Abstract
Geriatric depression is a costly health issue, but little is known about its physiological underpinnings. Systemic inflammation sensitizes the innate immune system of aged animals and humans, but it is unknown if chronic, low-grade infections affect the duration of depressive-like behaviors. In this report, we infected adult (4–6 months) and aged (20–24 months) Balb/c mice with an attenuated strain of Mycobacterium bovis, Bacillus Calmette-Guérin (BCG), to induce a chronic infection. We then measured depression-like behaviors that have construct, face and predictive validity for human inflammation-associated clinical depression. Exposure to BCG caused acute sickness responses in both adult and aged mice. However, sickness behavior was prolonged in aged mice, as assessed by both locomotor and rearing activity. Two measures of depression-like behavior, which were tests involving sucrose preference and tail suspension, both showed that adult mice displayed depression-like behaviors at one day and seven days after exposure to BCG. However, aged mice continued to express both of these depression-like behaviors at three weeks following infection. Infection with BCG caused an increase in tryptophan catabolism, as evidenced by a significant rise in the plasma kynurenine/tryptophan ratio that peaked at 7 days post-infection. In aged mice, greater tryptophan catabolism persisted longer and remained elevated at 21 days post-infection. This finding is consistent with the prolonged duration of depression-like behaviors in aged mice. These are the first data using a chronic infection model to establish that recovery from inflammation-induced depression-like behavior and tryptophan catabolism are prolonged in aged animals.
Keywords: inflammation, aging, sucrose preference, sickness behavior, depression-like behavior
INTRODUCTION
The prevalence of depressive symptoms is higher in the aged population compared to young adults. Although major depression, as assessed by the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM), may not be consistently elevated (Snowdon, 2001), the proportion of subjects with sub-threshold symptoms of depression rises with advancing age (Beekman et al., 2002; Meeks et al., 2011). In one longitudinal study with 277 subjects between the ages of 55–85, the incidence of depression as assessed by DSM criteria was low at 14 percent. However, average symptom duration in aged patients classified as suffering from depression was long and extended, as assessed by the Center for Epidemiological Studies Depression Scale (CES-D). Symptoms of depression remained above the 85th percentile for the entire six-year study, and most of them were long-lived (Beekman et al., 2002). This issue is exacerbated in aged subjects with low socioeconomic status (Koster et al., 2006). Similarly, symptoms of depression increase if the elderly become frail or disabled and moves from the community into a long-term health care facility (Llewellyn-Jones and Snowdon, 2007; Simonsick et al., 1998). Effective preventive strategies are needed for the aging world population (Madhusoodanan et al., 2010), but this problem is compounded by the lack of fundamental knowledge about the biological cause of prolonged depression in the aged (Tiemeier, 2003).
One common and striking feature of the aging process is an increase in health problems across a wide spectrum, including increased susceptibility to infections, heart and vessel diseases, cancers, obesity, diabetes, lung diseases and unrelenting fatigue. Indeed, these and other comorbid risk factors can be modeled to effectively predict late life depression (Almeida et al., 2011). Many of these health-related issues share a common underlying feature of chronic, systemic low-level inflammation. Inflammation can increase sensitivity of the brain to subsequent inflammatory stimuli (Perry et al., 2003). For example, microglia from prion-diseased mice, but not control mice, synthesize abundant IL-1β protein following exposure to LPS (Perry et al., 2007). These neuroimmune changes can prolong sickness behavior, particularly in aged mice (Dilger and Johnson, 2008; Kelley et al., 2003). A number of experiments have established that the acute inflammatory response induced by LPS in aged mice results in a slower recovery from sickness behaviors, such as anorexia, decrease locomotor activity, and diminished social interactions, with no significant increase in degree of the response (Abraham and Johnson, 2009; Godbout et al., 2005; Huang et al., 2008). Similarly, peripheral injection of LPS lengthens duration of depression-like behavior, with no increase in the amount of immobility, in aged as compared to younger adult mice (Godbout et al., 2008).
Although a single systemic injection of LPS causes the appearance of depression-like behaviors that disappear within a few days, even in aged animals, the effect of chronic inflammation induced by a live infection remains unknown. We have established that an attenuated strain of Mycobacterium bovis, Bacillus Calmette-Guérin (BCG), administered to young adult mice causes a chronic, systemic inflammatory response that endures for several days (Moreau et al., 2005). The acute sickness response to BCG disappears in less than a week, but depression-like behaviors persist after symptoms of sickness disappear (Moreau et al., 2008). Expression of BCG-induced depression-like behavior requires the induction of indoleamine 2,3 dioxygenase (IDO1) activity, resulting in a reduction in blood tryptophan and increase in the first product in this catabolic pathway, kynurenine (O’Connor et al., 2009c). Here we tested the hypothesis that BCG-induced depression-like behaviors would last longer in aged than in young adult mice and that this longer persistence would be mirrored by an increase in the ratio of kynurenine/tryptophan in blood. Two different behavioral tests of depression-like behavior unequivocally established that aging prolongs the duration of these behaviors than in young adult mice following chronic infection with BCG.
MATERIALS AND METHODS
Animals
All experiments were conducted in accordance with the NIH Policies for Animal Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. All mice used in these experiments were produced at the University of Illinois under housing conditions as previously described (Kelley et al., 2003). Young adult Balb/c mice ranged in age from 4–6 months and had a mean body weight of 29.2 ± 0.4 g at time of infection, whereas aged mice ranged in age from 20–24 months with a mean body weight of 31.3 ± 0.5 g at the time of infection with BCG or the control injection with saline. All mice were individually housed in standard polypropylene cages with corn cob litter in a temperature- (23°C) and humidity- (45–55%) controlled environment and a 12/12-h modified dark-light cycle (lights on 10:00 PM–10:00 AM). Food and water were available ad libitum. Mice were acclimated to the light cycle and facility for at least 3 weeks prior to infection with BCG or injection with saline. All mice were individually handled for a few minutes once daily for at least 7 days before treatments were initiated. Behavioral tests began during the start of the dark phase of the light cycle under red lighting. Body weights were recorded just prior to treatment and at various times afterwards.
Infection with Bacillus Calmette-Guérin
On the day of injection, fresh solutions were prepared by dispersing lyophilized cultures of BCG (Theracys, Sanofi Pasteur, Ltd., Toronto, Canada) into sterile endotoxin-free isotonic saline. Mice were inoculated by intraperitoneal (i.p.) administration of ~108 CFU per mouse, as previously described (O’Connor et al., 2009b). Control mice were injected i.p. with an equivalent volume of saline.
Culture of Bacillus Calmette-Guérin
Following euthanasia of mice at 1, 7, 14 or 21 days post-treatment, BCG was cultured from lungs and spleen essentially as described (Soudi et al., 2011). The right inferior lung lobe and whole spleens were rapidly collected, weighed and placed into ice-cold physiological saline. Mycobacteria were dispersed from tissue by mechanical disruption for 30 s using a motorized handheld tissue homogenizer with disposable generator probes (Omni International, Kennesaw, GA). Protein concentration was determined for each sample using a commercially available protein assay kit based on the Lowry assay (Bio-Rad, Hercules, CA). Serial dilutions were prepared in triplicate for each sample and suspensions were plated onto Middlebrook 7H10 Agar plates supplemented with 10% OADC (oleic acid, albumin, dextrose, catalase) enrichment medium (BD, Franklin Lakes, NJ). Plates were incubated at 37°F in 5% CO2 for approximately 2 weeks (until colony formation was visible). Colonies were manually counted under low magnification using a dissecting microscope, and the average number of colony forming units (CFU) from the triplicates was normalized to the protein concentration for statistical comparisons.
Plasma Tryptophan and Kynurenine
Tryptophan (Trp) and kynurenine (Kyn) concentrations were quantified in heparinized plasma as previously described (O’Connor et al., 2009b; O’Connor et al., 2009c). In brief, plasma samples were mixed 5:1 v/v with 10% sulfosalicylic acid to precipitate proteins, followed by centrifugation at 12,000 × g. Supernatant was collected and diluted 1:50 with mobile phase before analysis by high performance liquid chromatography (HPLC). The HPLC system consisted of a 5041 Enhanced Analytical amperometric cell and ESA Coulochem III detector (Thermo Scientific, Sunnyvale, CA). The mobile phase contained 75 mM NaH2PO4 (pH 4.6), 25 μM EDTA, 0.01 % (v/v) triethylamine in 94:6 (v/v) water:acetonitrile. Chromatograms were integrated and peaks quantified using EZ Chrom SI software in reference to a standard curve that was generated on each day of analysis. The plasma Kyn/Trp ratio, an index of peripheral IDO activity, was calculated for each sample.
Behavioral Measurements
On the day of assessment of animal behavior, locomotor and rearing activities were measured first, beginning at 10:00 AM. These measurements were followed by the tail suspension test. The sucrose preference test was then conducted until 10:00 AM the next day. Because of potential habituation to the test paradigm, separate cohorts of mice were tested only once at 1, 7, 14 or 21 days post-treatment.
Locomotor Activity
Exploratory locomotor activity (eLMA) was determined by placing mice individually into a cage that was identical to the home cage but lacking litter. Animals were videotaped and subsequently scored by a trained observer blind to treatment. The cage was divided into four virtual quadrants and eLMA was measured by counting the number of quadrant entries over a five-min session in which the mouse was allowed to freely explore the novel cage. Rearings were determined as the number of times mice assumed an upright posture on two paws during the five-min observation period. Counting was done by a trained observer who was blind to the treatments.
Depression-like Behaviors
Two measures of depression-like behavior were measured: sucrose preference and the tail suspension test (TST). Mice prefer to imbibe a sucrose solution rather than water, and failure to display this behavior is viewed as an anhedonic response that has face validity as a pre-clinical model of human depression (Chadman et al., 2009; Nestler and Hyman, 2010). The TST was originally developed as a convenient behavioral assay to screen for new antidepressant drugs (Nestler and Hyman, 2010). As such, it has predictive validity as a preclinical animal model of depression-like behavior. In these experiments, the sucrose preference test was conducted as described earlier (Moreau et al., 2008). A water bottle containing freshly prepared 2% sucrose solution was placed in the shoebox cage next to an identical bottle that contained tap water. All mice were exposed to the novelty of the sucrose preference procedure several times before they were infected with BCG, which served to establish a stable baseline of sucrose consumption. The training and testing began just prior to lights off at 10:00 AM and continued for 24 h. The water and sucrose bottles were weighed before and after the preference test and bottle weight change was used to determine the amount of water and sucrose fluids consumed. The TST test was conducted as previously described (O’Connor et al., 2009c). Briefly, mice were suspended by their tails to a computerized strain gauge that automatically collected and analyzed the movements of each mouse over a 10-min period (Mouse Tail Suspension Package, MED-TSS-MS; Med Associates, St Albans, VT, USA).
Statistical Analysis
Experiments were conducted as a completely randomized design. The response variables (behavioral measurements and the Kyn/Trp ratio) were tested for normality. The comparisons of primary interest were within each age group because aging is already known to delay behavioral recovery following treatment with LPS (Abraham and Johnson, 2009; Godbout et al., 2005; Godbout et al, 2008; Huang et al., 2008). Therefore, data were analyzed using a two-way (BCG × time) ANOVA within age groups and results are presented as the mean ± SEM. If the interaction was significant (P<0.05), differences between treatments at each time point were determined using the Fisher’s least significant difference post-hoc multiple pairwise comparisons.
RESULTS
Aged mice recover locomotor activity but not body weight following infection with BCG
To investigate the sickness response of adult (4–6 mo) and aged (20–24 mo) mice to chronic peripheral immune activation, male Balb/c were infected i.p. with BCG or given an equivalent volume of saline. Mice were monitored for metabolic and behavioral signs of sickness up to 3 weeks following inoculation, with separate groups of mice for each time point. In adult mice, infection with BCG induced a transient reduction in body weight (Fig. 1; time × BCG interaction, F3,42 = 12.5, P<0.001) that returned to pre-infection baseline levels by two weeks. Aged mice, however, exhibited a persistent reduction in body weight in response to the infection (Fig. 1; BCG effect, F1,35 = 46.8, P<0.001) that did not resolve during the three weeks of observation. In contrast, when eLMA was assessed to measure sickness-related behavior, infection with BCG caused a transient but non-significant reduction in locomotor activity of adult mice only on the first day after exposure (Fig. 2). However, in aged mice, locomotor activity depended upon both time (F3,34 = 4.0, P<0.05) and exposure to BCG (F1,34 = 11.6, P<0.01) in the absence of a significant BCG × time interaction. The infection had a prolonged effect on locomotor activity in aged mice because it was reduced at 1 and 7 days but fully recovered at both 14 and 21 days post-infection. When measured by number of rearings, very similar results were obtained (Fig. 3). In this case, rearing behavior depended upon both exposure to BCG and time following exposure in adult mice (BCG × time interaction, F3,42 = 4.6, P<0.01). Post-hoc analysis established that this reduction in rearing activity occurred at day 1 only (P<0.01) and was short-lived because it disappeared at 7 days and remained similar to controls for the rest of the experiment. In aged mice, number of rearings also was affected by both BCG and time after infection (BCG × time interaction, F3,32 = 3.7, P<0.05). Similar to locomotor activity, BCG caused a reduction in rearings at 1 day post-infection (P<0.001), and this reduction remained evident at 7 days after exposure to BCG (P<0.05) before returning to activity levels observed in control aged mice at 14 and 21 days post-infection. Collectively, these data indicate that there is a clear dissociation between a metabolic measure of sickness (loss of body weight) and behavioral parameters of sickness (locomotor activity and rearings) following infection in aged but not adult mice. Importantly, these data extend the concept of delayed behavioral recovery of sickness behaviors in aged mice that has been previously reported with an acute response to peripheral LPS (Abraham and Johnson, 2009; Godbout et al., 2005; Godbout et al., 2008) to the sickness-inducing properties of a live infection with BCG.
Figure 1. Aged mice do not recover loss of body weight in response to infection with BCG.
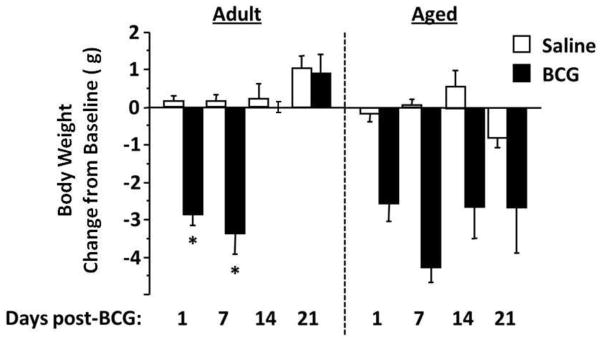
Changes in body weight from pre-inoculation baselines were calculated at the indicated times. Adult mice lost weight at 1 and 7 days post-infection (*P<0.001) but regained their weight by 14 days and maintained it through 21 days. In contrast, aged mice lost body weight post-infection (*P<0.001) and failed to recover this loss over the 3-week experiment. Asterisks denote a statistical difference (P<0.05) between saline and BCG-treated mice at a given time in either adult or aged mice. Data represent the mean ± SEM (n=5–7 mice per treatment group and for each time point).
Figure 2. Adult and aged mice recover exploratory locomotor activity within two weeks following infection with BCG.
Exploratory locomotor activity (eLMA) was measured as an index of sickness behavior. Although adult mice were less active on the first day post-infection, there was no significant effect of BCG on eLMA activity at any other time that was measured. In contrast, eLMA activity of aged mice was reduced at both 1 and 7 days following infection (P<0.05), but recovered two weeks after infection. Data represent the mean ± SEM (n=5–7 mice per treatment group and for each time point).
Figure 3. BCG reduces rearing behavior for a longer time in aged than in adult mice.
Rearing behavior was reduced in adult mice in both a BCG- and time-dependent fashion (BCG × time interaction, P<0.01). This reduction could be detected only at 1 day post-infection (P<0.01) in adult mice. In aged mice, both BCG and time after infection affected rearing behavior (BCG × time interaction, P<0.05). In contrast to adult mice, the reduction that was apparent at 1 day (P<0.001) remained evident a 7 days post-infection (P<0.05). Rearing behavior returned to that of control aged mice at 14 and 21 days post-infection. Asterisks denote a statistical difference (P<0.05) between saline and BCG-treated mice at a given time in either adult or aged mice. Data represent the mean ± SEM (n=5–7 mice per treatment group and for each time point).
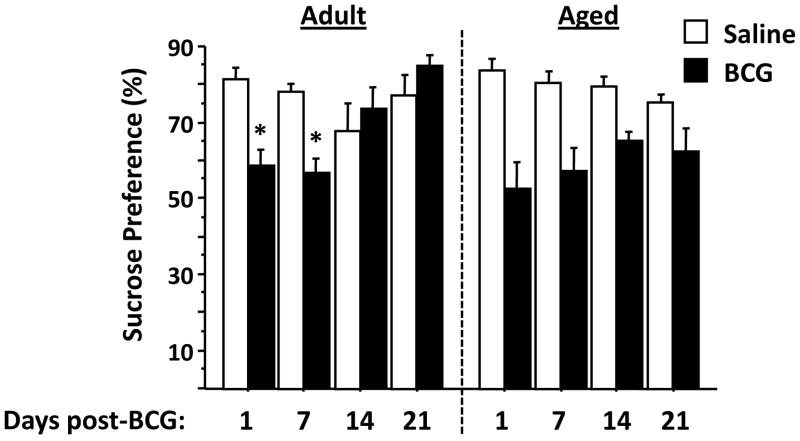
Anhedonic behavior is longer-lived in aged than in young adult mice
As expected, there was a clear preference for sucrose in both adult and aged mice that had been injected with saline (Fig. 4). However, exposure to BCG caused a time-dependent change (BCG × time interaction, F3,43 = 6.7, P<0.001) in adult but not in aged mice. Adult mice showed a significant reduction in preference for sucrose at one (P<0.01) and seven (P<0.001) days post-infection, and this reduction disappeared at both 14 and 21 days. In contrast, BCG caused a significant reduction in preference for sucrose at all four time points that were measured (BCG effect, F1,36 = 37.2, P<0.001). These data are consistent with the notion that aged mice display a delayed recovery in sucrose preference following infection with BCG.
Figure 4. Decreased preference for sucrose induced by BCG does not recover in aged mice.
Sucrose preference was calculated as a ratio of the volume of sucrose solution ingested divided by the total volume of both water and sucrose solution ingested. Both adult and aged mice treated with saline i.p. imbibed more of a 2% solution of sucrose than water at all times. Although adult mice consumed less sucrose relative to water at 1 (P<0.01) and 7 (P<0.001) days following infection with BCG, their preference for sucrose returned to that of control, uninfected mice at 14 and 21 days following infection (BCG × time interaction, P<0.001). In contrast, sucrose preference was reduced (P<0.001) in aged mice at all times. Asterisks denote a statistical difference (P<0.05) between saline and BCG-treated mice at a given time in either adult or aged mice. Data represent the mean ± SEM (n=5–7 mice per treatment group and for each time point).
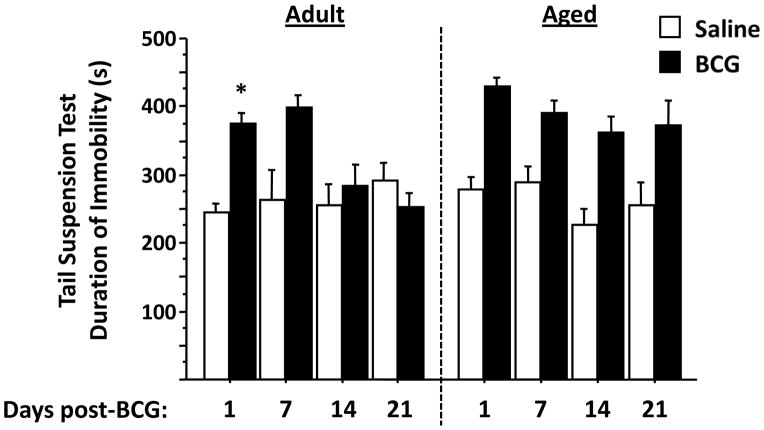
Aged mice fail to recover activity in the tail suspension test than do adult mice
In adult mice, BCG caused a time-dependent increase in immobility (BCG × time interaction, F3,41 = 4.0, P<0.05) in the tail suspension test (Fig. 5). This inactivity occurred at 1 day (P<0.001) post-infection but was not significant at 14 and 21 days post-infection. In contrast, in aged mice, BCG caused an increase in immobility at one and seven days, as well as 14 and 21 days after infection (BCG effect, F1,35 = 27.6, P<0.001). These data, taken in conjunction with the results of sucrose preference, confirm that depression-like behaviors persist in aged mice following infection with BCG.
Figure 5. Aged mice recover more slowly than adult mice in the tail suspension test following infection with BCG.
Immobility in adult mice was increased at 1 day (P<0.001) following infection (BCG × time interaction, P<0.05), whereas immobility remained elevated for the entire 21 days in aged mice (P<0.001). Asterisks denote a statistical difference (P<0.05) between saline and BCG-treated mice at a given time in either adult or aged mice. Data represent the mean ± SEM (n=5–7 mice per treatment group for each time point).
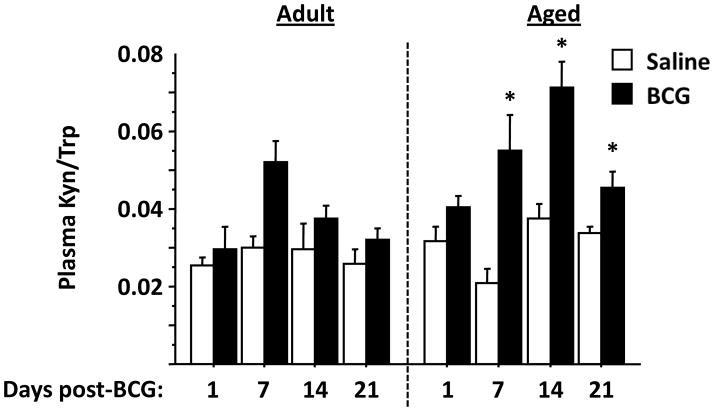
Tryptophan Catabolism Caused by BCG Infection Persists in Aged Mice
Depression-like behavior of adult mice that was induced by BCG requires IDO activity, which is reflected by an increase in the Kyn/Trp ratio (O’Connor et al., 2009b; O’Connor et al., 2009c). To determine if tryptophan catabolism was also more prolonged in aged than in adult mice infected with BCG, plasma was analyzed to determine the Kyn/Trp ratio (Fig. 6). As expected and previously reported (O’Connor et al., 2009b; O’Connor et al., 2009c), BCG caused an increase the ratio of Kyn/Trp in the plasma of adult mice in a BCG- (F1,42 = 3.7, P<0.01) and time-dependent manner (F3,42 = 9.5, P<0.05). Peak Kyn/Trp of adult BCG-treated mice occurred at 7 d post-treatment. In contrast, aged mice displayed a BCG- and time-dependent increase in the Kyn/Trp ratio (BCG × time interaction, F3,35 = 3.3, P<0.05). Post-hoc analysis revealed that the Kyn/Trp was elevated by BCG at 7, 14 and 21 d by BCG infection with peak levels occurring on day 14, a full week later than the peak ratio of adult mice. These data confirm that the long-lasting depression-like behaviors that occur in aged mice following infection with BCG occurs in parallel with an increase in tryptophan catabolism.
Figure 6. Aged mice have an extended peak increase in plasma kynurenine/tryptophan (Kyn/Trp) ratio following infection with BCG.
The Kyn/Trp ratio is elevated following infection of adult mice with BCG (P<0.05) with a peak at day 7. Aged mice displayed a significantly elevated Kyn/Trp ratio following BCG. Levels were elevated with BCG infection at day 7, were highest at day 14 and remained elevated at day 21. Asterisks denote a statistical difference (P<0.05) between saline and BCG-treated mice at a given time in either adult or aged mice. Data represent the mean ± SEM (n=5–7 mice per treatment group for each time point).
The simple Pearson correlation coefficient between the kynurenine/tryptophan ratio and sucrose preference was −0.33 (P<0.01; n=93) and for the TST it was 0.26 (P<0.05; n=93). After adjustment for age, BCG infection and time, the partial Spearman correlation coefficients were −0.22 (P<0.05) and 0.21 (P<0.05). There was no significant relationship between the kynurenine/tryptophan ratio and TST, locomotor activity or rearings.
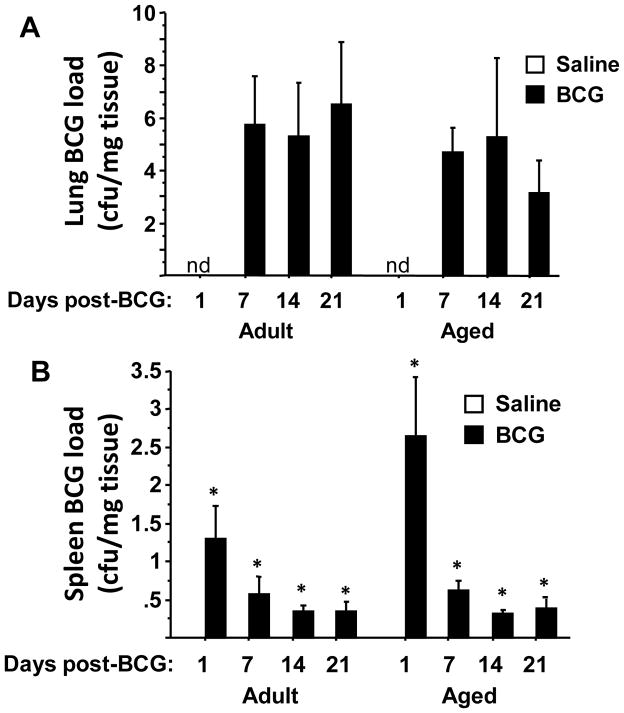
Number of BCG Colonies From Lungs and Spleens of Adult and Aged Mice
Lymphocytes undergo a number of age-related changes, and most notable is a reduction in the relative frequency of naïve versus memory CD4+ and CD8+ T lymphocytes. These cells are critical for secreting interferon (IFN)-γ to activate lung macrophages where mycobacteria are most prone to survive (Lee et al., 2012; Torrado et al., 2011). These and other age-associated changes in adaptive and innate immunity could alter the proliferation or survival of BCG. To determine whether the protracted depression-like behavioral responses of aged mice was associated with increased mycobacterial load of BCG, the number of BCG CFUs in the lungs and spleen was measured 1, 7, 14 and 21 days after infection. BCG was not detected in either lung or spleen of any saline-treated control mouse (Fig. 7A–B). As expected, the lungs of both young adult and aged mice contained the highest concentration of CFUs. A significant increase in BCG mycobacterial load was detected in lungs of adult mice beginning at 7 days post-infection (Fig. 7A; F1,40 = 14.0, p<0.001) that was independent of time. Similarly, the number of BCG CFU was also significantly increased in the lungs of aged mice from 7 to 21 days (Fig. 7A; F1,34 = 19.1, p<0.001) that was also independent of time. In the spleens of adult mice, BCG mycobacterial load peaked one day after inoculation, followed by diminished, but measurable, colony formation at 7, 14 and 21 days post-inoculation (Fig. 7B; BCG × time interaction F3,40 = 5.8, P<0.01). A similar BCG × time interaction (Fig. 7B, F3,34 = 3.0, P<0.05) was detected in splenic cultures from aged BCG infected mice. The number of splenic CFUs was similar in young adult and aged mice at 7, 14 and 21 days post-infection. Taken together, these data indicate that BCG load in the lungs and spleen is not markedly affected by age during the first 21 days following infection.
Figure 7. Mycobacterial Load in the lungs and spleen of adult and aged mice.
The number of CFUs in lung was increased (P<0.001) by infection with BCG at 7, 14 and 21 days post-infection in both adult and aged mice. In the spleen, there was a BCG × time interaction (P<0.05) for both young adult and aged mice, with the number of BCG colonies declining from 1 to 21 days. No CFUs were detected in either adult or aged mice injected with only saline. Asterisks denote a statistical difference (P<0.05) between saline and BCG-treated mice at a given time in either adult or aged mice. nd = Not Detectable. Data represent the mean ± SEM (n=5–7 mice per treatment group for each time point).
DISCUSSION
The discovery of a positive relationship between systemic inflammation and incidence of clinical depression created a new approach for understanding this disorder (Dantzer et al., 2008; Kelley and Dantzer, 2011; Miller, 2009). We have developed preclinical animal models in which depression-like symptoms appear following injection of either acute (LPS) (Frenois et al., 2007; Fu et al., 2010; O’Connor et al., 2009c) or chronic (BCG) (Moreau et al., 2008; O’Connor et al., 2009a; O’Connor et al., 2009b) inflammatory stimuli. Since clinical depression develops in a large proportion of patients treated with recombinant cytokines or afflicted with chronic inflammatory disorders, the BCG model that we employed in these experiments has construct validity for this costly human disorder. Furthermore, the two behavioral tests employed in these experiments have face (sucrose preference) and predictive (tail suspension) validity for clinical depression (Nestler and Hyman, 2010). These new data confirm that BCG infection induces depression-like behaviors, as we have previously demonstrated. More importantly, we extend these observations by showing that aged mice exhibit depression-like behaviors long after adult mice have recovered. Further, we confirm that the extended depression-like behavior that occurs in aged mice parallels an extended period of tryptophan catabolism, as shown by the sustained elevation of the Kyn/Trp ratio. By using this chronic infection model with BCG, data in this report are the first to show that recovery from infection-induced depression is prolonged in aged animals.
Using LPS to activate cytokine signaling in the periphery and in the brain, it has been established that aged animals require more time to recover from both sickness and depression- like behavior (Abraham and Johnson, 2009; Godbout et al., 2005; Godbout et al., 2008; Huang et al., 2008). In the LPS model, loss of body weight and reduction in both social behavior and locomotor activity appear within a few hours of injection. By 24 h after injection of LPS, these signs of sickness disappear and only behaviors characteristic of depression remain, such as those measured in this report. In aged mice, we have reported that following a single acute LPS injection via the i.p. route, recovery of locomotor activity, body weight and depression-like behaviors requires more time than in adult mice (Godbout et al., 2008). Although more prolonged in aged mice than in young adults, none of the LPS-induced changes reported by Godbout et al (2008) extended past 72 h following injection of LPS. Here we report prolonged (7 day) sickness and depression-like responses (21 days) in aged mice using BCG as a chronic model of inflammation. In accord with a major need to use chronic rather than acute preclinical models of depression (Nestler and Hyman, 2010), we found that body weight loss (Fig. 1), locomotor activity (Fig. 2), number of rearings (Fig. 3) and depression-like behaviors (Figs. 4 and 5) lasted longer in aged than in adult mice. These behavioral changes caused by BCG lasted for days to weeks rather than hours as occurs with LPS (O’Connor et al., 2009c). However, aged mice eventually recovered from the behavioral changes caused by BCG because both locomotor activity and rearing behavior fully recovered to that of control, non-infected mice at both 14 and 21 days following infection. Consistent with our earlier findings, aging increased duration of the depression-like behaviors. A second important observation was that depression-like behaviors of aged mice continued for at least three weeks following infection with BCG whereas they were apparent in adult mice at only 1 and 7 days following infection. Shorter duration of BCG-induced depression-like behaviors in control adult Balb/c mice used in these experiments than observed in CD1 mice (Moreau et al, 2008) is probably associated with genetic differences between inbred Balb/c and outbred CD1 mice. Collectively, these data offer unequivocal support for the idea that duration of depression-like behaviors is extended in aged animals following exposure to a chronic inflammatory insult.(Godbout et al., 2008).
The incidence of mycobacterial infections such as Mycobacterium tuberculosis increases with aging in the United States (Mori and Leung, 2010). Coupled with the decline in protective T cell immunity in the aged, we considered the possibility that the prolonged depression-like behaviors of aged mice might be due to inability of aged mice to clear the BCG microorganisms. At least three points argue against this possibility. The first is that BCG resides mostly in macrophages of the lung. However, the number of BCG colonies in the lungs, as well as the spleen, was similar in adult and aged mice. Second, aged mice appeared to clear the BCG from their lungs and spleens as well as adult mice. Indeed, the absolute number of BCG colonies in the lungs of aged mice was numerically less than in adult mice at 7 and 21 days following infection. Finally, both locomotor and rearing activity was comparable to saline-injected adult and aged mice at 14 and 21 days, indicating that these symptoms of sickness had fully recovered in both young adult and aged mice. However, at the same time points, both sucrose preference and the TST behavioral responses were impaired in only the aged mice. Infection with BCG initiated some physiological event, such as an increase in tryptophan catabolism, which caused the prolonged increase in depression-like behaviors that was independent of the number of BCG CFUs. These data are inconsistent with the hypothesis that a greater mycobacterial load is responsible for the prolonged depression-like behaviors and increase in tryptophan catabolism of aged mice.
Current understanding of aging and neurodegeneration offers some insights into how aging can influence chronic inflammation-induced depression-like behaviors. It is now well-accepted that peripheral activation of the immune system conveys that information to the brain to elicit behavioral changes (Dantzer et al., 2008; Kelley and Dantzer, 2011) and that aging somehow distorts this process (Lucin and Wyss-Coray, 2009). Part of this communicative process is mediated directly by peripherally produced cytokines. The peripheral cytokine response to an inflammatory signal such as LPS is elevated in aged mice compared to adults (Godbout et al., 2008). The greater change in peripheral cytokines results in an exaggerated inflammatory response in aged animals, as illustrated by heightened cytokine expression in the brains of aged mice (Huang et al., 2008; Kelley et al., 2003). In addition, part of the communicative process between the innate immune system and the brain is mediated by cytokine-induced changes in metabolism of precursors of neurotransmitters. One of the downstream mediators of inflammation-dependent depression-like behaviors of mice is the induction of IDO1 activity. Cytokines induce the expression of IDO1 with a resultant increase in tryptophan metabolism along the kynurenine pathway in peripheral tissues. This shift in metabolism induced by BCG infection results in an elevated Kyn/Trp ratio in the systemic circulation (O’Connor et al., 2009a). Inflammation-induced tryptophan catabolism is highly associated with depression (Dantzer et al., 2011; Kelley and Dantzer, 2011; McCusker and Kelley, 2012) and occurs in aged mice following acute activation of the immune system with LPS (Godbout et al., 2008). We have previously shown that depression-like behavior of mice is attenuated by 1-methyl-tryptophan (a competitive IDO inhibitor) (O’Connor et al., 2009c), minocycline (an inflammation inhibitor that indirectly decreases IDO activity) (O’Connor et al., 2009c), genetic deletion of IFN-γ receptor (to diminish IDO1 induction) (O’Connor et al., 2009a; O’Connor et al., 2009b) and importantly by genetic deletion of IDO1 activity (O’Connor et al., 2009b). In these models, diminishing inflammation-induced IDO1 expression resulted in lower Kyn/Trp ratios and loss of depression-like behaviors. In contrast, peripheral administration of kynurenine elevated the circulating Kyn/Trp ratio and induced depression-like behavior of mice in the forced swim test (O’Connor et al., 2009c), TST (O’Connor et al., 2009c) and sucrose preference test (Salazar et al., 2012). In our current experiments, we have extended the relationship between tryptophan catabolism and depression by showing that both depression-like behaviors and the increased Kyn/Trp ratio persist longer in aged mice than in adult mice following BCG infection. Together, these data strongly suggest that an IDO-dependent mechanism(s) is involved in the extended depression-like behaviors of aged mice following infection with BCG.
A major unresolved issue is whether it is the chronic proinflammatory response that is responsible for aged-associated changes in the brain or whether there are ineffective anti-inflammatory responses to prevent these changes. It is also unknown whether aberrant regulation in the periphery or in the brain is responsible for the age-associated increase in time to behaviorally recover from a chronic inflammatory insult. However, it is known that microglia are important targets in this process because these cells are central sensors of peripheral inflammation (Perry et al., 2007). Microglia are hypothesized to be responsible for increased behavioral responsiveness to peripheral inflammatory stimuli, a process that has been termed as priming (Dilger and Johnson, 2008). Recent evidence supports this possibility. Microglia isolated from the brains of aged rats display a heightened response to LPS in vitro, as determined by increased expression of steady-state transcripts for IL-1β and IL-6, as well as the anti-inflammatory cytokine IL-10 (Frank et al., 2010). A potential mechanism for prolonged expression of IL-1β and slower recovery from sickness behaviors in the aged, and possibly inflammation-induced depression-like behavior, is a prolonged reduction in fractalkine receptor (CX3CR1) expression in microglia (Wynne et al., 2010). Fractalkine, a chemokine, is expressed by neurons and binds to its receptor, CX3CR1, on microglia. When microglia from mice injected peripherally with LPS were isolated, these cells showed a large reduction in expression of the fractalkine receptor, and this reduction was sustained longer if the microglia were derived from aged mice (Wynne et al., 2010). These data are consistent with the idea that microglial cells in the central nervous system become dysregulated during the aging process and support the emerging concept that hyperactivity of microglia mediates inflammation-induced behavioral changes.
Prior to the present report, there was no direct evidence that a chronic infection would affect the behavior of aged mice any differently than adults. Here we offer persuasive evidence that BCG infection not only induces depression-like behaviors in both adult and aged mice, but that these behaviors are sustained much longer in aged mice. As such, these are the first data to use a chronic live infection model with BCG to show that aged mice recover more slowly than young adult mice from inflammation-induced depression-like behavior.
Acknowledgments
Supported by NIH grants to KWK (R01 AG 029573 and R01 AG 029573-04S1), SRZ (R21 MH096030), JCO (R01 MH 090127) and RHM (MH083767).
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain, behavior, and immunity. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Alfonso H, Pirkis J, Kerse N, Sim M, Flicker L, Snowdon J, Draper B, Byrne G, Goldney R, Lautenschlager NT, Stocks N, Scazufca M, Huisman M, Araya R, Pfaff J. A practical approach to assess depression risk and to guide risk reduction strategies in later life. Int Psychogeriatr. 2011;23:280–291. doi: 10.1017/S1041610210001870. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Geerlings SW, Deeg DJ, Smit JH, Schoevers RS, de Beurs E, Braam AW, Penninx BW, van Tilburg W. The natural history of late-life depression: a 6-year prospective study in the community. Archives of general psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. Journal of neuroimmunology. 2010;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zunich SM, O’Connor JC, Kavelaars A, Dantzer R, Kelley KW. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. Journal of neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain, behavior, and immunity. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Dantzer R. Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain, behavior, and immunity. 2011;25(Suppl 1):S13–20. doi: 10.1016/j.bbi.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Bosma H, Kempen GI, Penninx BW, Beekman AT, Deeg DJ, van Eijk JT. Socioeconomic differences in incident depression in older adults: the role of psychosocial factors, physical health status, and behavioral factors. J Psychosom Res. 2006;61:619–627. doi: 10.1016/j.jpsychores.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Lee N, Shin MS, Kang I. T-cell biology in aging, with a focus on lung disease. The journals of gerontology. Series A, Biological sciences and medical sciences. 2012;67:254–263. doi: 10.1093/gerona/glr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Jones RH, Snowdon J. Depression in nursing homes: ensuring adequate treatment. CNS drugs. 2007;21:627–640. doi: 10.2165/00023210-200721080-00002. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusoodanan S, Ibrahim FA, Malik A. Primary prevention in geriatric psychiatry. Ann Clin Psychiatry. 2010;22:249–261. [PubMed] [Google Scholar]
- McCusker RH, Kelley KW. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. J Experimental Biology. 2012 doi: 10.1242/jeb.073411. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. Journal of affective disorders. 2011;129:126–142. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Andre C, O’Connor JC, Dumich SA, Woods JA, Kelley KW, Dantzer R, Lestage J, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain, behavior, and immunity. 2008;22:1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. The Journal of infectious diseases. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Mori T, Leung CC. Tuberculosis in the global aging population. Infectious disease clinics of North America. 2010;24:751–768. doi: 10.1016/j.idc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nature neuroscience. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009a;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009b;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular psychiatry. 2009c;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nature reviews. Neuroscience. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.03.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Kasper JD, Phillips CL. Physical disability and social interaction: factors associated with low social contact and home confinement in disabled older women (The Women’s Health and Aging Study) The journals of gerontology. Series B, Psychological sciences and social sciences. 1998;53:S209–217. doi: 10.1093/geronb/53b.4.s209. [DOI] [PubMed] [Google Scholar]
- Snowdon J. Is depression more prevalent in old age? Aust N Z J Psychiatry. 2001;35:782–787. doi: 10.1046/j.1440-1614.2001.00968.x. [DOI] [PubMed] [Google Scholar]
- Soudi S, Hosseini AZ, Hashemi SM. Co-administration of rectal BCG and autoclaved Leishmania major induce protection in susceptible BALB/c mice. Parasite immunology. 2011;33:561–571. doi: 10.1111/j.1365-3024.2011.01318.x. [DOI] [PubMed] [Google Scholar]
- Tiemeier H. Biological risk factors for late life depression. Eur J Epidemiol. 2003;18:745–750. doi: 10.1023/a:1025388203548. [DOI] [PubMed] [Google Scholar]
- Torrado E, Robinson RT, Cooper AM. Cellular response to mycobacteria: balancing protection and pathology. Trends in immunology. 2011;32:66–72. doi: 10.1016/j.it.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]