Abstract
Dietary restriction extends lifespan in a variety of organisms, but the key nutritional components driving this process and how they interact remain uncertain. In Drosophila, while a substantial body of research suggests that protein is the major dietary component affecting longevity, recent studies claim that carbohydrates also play a central role. To clarify how nutritional factors influence longevity, nutrient consumption and lifespan were measured on a series of diets with varying yeast and sugar content. We show that optimal lifespan requires both high carbohydrate and low protein consumption, but neither nutrient by itself entirely predicts lifespan. Increased dietary carbohydrate or protein concentration does not always result in reduced feeding—the regulation of food consumption is best described by a constant daily caloric intake target. Moreover, due to differences in food intake, increased concentration of a nutrient within the diet does not necessarily result in increased consumption of that particular nutrient. Our results shed light on the issue of dietary effects on lifespan and highlight the need for accurate measures of nutrient intake in dietary manipulation studies.
Keywords: Aging, Dietary restriction, Drosophila, Feeding, Longevity, Nutrition
1. Introduction
Aging leads to increased susceptibility to metabolic diseases such as type 2 diabetes and cardiovascular disease. Hence, understanding the nutritional drivers of metabolic aging is of major interest for human health and evolutionary biology. Dietary restriction (DR), the reduction of nutrient intake short of malnutrition, appears to improve measures of human health and extends the lifespan of various organisms ranging from yeast to primates (Fontana et al. 2010). Therefore, the interaction between diet and lifespan may involve highly conserved mechanisms, highlighting the value of model organism research.
In mammals, the lifespan-extending effects of DR have been classically attributed to reduced caloric intake (Bordone and Guarente 2005). However, studies using isocaloric diets with altered nutrient composition also affect lifespan (Iwasaki et al. 1988). It remains unclear which nutrients have the greatest impact on longevity. In Drosophila, it has been claimed that protein is the main dietary determinant of lifespan, with carbohydrates having little or no effect (Grandison et al. 2009; Mair et al. 2005). In general, these studies suffer from (1) the lack of direct measurements of food intake, precluding interpretation of their results and (2) the use of very limited ranges of nutrient concentrations—typically only two concentrations per nutrient.
In contrast, a recent comprehensive study used a nutritional geometry approach, encompassing 28 experimental liquid diets, to investigate nutrient-specific effects on lifespan in individually housed female flies (Lee et al. 2008). In contrast to previous studies in Drosophila, the authors conclude that both carbohydrate and protein have pivotal effects. Flies were longest-lived at a relatively high dietary carbohydrate-to-protein ratio (C:P) of 16:1 (Lee et al. 2008). However, flies in this study were all significantly short-lived compared to those maintained under more commonly used laboratory conditions. Hence, the general applicability of their intriguing findings has remained uncertain and further studies are warranted—using different gender, strains, and laboratory conditions—to clarify these nutritional associations.
We measured nutrient consumption and lifespan in male flies of two Drosophila laboratory strains (Canton-S and Dahomey) fed up to eight different diets with varying yeast and sucrose content. We found that median lifespan is optimal at a relatively high C:P (10:1 to 20:1), consistent with previous studies (Lee et al. 2008). Low protein and high carbohydrate consumption maximize lifespan, while neither parameter alone is sufficient to ensure optimal longevity. Precise feeding measurements demonstrate that the dietary concentration of a particular nutrient correlates poorly with its actual consumption. Interestingly, our studies suggest that fly feeding behavior is driven by a daily caloric intake target and our results stress the importance of measuring food intake and considering macronutrient ratio in aging studies.
2. Materials and methods
2.1. Fly culture and reagents
Canton-S and Dahomey flies were developed in bottles (6 or 8 oz. sizes) on a standard food depending on site (Lewis medium (Lewis 1960) or Bloomington cornmeal-sucrose-yeast). Males were collected 2–4 days after eclosion on CO2 anesthesia and subsequently maintained on the experimental diet in vials (polypropylene, 25 × 95 mm). All flies were maintained in a humidity- and temperature-controlled incubator at 25 °C and 60% relative humidity under a 12/12-h light/dark cycle. Bacto™ agar and yeast extract were from BD Diagnostic Systems. All other fly food components were obtained from Fisher Scientific (Pittsburgh, PA), VWR (Radnor, PA), or Genesee Scientific (San Diego, CA).
2.2. Experimental diets
Fly food was prepared essentially as described (Ja et al. 2009). Solid food diets were based on a cornmeal-sugar-yeast medium containing a base of 0.5% (w/v) Bacto™ agar, 8.6% (w/v) cornmeal, 0.4% (v/v) propionic acid, 0.06% (v/v) phosphoric acid, and varying concentrations of sucrose (1%, 5%, and 20%, all w/v) and Bacto™ yeast extract (0.25%, 1%, 4%, or 5%, all w/v). Briefly, agar was boiled in water prior to adding yeast extract and sucrose with vigorous stirring. After the components were dissolved, cornmeal (8.6%, w/v) was slowly added to prevent clumping and the food was simmered for 3–5 min. After cooling to about 65 °C, the final volume was adjusted with ddH2O and propionic and phosphoric acids were added from a 100× stock solution. Food was dispensed into vials (2 mL) and stored at 4 °C. Fresh food was prepared every 1–2 weeks. Liquid diets for the CAFE assay did not include cornmeal or agar. These yeast extract/sucrose solutions were sterile-filtered before use.
2.3. Lifespan assays
Males (20–30 flies/vial) were scored for survival and transferred to fresh medium every 2–3 days. Trays of vials were placed randomly in the incubator and positions were rotated after each transfer to minimize the effects of microclimate. For experiments where water was provided, 400 µL of 1% agar was dispensed onto the vial wall approximately 3 cm from the bottom. Survival data are reported as the time since the flies were initially placed on the experimental diet. Comparisons of lifespan shown in individual figure panels are derived from a single initial population of flies.
2.4. Feeding rate determination
Food intake using radioisotope labeling was measured essentially as described (Ja et al. 2009). Flies were maintained identically as for the lifespan assays. After a period of habituation (typically 1–2 weeks), flies were transferred to medium supplemented with ~1 µCi/mL [α-32P]-dCTP. After 24 h of feeding on radiolabeled food, flies were transferred to empty vials, allowed to groom for 20 min, and then frozen. Flies were assayed in 5–10 mL of scintillation fluid (ScintiVerse™ BD Cocktail, Fisher Scientific, or equivalent) in a scintillation counter to measure accumulated 32P. Aliquots of labeled food were used to convert scintillation counts to mass or volume. For the plots where consumption is related to lifespan, food intake was typically measured from 4 to 6 vials each containing 10–20 flies. All measurements within an individual figure panel were derived from a common initial population of flies. Liquid food intake was measured using the CAFE assay, performed as described previously (Ja et al. 2007).
2.5. Statistical analyses
Protein and carbohydrate contents of diets were calculated using nutritional information from manufacturers (yeast extract: 51% protein + 16.33% carbohydrate; cornmeal: 7.5% protein + 78.8% total carbohydrate). Log-rank tests of survival data and correlation analyses (Pearson’s for linear or Spearman’s for non-linear relationships) were calculated using Prism (version 5.04, GraphPad Software, La Jolla, CA). Scatter plots were fitted using Excel (Microsoft, Redmond, WA) using linear (y = mx + b) or power (y = mxb) functions. Differences in feeding between diets were determined by one-way ANOVA and Tukey-Kramer post-hoc test for multiple comparisons.
3. Results
3.1. Nutrient ingestion and lifespan
In Drosophila, the effect of DR on lifespan has been claimed to be mediated largely by dietary protein, with other nutrients playing at most a small role (Grandison et al. 2009; Mair et al. 2005). However, Drosophila DR research has classically suffered from methodological issues that complicate the interpretation of previous work. In particular, diet manipulations are generally not accompanied by measurements of actual long-term food intake. A remarkably comprehensive study used the capillary feeder (CAFE) system (Ja et al. 2007) and 28 different diets in a geometric framework to relate nutrient intake to fitness parameters such as lifespan and fecundity (Lee et al. 2008). The authors found that low protein-high carbohydrate ingestion was essential for maximal lifespan, as neither low protein nor high carbohydrate consumption alone was sufficient to ensure longevity. The longest lifespan was observed on a dietary C:P of 16:1 in females (Supplementary Fig. 1). These findings are of great relevance, since they directly contradict previous claims that dietary carbohydrates play a lesser role in influencing lifespan (Grandison et al. 2009; Mair et al. 2005). The observations were previously overlooked because of the tendency to restrict DR studies to two food concentrations, as opposed to the experimental design of Lee et al., which explored a wide nutritional range.
Confirming the striking effect of carbohydrate ingestion on lifespan would represent a remarkable advance in the field and a departure from current dogma. Particularly, it remains unclear whether the observations can be confidently extrapolated to more common experimental settings. Most of the experiments conducted by Lee et al. were unconventional—flies were individually isolated in small vials and fed liquid foods throughout their entire lifespan, perhaps explaining the considerably short lives compared to those of traditionally housed flies (Supplementary Fig. 1). Although a small subset of diets was tested in traditional demography cages, food intake was not measured in these trials (Lee et al. 2008). Determining the role of carbohydrate and protein consumption on longevity in conventional rearing conditions, as well as in other strains and both genders, is crucial for informing future studies.
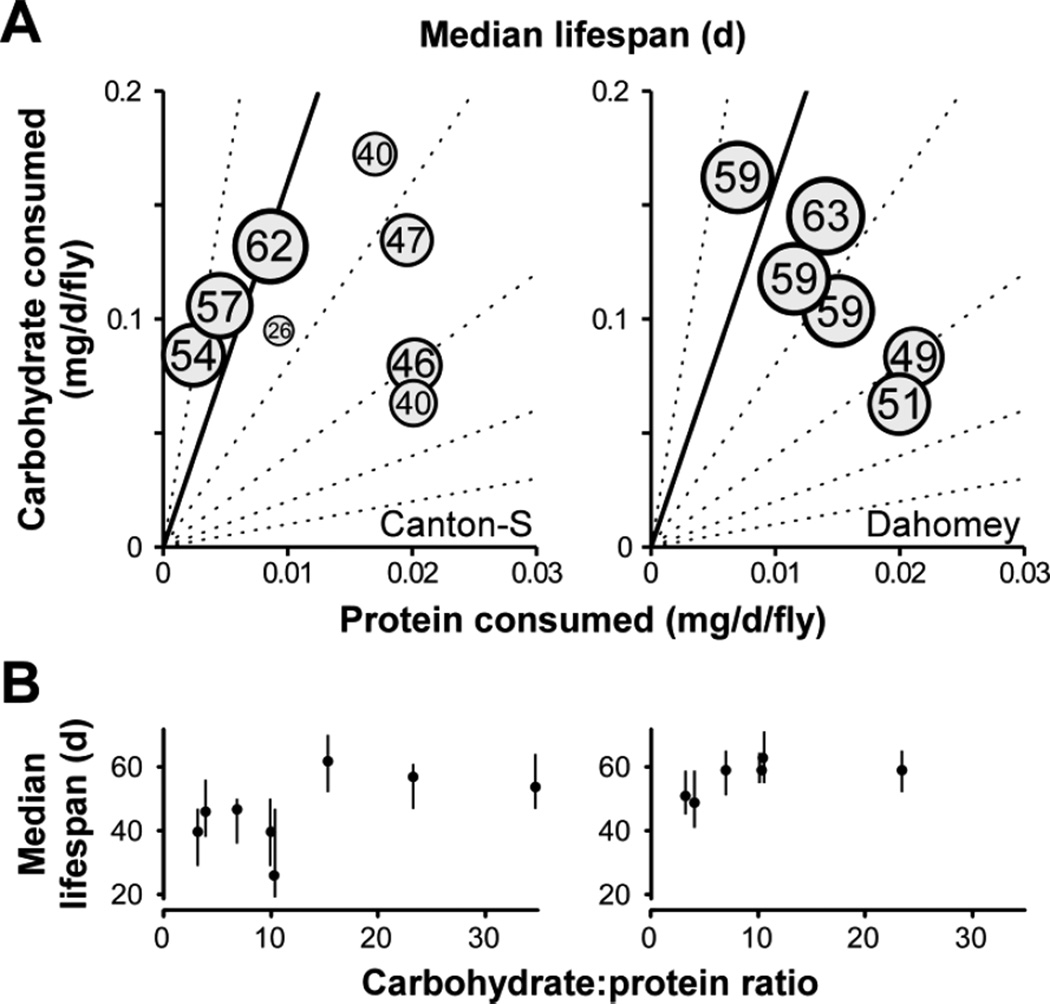
We fed male flies a series of different diets with varying yeast extract and sucrose content in a standard base containing agar and cornmeal. This agar-based solid medium is one of several regimes commonly used in Drosophila DR investigations (Katewa et al. 2012; Min et al. 2007; Zid et al. 2009). The lifespan of two commonly used laboratory strains, Canton-S and Dahomey, was measured and plotted as a function of both carbohydrate and protein consumption (Fig. 1A). In each genotype, the longest lifespan was recorded on diets where flies ingested high carbohydrate and low protein levels. Maximal longevity was seen at a C:P between 10:1 and 20:1 (Fig. 1B), consistent with the previously reported value of 16:1 (cf. Supplementary Fig. 1). Further increases in C:P shortened lifespan, a finding observed in studies using very high C:P diets (Lushchak et al. 2012). The median lifespan in this trial for Canton-S flies on a C:P diet of 10.2:1 was unusually short—the interquartile range is likely more representative of survival across independent replicate experiments with a subset of diets (Fig. 1B and data not shown). Our results extend published observations to an additional strain, gender, and dietary paradigm, demonstrating that the seminal findings of Lee et al. are not caused by the specific rearing conditions used in the original study and likely describe a phenomenon of general biological relevance. In agreement with their study, our results indicate that neither macronutrient (carbohydrate or protein) considered independently fully explains lifespan variation, supporting the notion that multi-dimensional interactions—namely the integration of both carbohydrate and protein intake—have by far the best predictive value in aging studies (Fig. 1) (Lee et al. 2008; Simpson and Raubenheimer 2009).
Figure 1.
Relationship between lifespan and macronutrient consumption. (A) Effects of protein and carbohydrate intake on median lifespan of Canton-S (left) and Dahomey (right) male flies. Protein and carbohydrate consumption are determined from accumulation of radiolabeled food over 24 h. Circle size scales with increasing longevity and the median lifespan at each point of protein and carbohydrate ingestion is shown. Longevity is maximized at an optimal C:P between 10:1 and 20:1, in agreement with previous studies on female Canton-S flies (Lee et al. 2008) that identified the optimum at 16:1 (solid line). Other carbohydrate:protein rails (1:1, 2:1, 4:1, 8:1, and 32:1) are shown as dashed lines. (B) Relationship between median lifespan and carbohydrate:protein ratio (C:P) for results from (A). Median lifespan is shown (solid points) and whiskers represent the interquartile range (1st and 3rd quartiles). Whiskers may be slightly offset horizontally for clarity.
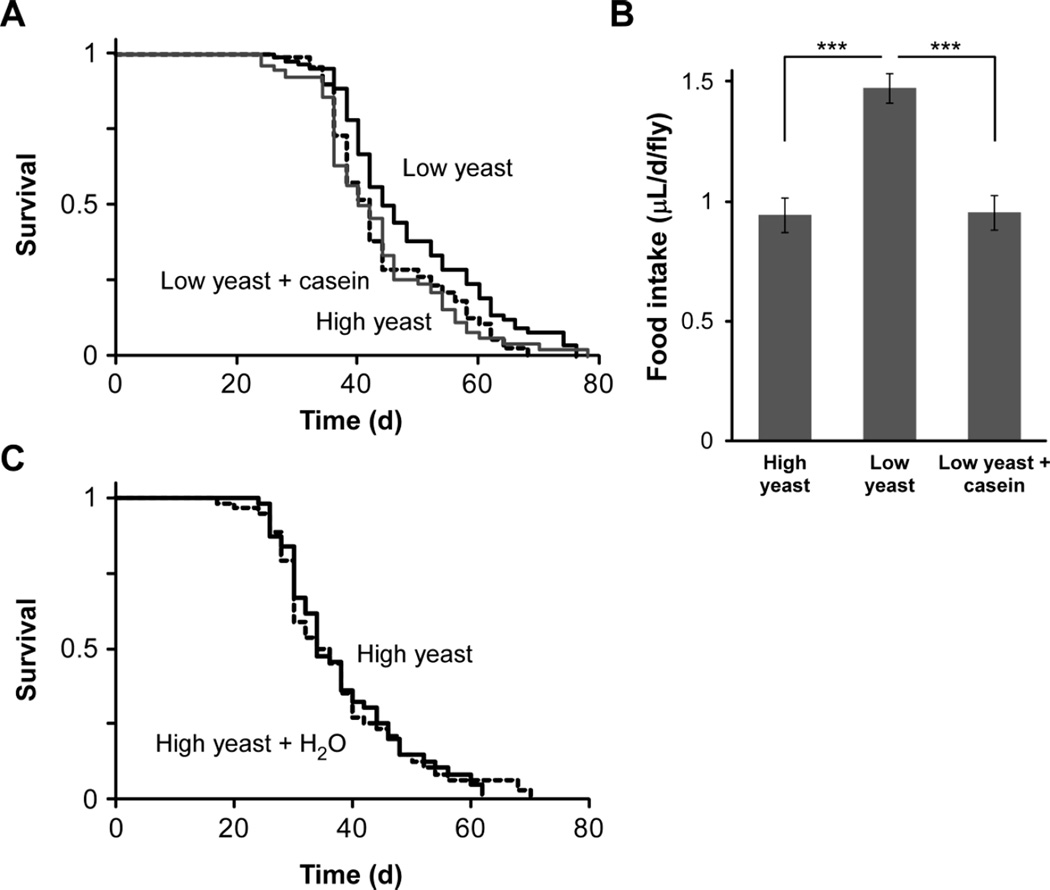
Although it is commonly assumed that the protein content of yeast is its main contribution to fly diet, yeast also contains vitamins, minerals, and carbohydrates. We measured the effect of increased dietary protein on longevity using casein as a pure protein source. Flies on casein-supplemented low yeast diet, matching the protein content of the high yeast diet, showed similar food intake and survival to that of flies on high yeast food (Fig. 2A and 2B). Hence, our data support previous work suggesting that protein is the main component of yeast responsible for lifespan modulation in DR (Grandison et al. 2009; Min and Tatar 2006b), although we cannot rule out that protein plays a lesser role in some of the other diets tested in this study. Importantly, the fact that the effect of yeast extract on both lifespan and feeding can be fully replicated using pure casein strongly indicates that yeast extract is a bona fide protein source for aging studies and that its effects are due to its protein content at the concentrations used in our diets, as opposed to possible nonspecific toxicity or micronutrient availability. This is important to establish, given that several different types of yeast are used in Drosophila DR research (Bass et al. 2007).
Figure 2.
Dietary casein mimics the effect of yeast extract on fly lifespan. (A) Dahomey males on a high yeast diet (5% yeast extract + 5% sucrose) are short-lived compared to those maintained on a low yeast diet (1% yeast extract + 5% sucrose; p = 0.002, log-rank). This effect is mimicked by supplementing the low yeast diet with casein (2%, w/v) to match the final protein content of the high yeast food (p = 0.0015, log-rank). There was no significant difference in lifespan between flies maintained on the high yeast and low yeast + casein diets (p = 0.73, log-rank). N = 84 (low yeast), 76 (high yeast), and 87 (low yeast + casein). (B) Food intake over 24 hours on high yeast, low yeast, and low yeast + casein diets. Ingestion by Dahomey males was determined using the radioisotope labeling method. Increased yeast concentration (high yeast diet) or the equivalent amount of protein (low yeast + casein) results in reduced consumption compared to that on low yeast food (***, p < 0.001; one-way ANOVA followed by Tukey post-hoc test). No difference in food intake was seen between the high yeast and low yeast + casein diets (p > 0.05, Tukey post-hoc). Results shown are averages ± s.d. of N = 6 vials of 10–13 flies each. (C) Observed lifespan differences are not due to dehydration. Lifespan of Dahomey males on a high yeast diet was measured with (N= 61) and without (N = 59) access to an independent water source. No difference in lifespan was observed (p = 0.96, log-rank).
Previously, we showed that changes in food concentration can lead to dehydration stress due to compensatory feeding and lack of access to an independent water source (Ja et al. 2009). By preventing flies from independently regulating their food and water intake, typical laboratory housing regimens may induce morbidity. In contrast, the yeast extract-sucrose-cornmeal regime used here has been shown to provide sufficient hydration (Ja et al. 2009). To confirm that the effect of diet on lifespan is not caused by differences in hydration, we compared the lifespan of flies maintained on one of our high-concentration diets with and without an independent water source. No differences in survival were observed following water supplementation (Fig. 2C). The presence of water does not affect daily food intake in these conditions (Ja et al. 2009). Flies on several other diets were long-lived despite consuming even less food (and therefore less water) than flies on the 5% yeast extract + 5% sucrose food, suggesting that dehydration is also not a factor in these conditions.
3.2. Regulation of food intake
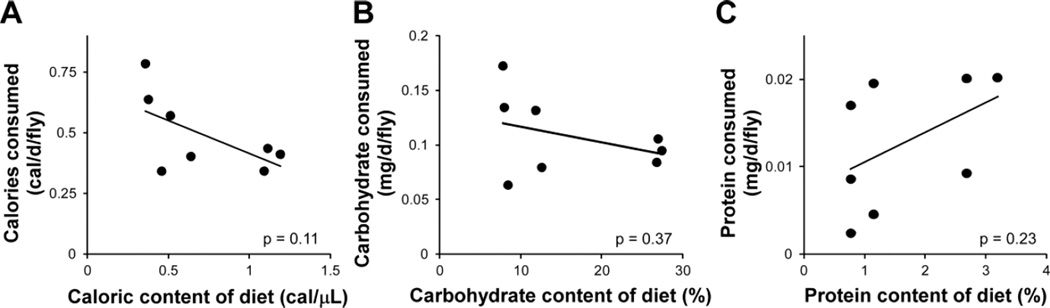
It has been traditionally assumed that fly nutrient intake could be directly inferred from the concentration of components in the diet. Recent observations challenge this assumption by demonstrating that flies can modify their food intake in response to changes in dietary composition (Carvalho et al. 2005; Ja et al. 2009; Lee et al. 2008; Skorupa et al. 2008; Vigne and Frelin 2010). We measured food intake by labeling diets with a radioactive tracer, allowing us to precisely infer consumption over a 24-hour period and determine the relationship between food composition and macronutrient consumption. Measuring intake over prolonged periods is critical since feeding patterns change over time; richer foods tend to be consumed in larger meals in the short term, but this pattern is reversed over a longer period as nutritional targets are met (Simpson et al. 1989). Surprisingly, increasing the food concentration of a component showed no significant correlation with increased intake of that component (Fig. 3), with only protein showing a weak positive trend (no statistical significance) and calories and carbohydrate having, if anything, a negative trend. It is important to note that these trends to single nutritional parameters are largely uninformative since food intake is dependent on both carbohydrate and protein concentration in the diet.
Figure 3.
Relationship between dietary concentration of a nutrient and its consumption. Actual consumption of nutrients can differ greatly from predictions based solely on dietary composition. As (A) caloric and (B) carbohydrate content of the diet increases, actual consumption of calories and carbohydrate, respectively, does not concurrently increase. (C) Protein content of the diet shows a positive trend with actual protein intake, although the correlation is statistically insignificant. Two-tailed p-values for the Pearson correlation coefficient are shown along with a linear trendline. All data are for Canton-S males.
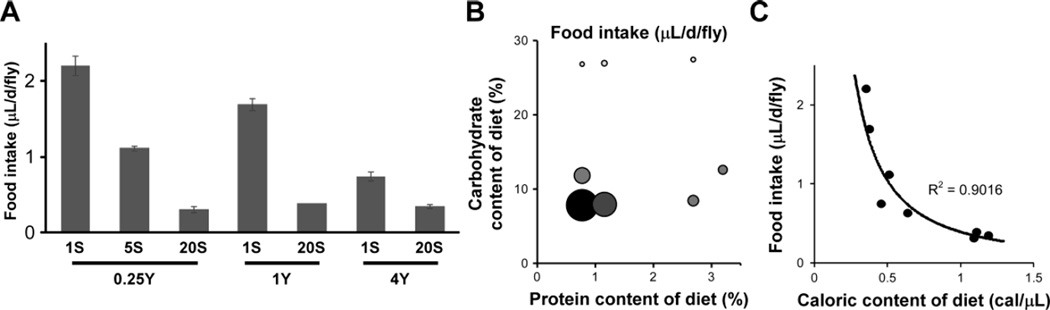
Hence, we next looked at the effect of nutrient concentration on overall intake. Flies reduce their consumption as dietary carbohydrate content is increased at all three levels of yeast tested (Fig. 4A), although the relative reduction is attenuated with increasing yeast concentration. Consumption is also decreased as yeast concentration rises, except at the highest level of sucrose tested (Fig. 4A). This may be due to a floor effect based on how little food (and water) a fly can consume and still survive. A two-dimensional representation clearly shows that both carbohydrate and protein dietary levels dramatically impact food intake (Fig. 4B). Generally, as the food concentration of a component increases, less total food by volume is ingested. The exact magnitude of the effect depends on the specific concentrations of all nutrients present, and it is possible to find conditions—such as in the presence of high concentrations of one nutrient—where varying the level of the other nutrient has little or no effect. Hence, consumption is not well-predicted by dietary carbohydrate or protein content alone (compare food intake to either axis individually in Fig. 4B). Much like lifespan, food intake is best predicted by the concerted variation in both nutrients. Accordingly, we found that food intake is well-described by a non-linear relationship with the caloric content of the diet, possibly because caloric content reflects the contributions of both protein and carbohydrate simultaneously (Fig. 4C). These findings illustrate the remarkable plasticity of Drosophila feeding behavior in response to nutrient availability and stress the importance of accurate measurements of actual intake in any studies involving food manipulation.
Figure 4.
Regulation of feeding rate by dietary nutrient concentration. (A) Food intake over 24 hours on radiolabeled medium (average ± s.d.). Consumption is reduced with increasing levels of sucrose and yeast extract (S and Y, respectively, all shown as w/v), except at the highest concentration of sucrose (20%), where the effect of yeast is muted. All pairwise comparisons are significantly different (p < 0.001, one-way ANOVA followed by Tukey post-hoc test) except between the three diets with 20% sucrose (p > 0.05). (B) Relationship between food intake volume and macronutrient content of the diet. The size and darkness of the circles are scaled with increasing food intake. In general, increasing the concentration of dietary protein or carbohydrate reduces intake. However, conditions can be found where a change in the concentration of a single nutrient does not trigger compensatory feeding. (C) Consumption shows a strong negative correlation with caloric value of the diet, best described by a power model, suggesting an inverse relationship between the two parameters (y = m/xb; m = 0.40, b = 1.39). The power fit and R2 value are shown. When b = 1, the product of food intake and dietary caloric content is a constant, m, which describes a daily caloric intake target. When b > 1, over-feeding occurs as the caloric value of the diet decreases. The non-linear relationship between food intake and dietary caloric content was also tested using Spearman’s correlation coefficient and confirmed a strong negative correlation (r = −0.90, p = 0.005). All results are for Canton-S males.
One shortcoming of feeding assays that rely on food-labeling is that different diets may alter the dynamics of the label—namely its absorption, metabolism, or excretion—which would confound consumption measurements. Cumulative feeding measurements made over two days demonstrate that signal accumulation is reasonably linear and, more importantly, relative food intake is consistent at multiple time points (Supplementary Fig. 2A). Hence, our typical measurements at 24 h are within the initial linear range of label accumulation preceding signal saturation, suggesting that they reflect true consumption (Wong et al. 2008).
Nonetheless, we sought to rule out caveats with label dynamics by using an independent feeding assay that does not rely on food labeling. Similar relative differences in food intake were observed using the CAFE assay (Ja et al. 2007), confirming our observations with radioactive isotopes (Supplementary Fig. 2B). Since the CAFE method employs liquid foods, we used identical recipes to the ones used for all other experiments, with the exception of cornmeal, which is not soluble and was therefore excluded from the media in the CAFE trials. The lack of cornmeal explains the difference in absolute feeding seen between the two techniques (cf. Supplementary Fig. 2A and B), although the relative differences are remarkably reproducible. Indeed, food intake measurements from both assays, which were performed on a common initial population of flies, perfectly fit the same non-linear model when the caloric contribution of cornmeal is accounted for in the diet (Supplementary Fig. 2C).
4. Discussion
Understanding the interactions between diet and longevity is critical for protecting against the rising incidence of age-related metabolic disease. Although it is fairly well-established that dietary restriction (DR) can affect longevity in many animals (Fontana et al. 2010), methods used to implement DR vary greatly across model organisms. In mammals, DR and time-restricted feeding have both been used to improve health throughout the life course (Bordone and Guarente 2005; Hatori et al. 2012). For example, DR has been shown to reduce the incidence of diabetes, cancer, and cardiovascular disease in non-human primates (Colman et al. 2009). However, unequivocal evidence regarding the effects of DR on mammalian lifespan is lacking. Since many of the core metabolic and molecular mechanisms involved in the response to DR are highly conserved, Drosophila has become an extensively used model to investigate the interaction between nutrition and lifespan. In the fruit fly, the effect of individual nutrients on lifespan remains less clear. While most studies agree that high protein intake is pernicious, the role of carbohydrates is controversial. Our observations support a central role for carbohydrates in longevity and highlight the importance of carbohydrate-protein balance for optimal lifespan.
4.1. Carbohydrate and protein ingestion determine longevity in Drosophila
In invertebrates, a convincing body of research argues that the ratio between protein and non-protein intake, rather than calories, is fundamental to the relationship between diet and longevity (Simpson and Raubenheimer 2009). Recent studies in organisms including Drosophila melanogaster, Queensland fruit fly (Bactocera tryoni), the Tephritid fruit fly (Anastrepha ludens), and the field cricket (Teleogryllus commodus) increasingly support the view that consumption of an optimal ratio of carbohydrate to protein, with or without changes in caloric intake, is the key determinant of lifespan (Carey et al. 2008; Fanson et al. 2009; Lee et al. 2008; Maklakov et al. 2009). Using Drosophila, we demonstrate that a relatively high C:P (10:1 to 20:1) maximizes longevity in males (Fig. 1), consistent with previous findings on females reporting an optimal C:P of 16:1 (Lee et al. 2008). Our results indicate that high protein consumption limits longevity, as previously suggested (Mair et al. 2005) and shown convincingly by Lee and colleagues. They also support the view that longevity is controlled by the interplay between carbohydrate and protein intake, in sharp contrast to the claim that carbohydrates have little to no effect in DR-mediated lifespan extension (Grandison et al. 2009; Mair et al. 2005).
The inaccurate conclusions drawn by previous studies regarding the role of carbohydrates may be due to the experimental paradigms used. The practice of using two dietary values chosen arbitrarily from a wide nutritional range inevitably leads to an incomplete picture. Classically, it has been common practice to assume that differences in lifespan observed upon varying yeast concentration in the food result from altered protein consumption. The reality, however, is that modifying the concentration of one food component often leads to a change in actual intake of all nutrients. The broad nutritional range characterized by Lee et al. shows that specific dietary sub-ranges can be found where lifespan is greatly affected by varying protein consumption alone (Lee et al. 2008)—a trend reflected here (Fig. 1A). However, other sub-ranges exist where changes in protein intake have no effect; while others yet show changes in carbohydrate intake at constant protein levels dramatically impacting longevity. Thus, studies drawing general claims from manipulations in a limited, arbitrary range are likely to be incomplete at best. The work of Lee et al. provides the most complete description to date of the influence of diet on longevity; nutritional geometry, the analytical technique employed by the authors, is the most powerful approach available to elucidate the interaction between nutrition and physiological parameters such as lifespan (Lee et al. 2008; Simpson and Raubenheimer 2007).
Our results extend previous findings on females fed liquid diets to males of two different strains maintained in more common rearing conditions. The importance of examining multiple genotypes is increasingly emphasized in aging studies. Although we observe that Dahomey is somewhat less sensitive to dietary changes than Canton-S, we observe identical trends in the dietary modulation of lifespan in both strains (Fig. 1).
Although lower C:P diets shorten lifespan, they may be beneficial for other physiological functions such as growth and fecundity (Lee et al. 2008). This phenomenon is not species-specific—in Queensland fruit flies, high dietary C:P is associated with increased lifespan while low C:P is associated with increased egg production (Fanson et al. 2009). Intermediate C:P is associated with maximal egg-laying over the life course, demonstrating that macronutrient ratio can be optimized for specific physiological functions.
Since the majority of Drosophila DR studies modulate dietary yeast concentration as a surrogate for protein, it is important to consider that yeast is not only a source of amino acids but also vitamins, minerals, and carbohydrates. A recent study on Queensland fruit flies used chemically defined diets to manipulate the dietary amino acid content—while maintaining constant macro- and micronutrient consumption—and showed that the effect of yeast on lifespan can be attributed to changes in dietary C:P (Fanson and Taylor 2012). Our work using casein as a pure protein source (Fig. 2A), taken together with previous studies pointing to the role of amino acids in regulating Drosophila lifespan (Grandison et al. 2009; Min and Tatar 2006b), supports the view that protein is the main component of yeast affecting longevity—at least, at the concentrations used. However, both protein and carbohydrate ingestion must be considered to fully understand the nutritional effects on longevity.
4.2. Food intake measurements are necessary to interpret nutritional effects
Constant food intake in response to changes in nutrient concentration has previously been observed (Grandison et al. 2009; Mair et al. 2005; Min and Tatar 2006a; Wong et al. 2009). The majority of these studies rely on methods with extremely low sensitivity. Counting the fraction of flies eating at a given time (Mair et al. 2005) does not address food intake volume. Although bona fide changes in feeding volume may in some cases be inferred with this assay (Barnes et al. 2008; Wong et al. 2009), provided they are accompanied by sufficiently dramatic changes in feeding frequency, this technique simply cannot be used to rule out a change in food consumption (Fanson et al. 2012). Such claims are uninformative and potentially erroneous. Dye-labeling, on the other hand, does measure intake and can be used to indicate some changes in consumption (Carvalho et al. 2006; Edgecomb et al. 1994), but lacks the sensitivity of radioisotope-labeling and the CAFE assay, and thus is also inappropriate to rule out feeding changes. Moreover, dye-labeling is a short-term technique, since dye excretion begins 30–60 min after initial ingestion (Wong et al. 2009). Dye feeding is therefore not necessarily reflective of long-term consumption.
We used a radioactive tracer as the label for food intake, permitting us to measure consumption under identical conditions to those used for the lifespan trials. While label dynamics—including absorption, elimination, and metabolism—are a theoretical concern, previous studies have shown that accumulation of the radioactive tracer over 24 hours is nearly equivalent to the actual rate of ingestion (Carvalho et al. 2006; Carvalho et al. 2005; Ja et al. 2009). Additionally, it has been shown on multiple diets that only ~5% of the consumed tracer is excreted within the 24-h feeding period (Zeng et al. 2011). We show that tracer accumulation is reasonably linear over two days and relative differences in consumption between diets remain stable during this period (Supplementary Fig. 2A), supporting the reliability of the results we report here using radioisotope labeling. Regardless, for the sake of rigor it is ideal to validate results obtained with food labels using an independent assay. Using the CAFE (Ja et al. 2007) to directly measure the consumption of yeast/sucrose liquid diets, we observe relative changes in food intake consistent with those seen with the radiolabeled media (Supplementary Fig. 2B). Moreover—and irrespective of the feeding assay used—absolute intake volume is predicted with remarkable accuracy when considering the total caloric content of the food (Supplementary Fig. 2C). These results support the validity of the radioactive tracer measurements and rule out that our observations are a product of technique-specific artifacts.
Surprisingly, protein and carbohydrate content of the food are poor predictors of protein and carbohydrate consumption, respectively. Indeed, increasing the protein or carbohydrate content of the diet can in certain cases lead to lower intake of that particular nutrient (Fig. 4). This counterintuitive finding is due to total food intake being dependent on the concentrations of all nutrients, not simply protein or carbohydrate alone. The lack of a correlation between dietary composition and actual nutrient consumption further supports the need for accurate feeding measurements in nutritional studies.
Although caloric consumption does not correlate well with lifespan, calorie content of the diet predicts feeding rate with remarkable accuracy (Supplementary Fig. 2C), suggesting that food intake may be determined, at least in some conditions, by caloric content of the diet. If this turns out to be the case, our measurements during the lifespan trials indicate that young Canton-S males have an intake target of 0.40 cal/d (Fig. 4C), which compares well to the 0.36 cal/d calculated from our recent measurements using CAFE and radiolabeling (Supplementary Fig. 2C). Further studies are required to validate the conditions where flies compensate feeding based on calories regardless of the source of those calories. Protein, for example, likely has a much higher value to females, given the different protein demands for reproduction and general energy needs. Additionally, different sources of protein and carbohydrate may have varied physiological values due to direct effects on feeding behavior (i.e., taste and palatability) and metabolism.
Our findings stress the central point that directly measuring long-term food intake should be an integral element of any studies on food manipulation. Further work will be required to fully elucidate the nutritional drivers of compensatory feeding. Regardless, it is firmly established that flies have the capacity to dramatically adapt their feeding behavior in response to dietary changes. Naturally, it remains a possibility that in some experimental paradigms this phenomenon does not occur. It behooves each investigator manipulating diet to rigorously measure food intake in the various conditions utilized.
4.3. Metabolism and aging
Our results suggest the possibility of a threshold, over which an excessive proportion of carbohydrate intake shortens life (Supplementary Fig. 1B). High dietary sugar content promotes measures of obesity, which are magnified with aging (Skorupa et al. 2008). Bertrand’s rule—historically applied to micronutrients—may also apply to dietary carbohydrate and protein consumption. This rule states that increasing benefits are observed with increased consumption, but excessive intake is toxic (Raubenheimer et al. 2005). In agreement with this, nutrient excess raises triglyceride stores and can contribute to “lipotoxicity” (Birse and Bodmer 2011).
Nutrient stores also correlate with fecundity and survival under starvation (Djawdan et al. 1998; Schwasinger-Schmidt et al. 2012). In Drosophila DR, increased endogenous energy storage and mobilization are associated with longevity (Katewa et al. 2012). DR may constitute a physiological stress, similar to but not as extreme as starvation, and in this context maximal lifespan may only be achievable with sufficient energy.
5. Conclusion
We emphasize the importance of carbohydrate and protein intake for optimal lifespan. Our results support previous work relating longevity with reduced protein and high carbohydrate intake. The complex interplay between nutrient balance and aging highlights the necessity of accurately measuring food intake when conducting lifespan studies. Further studies are required to obtain a clearer understanding of optimal nutrient requirements throughout life to identify preventative dietary interventions.
Supplementary Material
Relationship between median lifespan and carbohydrate:protein ratio (C:P) for Canton-S female flies derived from the results of Lee et al. (Lee et al. 2008). Median lifespan is shown (solid points) and whiskers represent the interquartile range (1st and 3rd quartiles). Although the relationship between longevity and C:P is similar to the one observed in our study (Fig. 1B), Lee et al. measured significantly shortened lifespan overall. The highest C:P used was a pure sucrose diet. This point is shown offset to the right of the plot. (B) Model of lifespan variation with dietary C:P. The effect of diet on longevity can deviate from the model depending on actual consumption, which is depicted when lifespan is plotted against actual protein and carbohydrate ingestion (cf. Fig. 1).
Food intake measured by radioactive tracer accumulation is likely to reflect actual consumption. (A) Cumulative feeding over 48 h shows near-linear isotope accumulation (left panel). Male Dahomey flies (2–4 d old) were habituated for 4 days on the indicated yeast extract/sucrose diets (Y/S, shown as w/v) in the standard cornmeal/agar base and subsequently switched to radiolabeled food for 0.5, 1, or 2 days. Shown are averages ± s.d. of N = 6 vials of 4–9 flies each. All dietary groups were significantly different from each other at each time point (p < 0.001, one-way ANOVA followed by Tukey post-hoc test). Importantly, the relative differences in feeding between diets remain consistent over the same period (right panel). Between days 1 and 2, only the relative feeding between flies on the 4/20 and 1/1 diets changes significantly (p < 0.05, t-test), and the change in relative feeding is less than 11%. (B) Similar differences in feeding between diets are observed using the CAFE assay with liquid food lacking cornmeal. Due to the lack of cornmeal, the 1/1 diet is a true 5-fold dilution of the 5/5 diet, and it is clear that flies are unable to compensate by consuming 5 times more food. In contrast, flies perfectly compensate between the 1/5 and 4/20 diets—consumption of the diluted food was 4.3 times greater than that of the 4-fold concentrated 4/20 diet. Results are averages ± s.d. of N = 5 CAFE chambers housing 3–4 flies each. All pairwise comparisons are significantly different (p < 0.001, one-way ANOVA followed by Tukey post-hoc test) except between the 1/1 and 1/5 diets (p > 0.05). (C) Food intake is accurately predicted by dietary caloric content. Aggregate data set from the different assays in (A) and (B). CAFE, squares; radioisotope-labeling assay, circles. The relationship between consumption and dietary caloric content is well-modeled by a power curve (y = m/xb; m = 0.43, b = 0.71, R2 = 0.88). The b value less than 1 suggests that underfeeding occurs as the caloric value of the diet decreases. This is likely due to the inability of flies on the 1/1 (CAFE) diet to consume sufficient volume to reach their caloric target. When feeding on this diet is excluded from the fit, the power model shows an almost perfect inverse relationship between caloric content and consumption (m = 0.36, b = 1.03). The power fit and R2 value of this model are shown.
Highlights.
Both high carbohydrate and low protein consumption are required for maximal longevity.
Diet concentration does not predict actual nutrient ingestion due to compensatory feeding.
Volume of food consumption may be driven by a target caloric intake.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1R15AG027749, T.B.; R01 AG031337 and AG038012, P.K.; and R00AG030493 and R21DK092735, W.W.J.), National Science Council (NSC 100-2311-B-007-006-, H.-D.W.), The Ellison Medical Foundation (P.K. and W.W.J.), and the Glenn Foundation for Medical Research/American Federation for Aging Research (P.K. and W.W.J.).
Abbreviations
- C:P
Carbohydrate:protein ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes AI, Wigby S, Boone JM, Partridge L, Chapman T. Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc. Biol. Sci. 2008;275:1675–1683. doi: 10.1098/rspb.2008.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MD. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse RT, Bodmer R. Lipotoxicity and cardiac dysfunction in mammals and Drosophila. Crit. Rev. Biochem. Mol. Biol. 2011;46:376–385. doi: 10.3109/10409238.2011.599830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Carey JR, Harshman LG, Liedo P, Muller HG, Wang JL, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit, fly Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr. Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat. Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djawdan M, Chippindale AK, Rose MR, Bradley TJ. Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiol. Zool. 1998;71:584–594. doi: 10.1086/515963. [DOI] [PubMed] [Google Scholar]
- Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J. Exp. Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- Fanson BG, Taylor PW. Protein:carbohydrate ratios explain life span patterns found in Queensland fruit fly on diets varying in yeast:sugar ratios. Age. 2012;34:1361–1368. doi: 10.1007/s11357-011-9308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson BG, Weldon CW, Perez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8:514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Fanson BG, Yap S, Taylor PW. Geometry of compensatory feeding and water consumption in Drosophila melanogaster. J. Exp. Biol. 2012;215:766–773. doi: 10.1242/jeb.066860. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J. Gerontol. 1988;43:B5–B12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Zid BM, Mak EM, Brummel T, Benzer S. Water- and nutrientdependent effects of dietary restriction on Drosophila lifespan. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18633–18637. doi: 10.1073/pnas.0908016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, Perrimon N, Melov S, Kapahi P. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 2012;16:97–103. doi: 10.1016/j.cmet.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EB. A new standard food medium. Drosophila Inf. Serv. 1960;34:117–118. [Google Scholar]
- Lushchak OV, Gospodaryov DV, Rovenko BM, Glovyak AD, Yurkevych IS, Klyuba VP, Shcherbij MV, Lushchak VI. Balance between macronutrients affects life span and functional senescence in fruit fly Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:118–125. doi: 10.1093/gerona/glr184. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklakov AA, Hall MD, Simpson SJ, Dessmann J, Clissold FJ, Zajitschek F, Lailvaux SP, Raubenheimer D, Bonduriansky R, Brooks RC. Sex differences in nutrient-dependent reproductive ageing. Aging Cell. 2009;8:324–330. doi: 10.1111/j.1474-9726.2009.00479.x. [DOI] [PubMed] [Google Scholar]
- Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Exp. Gerontol. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Drosophila diet restriction in practice: do flies consume fewer nutrients? Mech. Ageing Dev. 2006a;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech. Ageing Dev. 2006b;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Lee KP, Simpson SJ. Does Bertrand's rule apply to macronutrients? Proc. Biol. Sci. 2005;272:2429–2434. doi: 10.1098/rspb.2005.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwasinger-Schmidt TE, Kachman SD, Harshman LG. Evolution of starvation resistance in Drosophila melanogaster: measurement of direct and correlated responses to artificial selection. J. Evol. Biol. 2012;25:378–387. doi: 10.1111/j.1420-9101.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Barton Browne L, van Gerwen ACM. The patterning of compensatory sugar feeding in the Australian sheep blowfly. Physiol. Entomol. 1989;14:91–105. [Google Scholar]
- Simpson SJ, Raubenheimer D. Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Macronutrient balance and lifespan. Aging. 2009;1:875–880. doi: 10.18632/aging.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P, Frelin C. Food presentation modifies longevity and the beneficial action of dietary restriction in Drosophila. Exp. Gerontol. 2010;45:113–118. doi: 10.1016/j.exger.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Wong R, Piper MD, Blanc E, Partridge L. Pitfalls of measuring feeding rate in the fruit fly Drosophila melanogaster. Nat Methods. 2008;5:214–215. doi: 10.1038/nmeth0308-214. author reply 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Du Y, Alberico T, Seeberger J, Sun X, Zou S. Gender-specific prandial response to dietary restriction and oxidative stress in Drosophila melanogaster. Fly (Austin) 2011;5:174–180. doi: 10.4161/fly.5.3.15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between median lifespan and carbohydrate:protein ratio (C:P) for Canton-S female flies derived from the results of Lee et al. (Lee et al. 2008). Median lifespan is shown (solid points) and whiskers represent the interquartile range (1st and 3rd quartiles). Although the relationship between longevity and C:P is similar to the one observed in our study (Fig. 1B), Lee et al. measured significantly shortened lifespan overall. The highest C:P used was a pure sucrose diet. This point is shown offset to the right of the plot. (B) Model of lifespan variation with dietary C:P. The effect of diet on longevity can deviate from the model depending on actual consumption, which is depicted when lifespan is plotted against actual protein and carbohydrate ingestion (cf. Fig. 1).
Food intake measured by radioactive tracer accumulation is likely to reflect actual consumption. (A) Cumulative feeding over 48 h shows near-linear isotope accumulation (left panel). Male Dahomey flies (2–4 d old) were habituated for 4 days on the indicated yeast extract/sucrose diets (Y/S, shown as w/v) in the standard cornmeal/agar base and subsequently switched to radiolabeled food for 0.5, 1, or 2 days. Shown are averages ± s.d. of N = 6 vials of 4–9 flies each. All dietary groups were significantly different from each other at each time point (p < 0.001, one-way ANOVA followed by Tukey post-hoc test). Importantly, the relative differences in feeding between diets remain consistent over the same period (right panel). Between days 1 and 2, only the relative feeding between flies on the 4/20 and 1/1 diets changes significantly (p < 0.05, t-test), and the change in relative feeding is less than 11%. (B) Similar differences in feeding between diets are observed using the CAFE assay with liquid food lacking cornmeal. Due to the lack of cornmeal, the 1/1 diet is a true 5-fold dilution of the 5/5 diet, and it is clear that flies are unable to compensate by consuming 5 times more food. In contrast, flies perfectly compensate between the 1/5 and 4/20 diets—consumption of the diluted food was 4.3 times greater than that of the 4-fold concentrated 4/20 diet. Results are averages ± s.d. of N = 5 CAFE chambers housing 3–4 flies each. All pairwise comparisons are significantly different (p < 0.001, one-way ANOVA followed by Tukey post-hoc test) except between the 1/1 and 1/5 diets (p > 0.05). (C) Food intake is accurately predicted by dietary caloric content. Aggregate data set from the different assays in (A) and (B). CAFE, squares; radioisotope-labeling assay, circles. The relationship between consumption and dietary caloric content is well-modeled by a power curve (y = m/xb; m = 0.43, b = 0.71, R2 = 0.88). The b value less than 1 suggests that underfeeding occurs as the caloric value of the diet decreases. This is likely due to the inability of flies on the 1/1 (CAFE) diet to consume sufficient volume to reach their caloric target. When feeding on this diet is excluded from the fit, the power model shows an almost perfect inverse relationship between caloric content and consumption (m = 0.36, b = 1.03). The power fit and R2 value of this model are shown.