Abstract
The perinexus is a recently identified microdomain surrounding cardiac gap junctions that contains elevated levels of connexin43 and the sodium channel protein Nav1.5. Ongoing work has established a role for the perinexus in regulating gap junction aggregation. However, recent studies have raised the possibility of a perinexal contribution at the gap junction cleft to intercellular propagation of action potential via non-electrotonic mechanisms. The latter possibility could modify current theoretical understanding of cardiac conduction, help explain paradoxical experimental findings, and open up entirely new avenues for antiarrhythmic therapy. We review recent structural insights into the perinexus and its potential novel functional role in cardiac excitation spread, highlighting presently unanswered questions, the evidence for ephaptic conduction in the heart and how structural insights may help complete this picture.
Keywords: Perinexus, Gap Junction, Connexin, Sodium Channel, Nav1.5, Ephaptic, Intercalated Disk, Conduction
Introduction
The gap junction (GJ) is a specialization of cardiomyocyte membranes that has long been recognized as vital to conduction of action potential in the heart. Recently, we provided evidence that non-junctional membrane bordering GJs may itself represent a specialized sub-structure, in that it contains a unique cohort of protein-protein interactions between scaffolding and channel proteins including, ZO-1, connexin43 (Cx43), and Nav1.5 (Rhett et al., 2012; Rhett et al., 2011). This newly identified sarcolemmal microdomain has been called the perinexus owing to its proximity to the GJ. Our work on the perinexus has demonstrated its role in regulating gap junction (GJ) assembly. Interestingly, other recent experimental results vis-à-vis cardiac conduction suggest a role for the perinexus in intercellular propagation of the action potential. This review will summarize recent findings regarding the perinexus and then examine the possible functional implications of this structure in the light of ongoing functional investigations of the basis of cardiac conduction.
Historical Conception of Cardiac Conduction
Since the first demonstration of cardiac conduction by Engelmann in the late 19th century (Engelmann, 1875), the myocardium has been considered an electrical syncytium. Indeed, pioneering work by Silvio Weidmann (Weidmann, 1952; Weidmann, 1970), Lloyd Barr (Barr et al., 1965; Dewey and Barr, 1962) and others later connected the apparent syncytial nature of the myocardium to GJs at intercalated disks - the end-to-end abutments of cardiomyocytes. Thus, the mechanism of excitation propagation in the myocardium was viewed as distinct from that in nerves, in which cell-to-cell transmission of action potential occurs via neuro-chemical signaling at synapses without the need for low resistance electrical coupling between cells. In the heart, the structure of the GJ was seen as affording cytoplasmic continuity between cells thereby enabling electrical conduction of action potential through myocardial tissues. GJs are composed of subunit proteins called connexins, which form channels in the plasma membrane. Connexin channels directly couple the cytoplasms of connected cells, allowing for the passage of ions and molecules <1000Da in size. It is thought that the passage of ions through GJs is the mechanism by which electrical coupling between cardiomyocytes is achieved. For a detailed review on the biology of cardiac connexins, the reader is referred to the review by Desplantez et al (Desplantez et al., 2007).
The picture of the intercalated disk that emerged from early studies supported a purely electrical model of cardiac conduction, wherein the tissue could be represented as a network of resistors and capacitors. For in-depth descriptions of the history of the field the reader is referred to excellent reviews by Spach and Kootsey (Spach and Kootsey, 1983) and Kleber and Rudy (Kleber and Rudy, 2004). The above being said, recent studies have suggested that the intercalated disk and its constituent intercellular junctions are more complex, interdependent, and dynamic structures than previously conceived. Additionally, recent experimental data has raised questions about the conventional view of how cardiac conduction works. Such new perspectives raise the possibility that a new electrochemical model of cardiac conduction may be necessary. This review focuses on some structural and functional observations that are contributing to this emerging story.
GJ and Perinexus: Ultrastructure and Molecular Components
In general, GJs are large, semi-crystalline aggregates of intercellular channels arranged in a honeycomb-like hexagonal array (Fig. 1A). The works of Gaietta et al. (Gaietta et al., 2002) and Lauf et al. (Lauf et al., 2002) established the canonical pathway for GJ accretion by which half-channels (called connexons or hemichannels) composed of connexin subunits delivered to the plasma membrane diffuse laterally in the membrane to points of cell-cell contact where they are incorporated into the GJ plaque as intercellular channels. Questions of considerable interest in this model of GJ aggregation are the regions of membrane where connexons are delivered to and dock to form intercellular channels. Robin Shaw’s group has provided evidence that microtubules tethered to cadherin-complexes provide pathways for connexon trafficking (Smyth et al., 2010).
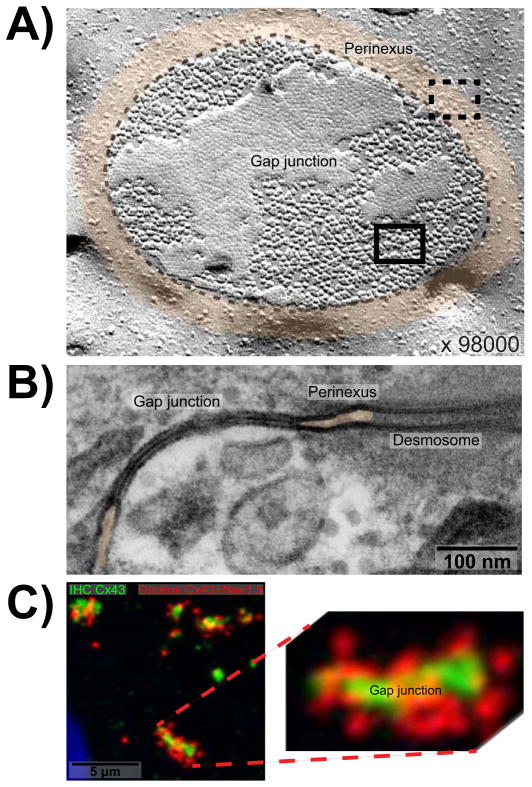
Figure 1. The perinexus.
A) Freeze fracture electron micrograph showing en face view of a gap junction plaque in ventricular myocardium (outlined by dashed black line) surrounded by perinexus (tinted orange) at 98000x magnification (Severs, 2002). Individual connexons, seen as particles, are densely packed in a hexagonal array within the gap junction (solid rectangle) but are less concentrated and organized within the perinexus (dashed rectangle). B) Transmission electron micrograph of gap junction in ventricular myocardium flanked on either side by perinexus. Space between perinexal membranes is tinted orange. A desmosome is also visible on the right hand side. C) Duolink labeling shows Cx43/Nav1.5 interaction at and surrounding GJs in neonatal rat ventricular myocytes. Cx43 is labeled in green, Cx43/Nav1.5 interaction is labeled by Duolink in red, and the nucleus is blue.
Data from the Gourdie lab provided insight into how the process of GJ accretion is regulated once connexons are trafficked to the cell membrane. Work by Hunter, Barker et al. (Hunter et al., 2005) first demonstrated that zonula occludens-1 (ZO-1) interaction with the Cx43 carboxyl-terminus regulates GJ size. Colocalization of ZO-1 with the GJ edge, combined with biochemical data showing that inhibiting Cx43/ZO-1 interaction increased the proportion of junctional to nonjunctional Cx43, indicated that ZO-1 could be controlling the transition of hemichannels to GJ-associated intercellular channels. This mechanism was confirmed in subsequent work, which gave us the first view of the perinexus (Rhett et al., 2011). In this 2011 paper, the in-situ protein-protein interaction assay Duolink provided visualization of an unanticipated phenomenon wherein ZO-1 interaction with Cx43 gave a robust signal in the region around the GJ aggregate proper.
Interestingly, perinexal Cx43/ZO-1 Duolink signals around GJs were not co-localized with Cx43 immunolabeling. As such, it was hypothesized that Cx43 molecules in the perinexus were not at sufficient concentrations to be visualized by standard immunofluorescence. In order to increase the dynamic range of detection and directly label perinexal Cx43, Duolink assay was used in a single-detection, high-sensitivity mode – essentially assaying for Cx43-Cx43 interaction, as would occur within a connexon. The assay revealed that Cx43 is more concentrated in the perinexus than other non-junctional regions of the cell, but not nearly as concentrated as in the GJ plaque (Rhett et al., 2012). Biochemical and functional assays confirmed that the channels in the perinexus were likely to be in the form of connexon hemichannels (Rhett et al., 2012; Rhett et al., 2011).
At the same time, data began to emerge suggesting a relationship between Cx43 and the voltage-gated sodium channel, Nav1.5. Studies by the Abriel lab showed that both proteins localized to the intercalated disk (Petitprez et al., 2011). Additionally, it had previously been demonstrated that Cx43 and Nav1.5 co-precipitate from mouse heart lysates (Malhotra et al., 2004). We again used Duolink to assay for Cx43/Nav1.5 interaction and elucidated the subcellular location of putative complexes formed by these two proteins. It was found that, much like Cx43/ZO-1 interaction, Cx43/Nav1.5 interaction localized preferentially to the perinexus (Fig. 1C)(Rhett et al., 2012). Interestingly, in agreement with Pretiprez et al, Nav1.5/ZO-1 interaction was not detected (Petitprez et al., 2011). This indicated that ZO-1 likely did not serve as a molecular scaffold for Cx43/Nav1.5. Whether Cx43 and Nav1.5 interact directly or through a molecular mediator remains unknown, but both proteins have been reported to interact with the scaffolding proteins SAP97 (Petitprez et al., 2011; Macdonald et al., 2012) and ankyrin-G (Malhotra et al., 2002; Sato et al., 2011).
GJs and the Perinexus: Implications for Cardiac Conduction
While GJs have been established as a key determinant of cardiac conduction, the electrophysiological impacts of loss of GJ coupling remain to be fully understood. On the one hand, pharmacological GJ uncoupling uniformly slows conduction in experiments, suggesting a positive (direct) linear relationship between conduction velocity and GJ coupling (Rohr et al., 1998; de Groot et al., 2003; Kojodjojo et al., 2006; Delmar et al., 1987; Balke et al., 1988; Jalife et al., 1989; Callans et al., 1996). However, the results from mice heterozygous for a null mutation of the Cx43 gene GJA1 have not been as straightforward to interpret. Some studies reported conduction slowing in these mice compared to wild-type (WT) littermates (Eloff et al., 2001; Guerrero et al., 1997) while others didn’t - even in cases where the Cx43 heterozygote null mice were obtained from the same source (Morley et al., 1999; van Rijen et al., 2004; Thomas et al., 2003; Beauchamp et al., 2004; Vaidya et al., 2001; Danik et al., 2004; Stein et al., 2009). Likewise, while reduced expression and lateralization of Cx43 are associated with multiple pathologies, the relationship of such changes to conduction disturbance and arrhythmias is highly complex (Poelzing and Rosenbaum, 2004; Akar and Tomaselli, 2005; Cascio et al., 2005; Gard et al., 2005; Severs, 2002).
The recent experimental study of the electrophysiologic impact of changes in interstitial volume by two of the authors of this essay, Drs. Veeraraghavan and Poelzing further reinforced the notion that the conduction velocity-GJ relationship is not as straightforward as previously conceived (Veeraraghavan et al., 2012). In these studies, Langendorff preparations of isolated guinea pig ventricles were perfused with solutions designed to prompt predictable decreases or increases in interstitial volume - the volume of extracellular space excluding the vasculature. This experimental manipulation of interstitium in turn enabled measurable adjustments to the spacing between cardiomyocyte membranes. In this experimental setting a negative (inverse) relationship between conduction velocity and interstitial volume was observed: decreasing interstitial volume resulted in acceleration of conduction and vice versa. Also, interstitial volume changes preferentially affected transverse relative to longitudinal conduction. The acute time course of these effects argued against these effects being mediated by significant GJ remodeling.
The inverse relationship observed in our studies stood in contrast to prevailing theoretical models (e.g., the bidomain model), which predicted a positive relationship between conduction velocity and interstitial volume (Spach et al., 2004). Such models anticipate that increased interstitial volume should decrease longitudinal interstitial resistance and thereby speed up conduction. While experiments in cable-like papillary muscles have yielded results consistent with this concept (Fleischhauer et al., 1995), our observations in the intact ventricular myocardium were not consistent with the model’s prediction (Veeraraghavan et al., 2012). In the same study, we demonstrated that swelling of the interstitial space (i.e., interstitial edema) unmasked a steeper conduction velocity - GJ relationship than was observed under control conditions. Further, the combination of edema and GJ uncoupling was more pro-arrhythmic than either condition alone. The prevailing theoretical models could not explain these findings. Based on our observations, we concluded that a reassessment of our theoretical understanding of cardiac conduction in intact myocardium was in order.
Since the 1960s Sperelakis (Sperelakis and McConnell, 2002; Sperelakis et al., 1983) and others (Pertsov and Medvinskii, 1979; Zemlin et al., 2006) have argued that current flow via GJs may not be the sole mechanism mediating the intercellular propagation of action potential in the heart. Early work focused on the idea that mechanisms such as an electric field extending via the gap junctional cleft could electrically couple cardiomyocytes without a need for GJ-mediated electrotonic coupling (Sperelakis and McConnell, 2002). Given the wealth of data on the role of gap junctions in normal and pathological conduction (Rohr, 2004; Jongsma and Wilders, 2000), the idea was met with wide skepticism. However, mounting evidence of a complex conduction velocity - GJ relationship as detailed above has fueled a resurgence of interest in non-electrotonic mechanisms. In a theoretical study, Mori et al. demonstrated that an ‘ephaptic coupling’ mechanism may operate in tandem with GJ coupling during cardiac conduction, dubbing the resulting mechanism ’mixed mode’ coupling (Mori et al., 2008). This computational model further suggested that localization of Na+ channels to the intercalated disk and the width of the gap junctional cleft may be key factors in conduction.
Cardiac Conduction: Revising our Present Understanding
One missing underpinning for ephaptic coupling is a functional unit as structurally definitive as the neural synapse – an ephapse. Facilitation of any non-electrotonic mechanism for the intercellular transfer of action potential would require some definable element of cellular ultrastructure. The narrow gap junction cleft and the preferential localization of Nav1.5 at the plaque edge (Fig. 1A and B)(Rhett et al., 2012), raises the prospect that the perinexus is the myocardial ephapse. As yet there are too many unknowns to make definitive claims, but the data suggesting that the perinexus localizes the machinery necessary for ephaptic conduction provides a basis for further investigation.
More broadly the essential questions seem to come down to: a) how, specifically, are ion channels, particularly Nav1.5, organized at the intercalated disk and b) what are the functional implications of this organization with respect to intercellular conduction of action potential? It has been known for some time that the cardiac isoform of the voltage gated Na+ channel localizes to the intercalated disk (Maier et al., 2004). Since then Petitprez et al. have demonstrated the localization of Na+ channels to the intercalated disk and the lateral membrane via distinct sets of interaction involving PDZ proteins (Petitprez et al., 2011). Recently, Lin et al. demonstrated greater INa density at the intercalated disk relative to the lateral membrane (Lin et al., 2011). Furthermore, they found most Na+ channels in the lateral membrane to be inactive at normal resting potential and suggested that the initiation of the action potential primarily occurs at the intercalated disk. Parallel developments in theoretical models of conduction have suggested a mechanistic role for subcellular Na+ channel localization. Kucera et al. proposed that transient changes in Na+ concentration within the narrow intercellular cleft at the intercalated disk could modulate the magnitude of INa when Na+ channels are located at the intercalated disk (Kucera et al., 2002). Additionally, they suggested that when GJ coupling is reduced, activation of INa in the upstream cell could induce negative cleft potentials and thereby suprathreshold depolarization of the downstream cell. Such a mechanism would act to preserve conduction when GJ coupling is compromised.
More recent models incorporating ephaptic coupling theorized that Na+ channel localization and GJ cleft width to be major determinants of conduction velocity (Mori et al., 2008; Lin and Keener, 2010). Further, these studies inferred that Na+ channels located at the intercalated disk may participate in transmission of the action potential between myocytes, and that this mechanism may help sustain conduction when direct electrical coupling at GJs is reduced. As a result, determinants of ephaptic coupling modulating the conduction velocity - GJ relationship were anticipated in these theoretical treatments. Importantly, it was suggested that Na+ channels located at the intercalated disk face the small, restricted GJ cleft, which may represent a specialized extracellular compartment with important mechanistic implications for cardiac conduction.
The inclusion of subcellular Na+ channel localization and GJ cleft effects into models of conduction is driving a revision of our understanding of the phenomenon and may help answer some of the open questions posed earlier in this essay. In this context, new knowledge of the perinexus raises some interesting possibilities. For instance, within the perinexus Na+ channels from apposed cells face the same, narrow interstitial cleft (Fig. 2). Put another way, the GJ cleft is formed by the two cell membranes apposed at the perinexus. Such a structure would allow for, and even facilitate local effects such as ion depletion or accumulation, particularly over the time course of the action potential upstroke. Thus, perinexal Na+ channels could make significant contributions to conduction. Moving forward, it is vital to continue careful investigation of the ultrastructure of the intercalated disk and to allow this information to inform functional investigations of cardiac conduction. Changes in intermembrane spacing at the GJ cleft seem like a tractable experimental target for modulation of the conduction velocity - GJ relationship. Results from such studies could potentially explain the seemingly paradoxical results obtained in models of reduced GJ coupling as well as residual conduction observed in homozygous conditional Cx43 knockout mice (Gutstein et al., 2001). Furthermore, weakening of ephaptic coupling secondary to GJ cleft widening could by itself slow conduction (Mori et al., 2008; Kucera et al., 2002; Lin and Keener, 2010). Such a mechanism may explain the experimentally observed inverse relationship between conduction velocity and interstitial volume.
Figure 2. Structural organization of channels within the perinexus.
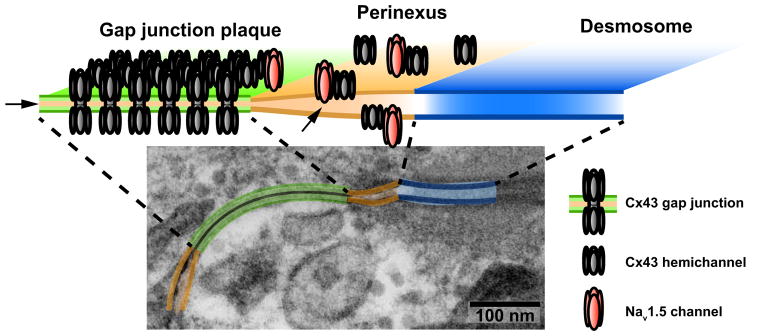
The transmission electron micrograph from figure 1B is repeated with the gap junction highlighted in green, the perinexus in orange and the desmosome in blue. The schematic cartoon above shows the regular organization of connexons within the gap junction and the localization of Cx43 hemichannels and Nav1.5 channels to the perinexus. Arrowheads point to the confined extracellular cleft between apposed cell membranes within the gap junction and the perinexus. While the cartoon shows a simplified view of the perinexus, the reader should remember that the structural organization of the channel proteins shown here is governed by their interactions with multiple structural proteins such as ZO-1, SAP97, that are not shown (Rhett and Gourdie, 2012). Furthermore, these structures are far from static, with connexons constantly flowing into the gap junction at its periphery.
Clinical implications
Aside from scientific value, revisions to our theoretical understanding of conduction could have important clinical implications. Given that arrhythmogenic conduction defects are a major cause of the 450,000+ cases of sudden cardiac death that occur annually in the U.S. (Zheng et al., 2001), there is an urgent need for new mechanistically-guided treatment options. Recent insights into trafficking mechanisms and inter-protein interactions of sarcolemmal channels have helped identify novel targets for antiarrhythmic therapy. In this respect, Cx43/Nav1.5 interaction in the perinexus merits investigation as a potential target for antiarrhythmic therapy - especially in the light of the recent report that Nav1.5 is downregulated in Cx43 HZ mice (Jansen et al., 2012). Further, O’Quinn et al. demonstrated the antiarrhythmic properties of a Cx43 mimetic peptide targeting Cx43/ZO-1 interaction in a murine infarction model (O’Quinn et al., 2011) while results from Rhett et al. indicate the Cx43/ZO-1 and Cx43/Nav1.5 interactions to be mutually exclusive (Rhett et al., 2012). One interpretation of these data is that blocking Cx43/ZO-1 interaction frees Cx43 molecules to interact with Nav1.5 (Rhett et al., 2011). The resulting sequestration of Nav1.5 in the perinexus and GJ cleft in such a model would in turn increase ephaptic contribution to conduction, thereby contributing to the antiarrhythmic effect observed in response to the Cx43 mimetic peptide. Other proteins that interact with Cx43 or Nav1.5 might warrant study along these lines.
Another new approach to antiarrhythmic therapy may involve targeting the intercalated disk microstructure itself rather than sarcolemmal proteins. Veeraraghavan et al. demonstrated the interstitial volume to be a potent modulator of not just the conduction velocity - GJ relationship but also of arrhythmia propensity during GJ uncoupling (Veeraraghavan et al., 2012). Therefore, targeting the vasculature (the endothelial glycocalyx in particular), with a view to preventing myocardial edema and the consequent increase in distance between cell membranes, may represent a viable antiarrhythmic strategy in pathologies where GJ coupling is compromised.
Conclusion
Although these are early days, the data already available underscores the need to revise theoretical models of conduction to include detailed structural information and careful accounting of electrochemical effects that may occur within extracellular microdomains such as that formed by the perinexus. Indeed, a parallel could be drawn here with the molecular machinery of cardiac Ca2+ cycling where the recognition of specialized structural microdomains and transient ion accumulation/depletion within these confined spaces has revolutionized our theoretical understanding of the phenomenon in recent years (Cannell and Kong, 2012).
In closing, it does not go unnoticed that one irony here is that the gap junction may turn out to be central to a non-electrotonic mechanism of action potential propagation in the heart - the rationale being that is there is no other location where the membranes of adjacent cells come closer together and where the extracellular space between cells is more narrowly confined. The gap junction or its immediate vicinity would thus be the logical place for a putative jump of action potential between myocytes to occur. This perhaps will be a wrinkle in the tale that not even the embattled early proponents of ephaptic conduction could have foreseen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akar FG, Tomaselli GF. Conduction abnormalities in nonischemic dilated cardiomyopathy: basic mechanisms and arrhythmic consequences. Trends Cardiovasc Med. 2005;15:259–64. doi: 10.1016/j.tcm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Balke CW, Lesh MD, Spear JF, Kadish A, Levine JH, Moore EN. Effects of cellular uncoupling on conduction in anisotropic canine ventricular myocardium. Circ Res. 1988;63:879–92. doi: 10.1161/01.res.63.5.879. [DOI] [PubMed] [Google Scholar]
- 3.Barr L, Dewey MM, Berger W. Propagation of Action Potentials and the Structure of the Nexus in Cardiac Muscle. J Gen Physiol. 1965;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circ Res. 2004;95:170–8. doi: 10.1161/01.RES.0000134923.05174.2f. [DOI] [PubMed] [Google Scholar]
- 5.Callans DJ, Moore EN, Spear JF. Effect of coronary perfusion of heptanol on conduction and ventricular arrhythmias in infarcted canine myocardium. J Cardiovasc Electrophysiol. 1996;7:1159–71. doi: 10.1111/j.1540-8167.1996.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 6.Cannell MB, Kong CH. Local control in cardiac E-C coupling. J Mol Cell Cardiol. 2012;52:298–303. doi: 10.1016/j.yjmcc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Cascio WE, Yang H, Muller-Borer BJ, Johnson TA. Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. J Electrocardiol. 2005;38:55–9. doi: 10.1016/j.jelectrocard.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–41. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groot JR, Veenstra T, Verkerk AO, Wilders R, Smits JP, Wilms-Schopman FJ, Wiegerinck RF, Bourier J, Belterman CN, Coronel R, Verheijck EE. Conduction slowing by the gap junctional uncoupler carbenoxolone. Cardiovasc Res. 2003;60:288–97. doi: 10.1016/j.cardiores.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Delmar M, Michaels DC, Johnson T, Jalife J. Effects of increasing intercellular resistance on transverse and longitudinal propagation in sheep epicardial muscle. Circ Res. 1987;60:780–5. doi: 10.1161/01.res.60.5.780. [DOI] [PubMed] [Google Scholar]
- 11.Desplantez T, Dupont E, Severs N, Weingart R. Gap Junction Channels and Cardiac Impulse Propagation. 1. Vol. 218. Springer; New York: 2007. pp. 13–28. [DOI] [PubMed] [Google Scholar]
- 12.Dewey MM, Barr L. Intercellular Connection between Smooth Muscle Cells: the Nexus. Science. 1962;137:670–2. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- 13.Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res. 2001;51:681–90. doi: 10.1016/s0008-6363(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann ThW. Pflügers Archiv European Journal of Physiology. 1. Vol. 11. Springer; Berlin/Heidelberg: 1875. Ueber die Leitung der Erregung im Herzmuskel; pp. 465–480. [Google Scholar]
- 15.Fleischhauer J, Lehmann L, Kleber AG. Electrical resistances of interstitial and microvascular space as determinants of the extracellular electrical field and velocity of propagation in ventricular myocardium. Circulation. 1995;92:587–94. doi: 10.1161/01.cir.92.3.587. [DOI] [PubMed] [Google Scholar]
- 16.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 17.Gard JJ, Yamada K, Green KG, Eloff BC, Rosenbaum DS, Wang X, Robbins J, Schuessler RB, Yamada KA, Saffitz JE. Remodeling of gap junctions and slow conduction in a mouse model of desmin-related cardiomyopathy. Cardiovasc Res. 2005;67:539–47. doi: 10.1016/j.cardiores.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–8. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–9. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–98. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalife J, Sicouri S, Delmar M, Michaels DC. Electrical uncoupling and impulse propagation in isolated sheep Purkinje fibers. Am J Physiol. 1989;257:H179–89. doi: 10.1152/ajpheart.1989.257.1.H179. [DOI] [PubMed] [Google Scholar]
- 22.Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV. Reduced heterogeneous expression of Cx43 results in decreased Nav1. 5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9:600–7. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000;86:1193–7. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- 24.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 25.Kojodjojo P, Kanagaratnam P, Segal OR, Hussain W, Peters NS. The effects of carbenoxolone on human myocardial conduction: a tool to investigate the role of gap junctional uncoupling in human arrhythmogenesis. J Am Coll Cardiol. 2006;48:1242–9. doi: 10.1016/j.jacc.2006.04.093. [DOI] [PubMed] [Google Scholar]
- 26.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res. 2002;91:1176–82. doi: 10.1161/01.res.0000046237.54156.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–51. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Keener JP. Modeling electrical activity of myocardial cells incorporating the effects of ephaptic coupling. Proc Natl Acad Sci U S A. 2010;107:20935–40. doi: 10.1073/pnas.1010154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL, Fishman GI, Delmar M. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm. 2011;8:1923–30. doi: 10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald AI, Sun P, Hernandez-Lopez H, Aasen T, Hodgins MB, Edward M, Roberts S, Massimi P, Thomas M, Banks L, Graham SV. A functional interaction between the MAGUK protein hDlg and the gap junction protein connexin 43 in cervical tumour cells. Biochem J. 2012;446:9–21. doi: 10.1042/BJ20111144. [DOI] [PubMed] [Google Scholar]
- 31.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–7. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL. Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin. J Biol Chem. 2002;277:26681–8. doi: 10.1074/jbc.M202354200. [DOI] [PubMed] [Google Scholar]
- 33.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279:40748–54. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 34.Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc Natl Acad Sci U S A. 2008;105:6463–8. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–75. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 36.O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. 2011;108:704–15. doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pertsov AM, Medvinskii AB. Effect of specialized contacts on fiber interaction during the spread of excitation in smooth muscle and myocardial tissues. Biofizika. 1979;24:293–8. [PubMed] [Google Scholar]
- 38.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1. 5 in cardiomyocytes. Circ Res. 2011;108:294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 39.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 40.Rhett JM, Gourdie RG. The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm. 2012;9:619–23. doi: 10.1016/j.hrthm.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 Associates with Na(v)1. 5 in the Cardiomyocyte Perinexus. J Membr Biol. 2012;245:411–22. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhett JM, Palatinus JA, Jourdan JA, Gourdie RG. Connexin43 Interacts with Voltage-Gated Sodium Channel 1. 5 in the Perinexus. Circulation. 2011;124:A9561. [Google Scholar]
- 44.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–22. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res. 1998;83:781–94. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 46.Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ Res. 2011;109:193–201. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Severs NJ. Gap junction remodeling in heart failure. J Card Fail. 2002;8:S293–9. doi: 10.1054/jcaf.2002.129255. [DOI] [PubMed] [Google Scholar]
- 48.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–79. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;1:500–15. doi: 10.1016/j.hrthm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Spach MS, Kootsey JM. The nature of electrical propagation in cardiac muscle. Am J Physiol. 1983;244:H3–22. doi: 10.1152/ajpheart.1983.244.1.H3. [DOI] [PubMed] [Google Scholar]
- 51.Sperelakis N, Marschall RA, Mann JE. Propagation down a chain of excitable cells by electric field interactions in the junctional clefts: effect of variation in extracellular resistances, including a “sucrose gap” simulation. IEEE Trans Biomed Eng. 1983;30:658–64. doi: 10.1109/tbme.1983.325068. [DOI] [PubMed] [Google Scholar]
- 52.Sperelakis N, McConnell K. Electric field interactions between closely abutting excitable cells. IEEE Eng Med Biol Mag. 2002;21:77–89. doi: 10.1109/51.993199. [DOI] [PubMed] [Google Scholar]
- 53.Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, van der Nagel R, Bezzina CR, Hauer RN, de Bakker JM, van Rijen HV. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovasc Res. 2009;83:52–60. doi: 10.1093/cvr/cvp124. [DOI] [PubMed] [Google Scholar]
- 54.Thomas SP, Kucera JP, Bircher-Lehmann L, Rudy Y, Saffitz JE, Kleber AG. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Circ Res. 2003;92:1209–16. doi: 10.1161/01.RES.0000074916.41221.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, Jalife J. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res. 2001;88:1196–202. doi: 10.1161/hh1101.091107. [DOI] [PubMed] [Google Scholar]
- 56.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–55. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 57.Veeraraghavan R, Salama ME, Poelzing S. Interstitial Volume Modulates the Conduction Velocity- Gap Junction Relationship. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00868.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weidmann S. The electrical constants of Purkinje fibres. J Physiol. 1952;118:348–60. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970;210:1041–54. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zemlin CW, Mironov S, Pertsov AM. Near-threshold field stimulation:intramural versus surface activation. Cardiovasc Res. 2006;69:98–106. doi: 10.1016/j.cardiores.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]