Abstract
Erythritol is a four-carbon sugar preferentially utilized by Brucella spp. The presence of erythritol in the placentas of goats, cows, and pigs has been used to explain the localization of Brucella to these sites and the subsequent accumulation of large amounts of bacteria, eventually leading to abortion. Here we show that B. melitensis will also localize to an artificial site of erythritol within a mouse, providing a potential model system to study the pathogenesis of Brucella abortion. Immunohistological staining of the sites of erythritol within infected mice indicated a higher than expected proportion of extracellular bacteria. Ensuing experiments suggested intracellular B. melitensis was unable to replicate within macrophages in the presence of erythritol and that erythritol was able to reach the site of intracellular bacteria. The intracellular inhibition of growth was found to encourage the bacteria to replicate extracellularly rather than intracellularly, a particularly interesting development in Brucella pathogenesis. To determine the effect of erythritol on expression of B. melitensis genes, bacteria grown either with or without erythritol were analyzed by microarray. Two major virulence pathways were up-regulated in response to exposure to erythritol (the type IV secretion system VirB and flagellar proteins), suggesting a role for erythritol in virulence.
Keywords: Brucella, erythritol, flagella, virulence
1. Introduction
Brucella spp. are facultative intracellular α-proteobacteria and the causative agent of the zoonotic disease brucellosis. Human infection with Brucella spp. results in an acute undulating fever before developing into a chronic disease potentially displaying endocarditis, meningitis, and osteomyelitis [1]. Brucellosis of domesticated animals (cows, goats, and pigs with B. abortus, B. melitensis, and B. suis, respectively) is also characterized by a chronic infection resulting in orchitis in infected males and spontaneous abortions in infected females [2]. Despite the endemic nature of Brucella spp. in many areas of the world, no suitable human vaccine is available and all current animal vaccines present a variety of potential downsides. A further knowledge of Brucella-host interactions is necessary to improve our understanding of the role of the host in Brucella infections.
A key site of this Brucella-host interface is the infected placenta. The spontaneous abortions that occur as a hallmark of animal brucellosis were thought to be due to the amount of Brucella “endotoxin” produced in the placenta as the bacteria replicate to levels as high as 1013 bacteria/gram of tissue [3]. This high level of bacteria was found to result from the presence of a growth-stimulatory factor in the fetal tissues that was later identified as the 4-carbon sugar erythritol [4]. Initially the ability of B. abortus to utilize erythritol was considered directly related to virulence, evidenced by the fact that the B. abortus vaccine strain S19 was known to be deficient in erythritol utilization [5]. While subsequent analysis found that the link between virulence and erythritol utilization was not as clear as it may have appeared, an erythritol-virulence connection is suggested by recent analysis [6–8].
Growth analysis of Brucella spp. in erythritol-containing media found that Brucella spp. will utilize erythritol as a carbon source preferentially over the presence of glucose in the media [9]. The genes responsible for erythritol utilization were found to be organized in a four-gene operon encoding three catabolic proteins and an erythritol-responsive repressor that controlled transcription of the operon [10]. While the catabolism of erythritol and its potential connection to virulence have been well studied, there are few studies on how the bacteria respond to the presence of erythritol. In the present study, we examined several aspects of how bacteria interact with erythritol. B. melitensis localized to artificial sites of erythritol in a mouse model of infection, resulting in a significant immune response. Further, the growth of intracellular B. melitensis is inhibited by the addition of erythritol to the cell culture medium. To determine a potential cause of this inhibition, microarray analysis of B. melitensis grown in erythritol was conducted. In addition to the expected activation of energy production and metabolism pathways, several virulence pathways (flagella, type IV secretion system VirB, quorum sensing) were also up-regulated in response to erythritol. From these data, erythritol may play a significant role in the regulation of virulence in B. melitensis.
2. Materials and Methods
2.1 Bacterial strains, plasmids, and growth conditions
The strains and plasmids used in this study are listed in Table S1. B. melitensis strain 16M (ATCC23456) was used as the wild type strain. A virulent strain of B. melitensis generated previously that contains the luxCDABE operon from Photorhabdus luminescens was used for mouse and cell infections [11]. E. coli strain DH5α was used for cloning and propagation. All B. melitensis strains were maintained on brucella agar and grown in brucella broth (Becton Dickinson) at 37°C. Microarray analysis and promoter activity levels of B. melitensis were conducted in yeast extract minimal medium (YEMM) [12]. E. coli was grown in Luria-Bertani broth at 37°C. Kanamycin (50 μg/ml) and ampicillin (100 μg/ml) were added to the medium as needed. RAW264.7 macrophages were grown in RPMI media containing 10% fetal bovine serum at 37C with 5% CO2.
2.2 Infection of mice containing sugar-matrigel mixtures
Analysis of the localization of B. melitensis to sites of erythritol in mice was examined using the non-immunogenic matrix substrate Matrigel (BD Biosciences). A 10% solution of glucose or erythritol was mixed with the high-concentration Matrigel substrate. Half a milliliter of glucose gel was injected into the lower left back of 4 BALB/c mice and the erythritol gel was injected into the lower right back. All 4 mice were then immediately infected intraperitoneally with 1×106 CFU of the virulent, luminescent B. melitensis strain GR023 in PBS [11]. Mice were imaged three days after infection using the in vivo imaging system and Living Image software (Caliper). This work was carried out in accordance with the protocol approved by the Animal Care and Use Committee at the University of Wisconsin-Madison (protocol #V554). All imaging was conducted under isoflurane anesthesia.
2.3 Histology and immunocytochemistry of sugar-matrigel mixtures from infected mice
After six days mice containing the sugar-matrigel mixtures and intraperitoneally infected with GR023 were euthanized and the gels removed. After luminescent imaging of both the glucose and erythritol gels, they were paraffin embedded and six micron sections were subjected to hematoxylin and eosin staining (University of Wisconsin-Madison School of Veterinary Medicine Histology Center). Additional sections were stained for B. melitensis by incubating with polyclonal antibody directed against B. melitensis (Tetracore, Inc.). Incubation of horse-radish peroxidase (HRP) conjugated secondary antibody and resulting treatment with 3,3′-Diaminobenzidine tetrahydrochloride produced characteristic brown precipitates at sites of antibody binding within gels.
2.4 B. melitensis infected RAW macrophages cultured with erythritol-containing media
To determine whether addition of erythritol to intracellular bacteria resulted in changes in intracellular growth, RAW 264.7 macrophages (ATCC) were infected with B. melitensis strain 16M. Bacterial cultures were grown overnight in brucella broth, diluted in RPMI, and used to infect RAW macrophages at a multiplicity of infection (MOI) of 100:1. Bacteria were allowed to infect for 1 hour at 37C, cells were washed twice with phosphate-buffered saline (PBS), and external bacteria were killed using RPMI containing 30 μg/ml gentamycin at 37C for 30 min. Media was replaced with RPMI containing 5 μg/ml gentamycin to ensure that any bacteria released from macrophages would be killed. Selected wells contained RPMI with an addition of 1% erythritol (w/v). Enumeration of bacteria within the cells was determined at 0, 24, and 48 hrs post infection by lysing cells with 0.1% Triton X-100. Colony forming units (CFUs) within each well were counted by diluting ten-fold within 96-well plates and plating on brucella agar as previously performed [13].
In addition to testing intracellular infection, the number of extracellular bacteria present during cell infections with erythritol was also tested. RAW 264.7 macrophages were infected with B. melitensis 16M as described above. After addition of 30 μg/ml gentamycin-containing RPMI to kill extracellular bacteria, cells were washed and media was replaced with RPMI without gentamycin and either with or without 1% erythritol. Supernatant samples were taken at 0, 24, and 48 hrs to determine the number of extracellular bacteria present in each sample. After supernatant samples were taken, cells were lysed and plated as described above to determine the number of intracellular bacteria present. The ratio of extracellular to intracellular bacteria in each condition was determined at each time point.
2.5 Activation of erythritol catabolism promoter in intracellular bacteria
Erythritol sensing by intracellular bacteria was analyzed by determining the level of promoter activity of the erythritol catabolism operon. Erythritol catabolism is controlled by a four gene operon encoding three catabolic proteins (BMEII0430–428) and a regulatory protein (BMEII0427). The promoter region (Pery) consisting of approximately 500bp upstream of the first gene in the operon (BMEII0430) was cloned into the luminescent reporter plasmid pEP3 [14, 15].. The resulting vector pEP4 (Pery-lux) was transformed into B. melitensis strain 16M.
The Pery -lux strain and the virulent, luminescent GR023 strain were separately used to infect RAW macrophages as described above. RAW cells were incubated with RPMI media either with or without 1% erythritol and 5ug/ml gentamycin to kill any extracellular bacteria. Luminescent measurements were taken at 0, 24, 48, and 72 hrs. The GR023 luminescent measurements were used to approximate the bacterial level under each condition. Luminescent levels of the ery promoter were standardized to the luminescent level of GR023 under RPMI and RPMI+erythritol conditions. All cellular assays were conducted in triplicate and analyzed using Student’s t test.
2.6 Microarray analysis of B. melitensis subjected to growth in erythritol
Microarray analysis was conducted on B. melitensis strain 16M to determine transcriptional changes during growth in erythritol. Cultures of B. melitensis strain 16M were inoculated into YEMM containing either 1% glucose or 1% erythritol and grown to an OD of 1.0. Total RNA was extracted using the Masterpure RNA extraction kit (Epicentre). Generation of cDNA, labeling, hybridization to microarray chips, and imaging of hybridized chips were conducted as performed previously [16]. Microarray scanned images were analyzed with Nimblescan software (Nimblegen) using robust multichip analysis (RMA). Gamma-gamma (GG) analysis was conducted on expression data using the R statistical program and EBarray package [17, 18]. Expression data for which the GG value was >0.5 were considered significant. Microarray data was submitted to the NCBI Gene Expression Omnibus (accession number GSE37819).
2.7 Analysis of promoter activity of B. melitensis during growth in erythritol
A subset of genes seen up-regulated in the microarray under growth in erythritol were selected for promoter analysis. The genes BMEI0095, BMEI0102, BMEI0347, BMEII0025, BMEII0159, BMEII1009, BMEII1089, BMEII1108, and BMEII1116 were selected to examine the effect of erythritol on promoter activity. A negative control infA (BMEI1671) and positive control ery (BMEII0430) were also used. Approximately 500bp upstream of each gene of interest was cloned into the luminescent reporter vector pEP3 as described above. Each reporter vector was transformed into wild type B. melitensis strain 16M. These strains were grown overnight in YEMM containing 1% glucose as a carbon source. Each strain was then diluted to 0.1 OD in YEMM containing either 1% glucose or 0.5% glucose and 0.5% erythritol. These cultures were grown at 37C in a 96-well plate for 14 hrs to an OD of 0.5. Luminescent values and the OD of each strain in both media were measured in a DTX 880 multimode plate reader (Beckman Coulter). Relative light units (RLU) were determined by the ratio of photons/OD600. Student’s t test was used to compare the levels of promoter activity in erythritol medium versus the glucose control. Fold change in promoter activity was determined by taking the RLU of the erythritol experimental culture over the control glucose culture.
3. Results
3.1 B. melitensis preferentially localized to erythritol sites during mouse infection and induced cell infiltration
The presence of erythritol in the placenta of cows, sows, and goats has been used to explain both the tropism of Brucella for these sites and the ability of the bacteria to replicate to very high numbers and contribute to abortion [3, 4, 19]. However, erythritol has not been found in any measurable quantities in the placentas of mice that are commonly used as model systems for Brucella spp. infection [19]. To determine if we could replicate the ability of B. melitensis to preferentially localize to sites of erythritol in mice simulating a placenta, we utilized the cellular substrate matrigel (BD Biosciences).
A sample of matrigel containing 10% glucose and one containing 10% erythritol were injected into the lower back of a BALB/c mouse (n=4) where it solidified into a solid gel. The glucose sample was injected into the left side of the mouse and the erythritol sample was injected into the right. The mice were then infected intraperitoneally with the luminescent, virulent B. melitensis strain GR023 and bacteria were localized using the in vivo imaging system (Caliper). At three days post infection, mice were imaged for the presence of bacteria in each gel site, and in all 4 mice the level of bacteria at the site of the erythritol injection was greater than the glucose site (Fig. 1A). After imaging, mice were euthanized and gels were removed for further imaging (Fig. 1B).
Fig. 1. B. melitensis preferentially localizes to sites of erythritol during mouse infection.
A) Gel solutions containing either 10% glucose (left) or 10% erythritol (right) was injected into the backs of BALB/c mice (n=4) where the gel solidified. The mice were then infected intraperitoneally with 106 CFU of virulent, luminescent B. melitensis GR023 and imaged three days post infection. In all four mice, the luminescent bacteria preferentially localizes at the site of the erythritol gel.
B) Gels from infected mice were extracted at day 6 and imaged for levels of luminescence. Gels containing erythritol (right gel) contained much higher levels of luminescent bacteria than gels containing glucose (left gel).
3.2 Both cell-associated and free B. melitensis were found within the erythritol matrigel and resulted in large cellular infiltration
To further analyze the effect of B. melitensis in the erythritol matrigel, gels were analyzed by histological staining. While glucose gels showed very little inflammatory reaction, erythritol gels showed a high level of cellular infiltration (Fig. 2A-B). In some cases, large masses of cells appeared to be forming adjacent to erythritol gels. These masses suggest that the higher level of bacteria in these sites may have stimulated a potent immune reaction and caused this cellular recruitment. These results are in contrast to erythritol gels isolated from uninfected mice, indicating that bacterial localization at the site of the erythritol gel was required (Fig. S2).
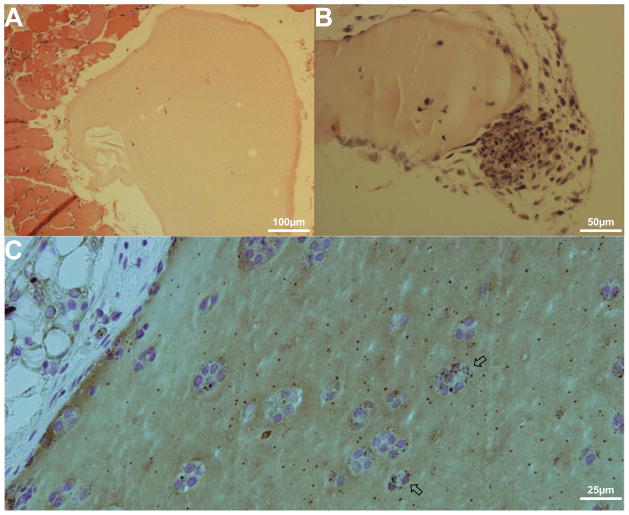
Fig. 2. B. melitensis at sites of erythritol induce a strong immune response.
Mice were prepared as for Fig. 1. Gels were removed at day 6 for histological analysis.
A) Glucose-containing gel from an infected mouse shows little to no immune cell recruitment.
B) Erythritol-containing gel from an infected mouse shows a high level of cellular recruitment (lower right), consistent with an increase in bacterial levels within the erythritol gel.
C) Immunocytochemistry of the erythritol-containing gel with HRP-conjugated anti-B. melitensis polyclonal antibody and reaction with diaminobenzidine tetrahydrochloride identifies high levels of bacteria within the gel. Arrows mark bacteria-associated cells.
The presence of bacteria in the erythritol gels was also analyzed using immunocytochemistry with a polyclonal antibody directed against B. melitensis (Fig. 2C). Bacteria in several locations can be seen localized to cells within the erythritol matrigel, indicating a possible intracellular infection. In addition to potential intracellular bacteria, there is also the presence of considerable extracellular bacteria within the gel. Large numbers of extracellular bacteria have also been observed within the placentas of Brucella-infected cows [20]. To determine if erythritol was playing any role in the presence large numbers of extracellular bacteria, the effect of erythritol on intracellular bacteria was examined.
3.3 Addition of erythritol to growth medium encourages extracellular growth of B. melitensis
The effect of erythritol on intracellular B. melitensis was examined by infecting RAW macrophages and monitoring infection by determining colony forming units (CFUs) present at 0, 24, and 48 hrs. Extracellular erythritol (1% w/v) was added to the growth medium and compared to RPMI growth medium without erythritol (Fig. 3A). In contrast to the control where the level of bacteria increased steadily through 48 hrs, in the erythritol-treated cells the same level of bacteria was observed throughout the entire infection. To confirm that erythritol-toxicity to the macrophages was not responsible for the observed phenotype, necrotic and apoptotic levels within macrophage cultures were tested, and erythritol was found to be non-toxic (Fig. S2).
Fig. 3. B. melitensis preferentially replicates extracellularly after incubation with erythritol in cell culture medium.
A) B. melitensis strain 16M was used to infect RAW 264.7 macrophages at an MOI of 100:1. Macrophage growth culture either consisted of RPMI or RPMI+1% erythritol. Bacteria grown in the presence of erythritol fail to replicate to wild type levels. Data presented is the mean of three individual experiments and analyzed via Student’s t test.
B) RAW macrophages were infected as described above. Extracellular bacteria were initially killed with gentamycin immediately after infection, and then gentamycin media was removed, allowing extracellular growth. The ratio of extracellular bacteria to intracellular bacteria in the presence of RPMI or RPMI+erythritol was determined. The addition of erythritol to the cell media causes B. melitensis to preferentially replicate extracellularly. Data presented is the mean of three individual experiments and analyzed via Student’s t test.
C) To measure the level of erythritol gaining access to intracellular bacteria, B. melitensis containing a luminescent reporter construct measuring activity of the erythritol-responsive ery promoter was used to infect RAW264.7 macrophages. Intracellular bacteria were incubated in either RPMI or RPMI containing 1% erythritol and luminescent output of the Pery reporter was measured. To correct for lower numbers of bacteria in erythritol-incubated cells, the virulent luminescent strain GR023 was infected alongside under identical conditions and promoter values were taken relative to GR023 values to correct for cell numbers. After incubation for 24 and 48 hours, levels of the ery promoter are higher in the erythritol-incubated cells, indicating that erythritol is able to access the intracellular bacteria in sufficient quantities to elicit a transcriptional response from B. melitensis. Data presented is the mean of three separate experiments and analyzed via Student’s t test.
Further analysis of the growth of intracellular B. melitensis under the presence of erythritol was conducted to determine if erythritol encouraged extracellular growth over intracellular growth. RAW macrophages were infected with B. melitensis strain 16M for one hour, and then cells were treated with gentamycin to kill all extracellular bacteria. Gentamycin was washed from the cells, and cell culture media without gentamycin and either with or without 1% erythritol was added to allow the growth of extracellular bacteria. Supernatant samples were taken at 0, 24, and 48 hrs and the number of bacteria present was determined. These values were compared to the level of intracellular bacteria to determine the extracellular-to-intracellular ratio of bacteria (Fig. 3B). At 48 hrs, the ratio of extracellular bacteria to intracellular bacteria was greater than 10-fold higher under the presence of erythritol, indicating that erythritol encourages extracellular growth over intracellular growth.
3.4 Extracellular erythritol reaches the intracellular niche of B. melitensis
Any effect of erythritol is dependent on erythritol being available to intracellular B. melitensis. To determine whether this occurs, expression of the erythritol-regulated ery promoter was examined under these conditions. The four gene ery operon (eryABCD) consists of three proteins required for breakdown of erythritol and a repressor of the operon (eryD) whose binding to erythritol allows for transcription of the promoter (Pery). The Pery promoter was cloned into a promoterless reporter vector containing the luxCDABE operon used previously to monitor promoter activity levels in B. melitensis [14, 15].
B. melitensis containing the Pery-lux reporter vector was used to infect RAW macrophages and cells were grown in either RPMI or RPMI containing 1% erythritol. Parallel to this infection was a set of RAW macrophages that were infected with the luminescent B. melitensis GR023 strain. Resulting levels of luminescent output from each condition were measured. Promoter activity levels were taken relative to the level of GR023 to compensate for the significantly higher level of bacteria found in the control (no-erythritol) condition. Levels of the Pery promoter activity were found to be 2–2.5 fold higher upon addition of erythritol to the growth medium at both 24 and 48 hrs, indicating that erythritol is able to reach the intracellular niche of B. melitensis (Fig. 3C).
3.5 Microarray analysis of B. melitensis exposed to erythritol indicated that the bacterial response to erythritol goes beyond simple metabolism
With the ability of B. melitensis to preferentially localize to sites with erythritol and the possibility that erythritol plays a strong role in the pathogenesis of Brucella spp., the transcriptional response to erythritol was determined using microarray analysis. Cultures of B. melitensis strain 16M grown in minimal medium containing either 1% glucose or 1% erythritol as a carbon source were compared to determine what effect erythritol had on transcription. Microarray analysis found that 95 genes were significantly up-regulated and 40 were significantly down-regulated in response to growth in erythritol (Table 1; full results in Table S3). Of these 135 genes, 30% are categorized in the cluster of orthologous groups (COGs) as general function prediction, unknown function, and hypothetical proteins. The remaining 70% of the genes represent 16 other COGs with a wide range of potential importance discussed below (Fig. 4).
Table 1.
Selected Genes Showing a Significant Change After Growth in Erythritol
| Gene Name | Product | Fold change | GG Probabilitya. |
|---|---|---|---|
| Type IV Secretion System VirB | |||
| BMEII0025 | Attachment mediating protein VirB1 homolog | 2.12 | 0.79 |
| BMEII0031 | Channel protein VirB7 homolog | 2.61 | 0.98 |
| BMEI0390 | Putative cytoplasmic protein – (VceA) | 2.40 | 0.92 |
| BMEII1116 | Transcriptional activator, LuxR family – (VjbR) | 2.14 | 0.66 |
| Flagella | |||
| BMEII0150 | Flagellin | 2.47 | 0.97 |
| BMEII0159 | Flagellar hook protein FlgE | 2.37 | 0.94 |
| BMEII0161 | Flagellar hook-associated protein FlgL | 3.72 | 1.00 |
| BMEII0162 | FlaF protein | 3.50 | 1.00 |
| BMEII0164 | Basal-body rod modification protein FlgD | 4.88 | 1.00 |
| BMEII0170 | Flagellar protein FlgJ | 2.97 | 1.00 |
| BMEII0171 | “FlgN protein” | 5.49 | 1.00 |
| BMEII0172 | “FliL protein” | 2.70 | 0.99 |
| BMEII1085 | Flagella basal body P ring formation protein FlgA | 2.16 | 0.83 |
| BMEII1086 | Flagellar basal-body rod protein FlgG | 5.82 | 1.00 |
| BMEII1087 | Flagellar hook-basal body complex protein FliE | 5.45 | 1.00 |
| BMEII1088 | Flagellar basal-body rod protein FlgC | 5.70 | 1.00 |
| BMEII1089 | Flagellar basal-body rod protein FlgB | 2.76 | 0.99 |
| BMEII1108 | Flagellar basal-body rod protein FlgF | 2.52 | 0.98 |
| BMEII1109 | Chemotaxis MotA protein | 6.65 | 1.00 |
| BMEII1110 | Flagellar motor switch protein FliM | 1.99 | 0.63 |
| BMEII1113 | Flagellar motor switch protein FliG | 2.09 | 0.75 |
| BMEII1009 | C-di-GMP phosphodiesterase A | 2.43 | 0.94 |
| Cytochrome Synthesis | |||
| BMEI0593 | SCO2 Protein | 4.03 | 1.00 |
| BMEI1463 | Cytochrome C oxidase assembly protein CtaG | −3.22 | 1.00 |
| BMEI1564 | Cytochrome C oxidase polypeptide I homolog, bacteroid | 3.59 | 1.00 |
| BMEI1565 | Cytochrome C oxidase, monoheme subunit, membrane-bound | 3.87 | 1.00 |
| BMEI1566 | Cytochrome C oxidase, diheme subunit, membrane-bound | 2.29 | 0.90 |
| BMEI1898 | Cytochrome O ubiquinol oxidase operon protein CyoD | 2.50 | 0.97 |
| BMEI1900 | Cytochrome O ubiquinol oxidase subunit I | 2.14 | 0.82 |
| BMEI1901 | Cytochrome O ubiquinol oxidase subunit II | 3.07 | 1.00 |
| BMEII0759 | Cytochrome D ubiquinol oxidase subunit II | 1.95 | 0.60 |
| BMEII1068 | Cytochrome C2 precursor | 2.99 | 1.00 |
| Nitrogen Fixation | |||
| BMEI1294 | Nitrogen fixation regulation protein FixK | 3.09 | 1.00 |
| BMEI1567 | Nitrogen fixation protein FixG | 2.64 | 0.99 |
| BMEI1967 | N utilization substance protein A | 2.02 | 0.68 |
| BMEII0246 | Nitroreductase | 2.31 | 0.88 |
| BMEII0948 | Nitrite extrusion protein | 2.35 | 0.93 |
| BMEII0975 | Regulatory protein NosR | 2.60 | 0.97 |
| Iron Metabolism | |||
| BMEI0673 | Thiosulfate-binding protein precursor | 2.73 | 0.99 |
| BMEI1591 | Ferredoxin-NADP reductase | 2.38 | 0.89 |
| BMEII0105 | Iron-regulated outer membrane protein FrpB | 2.02 | 0.54 |
| BMEII1002 | Calcium or iron-binding protein | 1.98 | 0.60 |
| Carbohydrate Metabolism | |||
| BMEII0424 | Ribose 5-phosphate isomerase | 2.51 | 0.95 |
| BMEII0425 | Triosephosphate isomerase | 1.97 | 0.61 |
| BMEII0590 | Sugar-binding protein | −20.38 | 1.00 |
| BMEII0591 | Sugar transport system permease protein | −6.93 | 1.00 |
| BMEII0592 | Sugar transport system permease protein | −7.56 | 1.00 |
| BMEII0593 | Glucose ABC transporter ATPase | −4.11 | 1.00 |
| Other | |||
| BMEI0095 | Succinoglycan biosynthesis regulator | 2.39 | 0.95 |
| BMEI0102 | Universal stress protein family | 4.16 | 1.00 |
| BMEI0347 | Phosphoserine aminotransferese | 2.27 | 0.90 |
| BMEI0454 | Outer membrane protein W precursor | 25.38 | 1.00 |
A GG probability of >0.5 was considered significant
Fig. 4. Microarray analysis of B. melitensis grown in the presence of erythritol identified a high number of regulated genes, including virulence factors.
Microarray analysis was conducted on B. melitensis strain 16M grown in either 1% glucose or 1% erythritol. A total of 135 genes were found to change in levels after growth in erythritol, and these genes fall within 19 clusters of orthologous groups (COGs). In addition to genes for erythritol metabolism, several virulence factors (flagellar and type IV secretion proteins) were found to be up-regulated.
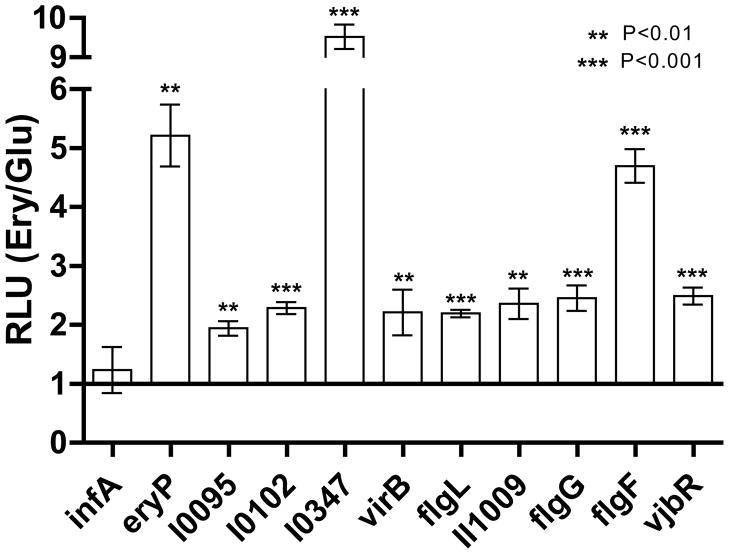
A subset of up-regulated genes identified by the microarray analysis was selected to confirm the microarray results. Nine experimental genes (BMEI0095, I0102, I0347, II0025, II0161, II1009, II1086, II1108, and II1116), a positive control ery promoter (BMEII0430), and the constitutive integration host factor 1 (infA) promoter (BMEI1671) negative control were examined via promoter-reporter assay.. All nine tested promoters and the positive control ery promoter were up-regulated upon addition of erythritol to the culture (Fig. 5).
Fig. 5. Erythritol up-regulates transcription of several virulence factors.
To confirm the results of the microarray on increased transcription, several promoters were assessed for their activity using a luminescent reporter assay. Cultures were grown in either 1% glucose or 0.5% erythritol to an OD595 of about 0.5 and photon output and OD595 were measured. Relative light units (RLUs) were calculated as the number of photons/OD595, and change in response to erythritol was calculated as the ratio to glucose-grown levels. The negative control initiation factor 1 (infA) and the positive control erythritol-responsive ery promoter were included to confirm the success of the experiment. All nine promoters tested were significantly up-regulated in response to growth in erythritol, including several promoters of genes encoding virulence factors (flagella, type IV secretion system VirB, cyclic-di-GMP regulation, quorum sensing). Data presented is the mean of three separate experiments each measured in triplicate and analyzed between erythritol and glucose RLUs for each promoter by Student’s t test.
4. Discussion
The connection between Brucella spp. and erythritol has been evident for some time [4, 21]. Initial studies identified a correlation between the ability of B. abortus strains to utilize erythritol and the virulence of the strain with lower utilization rates leading to lower virulence [5]. Subsequent studies with the same strains suggested that the decrease in erythritol utilization and virulence were both side effects of long passages during laboratory culture, but were not directly linked [6, 22]. This position was further strengthened with work on the primary example of the erythritol-virulence link, the B. abortus vaccine strain S19. Strain S19 possessed a well-documented defect in the utilization of erythritol that was considered a significant reason why it was successful in vaccination of animals without causing abortions [5, 23]. However, upon identification of the genes responsible for erythritol utilization, complementation of strain S19 with those genes did not restore virulence [7].
The only link between erythritol and virulence in B. abortus appeared to be the decrease in both erythritol utilization and virulence resulting from long periods spent passaged in laboratory culture, not a direct causation between erythritol utilization and virulence. However, recent work in B. suis found that deletion of one of the genes responsible for erythritol utilization (eryC) resulted in a strain attenuated during both macrophage and mouse infections [8]. Either this observation represents a significant difference between the species, or the link between virulence and erythritol is more significant than previously determined. Erythritol injection of guinea pigs infected with B. melitensis showed a dramatic increase in bacterial counts within the spleen, indicating that perhaps B. melitensis also showed a strong connection between virulence and erythritol [19].
Previously, the presence of erythritol was suggested as the primary cause for localization of Brucella spp. in the placenta [19, 24]. To determine if this effect could be replicated in mice with B. melitensis, a localized site of erythritol was introduced into mice. Using a luminescent strain of B. melitensis, we found that B. melitensis preferentially localized to the artificial site of erythritol (Fig. 1). Whether this is due to a preferential active process of the bacterium (i.e. a chemotactic system) or an increased level of replication upon reaching the site of erythritol is currently unknown. While B. melitensis is known to produce a sheathed flagellum, the lack of an identified chemotactic system and the presence of only a small initial placental infection suggests that it is the latter, but further work will be required to identify the precise mechanism [25, 26]. Not only does this result replicate what has been seen in natural infections, it presents a potential model system for the study of the role of erythritol in Brucella spp. during placental pathogenesis.
Histological analysis of the erythritol-containing gel indicated a high level of cellular infiltration around the infected gel (Fig. 2). Further analysis of the gel with an anti-Brucella antibody identified numerous intracellular bacteria within the erythritol-containing gel matrix. In addition to the intracellular bacteria, there were a large number of extracellular bacteria. A large colony of extracellular bacteria has also been observed in the placentas of Brucella-infected cows, suggesting a potential role for erythritol in this preference for extracellular over intracellular growth [20].
To identify a potential cause for this abundance of extracellular bacteria, the effect of erythritol on intracellular B. melitensis was examined. The addition of erythritol to cell culture medium of infected RAW macrophages significantly decreased the level of bacteria present during intracellular growth (Fig. 3A). Erythritol was also found to be able to reach the intracellular location of B. melitensis for use by the bacterium (Fig. 3C). This resulted in B. melitensis preferentially replicating extracellularly rather than intracellularly, consistent with the results of the histological analysis of the matrigel plug showing a high number of extracellular bacteria when erythritol was present. Extracellular growth in response to erythritol may be playing an important role in the pathogenesis of the bacterium, but further efforts to elucidate this role are needed.
If erythritol is able to stimulate the growth of B. melitensis in broth culture, it was interesting that it did not also stimulate bacterial growth inside of macrophages [27]. Previous work examining the effect of erythritol additions to the cell culture medium on intracellular B. abortus within bovine trophoblasts also found that erythritol was inhibitory to intracellular growth, although growth with erythritol in the culture medium was never compared to growth without erythritol [28]. In B. abortus, it was hypothesized that this intracellular growth defect in the presence of erythritol was due to the fact that iron played an important role in erythritol utilization, and iron may be limited in availability within the Brucella intracellular compartment [29, 30]. Further, it appears that several iron utilization genes are up-regulated within B. melitensis during intracellular growth, perhaps illustrating the lack of intracellular iron [31]. However, supplementation of iron (in the form of ferric citrate) to intracellular bacteria grown in the presence of erythritol was unable to restore replication to normal levels (data not shown). Due to difficulties in determining the level of available iron at the intracellular location of B. melitensis we are unable to exclude iron availability as the cause of this phenotype, but it is possible that erythritol is having an iron-independent effect upon intracellular replication.
In an effort to assess the impact of erythritol on B. melitensis, microarray analysis was conducted on bacteria grown with or without erythritol added to the media. Ninety-five genes were up-regulated during growth in erythritol while forty genes were down-regulated, constituting nineteen differ clusters of orthologous groups (COGs) (Fig. 5). Of those genes down-regulated, the four most significant constitute a four-gene operon that is potentially responsible for glucose transport and therefore down-regulated in the condition of growth in erythritol without glucose present.
Genes up-regulated in the presence of erythritol can be separated into six primary groups – sugar metabolism, iron utilization, nitrogen fixation, cytochrome synthesis, type IV secretion components, and flagellar proteins (Table 1). Sugar metabolism, iron utilization, nitrogen fixation, and cytochrome synthesis are all connected to the pathways used to convert erythritol into energy. Iron has been found to be an important component of erythritol utilization, and B. abortus mutants deficient in two iron siderophores have been found to utilize erythritol at much lower rates than wild type B. abortus [29, 30]. Similarly, the cytochrome electron transport system has been shown to be a crucial component of erythritol metabolism and nitrogen is coupled to the cytochrome system in Brucella spp. [32, 33]. The three genes encoding erythritol utilization proteins (eryABC) showed an increase of approximately 1.5-fold in the microarray but higher levels upon promoter testing (Fig. 5). This difference is likely due to experimental variation between the microarray conditions and promoter-testing conditions, such as growth time and erythritol:glucose ratio.
Of particular interest for this study were those groups of genes up-regulated during growth in erythritol that did not appear to play any role in the growth or energy production of the bacterium. These up-regulated genes can be separated into two groups, one consisting of genes encoding the type IV secretion system VirB and the other consisting of genes encoding flagellar proteins. Genes associated with VirB up-regulated during erythritol exposure were two structural proteins (VirB1 and VirB7), a protein shown to be secreted by the VirB secretion system (VceA), and a quorum sensing regulator that has been shown to regulate expression of the virB operon (VjbR) [34, 35]. Of the 12 structural proteins that make up the VirB type IV secretion system, VirB1 and VirB7 are two of the three that are required for in vitro infection of cell models but expendable in mouse models of infection [36, 37]. That both of these proteins are required for bacterial intracellular growth in cell culture but are not required for persistence in a mouse model suggests: 1) an additional protein not produced during cell culture conditions can replace their function, 2) the altered regulation of other VirB components in vivo makes VirB1 and VirB7 less important, or 3) that the secretion of VirB effector proteins required for cell culture infection is altered [36]. One such identified secreted effector protein (VceA) is also up-regulated in response to growth in erythritol [35]. To date, no function for VceA has been identified, but it is co-regulated along with the virB locus by the quorum sensing regulator VjbR, also up-regulated in response to erythritol [35]. It is possible that the up-regulation of VjbR via erythritol is responsible for the subsequent up-regulation of the VirB proteins and VceA.
Flagellar proteins are the third largest COG up-regulated by erythritol behind “energy production and conversion” and “hypothetical proteins” and constitute 13% of the up-regulated genes. Although B. melitensis has been shown to produce a sheathed flagellum, the precise role for the flagellum is currently unknown [25]. Historically classified as non-motile, the identification of flagellar genes in the sequenced genome of Brucella spp. was presumed to be cryptic artifacts of previous motility or a type III secretion system [38]. However, subsequent analysis of B. melitensis flagellar mutants showed them to be attenuated during mouse and goat infection (although not during in vitro cellular infection) indicating a potential role in virulence [25, 39]. While it is still unlikely that B. melitensis uses flagella for motility, the flagella may instead be used for secretion or attachment. Another possible role of flagellin is through the intentional activation of various immune system components through the host TLR5 and IpaF flagellin-sensing proteins [40]. In addition to the 17 flagellar proteins up-regulated, the diguanylate cyclase BMEII1009 is also up-regulated in response to erythritol. Diguanylate cyclases produce the secondary messenger cyclic-di-GMP, a signaling molecule responsible for flagellar regulation in several bacterial species and recently implicated in B. melitensis [14, reviewed in 41]. In addition to flagellar regulation, cyclic-di-GMP may also be playing a role in a host of other regulatory capacities or even acting as an immunostimulatory agent [42, reviewed in 43, 44].
The effect of erythritol on the expression of factors of the VirB type IV secretion and flagellar systems suggest a potential role in signaling for this carbon source, potentially providing a cause for the increase in virulence seen with the concomitant infection with Brucella and injection of erythritol into hosts [19, 24]. As erythritol has been connected repeatedly with virulence in Brucella spp. pathogenesis, this work may provide a potential explanation for these phenotypes. This link between carbon metabolism and virulence is currently a rapidly expanding field [reviewed in 45]. Of particular interest is the recent study indicating that Brucella may use the cellular autophagy pathway as a mechanism to induce release from an infected cell [46]. While the Brucella factors responsible for this effect have not been identified, it is possible that erythritol is activating these factors intracellularly, promoting the rapid release from the infected cell.
In a natural infection, the presence of erythritol may encourage Brucella to leave the intracellular niche of the trophoblasts in the placental region and replicate extracellularly, potentially through the activation of the cellular autophagy pathways by erythritol-stimulated bacterial factors. Once extracellular, the bacteria can rapidly grow to huge levels while simultaneously activating several virulence pathways that will be necessary upon transmission to a new host. These high levels of bacteria cause the inflammation that will eventually lead to abortion of the fetus and ejection of the placenta into the environment. Now within the environment, Brucella are already expressing virulence factors required to establish an infection due to their activation by erythritol. Upon uptake by a new host, these primed bacteria are able to swiftly and efficiently generate a successful infection. Each of these potential crucial steps signifies a new potential target in the search to unlock the secrets of Brucella pathogenesis.
Supplementary Material
Acknowledgments
We acknowledge the gift of antibody to Brucella melitensis provided by Tetracore, Inc. for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 2.Glynn MK, Lynn TV. Brucellosis J Am Vet Med Assoc. 2008;233:900–908. doi: 10.2460/javma.233.6.900. [DOI] [PubMed] [Google Scholar]
- 3.Alexander B, Schnurrenberger PR, Brown RR. Numbers of Brucella abortus in the placenta, umbilicus and fetal fluid of two naturally infected cows. Vet Rec. 1981;108:500. doi: 10.1136/vr.108.23.500. [DOI] [PubMed] [Google Scholar]
- 4.Smith H, Williams AE, Pearce JH, Keppie J, Harris-Smith PW, Fitz-George RB, Witt K. Foetal erythritol: a cause of the localization of Brucella abortus in bovine contagious abortion. Nature. 1962;193:47–49. doi: 10.1038/193047a0. [DOI] [PubMed] [Google Scholar]
- 5.Williams AE, Keppie J, Smith H. The relation of erythritol usage to virulence in the brucellas. J Gen Microbiol. 1964;37:285–292. doi: 10.1099/00221287-37-2-285. [DOI] [PubMed] [Google Scholar]
- 6.Meyer ME. Metabolic characterization of the genus Brucella. V. Relationship of strain oxidation rate of i-erythritol to strain virulence for guinea pigs. J Bacteriol. 1966;92:584–588. doi: 10.1128/jb.92.3.584-588.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangari FJ, Grillo MJ, Jimenez De Bagues MP, Gonzalez-Carrero MI, Garcia-Lobo JM, Blasco JM, Aguero J. The defect in the metabolism of erythritol of the Brucella abortus B19 vaccine strain is unrelated with its attenuated virulence in mice. Vaccine. 1998;16:1640–1645. doi: 10.1016/s0264-410x(98)00063-2. [DOI] [PubMed] [Google Scholar]
- 8.Burkhardt S, Jimenez de Bagues MP, Liautard JP, Kohler S. Analysis of the behavior of eryC mutants of Brucella suis attenuated in macrophages. Infect Immun. 2005;73:6782–6790. doi: 10.1128/IAI.73.10.6782-6790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson JD, Smith H. The metabolism of erythritol by Brucella abortus. J Gen Microbiol. 1965;38:109–124. doi: 10.1099/00221287-38-1-109. [DOI] [PubMed] [Google Scholar]
- 10.Sangari FJ, Aguero J, Garcia-Lobo JM. The genes for erythritol catabolism are organized as an inducible operon in Brucella abortus. Microbiology. 2000;146(Pt 2):487–495. doi: 10.1099/00221287-146-2-487. [DOI] [PubMed] [Google Scholar]
- 11.Rajashekara G, Glover DA, Krepps M, Splitter GA. Temporal analysis of pathogenic events in virulent and avirulent Brucella melitensis infections. Cell Microbiol. 2005;7:1459–1473. doi: 10.1111/j.1462-5822.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 12.Plommet M. Minimal requirements for growth of Brucella suis and other Brucella species. Zentralbl Bakteriol. 1991;275:436–450. doi: 10.1016/s0934-8840(11)80165-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, Nace GW, Irwin PL. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J Microbiol Methods. 2003;55:475–479. doi: 10.1016/s0167-7012(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 14.Petersen E, Chaudhuri P, Gourley C, Harms J, Splitter G. Brucella melitensis cyclic di-GMP phosphodiesterase BpdA controls expression of flagellar genes. J Bacteriol. 2011;193:5683–5691. doi: 10.1128/JB.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rambow-Larsen AA, Rajashekara G, Petersen E, Splitter G. Putative quorum-sensing regulator BlxR of Brucella melitensis regulates virulence factors including the type IV secretion system and flagella. J Bacteriol. 2008;190:3274–3282. doi: 10.1128/JB.01915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajashekara G, Glasner JD, Glover DA, Splitter GA. Comparative whole-genome hybridization reveals genomic islands in Brucella species. J Bacteriol. 2004;186:5040–5051. doi: 10.1128/JB.186.15.5040-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendziorski CM, Newton MA, Lan H, Gould MN. On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat Med. 2003;22:3899–3914. doi: 10.1002/sim.1548. [DOI] [PubMed] [Google Scholar]
- 18.Newton MA, Kendziorski CM, Richmond CS, Blattner FR, Tsui KW. On differential variability of expression ratios: improving statistical inference about gene expression changes from microarray data. J Comput Biol. 2001;8:37–52. doi: 10.1089/106652701300099074. [DOI] [PubMed] [Google Scholar]
- 19.Keppie J, Williams AE, Witt K, Smith H. The role of erythritol in the tissue localization of the Brucellae. Br J Exp Pathol. 1965;46:104–108. [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho Neta AV, Stynen AP, Paixao TA, Miranda KL, Silva FL, Roux CM, Tsolis RM, Everts RE, Lewin HA, Adams LG, Carvalho AF, Lage AP, Santos RL. Modulation of the bovine trophoblastic innate immune response by Brucella abortus. Infect Immun. 2008;76:1897–1907. doi: 10.1128/IAI.01554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mc CN, Beal GA. Growth and manometric studies on carbohydrate utilization of Brucella. J Infect Dis. 1951;89:266–271. doi: 10.1093/infdis/89.3.266. [DOI] [PubMed] [Google Scholar]
- 22.Meyer ME. Metabolic characterization of the genus Brucella. VI. Growth stimulation by i-erythritol compared with strain virulence for guinea pigs. J Bacteriol. 1967;93:996–1000. doi: 10.1128/jb.93.3.996-1000.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keppie J, Witt K, Smith H. The effect of erythritol on the growth of S19 and other attenuated strains of Brucella abortus. Res Vet Sci. 1967;8:294–296. [PubMed] [Google Scholar]
- 24.Williams AE, Keppie J, Smith H. The chemical basis of the virulence of Brucella abortus. III. Foetal erythritol a cause of the localisation of Brucella abortus in pregnant cows. Br J Exp Pathol. 1962;43:530–537. [PMC free article] [PubMed] [Google Scholar]
- 25.Fretin D, Fauconnier A, Kohler S, Halling S, Leonard S, Nijskens C, Ferooz J, Lestrate P, Delrue RM, Danese I, Vandenhaute J, Tibor A, DeBolle X, Letesson JJ. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell Microbiol. 2005;7:687–698. doi: 10.1111/j.1462-5822.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 26.Bosseray N. Kinetics of placental colonization of mice inoculated intravenously with Brucella abortus at day 15 of pregnancy. Br J Exp Pathol. 1983;64:612–616. [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi DV, Prakash O. Effect of erythritol on different strains of Brucella melitensis. Indian J Pathol Bacteriol. 1970;13:166–172. [PubMed] [Google Scholar]
- 28.Parent MA, Bellaire BH, Murphy EA, Roop RM, 2nd, Elzer PH, Baldwin CL. Brucella abortus siderophore 2,3-dihydroxybenzoic acid (DHBA) facilitates intracellular survival of the bacteria. Microb Pathog. 2002;32:239–248. doi: 10.1006/mpat.2002.0500. [DOI] [PubMed] [Google Scholar]
- 29.Bellaire BH, Elzer PH, Baldwin CL, Roop RM., 2nd Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro. Infect Immun. 2003;71:2927–2832. doi: 10.1128/IAI.71.5.2927-2932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain N, Rodriguez AC, Kimsawatde G, Seleem MN, Boyle SM, Sriranganathan N. Effect of entF deletion on iron acquisition and erythritol metabolism by Brucella abortus 2308. FEMS Microbiol Lett. 2011;316:1–6. doi: 10.1111/j.1574-6968.2010.02186.x. [DOI] [PubMed] [Google Scholar]
- 31.Eskra L, Covert J, Glasner J, Splitter G. Differential expression of iron acquisition genes by Brucella melitensis and Brucella canis during macrophage infection. PLoS ONE. 2012;7:e31747. doi: 10.1371/journal.pone.0031747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rest RF, Robertson DC. Characterization of the electron transport system in Brucella abortus. J Bacteriol. 1975;122:139–144. doi: 10.1128/jb.122.1.139-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperry JF, Robertson DC. Erythritol catabolism by Brucella abortus. J Bacteriol. 1975;121:619–630. doi: 10.1128/jb.121.2.619-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delrue RM, Deschamps C, Leonard S, Nijskens C, Danese I, Schaus JM, Bonnot S, Ferooz J, Tibor A, De Bolle X, Letesson JJ. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell Microbiol. 2005;7:1151–1161. doi: 10.1111/j.1462-5822.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 35.de Jong MF, Sun YH, den Hartigh AB, van Dijl JM, Tsolis RM. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol Microbiol. 2008;70:1378–1396. doi: 10.1111/j.1365-2958.2008.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.den Hartigh AB, Rolan HG, de Jong MF, Tsolis RM. VirB3 to VirB6 and VirB8 to VirB11, but not VirB7, are essential for mediating persistence of Brucella in the reticuloendothelial system. J Bacteriol. 2008;190:4427–4436. doi: 10.1128/JB.00406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.den Hartigh AB, Sun YH, Sondervan D, Heuvelmans N, Reinders MO, Ficht TA, Tsolis RM. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect Immun. 2004;72:5143–5149. doi: 10.1128/IAI.72.9.5143-5149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdallah AI, Commander NJ, Woodward MJ, Spencer S, Hart CA, Winstanley C. Type III secretion homologs are present in Brucella melitensis, B. ovis, and B. suis biovars 1, 2, and 3. Curr Microbiol. 2003;46:241–245. doi: 10.1007/s00284-002-3789-3. [DOI] [PubMed] [Google Scholar]
- 39.Zygmunt MS, Hagius SD, Walker JV, Elzer PH. Identification of Brucella melitensis 16M genes required for bacterial survival in the caprine host. Microbes Infect. 2006;8:2849–2854. doi: 10.1016/j.micinf.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe AJ, Visick KL. Get the message out: cyclic-Di-GMP regulates multiple levels of flagellum-based motility. J Bacteriol. 2008;190:463–475. doi: 10.1128/JB.01418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, Philpott D, Schroeder JT, Hyodo M, Hayakawa Y, Talbot BG, Brouillette E, Malouin F. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178:2171–2181. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 43.Yan H, Chen W. 3′,5′-Cyclic diguanylic acid: a small nucleotide that makes big impacts. Chem Soc Rev. 2010;39:2914–2924. doi: 10.1039/b914942m. [DOI] [PubMed] [Google Scholar]
- 44.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbier T, Nicolas C, Letesson JJ. Brucella adaptation and survival at the crossroad of metabolism and virulence. FEBS Lett. 2011;585:2929–2934. doi: 10.1016/j.febslet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Starr T, Child R, Wehrly TD, Hansen B, Hwang S, Lopez-Otin C, Virgin HW, Celli J. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe. 2012;11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.