlet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 74.Waterfield MR, Zhang M, Norman LP, Sun SC. NF-kappaB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol Cell. 2003;11:685–94. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 75.Gantke T, Sriskantharajah S, Ley SC. Regulation and function of TPL-2, an IkappaB kinase-regulated MAP kinase kinase kinase. Cell Res. 2011;21:131–45. doi: 10.1038/cr.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawrenz M, Visekruna A, Kuhl A, Schmidt N, Kaufmann SH, Steinhoff U. Genetic and pharmacological targeting of TPL-2 kinase ameliorates experimental colitis: a potential target for the treatment of Crohn's disease? Mucosal Immunol. 2012;5:129–39. doi: 10.1038/mi.2011.57. [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Pelaez M, Fumagalli S, Sanz C, Herrero C, Guerra S, Fernandez M, Alemany S. Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages. Mol Biol Cell. 2012;23:2982–92. doi: 10.1091/mbc.E12-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez-Pelaez M, Soria-Castro I, Bosca L, Fernandez M, Alemany S. Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: Implications for NO synthase 2 expression. Eur J Immunol. 2011;41:1733–41. doi: 10.1002/eji.201041101. [DOI] [PubMed] [Google Scholar]
- 79.Mielke LA, Elkins KL, Wei L, Starr R, Tsichlis PN, O'Shea JJ, Watford WT. Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1 beta production. J Immunol. 2009;183:7984–93. doi: 10.4049/jimmunol.0901336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rousseau S, Papoutsopoulou M, Symons A, Cook D, Lucocq JM, Prescott AR, O'Garra A, Ley SC, Cohen P. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci. 2008;121:149–54. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- 81.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–8. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y, Wang Z, Liu Z, Li H, He J. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia. 2012 doi: 10.1038/leu.2012.272. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, Epstein J. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–23. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 84.Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ, Blair HC, Roodman GD. Osteoclasts are important for bone angiogenesis. Blood. 2010;115:140–9. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pettit AR, Chang MK, Hume DA, Raggatt LJ. Osteal macrophages: a new twist on coupling during bone dynamics. Bone. 2008;43:976–82. doi: 10.1016/j.bone.2008.08.128. [DOI] [PubMed] [Google Scholar]
- 86.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–8. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–44. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 88.Rajkumar SV, Leong T, Roche PC, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Kyle RA, Gertz MA. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clin Cancer Res. 2000;6:3111–6. others. [PubMed] [Google Scholar]
- 89.Suyani E, Sucak GT, Akyurek N, Sahin S, Baysal NA, Yagci M, Haznedar R. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Ann Hematol. 2013 doi: 10.1007/s00277-012-1652-6. [DOI] [PubMed] [Google Scholar]
- 90.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–6. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 91.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–8. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 92.Vacca A, Ria R, Ribatti D, Semeraro F, Djonov V, Di Raimondo F, Dammacco F. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica. 2003;88:176–85. [PubMed] [Google Scholar]
- 93.Ribatti D, Nico B, Vacca A. Importance of the bone marrow microenvironment in inducing the angiogenic response in multiple myeloma. Oncogene. 2006;25:4257–66. doi: 10.1038/sj.onc.1209456. [DOI] [PubMed] [Google Scholar]
- 94.Sessa WC. Molecular control of blood flow and angiogenesis: role of nitric oxide. J Thromb Haemost. 2009;7(Suppl 1):35–7. doi: 10.1111/j.1538-7836.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 95.Ria R, Reale A, De Luisi A, Ferrucci A, Moschetta M, Vacca A. Bone marrow angiogenesis and progression in multiple myeloma. Am J Blood Res. 2011;1:76–89. [PMC free article] [PubMed] [Google Scholar]
- 96.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–44. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Ribatti D, Crivellato E, Molica S. Mast cells and angiogenesis in haematological malignancies. Leuk Res. 2009;33:876–9. doi: 10.1016/j.leukres.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 99.Vacca A, Ribatti D. Angiogenesis and vasculogenesis in multiple myeloma: role of inflammatory cells. Recent Results Cancer Res. 2011;183:87–95. doi: 10.1007/978-3-540-85772-3_4. [DOI] [PubMed] [Google Scholar]
- 100.Scavelli C, Nico B, Cirulli T, Ria R, Di Pietro G, Mangieri D, Bacigalupo A, Mangialardi G, Coluccia AM, Caravita T. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27:663–74. doi: 10.1038/sj.onc.1210691. others. [DOI] [PubMed] [Google Scholar]
- 101.Moschetta M, Di Pietro G, Ria R, Gnoni A, Mangialardi G, Guarini A, Ditonno P, Musto P, D'Auria F, Ricciardi MR. Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. Eur J Cancer. 2010;46:420–9. doi: 10.1016/j.ejca.2009.10.019. others. [DOI] [PubMed] [Google Scholar]
- 102.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012 doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 103.Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–32. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–10. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 106.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 107.Markovina S, Callander NS, O'Connor SL, Xu G, Shi Y, Leith CP, Kim K, Trivedi P, Kim J, Hematti P. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Mol Cancer. 2010;9:176. doi: 10.1186/1476-4598-9-176. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carter A, Merchav S, Silvian-Draxler I, Tatarsky I. The role of interleukin-1 and tumour necrosis factor-alpha in human multiple myeloma. Br J Haematol. 1990;74:424–31. doi: 10.1111/j.1365-2141.1990.tb06330.x. [DOI] [PubMed] [Google Scholar]
- 109.Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–20. [PubMed] [Google Scholar]
- 110.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–82. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 111.Xie JY, Li MX, Xiang DB, Mou JH, Qing Y, Zeng LL, Yang ZZ, Guan W, Wang D. Elevated expression of APE1/Ref-1 and its regulation on IL-6 and IL-8 in bone marrow stromal cells of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10:385–93. doi: 10.3816/CLML.2010.n.072. [DOI] [PubMed] [Google Scholar]
- 112.Sanz-Rodriguez F, Ruiz-Velasco N, Pascual-Salcedo D, Teixido J. Characterization of VLA-4-dependent myeloma cell adhesion to fibronectin and VCAM-1. Br J Haematol. 1999;107:825–34. doi: 10.1046/j.1365-2141.1999.01762.x. [DOI] [PubMed] [Google Scholar]
- 113.Michigami T, Shimizu N, Williams PJ, Niewolna M, Dallas SL, Mundy GR, Yoneda T. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–60. [PubMed] [Google Scholar]
- 114.Anton K, Banerjee D, Glod J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS One. 2012;7:e35036. doi: 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–53. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–54. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 118.Balkwill F, Joffroy C. TNF: a tumor-suppressing factor or a tumor-promoting factor? Future Oncol. 2010;6:1833–6. doi: 10.2217/fon.10.155. [DOI] [PubMed] [Google Scholar]
- 119.Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, Harview CL, Brunet JP, Ahmann GJ, Adli M. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–72. doi: 10.1038/nature09837. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guilloton F, Caron G, Menard C, Pangault C, Ame-Thomas P, Dulong J, De Vos J, Rossille D, Henry C, Lamy T. Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood. 2012;119:2556–67. doi: 10.1182/blood-2011-08-370908. others. [DOI] [PubMed] [Google Scholar]
- 124.Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B. CCR2-Dependent Recruitment of Macrophages by Tumor-Educated Mesenchymal Stromal Cells Promotes Tumor Development and Is Mimicked by TNFalpha. Cell Stem Cell. 2012;11:812–24. doi: 10.1016/j.stem.2012.08.013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mantovani A. MSCs, Macrophages, and Cancer: A Dangerous Menage-a-Trois. Cell Stem Cell. 2012;11:730–2. doi: 10.1016/j.stem.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 126.Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma. 2009;9:278–88. doi: 10.3816/CLM.2009.n.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–59. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immunemodulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2012.728597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitsiades CS. How “immunomodulatory” are IMIDs? Blood. 2011;117:1440–1. doi: 10.1182/blood-2010-11-317156. [DOI] [PubMed] [Google Scholar]
- 131.Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, Smyth MJ, Neeson P, Ritchie DS. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011;117:1605–13. doi: 10.1182/blood-2010-04-278432. [DOI] [PubMed] [Google Scholar]
- 132.Corral LG, Kaplan G. Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis. 1999;58(Suppl 1):I107–13. doi: 10.1136/ard.58.2008.i107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immunemodulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leukemia & Lymphoma. 0:1–5. doi: 10.3109/10428194.2012.728597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muller GW, Chen R, Huang SY, Corral LG, Wong LM, Patterson RT, Chen Y, Kaplan G, Stirling DI. Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett. 1999;9:1625–30. doi: 10.1016/s0960-894x(99)00250-4. [DOI] [PubMed] [Google Scholar]
- 135.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, 3rd, Antin JH, Comenzo R, Goodman S, Hari P. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–203. doi: 10.1016/S1470-2045(11)70243-1. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res. 2011;51:170–82. doi: 10.1007/s12026-011-8262-6. [DOI] [PubMed] [Google Scholar]
- 137.Serebrennikova OB, Tsatsanis C, Mao C, Gounaris E, Ren W, Siracusa LD, Eliopoulos AG, Khazaie K, Tsichlis PN. Tpl2 ablation promotes intestinal inflammation and tumorigenesis in Apcmin mice by inhibiting IL-10 secretion and regulatory T-cell generation. Proc Natl Acad Sci U S A. 2012;109:E1082–91. doi: 10.1073/pnas.1115098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ni Y, Gopalsamy A, Cole D, Hu Y, Denny R, Ipek M, Liu J, Lee J, Hall JP, Luong M. Identification and SAR of a new series of thieno[3,2-d]pyrimidines as Tpl2 kinase inhibitors. Bioorg Med Chem Lett. 2011;21:5952–6. doi: 10.1016/j.bmcl.2011.07.069. others. [DOI] [PubMed] [Google Scholar]
- 139.Teli MK, Rajanikant GK. Pharmacophore generation and atom-based 3D-QSAR of novel quinoline-3-carbonitrile derivatives as Tpl2 kinase inhibitors. J Enzyme Inhib Med Chem. 2012;27:558–70. doi: 10.3109/14756366.2011.603128. [DOI] [PubMed] [Google Scholar]
- 140.Hu Y, Cole D, Denny RA, Anderson DR, Ipek M, Ni Y, Wang X, Thaisrivongs S, Chamberlain T, Hall JP. Discovery of indazoles as inhibitors of Tpl2 kinase. Bioorg Med Chem Lett. 2011;21:4758–61. doi: 10.1016/j.bmcl.2011.06.065. others. [DOI] [PubMed] [Google Scholar]
- 141.Wu J, Green N, Hotchandani R, Hu Y, Condon J, Huang A, Kaila N, Li HQ, Guler S, Li W. Selective inhibitors of tumor progression loci-2 (Tpl2) kinase with potent inhibition of TNF-alpha production in human whole blood. Bioorg Med Chem Lett. 2009;19:3485–8. doi: 10.1016/j.bmcl.2009.05.009. others. [DOI] [PubMed] [Google Scholar]
- 142.Cusack K, Allen H, Bischoff A, Clabbers A, Dixon R, Fix-Stenzel S, Friedman M, Gaumont Y, George D, Gordon T. Identification of a selective thieno[2,3-c]pyridine inhibitor of COT kinase and TNF-alpha production. Bioorg Med Chem Lett. 2009;19:1722–5. doi: 10.1016/j.bmcl.2009.01.088. others. [DOI] [PubMed] [Google Scholar]
- 143.Hall JP, Kurdi Y, Hsu S, Cuozzo J, Liu J, Telliez JB, Seidl KJ, Winkler A, Hu Y, Green N. Pharmacologic inhibition of tpl2 blocks inflammatory responses in primary human monocytes, synoviocytes, and blood. J Biol Chem. 2007;282:33295–304. doi: 10.1074/jbc.M703694200. others. [DOI] [PubMed] [Google Scholar]
- 144.Kaila N, Green N, Li HQ, Hu Y, Janz K, Gavrin LK, Thomason J, Tam S, Powell D, Cuozzo J. Identification of a novel class of selective Tpl2 kinase inhibitors: 4-Alkylamino-[1,7]naphthyridine-3-carbonitriles. Bioorg Med Chem. 2007;15:6425–42. doi: 10.1016/j.bmc.2007.06.054. others. [DOI] [PubMed] [Google Scholar]
- 145.Luciano BS, Hsu S, Channavajhala PL, Lin LL, Cuozzo JW. Phosphorylation of threonine 290 in the activation loop of Tpl2/Cot is necessary but not sufficient for kinase activity. J Biol Chem. 2004;279:52117–23. doi: 10.1074/jbc.M403716200. [DOI] [PubMed] [Google Scholar]
- 146.Green N, Hu Y, Janz K, Li HQ, Kaila N, Guler S, Thomason J, Joseph-McCarthy D, Tam SY, Hotchandani R. Inhibitors of tumor progression loci-2 (Tpl2) kinase and tumor necrosis factor alpha (TNF-alpha) production: selectivity and in vivo antiinflammatory activity of novel 8-substituted-4-anilino-6-aminoquinoline-3-carbonitriles. J Med Chem. 2007;50:4728–45. doi: 10.1021/jm070436q. others. [DOI] [PubMed] [Google Scholar]
- 147.Kim JE, Son JE, Jang YJ, Lee DE, Kang NJ, Jung SK, Heo YS, Lee KW, Lee HJ. Luteolin, a novel natural inhibitor of tumor progression locus 2 serine/threonine kinase, inhibits tumor necrosis factor-alpha-induced cyclooxygenase-2 expression in JB6 mouse epidermis cells. J Pharmacol Exp Ther. 2011;338:1013–22. doi: 10.1124/jpet.111.179200. [DOI] [PubMed] [Google Scholar]
- 148.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. others. [DOI] [PubMed] [Google Scholar]
- 149.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr., van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14:149–54. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 151.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Struthers M, Pasternak A. CCR2 antagonists. Curr Top Med Chem. 2010;10:1278–98. doi: 10.2174/156802610791561255. [DOI] [PubMed] [Google Scholar]
- 153.Otjacques E, Binsfeld M, Noel A, Beguin Y, Cataldo D, Caers J. Biological aspects of angiogenesis in multiple myeloma. Int J Hematol. 2011;94:505–18. doi: 10.1007/s12185-011-0963-z. [DOI] [PubMed] [Google Scholar]
- 154.Pellegrino A, Ria R, Di Pietro G, Cirulli T, Surico G, Pennisi A, Morabito F, Ribatti D, Vacca A. Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells. Br J Haematol. 2005;129:248–56. doi: 10.1111/j.1365-2141.2005.05443.x. [DOI] [PubMed] [Google Scholar]
- 155.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F. Role of Macrophage Targeting in the Antitumor Activity of Trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. others. [DOI] [PubMed] [Google Scholar]