Abstract
Objectives
The impact of hot flashes on sleep is of great clinical interest, but results are inconsistent, especially when both hot flashes and sleep are measured objectively. Using objective and subjective measurements, we examined the impact of hot flashes on sleep by inducing hot flashes with a gonadotropin-releasing hormone agonist (GnRHa).
Methods
The GnRHa leuprolide was administered to 20 healthy premenopausal volunteers without hot flashes or sleep disturbances. Induced hot flashes were assessed objectively (skin-conductance monitor) and subjectively (daily diary) during one-month follow-up. Changes from baseline in objective (actigraphy) and subjective sleep quality (Pittsburgh Sleep Quality Index [PSQI]) were compared between women who did and did not develop objective hot flashes, and, in parallel analyses, subjective hot flashes.
Results
New-onset hot flashes were recorded in 14 (70%) and reported by 14 (70%) women (80% concordance). Estradiol was universally suppressed. Objective sleep efficiency worsened in women with objective hot flashes and improved in women without objective hot flashes (median decrease 2.6%, increase 4.2%, p=0.005). Subjective sleep quality worsened more in those with than without subjective hot flashes (median increase PSQI 2.5 vs. 1.0, p=0.03). Objective hot flashes were not associated with subjective sleep quality, nor were subjective symptoms linked to objective sleep measures.
Conclusions
This experimental model of induced hot flashes demonstrates a causal relationship between hot flashes and poor sleep quality. Objective hot flashes result in worse objective sleep efficiency, while subjective hot flashes worsen perceived sleep quality.
Keywords: hot flashes, vasomotor symptoms, sleep, quality-of-life, depressive symptoms, actigraphy
Introduction
Perimenopausal and early postmenopausal women report experiencing worse sleep quality more commonly than do premenopausal women of a similar age.1, 2 Although it might be expected that poor sleep quality is a direct result of hot flashes, data addressing this issue are inconsistent (Table 1). When both hot flashes and sleep are reported subjectively, hot flashes are consistently linked to poor sleep quality.1–8 In contrast, reports of the relationship between recorded hot flashes and reported sleep quality are much less consistent,8–10 as are studies examining the association of reported and objectively recorded (i.e., skin-conductance monitor) hot flashes with recorded sleep (polysomnography or actigraphy). Findings across studies of recorded sleep show increased wakefulness and reduced sleep efficiency in some,7, 8, 11–14 but not all,6, 10, 15, 16 reports.
Table 1.
Data on association of subjectively reported and objectively recorded hot flashes with subjectively reported and objectively recorded sleep measures.
| Reported hot flashes |
Recorded hot flashes |
|
|---|---|---|
| Reported sleep | ↑ chronic insomnia diagnosis3 ↓ sleep quality1,2,4–8 |
↓ sleep quality8,10 No association9 |
| Recorded sleep |
Polysomnography studies No association6, 15 Actigraphy studies ↑ wakefulness7 ↑ sleep fragmentation7,8 ↓ sleep efficiency8 |
Polysomnography studies ↑ wakefulness11–14 ↓ sleep efficiency12, 13 No association10,16 Actigraphy studies ↓ sleep efficiency8 |
Unlike laboratory settings,17 there is discrepancy between reported and recorded measures of hot flashes in ambulatory settings,9, 18–20 which occurs because psychological factors influence subjective reporting19 and recall of nocturnal events varies.18 While both under- and over-reporting of daytime hot flashes relative to objective assessments is seen,18, 19 nighttime hot flashes are consistently underreported relative to objective measures.9, 18 Perception of sleep quality and recorded sleep are also poorly correlated.21–23 Thus, while it might be expected that hot flash and sleep associations may differ depending on the way in which these core menopause-related symptoms are measured, only one study8 has examined these associations using both objective and subjective measures of hot flashes and sleep. Results of this study8 show that both reported and recorded hot flash measures were associated with both reported and recorded sleep measures. While this study is important because it is the only one to measure both hot flashes and sleep concurrently using subjective and objective methods, its cross-sectional design does not allow for causal inference.
Hot flashes are linked with reduced quality-of-life in midlife women,24 either directly because hot flashes cause daytime discomfort, or indirectly, through their effects on sleep1, 2, 4–6 and mood,25–28 each of which independently contributes to poor quality-of-life.24,29 Understanding the specific causal contribution of hot flashes to the development of poor sleep quality requires precise information on the temporal association between symptoms. The observational and primarily cross-sectional design of previous studies prevents causal inference about the specific impact of hot flashes on sleep quality, thereby limiting our ability to devise clinical strategies to treat these commonly co-occurring symptoms and improve quality-of-life.
We used a gonadotropin-releasing hormone agonist (GnRHa) which induces hypoestrogenism and mimics the hormonal changes of the menopause transition to isolate the specific effect of new-onset hot flashes on sleep quality. Hot flashes are induced rapidly on GnRHa in approximately two-thirds of women,30–34 providing a natural control group of women who undergo the same hormone changes but who do not develop hot flashes. We compared within-woman changes in each sleep parameter from before to one month after initiation of GnRHa between those who did and did not develop new-onset hot flashes, as recorded objectively and reported subjectively. We secondarily examined the effect of hot flashes on quality-of-life using the same approach.
Materials and Methods
Twenty premenopausal healthy volunteers received one open-label intramuscular injection of the depot GnRHa leuprolide 3.75-mg/day and were then followed for one month to determine whether they developed hot flashes, at which time post-treatment assessments were completed. Pre- and post-treatment measures included sleep quality and quality-of-life questionnaires, a 24-hour period of objective recording of hot flashes, two nights of actigraphic recording of sleep, and continuous reporting of hot flashes on a daily diary throughout the study period. Serum estradiol was measured weekly on GnRHa to confirm that ovarian function was suppressed and that differences in symptom response were not explained by differences in estradiol levels.
Study participants
Healthy premenopausal women 18–45 years-old with monthly menstrual cycles and no hot flashes, sleep disorders, depression, or other psychiatric illnesses were enrolled in the study. The absence of hot flashes at baseline was established both subjectively using a 7-day hot flash diary and objectively using a sternal skin-conductance monitor for 24 hours (Biolog ambulatory recorder, UFI, Morro Bay, CA). Participants were carefully screened to select healthy women with regular menstrual cycles and evidence of ovulation (mid-luteal serum progesterone >3 ng/dL). Women with major depression and significant depressive symptoms were excluded using a standardized psychiatric diagnosis instrument (Patient Health Questionnaire, PHQ-9).35 Eligible women were also required to have a score ≤16 on the clinician-rated Montgomery-Åsberg Depression Rating Scale (MADRS),36,37 a threshold suggesting the absence of clinically relevant depressive symptoms,38 and no previous psychotic symptoms, suicide attempt, or diagnosis of bipolar disorder, psychosis, anorexia nervosa, or alcohol/substance-use disorders.
Other exclusion criteria were pregnancy, lactation, abnormal prolactin, thyroid function, liver function, or renal function, primary sleep disorders (sleep apnea, periodic limb movement syndrome, narcolepsy), and use of centrally active medications (e.g., antidepressants, benzodiazepines, corticosteroids) or medications known to suppress hot flashes (e.g., birth control preparations, serotonergic agents, gabapentin).
Study Procedures
Participants received one open-label intramuscular injection of the depot GnRHa leuprolide 3.75-mg/day during the mid-luteal phase of their menstrual cycle to rapidly induce hypoestrogenism39–41 and maintain ovarian suppression for a one-month period.32, 33, 42 Serum estradiol, estrone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) were obtained immediately prior to and after 1, 2, and 4 weeks on GnRHa therapy.
Following GnRHa administration, participants were monitored closely for a one-month period to determine the onset of hot flashes using daily diaries and a 24-hour period of skin-conductance monitoring completed at the end of the study. The Biolog monitor is used widely and has good reliability and validity.43 Consistent with standard procedures, recorded hot flashes were defined as an increase in sternal skin conductance of ≥2µmho during a 30-second period using a 20-second lock-out period.44
Sleep patterns were recorded for 2 consecutive nights in each participant’s home environment with the Actiwatch-Score (Mini Mitter Co., Inc, Bend, OR). Actigraphy data were used to measure sleep efficiency (percent of time spent asleep between bedtime and wake-up time), time spent awake after sleep-onset (WASO), and sleep-onset latency (minutes) before and after one month on GnRHa. An awake state was defined for each 30-second epoch when the total activity count was greater than a sensitivity threshold of 80. Subjective sleep quality was documented using the widely used Pittsburgh Sleep Quality Index (PSQI; range 0–21, higher score indicates poorer quality sleep).45
Quality-of-life was assessed with the Menopause-Specific Quality-of-Life Questionnaire (MENQOL), which measures 4 quality-of-life domains (vasomotor, psychosocial, physical, and sexual) over the past month that are averaged to generate the overall MENQOL score.46 Daytime sleepiness and depressive symptoms, two domains of well-being commonly affected by hot flashes and sleep quality, were also measured using the Epworth Sleepiness Scale (ESS; range 0–24, higher score indicates more sleepiness),47 the self-reported Beck Depression Inventory (BDI; range 0–63, higher score indicates more depressive symptoms),48 and the clinician-rated MADRS (range 0–60, higher score indicates more depressive symptoms).36, 37
For safety monitoring purposes, participants were contacted by phone 2 and 3 months after receiving GnRHa to confirm that menses had resumed, that hot flashes had not subsequently developed, and, where previously present, that hot flashes had resolved. All participants provided written informed consent for study procedures, which were approved by the Partners HealthCare Institutional Review Boards.
Hormone Assays
Estradiol and estrone were measured using liquid chromatography, tandem mass spectrometry (Mayo Clinic, Rochester, NY),49, 50 in order to achieve accuracy and precision in the low range seen on endocrine therapy. The interassay coefficient of variation (CV) ranges for estradiol and estrone in the low range studied was 8.6% and 8.7%, respectively.49 The lower level of detectability for estradiol was 10 pg/ml.
Serum LH and FSH levels were measured in the Massachusetts General Hospital Reproductive Endocrine Laboratory (RELab) using a two-site monoclonal non-isotopic system according to the manufacturer’s directions (Axsym, Abbott Laboratories, Abbott Park, IL, USA), as previously described.51, 52 LH and FSH are expressed in IU per liter as equivalents of the Second International Pituitary Standard (80/552 and 92/510). The interassay coefficients of variation (CVs) for LH are 5.3, 5.5, and 7.4% for quality control sera containing 5.6, 26.2, and 69.0 IU/liter, respectively, and the interassay CVs for FSH are 5.2, 4.6 and 5.5% for quality control sera containing 7.4, 15.6 and 42.0 IU/liter, respectively.
Data Analysis
Analyses were conducted first using an objective hot flash classification and separately using subjective hot flash measurements. Baseline characteristics and serum levels of reproductive hormone at each time point were compared between women with and without hot flashes using Fisher’s exact tests for categorical variables and the non-parametric Wilcoxon rank-sum testing for continuous variables. For the purpose of analysis, serum estradiol levels below the level of detectability were set at 5 pg/ml (the midpoint between 0 and the lower level of detectability).
Analyses comparing changes in recorded and reported sleep parameters were conducted using the non-parametric Wilcoxon rank-sum test. For each recorded sleep measure (sleep efficiency, WASO, sleep-onset latency), pre-and post-treatment values were calculated using the average from the two actigraphic studies conducted at each time point. The association of hot flash frequency with each sleep and quality of life outcome was estimated using Spearman correlations. Because of differences in serum estradiol at week 2 between hot flash groups, ordinal regression models using quartile ranking for the sleep endpoints were used to adjust for the baseline sleep measure and change in estradiol. The same approaches were used to analyze the effect of hot flashes on quality-of-life measures. Analyses were conducted using STATA software (College Station, Texas) and SAS version 9.2 (Cary, NC). Statistical significance was established using a two-sided alpha of 0.05.
Results
Study Participants
After one month on GnRHa therapy, new-onset hot flashes were reported by 14 (70%) participants, beginning after a mean of 8.4 ± 2.0 days on leuprolide. Hot flashes were recorded objectively in 14 (70%) participants. Among women with hot flashes, the median number of hot flashes reported per day over the one-month period was 2.5 (IQR 1.3–5.1), and the median number of hot flashes recorded during the 24-hour monitoring period was 5.5 (IQR 2.0–9.1). There was 80% concordance between the number of women reporting hot flashes and the number of women whose hot flashes were recorded, with hot flashes recorded in 12 (85.7%) of those who reported hot flashes and in 2 (33.3%) of those who did not report hot flashes. Among those with hot flashes, the median number of hot flashes reported at night was 1.0 (IQR 0.6–1.9) and the median number recorded during the night was 2.2 (IQR 0.8–3.4).
For all participants together, the mean age was 30.6 ± 8.9 years, mean BMI was 27.1 ± 4.6 kg/m2, and half were African-American or Hispanic. There were no statistically significant differences between hot flash groups in baseline clinical characteristics, regardless of whether hot flashes were recorded (data not shown) or reported (Table 2), except for a trend association between age and reported hot flashes (p=0.07). However, analyses were not adjusted for age because age did not correlate with any sleep or quality-of-life endpoints.53
Table 2.
Study population characteristics at baseline according to subsequent development of subjectively reported hot flashes on gonadotropin-releasing hormone agonist.
| Subjective hot flash classification | ||
|---|---|---|
| Hot flashes reported (n=14) |
No hot flashes reported (n=6) |
|
| Age, mean ± SD | 32.8 ± 9.0 | 25.5 ± 6.7 |
| BMI (kg/m2) | 27.3 ± 4.7 | 26.6 ± 4.7 |
| Prior pregnancy, N (%) | 7 (50.0%) | 2 (33.3%) |
| Objective VMS, N (%) | 12 (85.7%) | 2 (33.3%) |
| Race | ||
| Non-Hispanic White, N (%) | 8 (57.1%) | 3 (50.0%) |
| Hispanic or African-American, N (%) | 6 (42.9%) | 3 (50.0%) |
| Marital Status | ||
| Married/living with partner | 4 (28.6%) | 0 |
| Single/divorced/separated | 10 (71.4%) | 6 (100.0%) |
| Employment Status | ||
| Working part time/full time | 11 (78.6%) | 3 (50.0%) |
| Student/Other | 3 (21.4%) | 3 (50.0%) |
| Education | ||
| College graduate or higher | 5 (35.7%) | 2 (33.3%) |
| High school graduate/college student | 9 (64.3%) | 4 (66.7%) |
|
Median (interquartile range) |
Median (interquartile range) |
|
| Baseline Objectively Measured Sleep Patterns | ||
| Sleep Efficiency (%) | 86.7 (85.1 to 90.4) | 89.3 (80.6 to 92.2) |
| Latency (minutes) | 13.8 (11.0 to 21.0) | 14.5 (6.0 to 24.5) |
| WASO (minutes) | 25.5 (20.0 to 42.0) | 24.5 (19.0 to 32.5) |
| Baseline Perceived Sleep Quality | ||
| PSQI Score | 2.5 (1.0 to 3.0) | 3.5 (2.0 to 5.0) |
| Baseline Menopause Quality-of-Life Measures | ||
| MENQOL Overall | 1.3 (1.2 to 1.4) | 1.2 (1.1 to 1.3) |
| MENQOL Psychological | 1.1 (1.0 to 1.6) | 1.4 (1.0 to 2.0) |
| MENQOL Physical | 1.2 (1.0 to 1.4) | 1.3 (1.0 to 1.6) |
| MENQOL Sexual | 1.0 (1.0 to 1.0) | 1.0 (1.0 to 1.0) |
| MENQOL Vasomotor | 1.0 (1.0 to 2.0) | 1.0 (1.0 to 1.0) |
| BDI Score | 1.0 (0 to 2.0) | 0 (0 to 1.0) |
| MADRS Score | 1.0 (0 to 1.0) | 0 (0 to 1.0) |
| ESS Score | 6.5 (3.0 to 8.0) | 3.5 (2.0 to 7.0) |
Normally distributed data are presented as mean ± standard deviation, non-normal data are presented as median (interquartile range), and categorical data are presented as N (%).
WASO = wake-time after sleep-onset; PSQI = Pittsburgh Sleep Quality Index; MENQOL = Menopause Quality of Life; MADRS = Montgomery-Åsberg Depression Rating Scale; BDI = Beck Depression Inventory; ESS = Epworth Sleepiness Scale
Baseline measures of reported sleep quality, recorded sleep patterns, quality-of-life, daytime sleepiness, and depressive symptoms did not differ between groups, regardless of whether hot flashes were reported (Table 2) or recorded (data not shown). In general, participants reported good sleep quality (median PSQI score = 3) and had good sleep efficiency (median 86.8%), minimal sleep-onset latency (median 14.0 minutes), and spent little time awake after sleep-onset (median WASO 25.3 minutes) on actigraphic assessment. Overall, participants had good quality-of-life, minimal daytime sleepiness, and low levels of depressive symptoms.
Effects of GnRHa on Reproductive Hormones
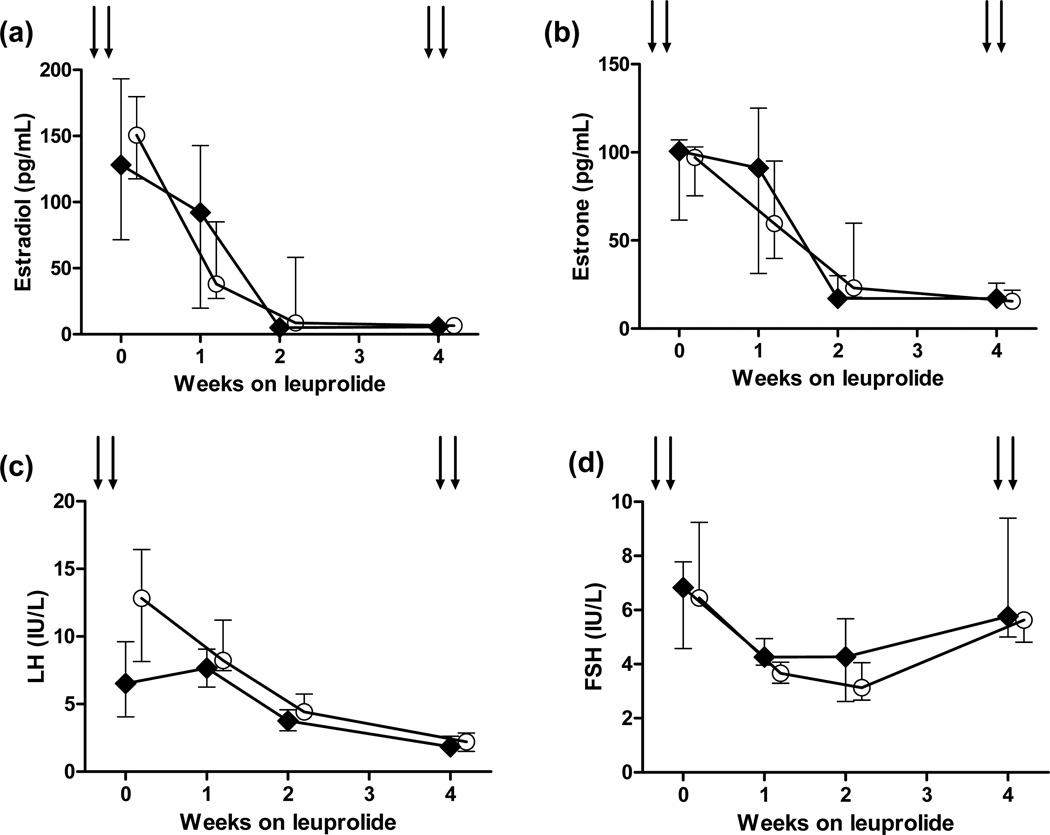
LH and FSH levels were rapidly suppressed on GnRHa (Figure 1c, 1d), consistent with the limited gonadotropin flare expected with mid-luteal GnRHa administration.39–41 Serum levels of reproductive hormones (estradiol, estrone, LH, FSH) did not differ between hot flash groups, regardless of whether subjective (Figure 1a–d) or objective hot flash categorizations (data not shown) were used. The only exception was the estradiol level at week 2 (median 5.0 vs. 8.5 pg/ml, p=0.03, for those reporting vs. not reporting hot flashes; Figure 1a). Within one month on GnRHa (Figure 1a), all women had serum estradiol levels <15 pg/ml (median 6.5, interquartile range [IQR] 6–11.5, pg/mL vs. median 11, IQR 8–12, pg/mL, for those with versus without hot flashes, p=0.26).
Figure 1.
Changes in serum estradiol, estrone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) for women who reported developing hot flashes versus those who did not secondary to treatment with the gonadotropin-releasing hormone agonist leuprolide. Data are shown as medians and interquartile ranges.
Effects of Recorded Hot Flashes on Recorded Sleep Patterns, Reported Sleep Quality, and Quality-of-Life
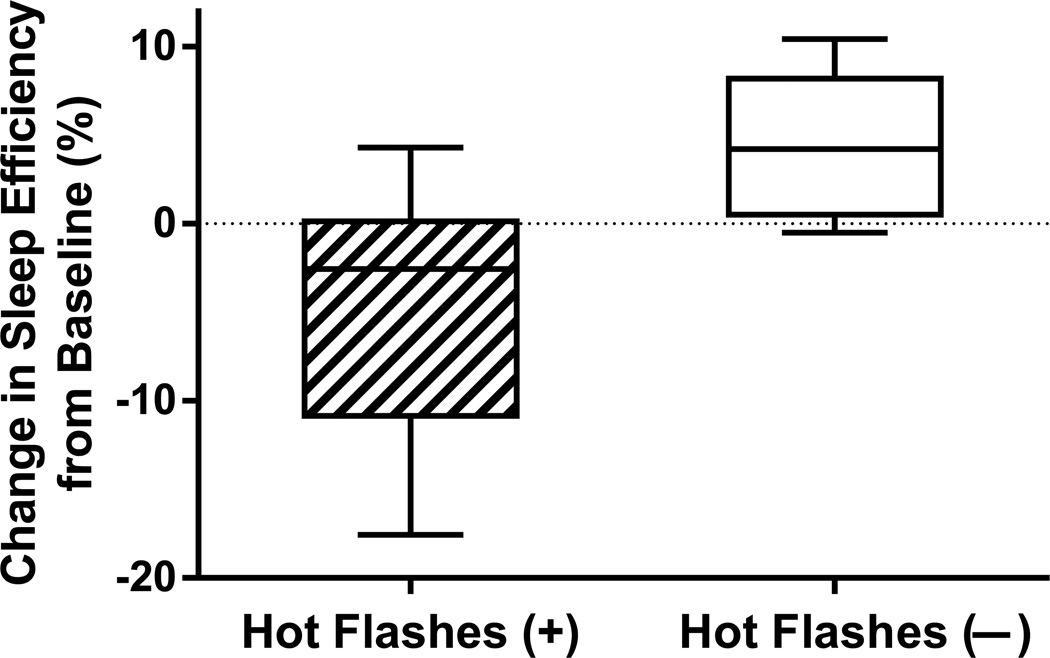
Using recorded hot flashes (Figure 2 and Table 3), women who developed hot flashes had worsening of recorded sleep efficiency, as indicated by a median reduction in sleep efficiency of 2.6 % (IQR 0.9% to 10.2% lower), whereas women who did not develop recorded hot flashes had a median increase/improvement in sleep efficiency of 4.2% (IQR 0.8% to 7.5% higher). Therefore, the difference between medians in the change in sleep efficiency was 6.8% between groups (p=0.005). The within-woman change in sleep efficiency between hot flash groups remained statistically significant after adjusting for changes in estradiol levels on GnRHa (p=0.01).
Figure 2.
Change in actigraphy-measured sleep efficiency from before (“pre”) to 4 weeks on treatment (“post”) with the gonadotropin-releasing hormone agonist leuprolide in women who did and did not develop objectively recorded hot flashes (p=0.005). Reduction in sleep efficiency indicates worsening.
Table 3.
Changes in objectively recorded sleep patterns, subjectively reported sleep quality, quality-of-life, and related domains from baseline to one month on gonadotropin-releasing hormone agonist therapy according to subsequent development of hot flashes, reported separately as medians (interquartile range) by (a) objectively recorded and (b) subjectively reported hot flashes, and (c) Spearman correlation with the number of hot flashes recorded or reported.
| (a) Objective hot flashes | (b) Subjective hot flashes | ||||||
|---|---|---|---|---|---|---|---|
| Hot flashes recorded (n=14) |
No hot flashes recorded (n=6) |
(c) Spearman correlation with # of hot flashes recorded ‡ |
Hot flashes reported (n=14) |
No hot flashes reported (n=6) |
(c) Spearman correlation with # of hot flashes reported ‡ |
||
| Objectively recorded sleep | |||||||
| Change in sleep efficiency† | −2.6 (−10.2 to −0.9) a | 4.2 (0.8 to 7.5) | 0.76 a | −1.8 (−7.7 to 1.2) | 1.9 (−2.5 to 7.5) | 0.47 c | |
| Change in sleep-onset latency | 7.0 (−0.5 to 16.5) | −1.3 (−4.0 to 3.0) | 0.32 | 7.0 (−2.5 to 16.5) | 3.0 (−3.0 to 3.0) | 0.35 | |
| Change in WASO | 2.0 (−5.0 to 9.0) b | 7.3 (4.0 to 11.5) | 0.32 | 3.5 (−5.0 to 11.0) | 4.0 (0 to 10.0) | 0.29 | |
| Subjectively reported sleep quality | |||||||
| Change in PSQI | 2.5 (1.0 to 4.0) | 1.0 (1.0 to 2.0) | 0.21 | 2.5 (1.0 to 4.0)c | 1.0 (0 to 1.0) | 0.53c | |
| Quality-of-Life Questionnaires | |||||||
| Change in overall MENQOL | 1.1 (0.4 to 1.8) | 0.3 (0 to 0.4) | 0.11 | 1.1 (0.5 to 1.8)d | 0 (−0.1 to 0.1) | 0.82d | |
| Change in BDI | 0 (0 to 4.0) | 0 (0 to 2.0) | 0.04 | 0.5 (0 to 4.0) | 0 (0 to 0) | 0.44 | |
| Change in MADRS | 2.0 (0 to 5.0) | 1.0 (0 to 2.0) | 0.15 | 2.0 (0 to 4.5) | 0.5 (0 to 1.0) | 0.68d | |
| Change in ESS | 0 (0 to 2.0) | 0.5 (0.5 to 1.0) | 0.11 | 0.5 (0 to 1.5) | 0 (−0.5 to 1.0) | 0.01 | |
WASO = wake-time after sleep-onset; PSQI = Pittsburgh Sleep Quality Index; MENQOL = Menopause Quality of Life; BDI = Beck Depression Inventory; MADRS = Montgomery-Åsberg Depression Rating Scale; ESS = Epworth Sleepiness Scale
Positive change score = worsening of symptoms from pre-treatment to post-treatment except for sleep efficiency, for which a negative change score = worsening of symptoms from pre-treatment to post-treatment.
Correlations between number of hot flashes recorded (or reported) and worsening of the sleep or quality-of-life outcome.
p≤0.005 and
p=0.09 for differences using objectively recorded hot flash classification.
p≤0.04 and
p≤0.001 for differences using subjectively reported hot flash classification.
There were no statistically significant differences between hot flash groups in the change in sleep-onset latency or WASO from baseline to one month on GnRHa. However, women with recorded hot flashes spent more time awake after sleep-onset (median 29.0 vs. 15.5 minutes, p=0.02) and had longer sleep-onset latency (median 24.5 vs. 10.3 minutes, p=0.05) at the end of the study. The number of recorded hot flashes correlated significantly with the magnitude of the reduction in sleep efficiency (rs=0.76, p=0.0001, Table 3).
Within-woman change in PSQI scores did not differ between recorded hot flash groups. There were no statistically significant effects of recorded hot flashes on quality-of-life (MENQOL), sleepiness (ESS), or mood (MADRS, BDI) except for the MENQOL vasomotor domain (p=0.02).
Analyses of changes in recorded sleep efficiency scores were similar when the subgroup of women that had both subjective and objective hot flashes (n=12) was compared with the group (n=4) that had neither (p=0.01).
Effects of Reported Hot Flashes on Recorded Sleep Patterns, Reported Sleep Quality, and Quality-of-Life
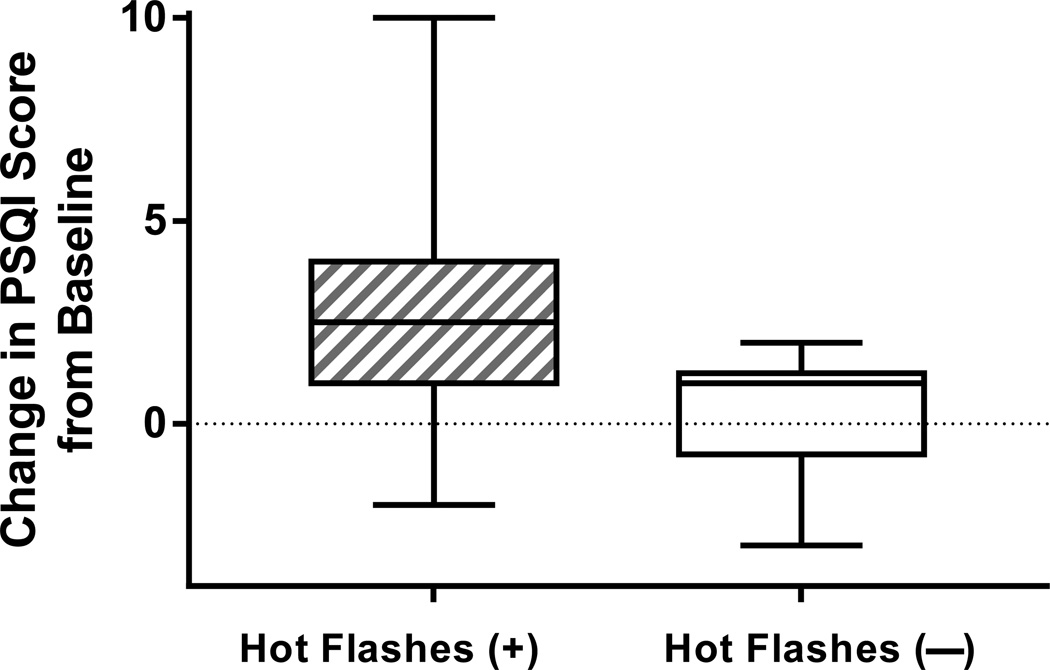
There were no effects of reported hot flashes on recorded sleep patterns when hot flash groups were defined subjectively, although an increasing number of reported hot flashes correlated with a greater reduction in sleep efficiency (rs=0.47, p=0.04, Table 3). PSQI scores increased more in women who reported hot flashes than in those who did not (median increase of 2.5, IQR 1.0 to 4.0, vs. median increase of 1.0, IQR 0 to 1.0, p=0.03, Figure 3 and Table 3). Adjusting for changes in serum estradiol did not alter the effect of reported hot flashes on within-woman change in PSQI scores (p=0.02). The number of hot flashes reported correlated with the extent of worsening in sleep quality, as measured by an increase in PSQI (rs=0.53, p=0.02). PSQI scores increased by ≥3 points in 50% vs. 0% of women with vs. without hot flashes (p=0.04), suggesting a meaningful change in perceived sleep quality among the majority of women who reported developing hot flashes.54
Figure 3.
Change in perceived sleep quality on the Pittsburgh Sleep Quality Index (PSQI) from before (“pre”) to 4 weeks on treatment (“post”) with the gonadotropin-releasing hormone agonist leuprolide in women who did and did not reported developing hot flashes (p=0.03). Increase in PSQI scores indicates worsening of perceived sleep quality.
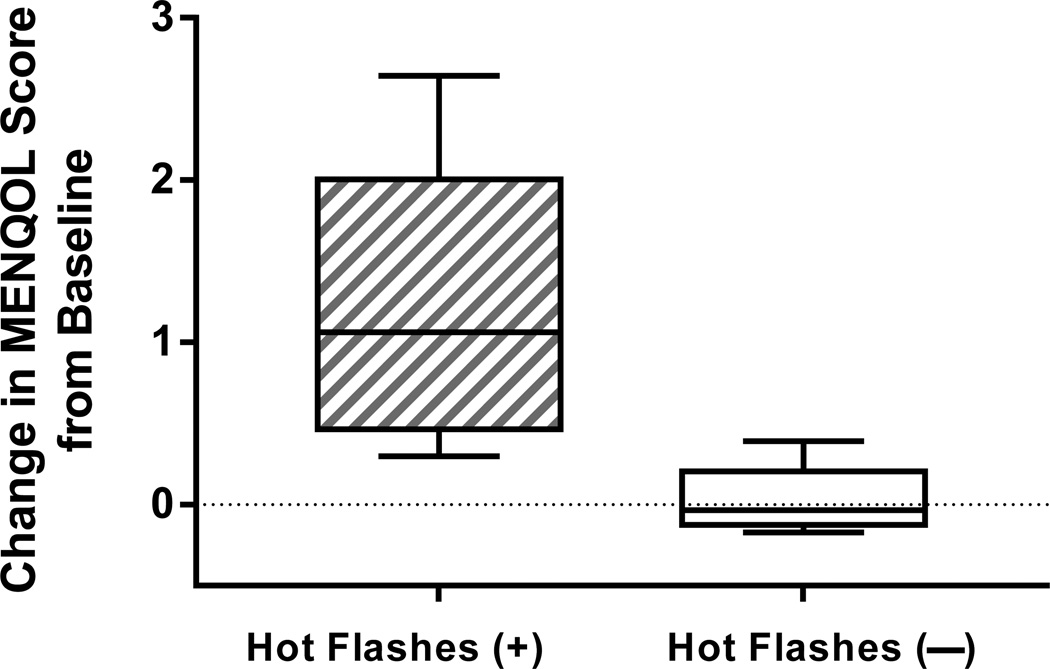
Quality-of-life was reduced in women who reported hot flashes but not in those who did not (Figure 4 and Table 3), as evidenced by an increase in overall MENQOL scores in only those with hot flashes (median increase 1.1, IQR 0.5–1.8 vs. 0, IQR 0.1–0.1, p<0.001). The MENQOL domains showing a significant change from baseline to one month on GnRHa between reported hot flash groups were the psychological subscore (p=0.04) and the vasomotor subscore (p<0.001), and there was a statistical trend toward a greater reduction in quality-of-life in those who reported hot flashes for the physical (p=0.06) and the sexual (p=0.09) subscores. The number of hot flashes reported correlated with the amount of worsening in quality-of-life (increase in overall MENQOL score rs=0.82, p<0.001).
Figure 4.
Change in quality-of-life on the Menopause Quality of Life scale (MENQOL) from before (“pre”) to 4 weeks on treatment (“post”) with the gonadotropin-releasing hormone agonist leuprolide in women who did and did not report developing hot flashes (p<0.001). Increase in MENQOL scores indicates worsening of quality-of-life.
Reduction in quality-of-life correlated with worsening in perceived sleep quality on the PSQI (rs=0.57, p=0.01) but not with changes in recorded sleep efficiency. However, the association between hot flashes and quality-of-life remained strong after adjusting for PSQI scores (p=0.02). Worsening of quality-of-life on the MENQOL also correlated with an increase in depressive symptoms on the MADRS (rs=0.72, p<0.001) and BDI (rs=0.72, p<0.001), but not in sleepiness on the ESS (rs=0.10, p=0.73). While within-woman changes in levels of depressive symptoms and sleepiness did not differ between subjective hot flash groups, women who reported developing hot flashes had higher levels of depressive symptoms after one month on GnRHa than those who did not on the BDI (median 2.0 vs. median 0, p=0.04) and on the MADRS (median 3.5 vs. median 1.0, p=0.07, statistical trend). As evidence of a meaningful change in depressive symptoms, BDI scores increased by ≥4 points in 28.6% and MADRS scores increased by ≥5 points in 35.7% of women who developed hot flashes (regardless of whether they were reported or recorded) and in 0% of those who did not develop hot flashes, but these differences did not reach statistical significance.
Analyses of changes in PSQI and of quality-of-life were similar when the subgroup of 12 women with both reported and recorded hot flashes was compared with the 4 women with neither (change in PSQI, p=0.07, and change in overall MENQOL, p=0.005).
Adverse Event Monitoring
The study intervention was well tolerated. Aside from the expected side effects of hot flashes, reduced sleep quality, and depressive symptoms, the most common side effects occurring in more than 5% of participants were fatigue (n=3), headache (n=2), upper respiratory tract infection (n=2), acne (n=1), bloating (n=1), irritability (n=1), malodorous urine (n=1), increased libido (n=1), and edema (n=1). No participant withdrew because of side effects. Within 3 months after GnRHa administration, hot flashes resolved and all women resumed their menstrual cycles. No one developed hot flashes after the first month on GnRHa.
Onset of clinically significant depressive symptoms was rare. Only one participant (5%) who developed hot flashes had a MADRS score on GnRHa that exceeded 15, suggesting clinically significant depression. Her depressive symptoms manifested one month after receiving the GnRH agonist (MADRS score = 28) and resolved spontaneously within 2 months.
Discussion
This study provides important evidence of a causal relationship between hot flashes and reduced sleep quality from a controlled experimental paradigm. Our results indicate that women with recorded hot flashes are more likely to have deterioration of recorded sleep, but they do not report a reduction in sleep quality or quality-of-life. In contrast, those who report hot flashes are more likely to report a reduction in sleep quality, but they do not have detectable changes in recorded sleep patterns, except when reported hot flashes are more frequent. Reported hot flashes and worsening of perceived sleep quality were associated with reduction in quality-of-life, although a similar relationship was not seen with recorded hot flashes and quality-of-life. Because changes in estradiol and gonadotropins were similar for those developing and not developing hot flashes, between-group differences in sleep parameters do not appear to be attributable to a divergent hormone response to the GnRHa. By inducing hot flashes in women without sleep disturbances at baseline and measuring hot flashes and sleep using both objective and subjective methodologies, findings from this small but rigorously controlled study provide evidence that hot flashes impair sleep.
It is not surprising that recorded hot flashes result in deterioration of recorded sleep efficiency but do not affect reported sleep quality because not all recorded nocturnal hot flashes are perceived or recalled in the morning.55 We hypothesize that nocturnal hot flashes may not be recalled if they result in only a brief arousal or lightening of sleep stage, as a more sustained awakening may be required for a hot flash to be remembered.53 If sleep is only minimally disturbed from repeated brief arousals or episodes of sleep stage lightening, recorded sleep efficiency might be reduced but reported sleep quality may not be impaired. Conversely, reported hot flashes may result in deterioration of perceived sleep quality but not influence recorded sleep efficiency because only those hot flashes associated with awakenings of sufficient length to generate memory are recalled in the morning.53 It is notable that the number of reported hot flashes correlated with a greater reduction in sleep efficiency (Table 3). Therefore, it is only when reported hot flashes are more frequent that recorded sleep is affected.
Given the relatively small number of hot flashes that we and others11–14 have recorded at night, it is unlikely that brief awakenings resulting from recorded hot flashes alone explain the observed reduction in sleep efficiency. We hypothesize that transient increases in core body temperature which precede objective hot flashes56–58 may also fragment sleep because increases in core temperature are known to induce awakenings.59 In women with hot flashes, the 1°C core temperature increase that commonly precedes a flash can last for as long as 20 minutes.57, 58 Such transient yet prolonged temperature increases may fragment sleep enough to reduce sleep efficiency. PSG studies are needed to confirm this hypothesis.
By inducing hot flashes, the experimental design of our study advances our understanding of the impact of new-onset hot flashes on sleep, although PSG or more actigraphy nights would provide a stronger method of recording sleep than our current approach. The optimal measurement of hot flashes is a matter of intense debate.18, 20, 60 It is increasingly clear that reported and recorded hot flashes are not identical and that, while subjective assessment is most important as a therapeutic target, objective measures play an important role for laboratory and sleep-related mechanistic studies.18, 20 The approach to measuring reported or recorded hot flashes differ in that, while diaries can be completed over a protracted period of time, objective hot flashes are typically recorded for only 1−7 days, which makes assumptions about the stability of this symptom recording over time. These different time frames, together with discrepancies observed between reported and recorded hot flashes due to psychological factors and recall of nocturnal events,9, 18–20 may contribute to differential effects of reported and recorded hot flashes on reported and recorded sleep quality. Our study is one of few8 that combine measurement approaches for both hot flashes and sleep, but provides the advantage of understanding causal relationships because of the experimental design.
Our data provides further support for studies showing that recorded hot flashes are linked to recorded reductions in sleep efficiency.8, 12, 13 Our work is also consistent with numerous studies showing that reported hot flashes are linked to a reduction in reported sleep quality.1, 2, 4–8 Studies examining the link between recorded hot flashes and reported sleep are mixed, with our data supporting the absence of an association,9 although differences in study design limit direct comparison. Our findings are consistent with some,6, 15 but not all,7, 8 studies showing the absence of an association between reported hot flashes and recorded sleep. Experimental studies of induced hot flashes using PSG to measure sleep would more definitively determine the effects of hot flashes on recorded sleep.
The baseline level of sleep efficiency we recorded and the magnitude of sleep efficiency reduction after hot flash developed are similar to that seen in other studies.12, 13, 15 While a median reduction in sleep efficiency of 3% in those developing hot flashes is modest, it is notable that pharmacologic interventions for insomnia have been approved by the Food and Drug Association for equally small changes,61 suggesting that relatively small changes in sleep time can have a meaningful impact on perceived sleep quality in insomniacs. Similar to changes in recorded sleep, the magnitude of change in perceived sleep quality we observed among those reporting hot flashes (PSQI score increased by ≥3 in 50%) is considered a meaningful change in perceived sleep quality.54 Moreover, our study population was not highly symptomatic, with a median of 2.5 reported and 5.5 recorded hot flashes on average each day. Because we found that those with more frequent hot flashes had larger deteriorations in sleep efficiency and perceived sleep quality, it is expected that midlife women with more frequent symptoms would also have more marked disruption of their sleep.
Our data provide further support that quality-of-life is reduced in women reporting hot flashes.24 While reduced perceived sleep quality correlated with impaired quality-of-life, sleep disruption alone does not appear to explain the negative impact of hot flashes on quality-of-life. These findings suggest that mechanisms such as episodic heat intolerance and sweating may be responsible for impaired quality-of-life in women with hot flashes.
This study uses an experimental model of hot flashes to isolate the effects of new-onset hot flashes on sleep and quality-of-life. By selecting young premenopausal women for this controlled investigation, we are by definition not studying a naturalistic population of midlife perimenopausal and postmenopausal women with hot flashes. Using the younger population has the advantage of eliminating the confound of age-related sleep changes from our study, but we may have underestimated the effect of hot flashes on sleep as this group of young women with good sleep at baseline may be more resilient to mild sleep perturbation. One important limitation of this and other studies7, 8 is that actigraphy is an indirect objective sleep measure which can underestimate wakefulness during sleep periods.62 Studies using PSG as the gold standard for objective sleep are needed to more definitively establish the impact of hot flashes on recorded sleep.
Consistent with other experimental studies,63 this study was small because of cost considerations and the intensity of study procedures. While analyses of these types of studies must consider chance associations and multiple comparisons, the within-woman subtraction of pre-treatment from post-treatment symptom levels reduces variability and makes for a more precise estimate of each sleep and quality-of-life measure. The consistency of associations—recorded hot flashes versus recorded sleep outcomes, and reported hot flashes versus reported sleep and quality of life outcomes—reduces the likelihood that the observed associations were found by chance. This prospective design using a model of induced hot flashes also has the important advantage of enabling causal inference about the consequences of hot flashes on well-being.
Conclusion
In summary, our experimental model of induced hot flashes uses a novel approach to show that new-onset objective hot flashes result in a rapid reduction in recorded sleep efficiency, while new-onset subjective hot flashes impair perceived sleep quality. The close temporal association of hot flashes with changes in sleep demonstrated with this GnRHa model serves as an important experimental paradigm for studying hot flash-related changes in sleep in midlife women. The observed reduction in recorded sleep efficiency validates the symptomatic concerns of perimenopausal and postmenopausal women with hot flashes and poor sleep quality. Identification of hot flashes as a cause of poor sleep quality suggests that hot flashes can be targeted to secondarily improve sleep quality and quality-of-life in women with multiple menopausal symptoms.
Acknowledgements
Funding source: Supported by 5R01MH082922 (HJ) and the Massachusetts General Hospital Claflin Distinguished Scholar Award (HJ).
We recognize the administrative support of Semmie Kim, BS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Joffe has received grant support from Cephalon/Teva, serves on the Advisory Board for Noven, and is an unpaid consultant for Sunovion. Dr. White is currently employed by Philips Respironics, and is supported by company stock/stock options and travel expenses related to his work with Philips. All other authors have nothing to disclose.
References
- 1.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Sleep. 2010 Apr 1;33(4):539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006 Jun 26;166(12):1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 4.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008 Jul 1;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 5.Burleson MH, Todd M, Trevathan WR. Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause. 2010 Jan-Feb;17(1):87–95. doi: 10.1097/gme.0b013e3181b20b2d. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003 Sep;26(6):667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 7.Ensrud KE, Stone KL, Blackwell TL, et al. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009 Mar-Apr;16(2):286–292. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 8.Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012 Jul;19(7):742–748. doi: 10.1097/gme.0b013e3182422973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. Int J Behav Med. 2006;13(2):163–172. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
- 10.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007 Sep-Oct;14(5):826–829. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 11.Erlik Y, Tataryn IV, Meldrum DR, Lomax P, Bajorek JG, Judd HL. Association of waking episodes with menopausal hot flushes. JAMA. 1981 May 1;245(17):1741–1744. [PubMed] [Google Scholar]
- 12.Savard J, Davidson JR, Ivers H, et al. The association between nocturnal hot flashes and sleep in breast cancer survivors. J Pain Symptom Manage. 2004 Jun;27(6):513–522. doi: 10.1016/j.jpainsymman.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Woodward S, Freedman RR. The thermoregulatory effects of menopausal hot flashes on sleep. Sleep. 1994;17(6):497–501. doi: 10.1093/sleep/17.6.497. [DOI] [PubMed] [Google Scholar]
- 14.Freedman RR, Benton MD, Genik RJ, 2nd, Graydon FX. Cortical activation during menopausal hot flashes. Fertil Steril. 2006 Mar;85(3):674–678. doi: 10.1016/j.fertnstert.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Shaver J, Giblin E, Lentz M, Lee K. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11(6):556–561. doi: 10.1093/sleep/11.6.556. [DOI] [PubMed] [Google Scholar]
- 16.Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004 Jul;82(1):138–144. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter JS, Newton KM, Sternfeld B, et al. Laboratory and ambulatory evaluation of vasomotor symptom monitors from the Menopause Strategies Finding Lasting Answers for Symptoms and Health network. Menopause. 2012;19(6):664–671. doi: 10.1097/gme.0b013e31823dbbe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter MS, Haqqani JR. An investigation of discordance between subjective and physiological measures of vasomotor symptoms. Climacteric. 2011 Feb;14(1):146–151. doi: 10.3109/13697131003735585. [DOI] [PubMed] [Google Scholar]
- 19.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosomatic Medicine. 2005;67(1):137–146. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter JS, Newton KM, Sternfeld B, et al. Laboratory and ambulatory evaluation of vasomotor symptom monitors from the MsFLASH Network. Menopause. 2012;19(4) doi: 10.1097/gme.0b013e31823dbbe3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker FC, Sassoon SA, Kahan T, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012 Mar 14; doi: 10.1111/j.1365-2869.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010 Apr;33(4):531–538. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okifuji A, Hare BD. Nightly analyses of subjective and objective (actigraphy) measures of sleep in fibromyalgia syndrome: what accounts for the discrepancy? Clin J Pain. 2011 May;27(4):289–296. doi: 10.1097/AJP.0b013e31820485db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women's Health Across the Nation. Menopause. 2009 May 8;16(5):860–869. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2008 Mar-Apr;15(2):223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 26.Joffe H, Hall J, Soares C, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002 Nov-Dec;9(6):392–398. doi: 10.1097/00042192-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004 Jan;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 28.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010 Jun;67(6):598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joffe H, Petrillo LF, Koukopoulos A, et al. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011 Apr 27; doi: 10.1210/jc.2010-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J Obstet Gynaecol Res. 2000 Oct;26(5):325–331. doi: 10.1111/j.1447-0756.2000.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T. Effects of herbal medicines on menopausal symptoms induced by gonadotropin-releasing hormone agonist therapy. Clin Exp Obstet Gynecol. 2001;28(1):20–23. [PubMed] [Google Scholar]
- 32.DeFazio J, Meldrum DR, Laufer L, et al. Induction of hot flashes in premenopausal women treated with a long-acting GnRH agonist. J Clin Endocrinol Metab. 1983 Mar;56(3):445–448. doi: 10.1210/jcem-56-3-445. [DOI] [PubMed] [Google Scholar]
- 33.Blamey RW, Jonat W, Kaufmann M, Bianco AR, Namer M. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer. 1992:810–814. doi: 10.1016/0959-8049(92)90120-q. [DOI] [PubMed] [Google Scholar]
- 34.Fellowes D, Fallowfield LJ, Saunders CM, Houghton J. Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for 'well-tolerated' treatments? Breast Cancer Res Treat. 2001;66(1):73–81. doi: 10.1023/a:1010684903199. [DOI] [PubMed] [Google Scholar]
- 35.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Asberg Depression Scale: Reliability and validity. Acta Psychiatr Scand. 1986;74:544–548. doi: 10.1111/j.1600-0447.1986.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 38.Rudd MD, Rajab MH. Specificity of the Beck Depression Inventory and the confounding role of comorbid disorders in a clinical sample. Cognitive Therapy Research. 1995;19(54-68) [Google Scholar]
- 39.San Roman GA, Surrey ES, Judd HL, Kerin JF. A prospective randomized comparison of luteal phase versus concurrent follicular phase initiation of gonadotropin-releasing hormone agonist for in vitro fertilization. Fertil Steril. 1992 Oct;58(4):744–749. doi: 10.1016/s0015-0282(16)55322-9. [DOI] [PubMed] [Google Scholar]
- 40.Meldrum DR, Wisot A, Hamilton F, Gutlay AL, Huynh D, Kempton W. Timing of initiation and dose schedule of leuprolide influence the time course of ovarian suppression. Fertil Steril. 1988 Sep;50(3):400–402. doi: 10.1016/s0015-0282(16)60121-8. [DOI] [PubMed] [Google Scholar]
- 41.Gelety TJ, Pearlstone AC, Surrey ES. Short-term endocrine response to gonadotropinreleasing hormone agonist initiated in the early follicular, midluteal, or late luteal phase in normally cycling women. Fertil Steril. 1995 Dec;64(6):1074–1080. doi: 10.1016/s0015-0282(16)57963-1. [DOI] [PubMed] [Google Scholar]
- 42.West CP, Baird DT. Suppression of ovarian activity by Zoladex depot (ICI 118630), a long-acting luteinizing hormone releasing hormone agonist analogue. Clin Endocrinol (Oxf) 1987 Feb;26(2):213–220. doi: 10.1111/j.1365-2265.1987.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 44.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26(5):573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CFd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas. 2005 Mar 14;50(3):209–221. doi: 10.1016/j.maturitas.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 48.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 49.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004 Feb;50(2):373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 50.Siekmann L. Determination of oestradiol-17 beta in human serum by isotope dilutionmass spectrometry. Definitive methods in clinical chemistry, II. J Clin Chem Clin Biochem. 1984 Aug;22(8):551–557. doi: 10.1515/cclm.1984.22.8.551. [DOI] [PubMed] [Google Scholar]
- 51.Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE. Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab. 2003 Apr;88(4):1766–1771. doi: 10.1210/jc.2002-021516. [DOI] [PubMed] [Google Scholar]
- 52.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–111. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 53.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000 Mar 25;355(9209):1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 54.Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for latelife insomnia: preliminary findings. J Clin Sleep Med. 2006 Oct 15;2(4):403–406. [PubMed] [Google Scholar]
- 55.Woodward S, Arfken CL, Ditri DW, Freedman RR. Ambient temperature effects on sleep and mood in menopausal women. Sleep. 1999;22(Suppl 1):S224–S225. [Google Scholar]
- 56.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70(2):332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 57.Freedman RR, Woodward S. Core body temperature during menopausal hot flushes. Fertil Steril. 1996;65(6):1141–1144. [PubMed] [Google Scholar]
- 58.Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause. 2004 Jul-Aug;11(4):375–381. doi: 10.1097/01.gme.0000113848.74835.1a. [DOI] [PubMed] [Google Scholar]
- 59.Van Someren EJ. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000 May;17(3):313–354. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- 60.Loprinzi CL, Barton DL. Gadgets for measuring hot flashes: have they become the gold standard? J Support Oncol. 2009 Jul-Aug;7(4):136–137. [PubMed] [Google Scholar]
- 61.Wang-Weigand S, McCue M, Ogrinc F, Mini L. Effects of ramelteon 8 mg on objective sleep latency in adults with chronic insomnia on nights 1 and 2: pooled analysis. Curr Med Res Opin. 2009 May;25(5):1209–1213. doi: 10.1185/03007990902858527. [DOI] [PubMed] [Google Scholar]
- 62.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011 Aug;15(4):259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 63.D'Ambrosio C, Stachenfeld NS, Pisani M, Mohsenin V. Sleep, breathing, and menopause: the effect of fluctuating estrogen and progesterone on sleep and breathing in women. Gend Med. 2005 Dec;2(4):238–245. doi: 10.1016/s1550-8579(05)80053-1. [DOI] [PubMed] [Google Scholar]