Abstract
Prenatal anxiety has been linked with altered immune function in offspring in animal studies, but the relevance for human health is unknown. We examined prenatal maternal anxiety as a predictor of adaptive immunity in infants at 2 and 6 months of age as part of a prospective longitudinal study. The humoral immune response to hepatitis B vaccine was assessed at 2 months (n=80) and 6 months (n=76) of age. Prenatal anxiety predicted lower hepatitis B antibody titers at 6 months of age independent of obstetric and socio-demographic covariates; the effects were limited to those infants who had not completed the 3-dose vaccine series (for transformed titer values, r= −.36, p<.05). Cell-mediated immune responses at 2 (n=56) and 6 (n=54) months of age were examined by ELISpot assays for interferon(IFN)-γ, interleukin(IL)-2, and IL-4 responder cell frequencies to three antigens: hepatitis B surface antigen, tetanus toxoid, and phytohaemagglutinin (PHA). Prenatal maternal anxiety was associated with reduced IFN-γ and increased IL-4 responder cell frequencies at 6 months of age, independent of obstetric and socio-demographic covariates. No effect of prenatal anxiety was found on adaptive immunity at 2 months of age. The findings provide the first demonstration in humans that prenatal anxiety alters adaptive immunity in the infant.
Keywords: prenatal anxiety, adaptive immunity, developmental programming
Several lines of empirical study and theory suggest that the prenatal environment may have lasting effects on the offspring’s behavioral and biological development (Barker, 2007; Gluckman et al., 2005). Prenatal stress is an exemplar. Experimental animal studies demonstrate that offspring of prenatally stressed mothers exhibit disturbances in behavior, stress physiology, cardiovascular function, neurological development, and immunity that persist into adulthood (Clarke and Schneider, 1993; Coe and Lubach, 2005; Diz-Chaves et al., 2012; Henry et al., 1994; Kay et al., 1998). Because of the potential public health implications of these observations, several research programs have sought to translate these findings to human development. Data so far reported suggest that a wide range of outcomes in humans is linked with prenatal anxiety or stress (both risk phenotypes have been used in human studies), including psychiatric symptoms (Gutteling et al., 2005; O’Connor et al., 2003), cognitive and language ability (Laplante et al., 2008), neurodevelopment (Buss et al., 2012; Glover et al., 2004; Obel et al., 2003), and stress physiology (Davis et al., 2011; O’Connor et al., in press; O’Donnell et al., in press). The current study provides further translation of this work by assessing the link between prenatal anxiety and adaptive immunity in infants.
Animal studies suggest that prenatal stress has lasting effects on the immune system of offspring (Coe et al., 2002; Coe et al., 2007; Couret et al., 2009; Kay et al., 1998). Although the kind of stress exposure and the particular index of immune function vary across investigation, results generally indicate that prenatal stress compromises immune responses in the offspring. Whether or not a similar link exists in humans is not known. Indirect support is suggested by studies demonstrating an association between prenatal maternal anxiety or other mood states with asthma (Khashan et al., 2012; Lefevre et al., 2011) and infectious diseases (Nielsen et al., 2011) in children; studies using cord blood samples report associations between lymphocyte subset distributions and mode of delivery as an index of perinatal stress (Duijts et al., 2008), and between maternal stress and select in vitro cytokine responses (Wright et al., 2010). The current study, the first to examine prenatal anxiety and specific immune responses in infants, extends this line of study by assessing both humoral immune responses and antigen specific T cell responses to vaccination in serial blood draws in 2- and 6-months-old infants.
Antibody responses to hepatitis B vaccine are an in vivo indicator of the infant immune response to a novel antigen. The examination of hepatitis B antibody responses provides several advantages for experimental study. First, maternal vaccination or infection with hepatitis B in pregnancy is rare (and subjects with either can be excluded); furthermore, unlike other immunizations such as measles, there is no maternal antibody interference of the infant immunization response for hepatitis B (Junqueira et al., 2011; Wang et al., 2011), and so the infant humoral immune response is a de novo response with no interference from maternal antibodies. A second advantage is that the hepatitis B vaccine is a 3-dose series. The Center for Disease Control and Prevention (CDC) guidelines in place at the start of the study recommended a birth dose, a second at 2 months, and a final dose of vaccine between 6–18 months of age for all infants. This schedule offers short-term longitudinal leverage to track the infant immune response to multiple vaccine doses. Third, although clinical effectiveness has been demonstrated, individual differences in antibody production are regularly reported, especially following the initial dose, in infants (Davis, 2005; Greenberg et al., 1996; Samandari et al., 2007) as well as adults (Marsland et al., 2001). In adults, this variation, particularly variation in response to an initial dose, has been linked with psychological factors and traits, such as stress and mood (Burns et al., 2002; Marsland et al., 2001, 2006). We examine variation in infant antibody response in relation to prenatal maternal anxiety.
In addition to measuring antibody production, T cell responses to vaccine were examined using standard in vitro Elispot techniques for measuring interferon (IFN)-γ, IL-2, and IL-4 responder cell frequencies to hepatitis B surface antigen, tetanus toxoid, and PHA. Previous data have shown that IFN-γ recall responses to both hepatitis B and tetanus antigen are due to CD4+ T cell responses almost exclusively, and therefore, these targets were chosen (Ota et al., 2004). We chose IFN-γ T cell responder cell frequencies as a measure of type 1 responses and IL-4 for type 2 responses. In addition, we measured IL-2 responder cell frequencies because of prior data suggesting that the dominant T cell response to hepatitis B vaccine is via cells producing IL-2 (De Rosa et al., 2004). Including markers of both type 1 and type 2 responses allowed us to test the hypothesis that prenatal maternal anxiety, an index of early stress exposure, would be associated with a reduced type 1 response in comparison to a type 2 response, which is the pattern that would be expected to underlie reports linking prenatal anxiety to asthma.
We applied the research paradigm used to explain variation in hepatitis B immune responses in adults to a study of prenatal maternal anxiety and child immune function. Our hypothesis was that children whose mothers experienced greater anxiety in pregnancy would exhibit less robust immune responses. We also examined a possible mechanism for this association. Prenatal glucocorticoid exposure is a leading candidate mechanism that might link prenatal maternal anxiety or stress and immune function in the child. Specifically, prenatal anxiety or stress may alter maternal hypothalamic-pituitary-adrenal (HPA) axis function which may influence the development of the fetal HPA axis (O’Connor et al., 2005; O’Donnell et al., in press) and possible infant immune responsiveness given the suppressive effect of HPA axis hormones, particularly cortisol, on the immune system (Glaser and Kiecolt-Glaser, 2005; Moynihan and Ader, 1996). Research utilizing direct measurement of fetal exposure to (or production of) glucocorticoids is limited; we include in this study maternal prenatal diurnal cortisol at two time points in pregnancy as an index of fetal glucocorticoid exposure.
Methods
Participants and procedures
Pregnant women attending a university medical center obstetrics clinic in a medium-sized urban setting were approached to participate in a study on mood in pregnancy and child development. The clinic serves a disproportionate percentage of low-income, urban, minority women. A total of 2,607 women were approached at their initial clinic visit; of these, 1,209 met the inclusion criteria by being 20–34 years of age, with no current or history of psychotic illness, having a normal medical risk pregnancy (as determined by obstetrics clinic staff and physicians), and being able to communicate in English.
Routine prenatal maternal screening included screening for hepatitis B surface antigen; all those who were positive (indicating active infection) were excluded from the study. Prenatal screening does not routinely include screening for maternal hepatitis B antibodies (anti-HBs), which may be positive if there was past immunization or prior resolved infection. Wang et al. (Wang et al., 2011) and Junqueira et al. (Junqueira et al., 2011) reported that maternal antibody to hepatitis B surface antigen does not interfere with hepatitis B vaccine response in the infant; Hu et al. (Hu et al., 2008) reported that there is interference but only at high maternal anti-HBs titer (> 1000 mIU/ml). Full hepatitis B vaccine history was available on a subset (40%) of mothers; of these, 23% had been immunized for hepatitis B, with an average of 9 years prior to the baby’s birth (see supplementary information).
Of those who met these inclusion criteria, 627 (52%) expressed an interest in participating in the study. For these women, we applied a screening process to over-sample women with significant prenatal anxiety. Screening for prenatal anxiety was based on the Penn State Worry Questionnaire (PSWQ) (Behar et al., 2003; Meyer et al., 1990); women completed the measure at the time of the initial clinic visit, usually 8–12 weeks gestation. We adopted a comparatively low cut-off score for screening for clinically significant anxiety: women scoring above 45 on the PSWQ and a subset of women scoring below 45 were invited to participate in the study; this resulted in over-sampling women with clinically significant anxiety (n=210). Because the enrollment time frame was approximately 24 months, there were 12 women with repeat pregnancies; we adjusted for >1 child from the same mother in the analyses below. Once enrolled, women were assessed at approximately 20 and 32 weeks gestation for psychiatric and psychosocial factors and diurnal cortisol; the mother and baby were assessed when the child was 2- and 6-months of age, at which time we conducted developmental assessments of the baby and collected a blood sample via venipuncture. All questionnaire measures were read aloud to the mother in an interview format. Mothers provided written informed consent to participate and the study was approved by the local Institutional Review Board.
Measures
Maternal anxiety
The key index of anxious symptoms in the study was the PSWQ (Meyer et al., 1990), a 16-item index of worry with considerable evidence for reliability and validity; the PSWQ has been used in the perinatal period (Swanson et al., 2011). We averaged scores at 20 and 32 weeks gestation to index prenatal anxiety in the absence of strong a priori prenatal anxiety timing effects (see below). An additional index of anxiety was the state subscale of the State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1983), a 20-item index of current anxiety-related items with considerable validity and reliability. To address limits of self-report questionnaires, we administered the Structured Clinical Inventory for DSM-IV(First, 1995). For analyses below we consider those women who met criteria for Generalized Anxiety Disorder at either prenatal assessment as having a prenatal diagnosis.
Maternal diurnal cortisol
At each prenatal visit, mothers were instructed to provide diurnal cortisol according to a standard protocol (MacArthur Research Network on Socioeconomic Status and Health, http:/www.macses.ucsf.edu/research/allostatic/notebook/salivarycort.html). Samples were collected at awakening and then 45 minutes, 2.5 hours, 8 hours, and 12 hours after awakening on an ordinary week-day. Women were provided pre-labeled, color-coded tubes, and a diary for reporting collection time. Collection was made using salivettes (Starstedt, Newton, NC). Women were trained in how to use the salivettes, instructed to avoid brushing teeth and eating 30 minutes prior to sampling, and instructed to store the samples in a refrigerator until the next regularly scheduled obstetrics visit. Reminder phone calls were used where needed to coordinate sample collection. Samples were kept at −20°C until assayed using commercially available high-sensitivity enzyme-linked immunoassay kits (Salimetrics, State College, PA) which have a range of sensitivity from 0.003 to 3 μg/dl. All samples were run in duplicate; average intra- and inter-assay coefficients of variation were 4.13% and 8.89%. Maternal prenatal diurnal cortisol data at 20 and 32 weeks gestation were analyzed using the area under the curve with respect to ground (Fekedulegn et al., 2007).
Hepatitis B antibody titer
Using serum separator tubes, peripheral whole blood from babies was obtained and the serum was separated by centrifugation and transferred to sterile cryovials and frozen at −80°C until time of assay, per the manufacturer’s protocol. Antibodies to hepatitis B surface antigen were detected using the MONOLISA enzyme-linked immunoassay kit from Bio-Rad Laboratories (Redmond, WA). The assay was read at 450nm using an automated SpectraMAX 340 microplate reader (Molecular Devices, Sunnyvale, CA). Samples were assayed in duplicate when possible. Hepatitis B antibody concentrations were assessed as a continuous measure and as a dichotomous measure of seroconversion (>10 mIU/ml).
The study visits at 2- and 6-months of age were planned to precede on the same day the well-baby visits when routine vaccines, including hepatitis B and diphtheria, tetanus and acellular pertussis (DTaP) were administered. That is, at the 2-month visit the infant hepatitis B titer would reflect the response to the birth dose; at the 6-month visit the hepatitis B titer would reflect the response following two doses. In practice, because of missed and rescheduled appointments, this was not possible in all cases. In addition, because some mothers refused the birth dose (<10%) and the pediatric clinic was immunizing “ahead” of schedule in some cases, there was variation in the number of hepatitis B immunizations received by the infants at the 2- and 6-month visits. We consider the number of immunizations and the timing of the last immunization as covariates in our analyses of hepatitis B antibody titer.
Cytokine secretion ELISpots
Fresh peripheral blood mononuclear cells (PBMCs) were obtained from 0.5–2ml of heparinized venous blood via density gradient centrifugation (same venipuncture used for measurement of antibody responses) and were used in ELISpot assays. Briefly, 96 well ELISpot plates (Millipore, MAIPS4510) were coated with anti-human IFN-γ (1 μg /mL, MabTech, Cincinnati, OH), IL-2 (1:60, R&D Systems, Minneapolis, MN) or IL-4 (1 μg /mL, MabTech, Cincinnati, OH), and blocked with RPMI with 8% fetal bovine serum. PBMC were loaded into wells and incubated overnight for 16–18 hours with hepatitis B antigen (10 μg /mL, Aldevron, South Fargo, ND), tetanus toxoid (1 Lf/mL, Cylex Incorporated, Columbia, MD), or phytohemagglutinin (PHA; 20 μg /mL, Sigma Aldrich). PBMCs plated in RPMI+8% FBS served as background controls. Additionally, an internal control was used for each assay. After washing, wells were incubated with alkaline phosphatase conjugated anti-human IFN-γ (1ug/mL, MabTech), IL-2 (1:60, R&D Systems) or IL-4 (1 μg /mL, MabTech), and spots were developed with substrate vector kit (Vector Laboratories, Burlingame, CA), counted on an Immunospot CTL reader (Cellular Technologies, Ltd) and was expressed as cells per 106 PMBC. Background control well values were subtracted from stimulated well values.
Circulating cytokines
Milliplex MAP high-sensitivity human cytokine magnetic beads (Millipore, Billercia, MA) in conjunction with the Bio-Plex 200 (Bio-Rad, Hercules, CA) Luminex platform was utilized, as per manufacturer’s instructions, to quantify circulating interleukin-12 (IL-12) using 50–100 μl of serum obtained as described above. Briefly, magnetic beads coated with antibodies to human IL-12 were washed in a 96-well plate using a magnetic plate washer. Serum samples diluted 1:2 with serum matrix (provided) along with the kit standards and controls were then added. All subject samples were run in duplicate when possible. The plate was incubated overnight at 4°C on an orbital plate shaker then the beads were washed followed by the addition of biotin- conjugated detection antibody. After incubating and shaking at room temperature for 60 minutes, streptavidin-phycoerythrin was added and the plate was incubated with further shaking for an additional 30 minutes. The beads were washed, re-suspended and streamed through the lasers in the Bio-Plex 200. Data were analyzed using the Bio-Plex Manager software, version 4.1 (Bio-Rad, Hercules, CA). The minimum detectable concentration of IL-12 with this kit is 0.34 pg/ml.
Covariates
Obstetric factors included maternal BMI, pregnancy complications (composite index based on obstetrician review), smoking (coded yes/no) and alcohol intake (coded yes/no) in pregnancy, as well as birth weight, gestational age, and mode of delivery (vaginal versus cesarean section). Psychosocial and demographic covariates included maternal education, race/ethnicity, health insurance status, marital status, income, and infant sex; for the hepatitis B antibody analyses we also considered the manufacturer of the hepatitis B vaccine at the most recent immunization (Greenberg et al., 1996).
Statistical analyses
For analyses of hepatitis B antibody titer we include the number of immunizations and the timing of the last immunization as covariates. Bivariate analyses examine the association between prenatal anxiety and antibody titer according to assessment age and number of vaccinations received; the final prediction model included a prenatal anxiety X dose interaction to test the hypothesis that the association between prenatal anxiety and infant hepatitis B antibody titer varied as a function of immunization history, after accounting for covariates. In analyses of T cell responses, we consider as potential confounders immunization history of hepatitis B vaccinations for responder cell frequencies to hepatitis B surface antigen; similarly, we consider history of tetanus toxoid vaccinations for responder cell frequencies to tetanus toxoid. We first examine correlations between prenatal maternal anxiety and IFN-γ, IL-2, and IL-4 responder cell frequencies to the hepatitis B surface antigen, tetanus toxoid, and PHA antigens at 2 and 6 months of age; this is followed by a regression model that includes covariates. We report raw values of antibody response to hepatitis B vaccination and responder cell frequencies for descriptive purposes, but correlation and prediction analyses are based on the square root transformation because of the non-normal distribution of the raw data (findings were substantially identical with the raw and transformed data using natural log or square root transformation). In addition to prenatal maternal anxiety as a predictor, we also consider maternal prenatal diurnal cortisol as a predictor of infant adaptive immune outcomes. Obstetric and socio-demographic covariates noted above are examined as possible confounds. Final prediction models are analyzed using generalized estimating equations.
Results
Of the 210 mothers originally recruited, 4 were excluded because of significant drug use or psychotic illness that was identified only after enrollment; we also subsequently excluded 8 children because of very low birth weight (<1500g), stillbirth, or significant health problems that would confound analyses of immune function (e.g., sickle cell disease), leaving 198 participants. Of these, 142 (72%) returned for a visit by 6 months of age (133 at 2 months, 123 at 6 months). Women and their babies who were seen for a postnatal visit did not differ from those who did not return for a postnatal visit on prenatal anxiety, socio-demographic factors, or obstetric outcomes; however, mothers lost to contact had a lower prenatal BMI than women who remained in the study (F(1,196)=4.72, p=.03). Descriptive data are supplied in Table 1.
Table 1.
Socio-demographic characteristics of mothers and babies.
| Mean (SD)/% | |
|---|---|
|
| |
| Maternal age | 24.39 (3.90) |
|
| |
| Ethnicity/Race | |
| African-American | 58% |
| White/Caucasian | 28% |
| Hispanic | 13% |
| Other | 1% |
|
| |
| Education (years) | 12.5 (1.77) |
| < high school | 24% |
| HS or equivalent | 37% |
| >HS < college degree | 34% |
| College/university degree | 6% |
|
| |
| Medicaid recipient | 73% |
|
| |
| Birth weight (g) | 3265.81 (490.26) |
| Gestational age (days) | 276.26 (8.97) |
|
| |
| Smoking (cigarettes) | |
| None | 84% |
| <10/day | 15% |
| >= 10/day | 1% |
|
| |
| Child sex (male) | 50% |
|
| |
| Anxiety disorder (any in pregnancy) | 46% |
Note. N=142.
Hepatitis B antibody response to immunization
At the 2-month visit, blood samples for hepatitis B antibody titers were collected on 86 (65%) infants; the primary reason for missing data was failure to obtain a sufficient sample (31, 23%). Of these 86 infants, 10 were subsequently excluded because of a measurable hepatitis B antibody titer in the mother; 8 were excluded because of technical problems with the blood sample; 1 sample was excluded because of uncertainty about the date of hepatitis B vaccine administration. The majority of infants, 54 (81%), had a single dose of vaccine (range: 0–2); the number of doses was not significantly associated with prenatal anxiety or covariates.
Variation in hepatitis B antibody titer concentration at 2 months of age was associated with the number of doses; for transformed data, r(67) = .36, p<.01, but there was variation among those with 1 dose (raw mean 61.01 mIU/ml [SD 183.40]) and 2 doses (raw mean 195.73 mIU/ml [SD 225.25]); seroconversion was detected in 23/54 (43%) infants with 1 dose and 7/9 (78%) infants with 2 doses, chi-square (2) = 8.94, p<.05. There was no evidence that hepatitis B antibody concentration at 2 months was predicted from prenatal anxiety.
At 6 months, hepatitis B antibody titers were available on 75 of 123 (61%) infants assessed; the primary reason for missing antibody concentrations was failure to obtain a sufficient sample (28, 23%). Of these 75 infants, 11 were excluded because of a measurable hepatitis B antibody titer in the mother; and a further 3 cases were excluded because of uncertainty about the timing of a hepatitis immunization, leaving 61 subjects for analysis. At the 6-month visit, 28 (46%) had completed the 3-dose series; these infants did not differ significantly from those who had not completed the immunization series on maternal prenatal anxiety or the covariates listed above. Antibody titers were significantly greater in infants who had completed the 3-dose series (raw mean 509.23 mIU/ml [SD 607.84]) compared to those who had not received the full series (raw mean 208.51 mIU/ml [SD273.93]; F(1,59)=6.530, p<.05). Also, whereas 27/28 (96%) infants who had received the full series demonstrated seroconversion, only 26/33 (79%) who had not received the full 3-dose series had seroconverted (chi-square(1)=4.67, p<.01). Time since last immunization was negatively associated with antibody concentration (for transformed data, r(61) = −.25, p=.054).
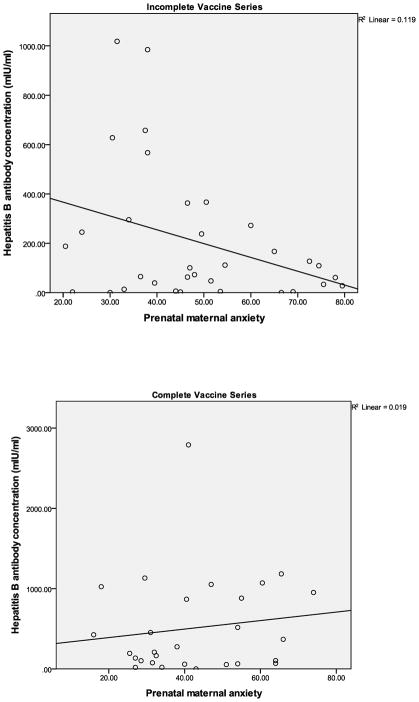
Among those infants who did not receive the full 3-dose series, maternal prenatal anxiety was significantly negatively associated with antibody titer (raw titer r(33)= −.35, p<.05; for transformed values, r=−.30); in contrast, there was no significant association between maternal prenatal anxiety and antibody titer in infants who received the full 3-dose series (raw titer r(28)=.14, ns; for transformed values, r=.16); see Figure 1. Regression model results confirmed the above analyses in showing an interaction between vaccine history and prenatal anxiety; the effect remained significant after adjusting for key covariates (B=.42, SE [.14]; Table 2).
Figure 1.
Association between maternal prenatal anxiety and hepatitis B antibody response in 6-month-olds according to immunization history.
Table 2.
Prenatal maternal anxiety predicts infant hepatitis B antibody concentration at 6 months.
| B | SE | 95%CI (B) | p | |
|---|---|---|---|---|
| Immunization history | 12.91 | 6.95 | −.72 – 26.53 | .06 |
| Time since last immunization | −.02 | .02 | −.06 – .02 | .41 |
| Maternal prenatal anxiety | −.12 | .08 | −.28 – .04 | .14 |
| Immunization history X Maternal prenatal anxiety | .42 | .14 | .15 – .69 | .01 |
Note. Estimates derived from final model for transformed hepatitis B antibody response. Immunization history is coded 0=incomplete series, 1=complete series. Covariates include obstetric complications, smoking, vaccine manufacturer, and birth weight.
T cell response to antigen/mitogen
The number of responder cells expressing IFN-γ, IL-2, or IL-4 varied across the antigen or mitogen used for stimulation in samples at 2- and 6-months of age (supplementary Table 1).
At 2 months of age, prenatal maternal anxiety was not significantly associated with responder cell frequencies by IFN-γ, IL-2, or IL-4 to any antigen/mitogen.
However, at 6 months of age, prenatal maternal anxiety was significantly associated with responder cells expressing IFN-γ and IL-4; this varied across antigen condition (supplementary Table 2). Prenatal maternal anxiety significantly predicted decreased IFN-γ producing cell frequencies to hepatitis B surface antigen at 6 months (r(54)= −.29, p<.05); this association was unchanged after adjusting for number of child hepatitis B vaccine doses, time since last vaccination, and presence of maternal hepatitis B titer. A regression model indicated that maternal prenatal anxiety predicted decreased IFN-γ responder cell frequencies to hepatitis B antigen (B=−.02 [.01], 95% CI −.04 −.001, p<.05) controlling for number of hepatitis B immunizations, time since last immunization, and birth weight.
Additionally, prenatal maternal anxiety predicted increased IL-4 responder cell frequencies in response to tetanus antigen (r(55)= .28, p<.05; supplementary Table 2); this association was unchanged after controlling for tetanus vaccine exposure (at 6 months, all infants had been exposed to at least one tetanus vaccine). A regression model for IL-4 T cell responses to tetanus antigen indicated that the effect of prenatal anxiety remained significant (B=.03 [.01], 95% CI .004 .05, p<.05) after accounting for birth weight and other covariates (although none of the covariates was a significant predictor or reduced the prenatal maternal anxiety effect).
IL-12 is critical for the induction of IFN-γ and type 1 responses (Hendrzak and Brunda, 1995; Heufler et al., 1996). Accordingly, we investigated if maternal prenatal anxiety was associated with a reduction of circulating concentrations of IL-12 in the infant. We were able to detect serum IL-12 concentrations in a subset of 30 children on whom we had Elispot data. In this subset of infants, a modest negative association between prenatal anxiety and IL-12 concentrations was observed at 2 months of age (r(30)= −.34, p=.065), however, prenatal anxiety was not significantly associated with serum IL-12 concentrations at 6 months of age. Additionally, serum IL-12 concentrations at 2 months of age were positively associated with IFN-γ Elispot responses to hepatitis B antigen at 6 months of age (r(16)=.40). Although the sample size in the latter analysis is too small to draw firm conclusions, the findings are consistent with the hypothesis that prenatal anxiety may impair type 1 responses to antigen via reduced concentrations of IL-12 in early infancy.
Supplementary analyses (see supplementary results) indicated that the prediction of adaptive immunity extends to other measures of anxiety collected in pregnancy.
Discussion
The role of adaptive immunity in conferring protection to the infant is of particular importance and has attracted considerable attention (Siegrist and Aspinall, 2009), but few studies assess sources of normative variation. Results from the current study provide the first evidence in humans that prenatal anxiety predicts reduced adaptive immune responses in infants, both humoral and cell-mediated. For antibody responses to hepatitis B vaccination, a significant negative association between prenatal anxiety and antibody titer was limited to those infants who did not receive the complete vaccine series at 6 months of age, suggesting that the relationship between prenatal anxiety and antibody response is only observable at times of suboptimal antigen stimulation. We also found that prenatal anxiety is associated with a reduced type 1 and increased type 2 response in the infant; that is, prenatal maternal anxiety may exaggerate the normal type 2 skewing and less robust type 1 cytokine response to specific antigens (Ota et al., 2004; PrabhuDas et al., 2011). These predictions were independent of multiple confounds, including obstetric and psychosocial factors.
Our data add to the growing list of outcomes in humans that have been linked with prenatal anxiety or stress. In addition, these data further translate the developmental programming model to immune function in humans. The current results inform and extend research on the developmental origins of adult disease model, which has typically been applied to metabolic and cardiovascular disease and mental illness rather than immune responsiveness or susceptibility to infectious disease (Hanson and Gluckman, 2011). Our findings show that prenatal maternal anxiety, which may partly underlie the developmental origins of adult disease hypothesis, is associated with decreased adaptive immune responses to vaccine and may explain increased rates of infectious and autoimmune disease.
The robustness of the prenatal anxiety effect on adaptive immunity is implied by the association with both humoral immunity, derived from in vivo analyses of infant production of antibodies, and from measures of immune memory, derived from in vitro analyses of T cell responses to antigen re-stimulation. Furthermore, the prenatal effect was generalized to symptom measures and clinical interviewer-rated symptoms. One other consistent finding between the humoral and T cell-mediated responses was that the effect of prenatal maternal anxiety was not evident in early infancy but was robust by 6 months of age.
These results represent an important extension of the animal work on prenatal stress because the translation of early immune function and response is difficult across species, and there are substantive differences in the early immune responses found in humans and rats (Hodgins and Shewen, 2012) – the species in which much of the animal prenatal stress work on immune function is based (Kay et al., 1998). Similarly, our data in infants are an important extension of the work reported on cord blood lymphocyte subsets because the phenotype of cells found in the circulation at birth may not be representative of the functional capability of individual cell types (Hodgins and Shewen, 2012). These results also begin to translate and encourage further research on early stress exposure and immune function in the child. Many studies show that early stress exposure is associated with increased rates of health problems in adults (Juster et al., 2010). Results from this investigation, and other recent research (Caserta et al., 2008; Caserta et al., 2011; Chen et al., 2006), imply that evidence of stress exposure on immune function is evident early in development, at least from 6 months of age.
Analyses of T-cell responses indicated that prenatal anxiety was negatively associated with a type 1 response to hepatitis B surface antigen and positively associated with a type 2 response to tetanus toxoid. Because these findings were in response to different vaccines it is difficult to draw any firm conclusions. One implication may be that prenatal anxiety is associated with differentiation of CD4+ T cells to further favor a type 2 response in the infant (Ota et al., 2004). That would help explain the links between prenatal anxiety and asthma that have been reported in the literature (Khashan et al., 2012).
Although significant associations between prenatal anxiety and hepatitis B antibody responses and IFN-γ T cell responses to hepatitis B vaccine were found, there was no association between the two types of responses. This is consistent with previous reports (Gans et al., 1999) of both infant and adult responses to hepatitis B vaccine (Ota et al., 2004). We did not find a predominant IL-2 CD4+T cell response to hepatitis B antigen as previously reported (De Rosa et al., 2004). One explanation for this may be differences in the methods of the two studies. Our study included young infants and not adult health care workers; in addition, we measured the vaccine responses several weeks to months following vaccination and not the short time period used in the previous study (De Rosa et al., 2004).
Glucocorticoid exposure is a leading candidate mechanism accounting for an association between prenatal maternal anxiety and immune outcomes in the offspring. We found no evidence for this link in the current study, in which fetal exposure was indexed by maternal prenatal diurnal cortisol. A lack of association may be because maternal salivary cortisol is only a weak indicator of fetal exposure, and so the current study may not have offered an adequate test of this hypothesis. It is noteworthy that experimental animal work has not yet provided compelling data that the prenatal stress/immune association is mediated by glucocorticoid exposure (Merlot et al., 2008). A second possibility is that maternal prenatal anxiety alters maternal prenatal immune responsiveness, which is transmitted to the fetus. We did not address this hypothesis in the current study, but we note that data are so far inconclusive about the impact of psychiatric symptoms on immune parameters in pregnancy (Blackmore et al., 2011). Third, prenatal anxiety may alter infant immune responses by exacerbating those immunological features that are known to be immature in the infant, including dendritic cell activation. For example, in the current study, analyses of a subset of infants indicated that prenatal anxiety was associated with less circulating IL-12, a known characteristic of the neonatal response, which may help explain the reduced IFN-γ responses to vaccine noted at 6 months of age. Future work, such as examining IL-12 production by dendritic cells in vitro, is needed to determine how maternal anxiety might affect activation of these cells in infants.
The lack of association between maternal prenatal anxiety and infant immune outcomes at 2 months but a robust effect at 6 months of age may reflect an important feature of the developing immune response in the infant. It has been appreciated for some time that the vaccine response in the neonate is different from that observed in older infants (Hodgins and Shewen, 2012; Siegrist, 2007). At the 2-month assessment, we were predicting variation in the neonatal response in the vast majority of cases, and it may be that any detectable effect of prenatal maternal anxiety would have dissipated with the very short-term antibody response in the neonate to the birth dose of vaccine. By 6 months, the infant has a more mature immune response and multiple vaccine exposures, and secondary effects on vaccine responses may be more detectable. Similarly, the lack of association between T cell responses and prenatal anxiety (or other covariates considered) at two months of age may be explained by a less robust CD4+ IFN-γ response in young infants, e.g.(Gans et al., 1999), although it is not yet clear from these studies why there would be a clear association detected by 6 months.
Limitations of the study should be noted. We were unable to completely control the timing and number of immunizations received by the infants during the study. We did not interfere with the primary care of the subjects but instead coordinated our research with providers based upon the routine immunization schedule; our analyses controlled for the uneven timing of vaccine administration. In addition, we capitalized on the experimental advantages of the hepatitis B vaccine paradigm to answer questions about the impact of prenatal anxiety on the infant humoral and cell mediated immune responses. Although we were not able to collect data on maternal immunization history in all cases, through routine prenatal care we were able to determine which mothers had measurable hepatitis B surface antigen and exclude them from our analyses. Additionally, we did not assess neonatal hepatitis infection, nor would we propose that prenatal anxiety would impact the risk of infection because a substantial majority of infants demonstrated sero-conversion and because maternal prenatal anxiety predicted variation in antibody concentrations but not sero-conversion (see supplementary information). A further consideration is that we were not able to control for postnatal exposures that may have interfered with infant adaptive immune function. Finally, it should be noted that the risk phenotype is likely broad, as the patterns of findings in the supplementary information suggest; that is consistent with the larger literature on the effects of prenatal maternal anxiety reviewed above.
Further follow-up of our sample will examine if hepatitis B antibody concentrations in children whose mothers experienced significant prenatal anxiety show a more rapid decline. The rate of measurable hepatitis B antibody titers evident 10 or more years after a complete infant immunization series varies from a minority to a strong majority of individuals, depending on the population (Samandari et al., 2007; Wu et al., 1999). Long-term follow-up of children who had received hepatitis B vaccine at birth indicate that a substantial minority do not have protective antibody responses measurable by adolescence, and a sizable minority of these do not show an anamnestic response to a single dose (Chaves et al., 2012).
Collectively, the findings in the current study extend human research on prenatal anxiety in important new directions and identify further targets for outcome studies and additional evidence for the implementation of preventive interventions.
Supplementary Material
Figure 2.
Association between maternal prenatal anxiety and cell-mediated immunity: IFN- γ T cell responder cell frequencies in response to hepatitis B antigen re-stimulation in 6-month-olds.
Acknowledgments
We thank Suzanne Coglitore, Carol Ferro, Michelle Gilchrist, Jessica Halliley, Mary Harper, Andrew Moscatiello, Bridget O’Connor, Meagan Saile, Bridget Szczypinski, Claire Wyman and staff at the Clinical Research Center for their assistance with the study, and the mothers and babies who participated. The Thoughts, Emotions, and Mood in Pregnancy study was funded by the National Institute of Health grant MH073019; support was also provided by NIH grants MH529173, K23MH080290 and K99HD070953; support was also provided through the Clinical Research Center from UL1 RR 024160 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Conflicts of Interest: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker DJ. The origins of the developmental origins theory. Journal of internal medicine. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Behar E, Alcaine O, Zuellig AR, Borkovec TD. Screening for generalized anxiety disorder using the Penn State Worry Questionnaire: a receiver operating characteristic analysis. Journal of behavior therapy and experimental psychiatry. 2003;34:25–43. doi: 10.1016/s0005-7916(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic medicine. 2011;73:656–663. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns VE, Carroll D, Ring C, Harrison LK, Drayson M. Stress, coping, and hepatitis B antibody status. Psychosomatic medicine. 2002;64:287–293. doi: 10.1097/00006842-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1312–1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, O’Connor TG, Wyman PA, Wang H, Moynihan J, Cross W, Tu X, Jin X. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain Behav Immun. 2008;22:933–940. doi: 10.1016/j.bbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Wyman PA, Wang H, Moynihan J, O’Connor TG. Associations among depression, perceived self-efficacy, and immune function and health in preadolescent children. Development and Psychopathology. 2011;23:1139–1147. doi: 10.1017/S0954579411000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves SS, Fischer G, Groeger J, Patel PR, Thompson ND, Teshale EH, Stevenson K, Yano VM, Armstrong GL, Samandari T, et al. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine. 2012;30:1644–1649. doi: 10.1016/j.vaccine.2011.12.106. [DOI] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev Psychobiol. 1993;26:293–304. doi: 10.1002/dev.420260506. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87:675–681. doi: 10.1210/jcem.87.2.8233. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Prenatal origins of individual variation in behavior and immunity. Neurosci Biobehav Rev. 2005;29:39–49. doi: 10.1016/j.neubiorev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61:520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- Couret D, Jamin A, Kuntz-Simon G, Prunier A, Merlot E. Maternal stress during late gestation has moderate but long-lasting effects on the immune system of the piglets. Veterinary immunology and immunopathology. 2009;131:17–24. doi: 10.1016/j.vetimm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JP. Experience with hepatitis A and B vaccines. The American journal of medicine. 2005;118(Suppl 10A):7S–15S. doi: 10.1016/j.amjmed.2005.07.011. [DOI] [PubMed] [Google Scholar]
- De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, Koup R, Miller CJ, Roederer M. Vaccination in humans generates broad T cell cytokine responses. Journal of immunology. 2004;173:5372–5380. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Pernia O, Carrero P, Garcia-Segura LM. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. Journal of neuroinflammation. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijts L, Bakker-Jonges LE, Labout JA, Jaddoe VW, Hofman A, Steegers EA, Van Dongen JJ, Hooijkaas H, Moll HA. Perinatal stress influences lymphocyte subset counts in neonates. The generation R study. Pediatric research. 2008;63:292–298. doi: 10.1203/PDR.0b013e318163a29f. [DOI] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic medicine. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV. New York: American Psychiatric Press; 1995. [Google Scholar]
- Gans HA, Maldonado Y, Yasukawa LL, Beeler J, Audet S, Rinki MM, DeHovitz R, Arvin AM. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. Journal of immunology. 1999;162:5569–5575. [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, Heron J, Golding J. Antenatal maternal anxiety is linked with atypical handedness in the child. Early Hum Dev. 2004;79:107–118. doi: 10.1016/j.earlhumdev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proceedings Biological sciences / The Royal Society. 2005;272:671–677. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DP, Vadheim CM, Wong VK, Marcy SM, Partridge S, Greene T, Chiu CY, Margolis HS, Ward JI. Comparative safety and immunogenicity of two recombinant hepatitis B vaccines given to infants at two, four and six months of age. The Pediatric infectious disease journal. 1996;15:590–596. doi: 10.1097/00006454-199607000-00006. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 2005;14:41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011 doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- Hendrzak JA, Brunda MJ. Interleukin-12. Biologic activity, therapeutic utility, and role in disease. Lab Invest. 1995;72:619–637. [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. European journal of immunology. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, Shewen PE. Vaccination of neonates: problem and issues. Vaccine. 2012;30:1541–1559. doi: 10.1016/j.vaccine.2011.12.047. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wu Q, Xu B, Zhou Z, Wang Z, Zhou YH. Influence of maternal antibody against hepatitis B surface antigen on active immune response to hepatitis B vaccine in infants. Vaccine. 2008;26:6064–6067. doi: 10.1016/j.vaccine.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Junqueira AL, Tavares VR, Martins RM, Frauzino KV, Silva AM, Rodrigues IM, Minamisava R, Teles SA. Presence of maternal anti-HBs antibodies does not influence hepatitis B vaccine response in Brazilian neonates. Memorias do Instituto Oswaldo Cruz. 2011;106:113–116. doi: 10.1590/s0074-02762011000100018. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kay G, Tarcic N, Poltyrev T, Weinstock M. Prenatal stress depresses immune function in rats. Physiol Behav. 1998;63:397–402. doi: 10.1016/s0031-9384(97)00456-3. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Wicks S, Dalman C, Henriksen TB, Li J, Mortensen PB, Kenny LC. Prenatal stress and risk of asthma hospitalization in the offspring: a Swedish population-based study. Psychosomatic medicine. 2012;74:635–641. doi: 10.1097/PSY.0b013e31825ac5e7. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–1072. doi: 10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- Lefevre F, Moreau D, Semon E, Kalaboka S, Annesi-Maesano I, Just J. Maternal depression related to infant’s wheezing. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2011;22:608–613. doi: 10.1111/j.1399-3038.2011.01155.x. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Associations between stress, trait negative affect, acute immune reactivity, and antibody response to hepatitis B injection in healthy young adults. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2001;20:4–11. [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Trait positive affect and antibody response to hepatitis B vaccination. Brain, behavior, and immunity. 2006;20:261–269. doi: 10.1016/j.bbi.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Merlot E, Couret D, Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav Immun. 2008;22:42–51. doi: 10.1016/j.bbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Moynihan JA, Ader R. Psychoneuroimmunology: animal models of disease. Psychosomatic medicine. 1996;58:546–558. doi: 10.1097/00006842-199611000-00003. [DOI] [PubMed] [Google Scholar]
- Nielsen NM, Hansen AV, Simonsen J, Hviid A. Prenatal stress and risk of infectious diseases in offspring. American journal of epidemiology. 2011;173:990–997. doi: 10.1093/aje/kwq492. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental Psychobiology. doi: 10.1002/dev.21007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Jenkins J, Browne D, Ben-Shlomo Y, Golding J, O’Connor TG. Prenatal Maternal Mood is Associated with Altered Diurnal Cortisol in Adolescence. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2013.01.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel C, Hedegaard M, Henriksen TB, Secher NJ, Olsen J. Psychological factors in pregnancy and mixed-handedness in the offspring. Dev Med Child Neurol. 2003;45:557–561. doi: 10.1017/s0012162203001014. [DOI] [PubMed] [Google Scholar]
- Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Whittle H, Lambert PH, McAdam KP, Siegrist CA, Marchant A. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine. 2004;22:511–519. doi: 10.1016/j.vaccine.2003.07.020. [DOI] [PubMed] [Google Scholar]
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- Samandari T, Fiore AE, Negus S, Williams JL, Kuhnert W, McMahon BJ, Bell BP. Differences in response to a hepatitis B vaccine booster dose among Alaskan children and adolescents vaccinated during infancy. Pediatrics. 2007;120:e373–381. doi: 10.1542/peds.2007-0131. [DOI] [PubMed] [Google Scholar]
- Siegrist CA. The challenges of vaccine responses in early life: selected examples. Journal of comparative pathology. 2007;137(Suppl 1):S4–9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nature reviews Immunology. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorusch I, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Swanson LM, Pickett SM, Flynn H, Armitage R. Relationships among depression, anxiety, and insomnia symptoms in perinatal women seeking mental health treatment. J Womens Health (Larchmt) 2011;20:553–558. doi: 10.1089/jwh.2010.2371. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang S, Luo C, Wu Q, Liu Q, Zhou YH, Hu Y. Transplacentally acquired maternal antibody against hepatitis B surface antigen in infants and its influence on the response to hepatitis B vaccine. PLoS One. 2011;6:e25130. doi: 10.1371/journal.pone.0025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, Lee-Parritz A, Wood RA, Kattan M, Bloomberg GR, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. American journal of respiratory and critical care medicine. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JS, Hwang LY, Goodman KJ, Beasley RP. Hepatitis B vaccination in high-risk infants: 10-year follow-up. The Journal of infectious diseases. 1999;179:1319–1325. doi: 10.1086/314768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.