Abstract
BACKGROUND
More than 80,000 postmenopausal breast cancer patients in the US each year are estimated to begin a five-year course of aromatase inhibitors (AIs) to prevent recurrence. AI-related arthralgia (joint pain and/or stiffness) may contribute to nonadherence, but longitudinal data are needed on arthralgia risk factors, trajectories, and background in postmenopause.
OBJECTIVES
To describe one-year arthralgia trajectories and baseline covariates among AI patients and a postmenopausal comparison group.
METHODS
Patients initiating AIs (n=91) were surveyed at the time of AI initiation and at six repeated assessments over one year. A comparison group of postmenopausal women without breast cancer (n=177) completed concomitantly-timed surveys. Numeric rating scales (0–10) were used to measure pain in eight joint pair groups (bilateral fingers, wrists, elbows, shoulders, hips, knees, ankles, and toes). Poisson regression models were used to analyze arthralgia trajectories and risk factors.
RESULTS
By week six, the AI-initiating group had more severe arthralgia than did the comparison group (ratio of means=1.8, (95% CI 1.2–2.7, p=0.002), adjusting for baseline characteristics. Arthralgia then worsened further over a year in the AI group. Menopausal symptom severity and existing joint-related comorbidity at baseline among women initiating AI were associated with more severe longitudinal arthralgia.
CONCLUSIONS
Patients initiating AI should be told about the timing of arthralgia over the first year of therapy, and advised that it does not appear to resolve over the course of a year. Menopausal symptoms and joint-related comorbidity at AI initiation can help identify patients at risk for developing AI-related arthralgia.
Keywords: Breast neoplasms, arthralgia, joint pain, aromatase inhibitors, postmenopause, longitudinal studies
INTRODUCTION
Randomized clinical trials have shown that aromatase inhibitors (AIs) improve breast cancer disease-free survival compared with tamoxifen among postmenopausal women with early-stage, hormone-receptor-positive breast cancer.1 Based on these findings AIs have, since 2006, become standard of care for adjuvant endocrine therapy in postmenopausal women. The American Cancer Society estimates that there are nearly three million breast cancer survivors in the United States (U.S.), and more than 226,000 new cases diagnosed each year.2 Each year more than 80,000 women in the U.S. are estimated to begin a course of five or more years of AI as adjuvant endocrine therapy.
AI-associated arthralgia (joint pain and/or stiffness) is of serious concern, in that it may be undermining effective cancer treatment in these patients. Adverse event data from clinical trials and observational studies have indicated that AIs are associated with arthralgia in some women.3;4 Arthralgia may negatively impact patients' health-related quality of life (HRQoL) and cause suboptimal adherence and/or discontinuation.3;5 Over half of patients taking adjuvant endocrine therapy (tamoxifen or AI) were found to discontinue early in one study.6 In another study, 24% discontinued AI specifically due to musculoskeletal symptoms.7 AI adherence is known to decline over time, with nearly 40% suboptimal adherence by the third year of AI therapy.8
Findings to date on arthralgia incidence, risk factors, and trajectories have been inconsistent.4;9–11 Prevalence estimates for AI-associated arthralgia vary widely, from 10–61%.9;12;13 Some estimates come from physician reports, while others are based on retrospective or small prospective studies lacking consistent definitions of arthralgia, lacking control groups, lacking HRQoL assessment, or nested within clinical trials. There is conflicting information about arthralgia risk factors.4;9 Associations between patient characteristics, treatments, and pain outcomes are highly variable across studies,4;9;11 reflecting variation in measurement and design methodologies.
Few prospective studies in clinical practice settings have followed arthralgia longitudinally along with other relevant clinical, demographic, and patient-reported outcomes. This prospective cohort was designed to assess arthralgia, clinical and patient-reported arthralgia predictors, and other clinical and patient-reported outcomes including HRQoL longitudinally using consistent measurement criteria to enable comparisons across groups and against future studies. The objectives of this study were to a) quantify differences in arthralgia trajectories between women initiating AIs and a comparison group of postmenopausal women without cancer over a 52-week observation period; b)identify baseline demographic, clinical, and HRQoL arthralgia risk factors (such as age, employment status, depression, or menopausal symptom severity); and c) identify arthralgia risk factors including cancer-relevant clinical characteristics among the subgroup of women taking AIs.
METHODS
Study design and setting
We conducted a prospective cohort study (NCT00954564) with 52 weeks' follow up per participant. The study was approved by the Vanderbilt University Institutional Review Board. To measure patient-reported data, we used paper questionnaires administered at baseline, 2, 4, 6, 8, 12, and 52 weeks. Clinical data were abstracted from electronic medical records. Study personnel at Vanderbilt Ingram Cancer Center, Vanderbilt Institute for Medicine and Public Health, and Vanderbilt Women's Health Research conducted screening, enrollment, data collection, and analysis. Data were collected using paper surveys and managed using Research Electronic Data Capture.14
Participants
We recruited participants into two groups: women initiating AIs and a comparison group of postmenopausal women who had never been diagnosed with breast cancer. Because arthralgia is common in postmenopausal women regardless of breast cancer or endocrine therapy,15 the comparison group was selected to assess the background rate of arthralgia in menopause, using the same measurement scale and timeframe. Starting in 2009, participants were recruited either by their treating physician at a Vanderbilt University Medical Center clinic or by community recruitment in greater Nashville, TN (flyers, email listserv, Metro Transit Authority bus ads). Participants in either group had to be female, postmenopausal (self-report of at least 12 months without a menstrual period, unrelated to surgery or medication), age 35 to 90, and have self-reported performance status16≤ three. AI group participants had to initiate anastrazole, exemestane, or letrozole within 30 days of baseline assessment. Comparison group respondents had to never have been told they had breast cancer by a doctor. Respondents were ineligible if currently undergoing treatment for another non-breast cancer, unable to provide informed consent, non-English speaking, pregnant, or had metastatic disease. Screening was either by telephone or self-administered via http://www.breastcancersurvivorstudy.com. Follow-up comprised phone and mail contact to assist participants in staying on schedule. Women were considered lost to follow-up if they missed more than two surveys in a row and could not be reached after six attempts.
Variables
We measured clinical, demographic, and HRQoL variables using existing validated scales when such instruments were available. Clinical characteristics included body mass index (BMI), time in menopause, analgesic use, and comorbidities collected via patient self-report. We categorized comorbidities as joint-related or not. The surveys also assessed depression,17 performance status,18 menopausal symptoms,19 physical function,20 sleep disturbance,21 exercise frequency,22 demographics (age, race, ethnicity, marital/partnered status, employment status, education, and income), comorbidities, and arthralgia. Among the AI group, we collected information on cancer treatments including surgery, radiation, chemotherapy, and stage at diagnosis. Table 1 shows how categorical variables were dichotomized for analysis.
Table 1.
Baseline demographic and clinical characteristics, overall and by group
| Overall (N=268)a | Comparison Group (n=177) | Aromatase Inhibitor Group (n=91) | Statistical test, P-valueb | |
|---|---|---|---|---|
| Age in years, mean (SD) | 58.7 (8.0) | 56.7 (6.2) | 62.2 (9.5) | −4.92 (<.0001) |
| Race, n (%) | ||||
| White, non-Hispanic | 231 (86) | 150 (85) | 81 (89) | |
| Black, non-Hispanic | 22 (8) | 19 (11) | 3 (3) | |
| Hispanic | 3 (1) | 2 (1) | 1 (1) | 5.56 (<0.001) |
| Other | 2 (1) | 1 (1) | 1 (1) | |
| Missing/Unknown/Refused | 10 (4) | 5 (3) | 5 (5) | |
| Married or partnered, n (%) | 171 (67) | 105 (62) | 66 (76) | 5.14 (0.026) |
| Employed at least part time, n (%) | 195 (77) | 145 (87) | 50 (57) | 28.84 (<.0001) |
| Education > 12 years, n (%) | 221 (86) | 156 (91) | 65 (77) | 8.48 (0.006) |
| Low income (< $40k), n (%) e | 36 (17) | 20 (13) | 16 (27) | 6.07 (0.023) |
| Body mass index, mean (SD) | 29.0 (6.4) | 28.8 (6.2) | 29.4 (6.8) | −0.75 (0.453) |
| Years since last menstrual period, median (IQR) | 8 (3–16) | 7 (3–14) | 13 (3–24) | 11337 (0.005) |
| Weak analgesic use, n (%)g | 232 (87) | 158 (89) | 74 (81) | 3.26 (0.088) |
| Strong analgesic use, n (%) h | 50 (19) | 14 (8) | 36 (40) | 39.67 (<.0001) |
| Joint-related comorbidity, n (%)c | 118 (44) | 80 (45) | 38 (42) | 0.29 (0.606) |
| Major depressive disorder (PHQ-2 ≥ 3), n (%)d | 21 (8) | 11 (6) | 10 (11) | 1.9 (0.229) |
| Sweat-inducing exercise often, n (%) | 68 (26) | 45 (26) | 23 (26) | 0 (1.000) |
| Active performance status (patient-reported), n (%) f | 172 (67) | 105 (62) | 66 (76) | 5.14 (0.026) |
| Menopausal symptoms (FACT-ES), median (IQR) (higher = better health)i | 60 (52–65) | 59 (52–65) | 60 (53–66) | 12216 (0.232) |
| Physical function (PROMIS-PF), median (IQR) (higher = better function) j | 51 (45–55) | 53 (48–55) | 48 (40–53) | 8859.0 (<.0001) |
| Sleep disturbance (PROMIS-Sleep SF-8), mean (SD) (higher = more disturbance)k | 51.5 (8.9) | 51.6 (9.1) | 51.3 (8.4) | 0.27 (0.784) |
| Arthralgia as mean pain: 0–10 (PRAI), median (IQR)l | 0 (0–1) | 0 (0–1) | 0 (0–1) | 10820 (0.162) |
| Arthralgia as sum pain: 0–160 (PRAI), median (IQR)l | 5 (0–15) | 5 (1–15) | 4 (0–16) | 10810 (0.157) |
| AI group only: chemotherapy | - | 30 (34) | - | |
| AI group only: radiation | - | 51 (58) | - | |
| AI group only: surgery | - | 86 (98) | - | |
| AI group only: stage II or greater at diagnosis | - | 32 (37) | - |
Notes: SD = standard deviation, IQR = interquartile range (25th–75th percentiles)
Overall percentage reported, unless otherwise noted
If means are reported, variable was normally distributed; test statistic for differences by group is T-test; p-value is Satterthwaite if unequal variances, pooled if equal variances. If medians are reported, variable was not normally distributed; test statistic is Wilcoxon signed-rank test. For categorical variables, test statistic is Chi-square, or Fisher Exact test if cell counts were low.
Joint-related comorbidity =“yes” if patient indicated she had osteoarthritis, rheumatoid arthritis, psoriatic arthritis, lupus, gout, ankylosing spondylitis, fibromyalgia, osteoporosis, osteopenia, or Sjogren's syndrome.
Major depressive disorder as assessed by the Patient Health Questionnaire-2 (PHQ-2)17
Variable dichotomized according to 2012 Women Infants and Children program eligibility income cutoff, corresponding with the question on household income falling into response category of less than $36,000 per year.
Patient-reported Eastern Cooperative Oncology Group (ECOG) status (Basch)16, with 0 or 1=active, and >1=not active, from 0–4 scale.
Weak analgesics included aspirin or non-steroidal anti-inflammatory agents
Strong analgesics included weak or strong narcotics.
Functional Assessment of Cancer Therapy – Excerpt of Endocrine Symptom Subscale (FACT-ES)19
Patient-Reported Measurement Information System (PROMIS) Physical Function short form20
PROMIS Sleep Disturbance short form21
Patient-Reported Arthralgia Inventory, adapted from Regional Pain Scale23
We measured arthralgia using the Patient-Reported Arthralgia Inventory (PRAI), an adaptation of the Regional Pain Scale23 that we are in the process of validating, as there is currently no validated tool to measure arthralgia in this clinical population. The PRAI follows best practices in patient-reported outcome measurement, using a numeric rating scale of 0–10 for pain over the preceding seven days in eight joint pair groups: bilateral fingers, wrists, elbows, shoulders, hips, knees, ankles, and toes. We scored the PRAI by computing the sum joint pain rating across all joints, resulting in a composite arthralgia score ranging from 0–160. For ease of interpretation, mean pain per joint (0–10) is reported.
Statistical methods
T-tests, Wilcoxon signed rank tests, Chi-square tests, and Fisher's exact tests were used to compare baseline characteristics and arthralgia composite scores between the AI and comparison groups. We used Poisson regression with generalized estimating equations (GEE) to regress longitudinal joint pain sum scores on group, time since enrollment, time*group interaction, and other baseline covariates. The arthralgia composite score was the sum score across all joints for an individual at a given week, with the Poisson model appropriately accounting for log number of joints with observed scores as the exposure offset. Such a structure allows modeling mean arthralgia score per joint, adjusting for relevant covariates. An exchangeable correlation structure was assumed for repeated measures, given that GEE methods are robust for misspecification of the covariance structure.
Baseline clinical and demographic variables selected for the model were those factors hypothesized to be associated with greater arthralgia over time: baseline composite arthralgia score, increased BMI, joint-related comorbidity, depression, less exercise, more severe menopausal symptoms, and greater sleep disturbance. We also included factors by which the groups differed (see Table 1) to account for potential confounding by these factors: age, years in menopause, education, marital status, income, employment, performance status, strong analgesic use, and physical function. Several baseline variables were missing observations for ~5% of the sample, resulting in ~30% missing data for at least one covariate. The median (continuous variables) or mode (binary/categorical variables) was imputed for these covariates such that the model included all patients with outcome data. Given that missing data was <5% per variable, more elaborate imputation strategies such as multiple imputation yielded similar results (results not shown).
A secondary and exploratory question was to identify arthralgia risk factors among the AI group. Because certain clinical characteristics of interest (chemotherapy, radiation, surgery, and stage at diagnosis) were specific to the AI group, a subgroup analysis with a restricted sample size was conducted. We used a Poisson regression model with GEE to regress longitudinal arthralgia composite scores on all those baseline factors included in the previous model, plus clinical characteristics specific to the AI group. Statistical analyses were conducted using R version 2.15.2 and SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
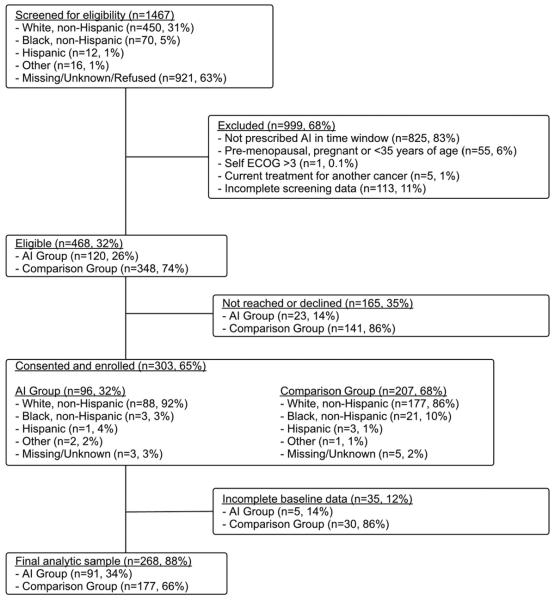
Figure 1 shows cohort screening and enrollment. The analytic sample comprised data from 91 women enrolled in the AI group and 177 in the comparison group. Attrition did not differ by group over time (p=0.99); attrition rates overall were 17% at week 2, 23% at week 4, 28% at week 6, 34% at week 8, 37% at week 12, and 47% at week 52.
Figure 1.
Cohort screening and enrollment flow chart
Baseline clinical, demographic, and HRQoL characteristics are shown in Table 1. At baseline the AI and comparison groups did not differ onthe outcome of composite arthralgia score. Factors on which the groups did differ were used to adjust the multivariable models. Lack of differences between groups led us to exclude race, ethnicity, and surgery in the multivariable models.
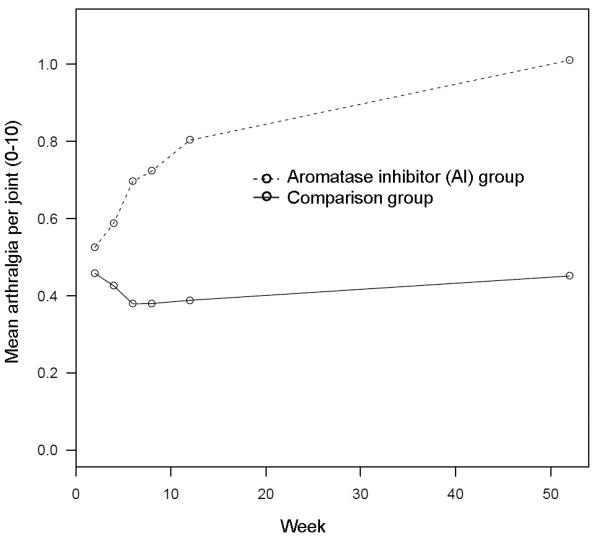
Figure 2 shows model-based mean trajectories of arthralgia by group over 52 weeks' observation. Model-based trajectories diverged by group at week six (see Figure 2). The trajectories also showed an increase in arthralgia severity over the 52 weeks' per-participant observation. Table 2 shows the estimated mean arthralgia per joint by group, corresponding to the trajectories in Figure 2. Table 3 shows the ratio of mean arthralgia per joint of AI versus comparison group, with corresponding CI and p-values (where values of 1.0 are consistent with no difference), showing a significant difference between groups starting at week six.
Figure 2.
Model-based mean composite arthralgia severity score by week and group, adjusted to medians and modes of numeric and categorical covariates, respectively
Table 2.
Model-based mean arthralgia per joint by week and group, given median baseline arthralgia
| Week | Estimate (95% confidence interval) | |
|---|---|---|
| Comparison group | 2 | 0.46(0.33,0.64) |
| 4 | 0.43(0.31,0.60) | |
| 6 | 0.38(0.28,0.53) | |
| 8 | 0.38(0.28,0.53) | |
| 12 | 0.39(0.28,0.55) | |
| 52 | 0.46(0.32,0.65) | |
|
| ||
| Aromatase inhibitor group | 2 | 0.52(0.30,0.91) |
| 4 | 0.59(0.34,1.03) | |
| 6 | 0.69(0.39,1.22) | |
| 8 | 0.72(0.40,1.30) | |
| 12 | 0.80(0.46,1.38) | |
| 52 | 1.00(0.57,1.76) | |
Table 3.
Ratio of mean arthralgia per joint of AI versus comparison group by week
| Week | Group difference estimate (95% confidence interval) | p-value |
|---|---|---|
| 2 | 1.1(0.8,1.7) | 0.548 |
| 4 | 1.4(0.9,2.0) | 0.123 |
| 6 | 1.8(1.2,2.7) | 0.002 |
| 8 | 1.9(1.2,2.9) | 0.004 |
| 12 | 2.0(1.4,3.1) | <0.001 |
| 52 | 2.2(1.5,3.3) | <0.001 |
Table 4 shows exponentiated parameter estimates from the multivariable model including both groups, giving the ratio of mean arthralgia per joint between each level of categorical variables, and multiplicative mean change for each one-unit change in continuous variables. No baseline demographic or clinical covariates (other than baseline arthralgia) were significantly associated with longitudinal arthralgia.
Table 4.
Multivariable model of 52-week arthralgia trajectories and risk factors
| Effect |
|
95% Confidence Interval | p | |
|---|---|---|---|---|
| Age (continuous) | 1.00 | (0.99,1.02) | 0.77 | |
| Physical function (continuous) | 0.99 | (0.97,1.01) | 0.18 | |
| Depression | 1.04 | (0.79,1.38) | 0.77 | |
| Body mass index (continuous) | 0.99 | (0.97,1.01) | 0.19 | |
| Sleep disturbance (continuous) | 1.01 | (1.00,1.02) | 0.18 | |
| Menopausal symptoms (continuous) | 0.99 | (0.97,1.00) | 0.08 | |
| At least some college | 1.35 | (0.84,2.19) | 0.22 | |
| Low income | 0.99 | (0.69,1.42) | 0.95 | |
| Employed | 1.09 | (0.82,1.45) | 0.54 | |
| Active performance status | 1.03 | (0.72,1.48) | 0.87 | |
| Joint-related comorbidity | 1.26 | (0.97,1.63) | 0.08 | |
| Married or partnered | 0.94 | (0.73,1.20) | 0.62 | |
| Exercise often | 0.75 | (0.54,1.05) | 0.09 | |
| Strong analgesic use | 0.79 | (0.58,1.08) | 0.14 | |
| Baseline arthralgia severity (continuous) | 1.49 | (1.32,1.69) | <0.01 | |
| AI Group: baseline (Group * week 4) | 1.21 | (0.98,1.50) | 0.08 | |
| Group * week 6 | 1.60 | (1.29,1.99) | <0.01 | |
| Group * week 8 | 1.67 | (1.23,2.27) | <0.01 | |
| Group * week 12 | 1.81 | (1.35,2.42) | <0.01 | |
| Group *week 52 | 1.95 | (1.37,2.77) | <0.01 |
Notes: All effect variables are baseline values coded dichotomously except as otherwise noted.
Exponentiated β estimates represent ratio of mean arthralgia per joint between each level of categorical variables, and multiplicative mean change for each 1-unit change in continuous variables. Estimates for intercept and main effects of week and group were included in the model but are not shown above.
As shown in Table 5, a multivariable model among only the AI group showed that baseline severity of menopausal symptoms (ratio of means 0.97 [0.95,0.99]) and presence of joint-related comorbidity (ratio of means 1.71 [1.12,2.61], including osteoarthritis, rheumatoid arthritis, psoriatic arthritis, lupus, gout, ankylosing spondylitis, fibromyalgia, osteoporosis, osteopenia, or Sjogren's syndrome) were associated with increased longitudinal arthralgia severity. We did not observe differences in arthralgia severity according to cancer treatment or stage.
Table 5.
Multivariable model of 52-week arthralgia trajectories and risk factors, AI group only
| Effect |
|
95% Confidence Interval | p | |
|---|---|---|---|---|
| Age (continuous) | 0.99 | (0.97,1.02) | 0.44 | |
| Physical function (continuous) | 0.99 | (0.97,1.01) | 0.47 | |
| Depression | 0.86 | (0.57,1.30) | 0.47 | |
| Body mass index (continuous) | 1.00 | (0.97,1.02) | 0.85 | |
| Sleep disturbance (continuous) | 0.99 | (0.97,1.02) | 0.62 | |
| Less severe menopausal symptoms (continuous) | 0.97 | (0.95,0.99) | 0.04 | |
| At least some college | 1.42 | (0.83,2.41) | 0.20 | |
| Low income | 1.13 | (0.75,1.70) | 0.56 | |
| Employed | 1.02 | (0.58,1.77) | 0.95 | |
| Active performance status | 1.12 | (0.63,2.00) | 0.70 | |
| Joint-related comorbidity | 1.71 | (1.12,2.61) | 0.01 | |
| Married or partnered | 0.94 | (0.62,1.42) | 0.77 | |
| Exercise often | 0.73 | (0.46,1.14) | 0.17 | |
| Strong analgesic use | 0.71 | (0.49,1.03) | 0.07 | |
| Chemotherapy | 0.99 | (0.69,1.41) | 0.93 | |
| Radiation | 0.73 | (0.43,1.23) | 0.23 | |
| Stage II or greater at diagnosis | 0.89 | (0.60,1.32) | 0.56 | |
| Baseline arthralgia severity (continuous) | 1.22 | (0.92,1.63) | 0.17 | |
| Week 4 | 1.10 | (0.92,1.31) | 0.32 | |
| Week 6 | 1.28 | (1.08,1.52) | <0.01 | |
| Week 8 | 1.35 | (1.03,1.77) | 0.03 | |
| Week 12 | 1.43 | (1.14,1.78) | <0.01 | |
| Week 52 | 1.80 | (1.34,2.41) | <0.01 |
Notes: see notes for Table 4; ibid.
With regard to adherence, over one year, 78 (83%) of the women initiating AI were reported as still taking either the AI they initiated or another AI; 11 (12%) were reported switched to tamoxifen, and 5 (5%) were reported as having discontinued adjuvant endocrine therapy entirely.
DISCUSSION
Prospective patient-reported longitudinal data are needed to understand the course of arthralgia secondary to AI treatment. We charted arthralgia trajectories over the first year of AI treatment, in comparison to a background rate of arthralgia among postmenopausal women without breast cancer. By week six, women in the AI group experienced a statistically significant increase in arthralgia severity. This finding is consistent with other recent studies.24;25 Our trajectory analysis indicated that after this 6-week worsening, arthralgia does not appear to resolve, and in fact worsens further over the first year of AI therapy. Future research should extend the observation period beyond a year and should also address the clinical meaningfulness of observed increases in arthralgia severity, using larger cohorts to enable more precise estimates of the outcome over time.26 Future research should also compare the AIs with each other and with tamoxifen, as switching appears to be a strategy for managing AI-related arthralgia, yet little is known about the relative benefits of switching with regard to arthralgia severity.
We found not only that over time women taking AI had more severe arthralgia than women in the comparison group, but also that women taking AI with more severe menopausal symptomsor existing joint-related conditions at the time of AI initiation had worse arthralgia over time. Targeted intervention in these at-risk groups may improve AI adherence. To date no single treatment strategy has emerged as satisfactory.3 While analgesics may be efficacious,13 it is feared that opioids may mask the pain associated with actual degeneration of joints.3 Soy/genestein may alleviate symptoms by raising estrogen levels, but in doing so it interferes with the efficacy of AIs.27 Vitamin D,28;29 calcium, and bisphosphonates30 have been proposed for study on the basis of bone loss underlying AI-associated arthralgia. Other therapies include antidepressants, hypnotics, duloxetine, gabapentin, anti-irritants, omega essential fatty acids, steroidal injections, targeted heat, weight loss, yoga, resistance exercise, cardiovascular aerobics, and water aerobics.3;31;32 More research assessing arthralgia, HRQoL, adherence, and switching should be done to aid in the development of effective intervention strategies.33–35
Our findingsalso suggest that arthralgia may be part of a cluster of menopausal/vasomotor symptoms including hot flashes, sweats, vaginal discharge, loss of interest in sex, diarrhea, headaches, and irritability; these factors should also be assessed as potential AI secondary effects. None of the following showed evidence of being arthralgia risk factors: age, race, physical function, education, income, employment, performance status, body mass index, marital/partnered status, exercise frequency, or sleep disturbance. This is consistent with other recent studies that have lacked evidence for a clear set of arthralgia predictors including biomarkers.24;25 We propose that menopausal symptom severity and the presence of joint-related conditions at AI initiation should be points of focus, and that future studies of arthralgia in this population measure these potentially important predictive factors.
Our findings have specific implications for clinical practice. First, women initiating AI should be advised that arthralgia worse than what one could expect in postmenopause appears to develop six weeks post AI-initiation, and that it does not appear to resolve over the first year. They should be monitored for arthralgia development and/or worsening; follow-up visits could be timed so that patients are seen within this interval to address emergent arthralgia. Women initiating AI therapy should be evaluated for the presence of baseline menopausal symptoms and joint-related conditions and advised about increased arthralgia risk; these patients should also be targeted in future AI adherence interventions. Finally, all patients initiating AI should be warned they may develop arthralgia, and strategies for managing arthralgia or switching should be discussed to reduce nonadherence.
Study limitations include selection bias as evidenced by differences in baseline characteristics between the AI and comparison groups. These differences were likely due to the fact that more AI group participants than comparison group participants were recruited in-clinic versus in the community, respectively. To minimize the impact of these differences on our trajectory estimates, we included the differing factors as adjustment variables in our multivariable models.36 With few African-American women enrolled in the AI group, lack of variation led us to exclude race as a risk factor, and comparisons in trajectories by race were not possible; oversampling of African-American women is warranted to detect potential differences by race. Missing outcome data due to loss to follow up was also a potential source of bias, however, attrition rates did not differ by group. Lastly, the subset model of the AI group lacked sufficient sample size and was therefore overfitted; future research is needed to confirm findings from this model.
By characterizing arthralgia in women initiating AI and its background rate in women without breast cancer, these findings can help oncologists advise their patients about what to expect over time. Improved understanding and management of AI-associated arthralgia are keys toward improving AI adherence and minimizing cancer recurrence.3;37 Further research is needed not only on longer-term arthralgia trajectories, but also on the relationships of arthralgia with HRQoL, AI adherence, recurrence, and mortality in women taking AIs.
ACKNOWLEDGEMENTS
We gratefully acknowledge the BCAT Study Team: Tonya Brown, Jessica Islam, Danielle LaMorte, Ashley Pasquariello, Angel Sherrill, Bradley Shields, and Angela Zito. We gratefully acknowledge programming support from Joseph Burden, Gregory Todd Salter, Mikhail Zemmel, and Scott Sobecki, contributions to screening provided by the Vanderbilt Cancer Trials Information Program, and statistical expertise provided by Chiu-Lan (Heidi) Chen and Jonathan Schildcrout. This study was conducted in collaboration with the Vanderbilt Ingram Cancer Center, Vanderbilt Internal Medicine, Vanderbilt Institute for Medicine and Public Health, Vanderbilt Division of Rheumatology and Immunology, Vanderbilt Epidemiology Center, Vanderbilt Bioinformatics, Vanderbilt Biostatistics, Vanderbilt REACH for Survivorship, Meharry Medical College/Nashville General Hospital, and Northwestern University.
FUNDING
This study was funded by the American Cancer Society (119475-MRSG-10-169-01-PCSM), Vanderbilt Institute for Clinical and Translational Research (UL1RR024975-01 and UL1TR000011 from National Institutes of Health [NIH]), and 5K12HD43483-10 (Hartmann, PI) from NIH Building Interdisciplinary Careers in Women's Health Research.
Footnotes
FINANCIAL DISCLOSURES The authors have no conflicts of interest to declare.
REFERENCES
- (1).Buzdar A. Anastrozole as adjuvant therapy for early-stage breast cancer: implications of the ATAC trial. Clin Breast Cancer. 2003;4(Suppl 1):S42–S48. doi: 10.3816/cbc.2003.s.014. [DOI] [PubMed] [Google Scholar]
- (2).American Cancer Society [Accessed June 08. 2012]; Report available at http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/docum ent/acspc-033876.pdf.
- (3).Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107(2):167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- (4).Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111(2):365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Cella D, Fallowfield L, Barker P, et al. Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for early breast cancer. Breast Cancer Res Treat. 2006;100(3):273–284. doi: 10.1007/s10549-006-9260-6. [DOI] [PubMed] [Google Scholar]
- (6).van Herk-Sukel MP, van de Poll-Franse LV, Voogd AC, et al. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat. 2010;122(3):843–851. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- (7).Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- (9).Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25(25):3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- (10).Moy B, Tu D, Pater JL, et al. Clinical outcomes of ethnic minority women in MA.17: a trial of letrozole after 5 years of tamoxifen in postmenopausal women with early stage breast cancer. Ann Oncol. 2006;17(11):1637–1643. doi: 10.1093/annonc/mdl177. [DOI] [PubMed] [Google Scholar]
- (11).Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9(9):866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- (12).Donnellan PP, Douglas SL, Cameron DA, et al. Aromatase inhibitors and arthralgia. J Clin Oncol. 2001;19(10):2767. [PubMed] [Google Scholar]
- (13).Presant CA, Bosserman L, Young T, et al. Aromatase inhibitor-associated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer. 2007;7(10):775–778. doi: 10.3816/CBC.2007.n.038. [DOI] [PubMed] [Google Scholar]
- (14).Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. Am J Public Health. 1984;74(6):574–579. doi: 10.2105/ajph.74.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23(15):3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- (17).Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- (18).Basch E, Iasonos A, Barz A, et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007;25(34):5374–5380. doi: 10.1200/JCO.2007.11.2243. [DOI] [PubMed] [Google Scholar]
- (19).Fallowfield LJ, Leaity SK, Howell A, et al. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55(2):189–199. doi: 10.1023/a:1006263818115. [DOI] [PubMed] [Google Scholar]
- (20).Rose M, Bjorner JB, Becker J, et al. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2008;61(1):17–33. doi: 10.1016/j.jclinepi.2006.06.025. [DOI] [PubMed] [Google Scholar]
- (21).Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- (23).Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol. 2003;30(2):369–378. [PubMed] [Google Scholar]
- (24).Helzlsouer KJ, Gallicchio L, Macdonald R, et al. A prospective study of aromatase inhibitor therapy, vitamin D, C-reactive protein and musculoskeletal symptoms. Breast Cancer Res Treat. 2012;131(1):277–285. doi: 10.1007/s10549-011-1729-2. [DOI] [PubMed] [Google Scholar]
- (25).Robidoux A, Rich E, Bureau NJ, et al. A prospective pilot study investigating the musculoskeletal pain in postmenopausal breast cancer patients receiving aromatase inhibitor therapy. Curr Oncol. 2011;18(6):285–294. doi: 10.3747/co.v18i6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zlowodzki M, Bhandari M. Outcome measures and implications for sample-size calculations. J Bone Joint Surg Am. 2009;91(Suppl 3):35–40. doi: 10.2106/JBJS.H.01602. [DOI] [PubMed] [Google Scholar]
- (27).Ju YH, Doerge DR, Woodling KA, et al. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29(11):2162–2168. doi: 10.1093/carcin/bgn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Prieto-Alhambra D, Javaid MK, Servitja S, et al. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat. 2011;125(3):869–878. doi: 10.1007/s10549-010-1075-9. [DOI] [PubMed] [Google Scholar]
- (29).Prieto-Alhambra D, Javaid MK. Aromatase inhibitor-induced arthralgia: is vitamin D deficiency responsible? Maturitas. 2011;69(1):3–4. doi: 10.1016/j.maturitas.2011.01.001. [DOI] [PubMed] [Google Scholar]
- (30).Boonen S, Haentjens P, Vandenput L, et al. Preventing osteoporotic fractures with antiresorptive therapy: implications of microarchitectural changes. J Intern Med. 2004;255(1):1–12. doi: 10.1046/j.0954-6820.2003.01258.x. [DOI] [PubMed] [Google Scholar]
- (31).Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16(3):223–234. doi: 10.1016/j.breast.2007.01.011. [DOI] [PubMed] [Google Scholar]
- (32).Thorne C. Management of arthralgias associated with aromatase inhibitor therapy. Curr Oncol. 2007;14(Suppl 1):S11–S19. doi: 10.3747/co.2007.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dent SF, Gaspo R, Kissner M, et al. Aromatase inhibitor therapy: toxicities and management strategies in the treatment of postmenopausal women with hormone-sensitive early breast cancer. Breast Cancer Res Treat. 2011;126(2):295–310. doi: 10.1007/s10549-011-1351-3. [DOI] [PubMed] [Google Scholar]
- (34).Fallowfield L. Acceptance of adjuvant therapy and quality of life issues. Breast. 2005;14(6):612–616. doi: 10.1016/j.breast.2005.08.012. [DOI] [PubMed] [Google Scholar]
- (35).Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pocock SJ, Assmann SE, Enos LE, et al. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21(19):2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- (37).Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]