Abstract
Cord Colitis Syndrome (CCS) is a recently described diarrheal illness of uncertain pathogenesis that affects recipients of umbilical cord blood transplant (CBT) and is associated with negative cultures. CCS exhibits a peculiar histopathological appearance, as it commonly manifests as granulomatous inflammation involving the upper and lower gastrointestinal tract, with features of chronicity in the colon. Importantly, the treatment for CCS differs from that of acute Graft-versus-Host Disease (GvHD), which is commonly in the clinical differential diagnosis: CCS responds to antibiotic treatment, while acute GvHD responds to immunosuppression. We describe here the case of a 36-year-old woman with a history of acute myeloid leukemia who developed refractory diarrhea following CBT. Endoscopic biopsies of the stomach and colon revealed granulomatous inflammation, consisting of scattered ill-defined aggregates of epithelioid histiocytes, with associated mild neutrophilic inflammation and mildly increased epithelial cell apoptosis. In the colon, the granulomatous inflammation was associated with surface epithelial injury (including surface erosions) and contained occasional multinucleated epithelioid giant cells. Paneth cell metaplasia was present in the distal colon, but crypt architecture was preserved and there was no basal lymphoplasmacytosis. Special stains and immunohistochemical stains for infectious organisms were negative. A diagnosis of CCS was made, and the patient promptly responded to treatment with ciprofloxacin and metronidazole. We present this case to raise awareness of this newly described entity among pathologists, in order to facilitate its timely diagnosis and treatment.
Keywords: cord colitis syndrome, graft-versus-host disease, granulomatous gastritis, granulomatous colitis, umbilical cord blood transplant
Introduction
Umbilical cord blood transplantation (CBT) is an alternative source of hematopoietic cells for individuals who need hematopoietic stem-cell transplantation (HSCT) and do not have a matched related or unrelated donor(1-6). CBT, when compared to other forms of marrow transplantation, typically results in less severe symptoms of graft-versus-host disease (GvHD)(1-6). However, a peculiar form of culture-negative, non-steroid responsive diarrhea in patients who have undergone CBT has recently been described(7). This diarrheal illness has been termed Cord Colitis Syndrome (CCS), and is associated with a favorable prognosis, as it responds to a short course of antibiotics, although relapse is common(7). Interestingly, CCS patients commonly exhibit granulomatous inflammation on biopsies from the upper and lower GI tract, as well as features of chronic injury such as Paneth cell metaplasia in the distal colon(7). The precise pathogenesis of CCS remains unclear at this time, but may be related to immunological and/or microbiological alterations in the gut in the setting of CBT.
We present here the case of a 36-year-old woman with acute myeloid leukemia (AML) who developed CCS following CBT, with characteristic histopathologic findings on endoscopic biopsies of the stomach and colon. We describe this case to increase awareness among pathologists of this newly described condition, in order to promote prompt diagnosis and treatment.
Case Report
Clinical History
A 36-year-old woman with a history of AML was admitted with symptoms of persistent nausea, vomiting, and diarrhea. Her past history was significant for induction chemotherapy with daunorubicin and cytarabine two years prior. She then received consolidation with etoposide and high dose arabinofuranosyl cytidine (Ara-C) followed by autologous stem cell transplant. She did well until eight months prior to admission, when she was found to have recurrent AML. She then underwent induction therapy with high dose cytarabine and consolidation chemotherapy. There were no matched related or unrelated donors. She was subsequently admitted for double CBT. She received a conditioning regimen of fludarabine, melphalan and total body irradiation and underwent CBT. She then developed engraftment syndrome, which responded to low dose prednisone therapy.
Two months prior to admission, she developed symptoms of diarrhea and elevated serum transaminases and alkaline phosphatase [aspartate aminotransferase (AST) 205 U/L, normal 9 to 32 U/L; alanine aminotransferase (ALT) 584 U/L, normal 7 to 32 U/L, and alkaline phosphatase (AP) 137 U/L, normal 30 to 100 U/L], with a normal bilirubin. Stool studies, including cultures, Clostridium difficile, and ova and parasite testing were negative. She was empirically started on steroids for presumed acute GvHD. A flexible sigmoidoscopy was performed up to the splenic flexure and was unremarkable. Colonic biopsies did not show any evidence of acute GvHD and were otherwise unremarkable. A liver biopsy was performed. The biopsy showed moderate to severe iron deposition in a mixed (parenchymal and reticuloendothelial) pattern and scattered ceroid-laden macrophages, consistent with recent injury to hepatocytes. A single small parenchymal granuloma was noted. There was minimal portal and lobular inflammation, with no apoptotic hepatocytes, and unremarkable bile ducts. An immunohistochemical stain for cytomegalovirus was negative, an acid fast bacilli (AFB) stain was negative, and in-situ hybridization for Epstein Barr Virus-encoded RNA was negative. The findings were considered non-specific and interpreted as evidence of resolving injury to hepatocytes, possibly related to the use of fluconazole. There was no evidence of acute GvHD.
Upper and lower gastrointestinal tract endoscopic evaluation with biopsies was then performed. The duodenum showed increased mitoses suggestive of regenerative changes, and the colon showed regenerative colonic mucosa with patchy inflammation and ulceration. The inflammation in the colon was predominantly histiocytic with a few areas of neutrophils. There were no granulomas or viral inclusions and only very rare apoptotic bodies. An immunohistochemical stain for cytomegalovirus was negative. The findings were considered non-specific with possible etiologies including an infectious or drug exposure. After several days of non-response to steroids, the patient was given an empiric trial of antibiotics and had improvement in her diarrhea. The serum transaminases improved with cessation of fluconazole. Her dose of sirolimus was also adjusted for the appropriate level of immunosuppression.
A follow-up bone marrow biopsy was unremarkable and consistent with remission of disease. The patient was then started on sorafenib, a small molecule inhibitor of several tyrosine kinases, per a study protocol. Two weeks prior to admission, the patient started to experience refractory symptoms of nausea, vomiting, and diarrhea. Her symptoms were so severe that she required initiation of total parenteral nutrition (TPN). She had no other risk factors for her symptoms based on review of her medication list or social history. Her physical exam was also unremarkable. Stool studies were negative for infectious etiologies. The remainder of her laboratory tests, including liver function tests, was all within normal limits. She was subsequently admitted due to her continued symptoms and empirically started on steroids for presumed acute GvHD.
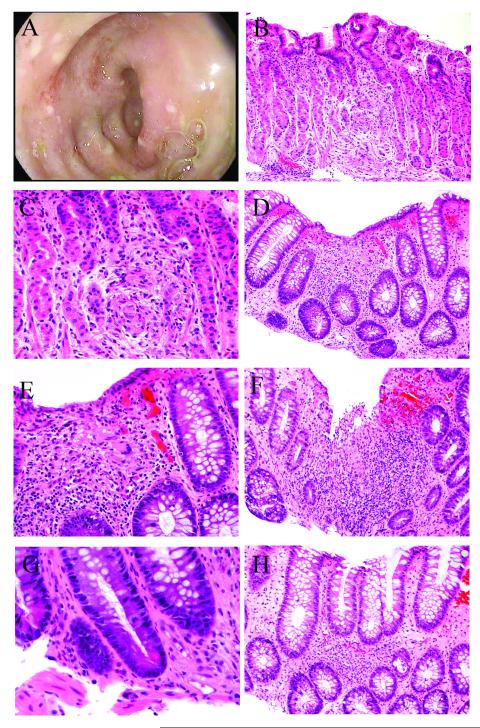
An upper endoscopy was performed and revealed a normal esophagus, a diffuse moderate gastritis, and a normal duodenum. Biopsies were taken from the stomach and duodenum. A colonoscopy was performed and revealed a diffuse area of moderately erythematous mucosa with superficial erosions in the rectum, sigmoid, and descending colon (Fig. 1A). The procedure was intentionally stopped at the descending colon, and biopsies were obtained from the sigmoid colon and rectum.
Figure 1.
Cord colitis syndrome presenting with granulomatous inflammation in the upper and lower gastrointestinal tract. A) Endoscopic appearance of rectosigmoid colon, with erythematous mucosa and surface erosions. B-C) Granulomatous inflammation involving the stomach (hematoxylin and eosin, 200X [B] and 400X [C]). D-E) Granulomatous inflammation involving the colon, with associated surface epithelial injury and mild increase in crypt epithelial cell apoptosis. Note multinucleated giant cell (hematoxylin and eosin, 200X [D] and 400X [E]). F) Granulomatous inflammation with surface erosion in the colon (hematoxylin and eosin, 400X). G) Paneth cell metaplasia in the colon (hematoxylin and eosin, 600X). H) Patchy lymphohistiocytic inflammation and foci of neutrophilic cryptitis in the colon (hematoxylin and eosin, 200X).
Histopathologic findings
The stomach and colonic biopsies both exhibited patchy lymphohistiocytic to granulomatous inflammation, consisting of ill-defined aggregates of epithelioid histiocytes (Fig. 1). The inflammation was centered within the lamina propria but was associated with occasional foci of neutrophilic cryptitis/”glanditis” and epithelial cell injury. The changes were more prominent in the colon, which also exhibited multinucleated epithelioid giant cells. Mildly increased epithelial cell apoptosis was noted in the areas of inflammation; however, this was not a prominent feature. Other reactive epithelial changes were noted in both the stomach and colon, including increased mitotic activity and mucin depletion. In the colon, the granulomatous inflammation was associated with surface epithelial injury, including occasional erosions (Fig. 1). The inflammation, however, was not exclusively superficial, as it was also seen in a basal location. Paneth cell metaplasia was present in the colon (Fig. 1). Crypt architecture was preserved, and there was no basal lymphoplasmacytosis. No distinct, well-formed granulomas were seen in any of the biopsies. No viral cytopathic changes were identified. The duodenum was largely unremarkable, with preserved villous architecture, no significant active inflammation, and only rare foci of lymphohistiocytic inflammation.
The following special stains for organisms were performed, and were negative: acid fast bacilli (AFB), Gomori methenamine silver (GMS), periodic acid-Schiff with diastase digestion (PAS/d), Giemsa, and Brown-Hopps. The following immunohistochemical stains for viruses were performed, and were negative: cytomegalovirus, adenovirus, Herpes simplex virus I and II, and Varicella Zoster Virus. From a histopathological standpoint, the differential diagnosis was considered to be broad and included infection, medication effect, and a manifestation of systemic illness. However, given the negative infectious work-up and the clinical context of the case, the findings were judged to be consistent with CCS. The patient was started on intravenous ciprofloxacin and metronidazole with an immediate improvement in her gastrointestinal symptoms. After a few days, the patient was discharged on a course of oral antibiotics.
Discussion
The development of culture-negative and steroid non-responsive diarrhea following CBT has been previously described as CCS. This illness is important to distinguish from acute GvHD, as the symptoms overlap but the treatment is different: CCS responds to antimicrobial therapy, either a combination of fluroquinolones and metronidazole, as in our patient, or metronidazole alone(7), whereas GvHD responds to immunosuppression. Given that CCS occurs in patients who have many other potential etiologies for diarrhea, including acute GvHD, infection, or medication effect, it is likely to be under appreciated. For example, the patient described here was admitted on two separate occasions with various symptoms, including diarrhea, without an identifiable etiology. We aim to raise awareness of this newly described entity among pathologists, because CCS exhibits relatively characteristic histopathological features, as recently described by Herrera and coworkers in a case series of 11 patients(7). Although neither of these features is specific by itself, we believe that in the appropriate clinical scenario, pathologists should be able to recognize this constellation of findings and suggest CCS as the appropriate diagnosis.
In the series by Herrera and coworkers(7), granulomatous inflammation was present in a majority of patients (64%) and could affect both the upper and lower gastrointestinal tract, as it was observed in the stomach, duodenum, or colon. Surface epithelial injury in the colon was noted in 64% of patients. Neutrophilic cryptitis was seen in all cases and was focal or mild in 72% of cases. Paneth cell metaplasia in the distal colon was very common, and was seen in 72% of cases. Basal lymphoplasmacytosis and substantial crypt architectural distortion, however, were rare, as they were noted only in 1 patient each (9%)(7). Mildly increased crypt or gland apoptosis was also common, but was not a prominent feature: 64% of cases exhibited Grade 1 apoptosis, and only one case (9%) exhibited Grade 2 apoptosis(7). The case described here exhibits each of the histopathological features identified by Herrera and coworkers as typical of CCS (Table 1).
Table 1.
Histopathologic features of Cord Colitis Syndrome
1) Granulomatous inflammation associated with:
|
| 2) Involvement of upper and lower gastrointestinal tract |
| 3) Paneth cell metaplasia in the distal colon |
Given the clinical and pathologic differential diagnosis, we recommend excluding infectious etiologies with available special and immunohistochemical stains. In particular, Whipple’s disease should be excluded by a PAS/d stain, as some authors have speculated that CCS may represent an atypical presentation of Whipple’s disease(8).
Although the second set of endoscopic biopsies in our patient showed only subtle non-specific changes (foci of lymphohistiocytic inflammation and neutrophilic cryptitis in the colon biopsy), in retrospect it appears likely that these were already a manifestation of CCS. Pathologists should therefore raise the possibility of CCS even if the histopathologic features are not well developed. In the case described here, the distal colon exhibited more prominent changes than the stomach. However, we recommend tissue sampling from multiple gastrointestinal locations when CCS is clinically in the differential, given that findings may be seen in the stomach, duodenum, or colon(7).
Epithelial cell apoptosis is only mildly increased in CCS, which, in conjunction with the other findings seen in CCS, should allow pathologists to distinguish CCS from acute GvHD(9). Etiologically, however, CCS may possibly overlap with the disease spectrum of acute GvHD, given that there is some suggestion that the use of antibiotics or antifungals improves GI symptoms in acute GvHD(10-12). However, definitive treatment of acute GvHD typically requires a form of immunosuppression(13), which is not required for treatment of CCS. Likewise, some authors have speculated whether CCS represents a form of chronic GvHD(14). Chronic GvHD may exhibit a range of non-specific pathological findings, such as lamina propria fibrosis and crypt architectural irregularity(15, 16), but it is not known to cause granulomatous inflammation. In the case of our patient, chronic GvHD appears unlikely given the she never had an episode of acute GvHD during her clinical course and lacked features suggestive of GvHD in her liver biopsy Although the precise nature of CCS remains unclear, we believe that it is reasonable to consider it as a distinct entity due to its predictable histopathological findings and clinical response to antibiotics.
A drug effect may be in the differential diagnosis, although this should not be favored if there have been no recent changes in medications. In our patient, there was clinical suspicion that sorafenib was contributing to the symptoms of diarrhea, as this has been seen in up to 82% of patients while on sorafenib for hepatocellular disease(17-19). However, in our patient, the timing is not supportive of this, as sorafenib was stopped during the patient’s hospitalization, and there was no improvement in her symptoms. Additionally, the reported histopathologic findings in sorafenib-induced colonic injury are different from those in CCS. Specifically, sorafenib is associated with development of discrete ulcers and perforations with thrombotic occlusion of vessels, presumably indicating an ischemic etiology(20-22). While a contribution of sorafenib to the patient’s symptoms cannot be definitively excluded, resolution of the diarrhea following antibiotic treatment strongly supports CCS as the overriding etiology.
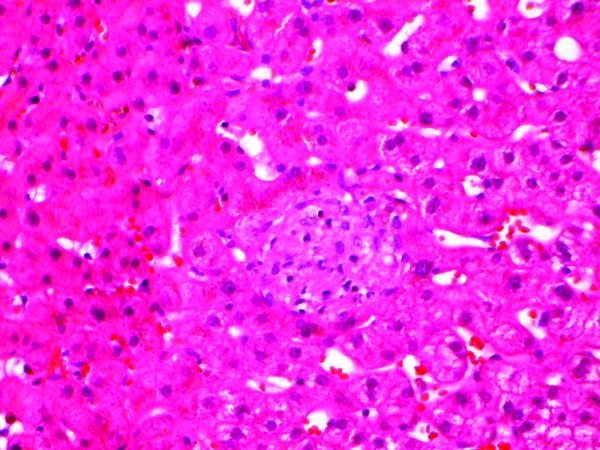
It is interesting to speculate whether the small granuloma in the liver biopsy (Fig. 2A), as well as the evidence of hepatocytic injury, were also a manifestation of CCS. However, granulomas in the liver may be seen in association with a wide range of etiologies or simply be an incidental finding. The clinical suspicion that the liver injury was due to fluconazole was supported by improvement in transaminases with drug cessation. In rats, injection of the related antifungal itraconazole is associated with development of granulomas in addition to prominent centrilobular hepatocellular injury(24). Thus, it is plausible that the liver biopsy findings in our patient may be attributable to fluconazole, although an overlap of CCS remains a possibility.
Figure 2.
Small non-caseating granuloma in liver biopsy (hematoxylin and eosin, 400X).
The presence of granulomatous inflammation in conjunction with Paneth cell metaplasia in the distal colon may raise the possibility of idiopathic inflammatory bowel disease (IBD); specifically, Crohn’s disease(25-29). Herrera and coworkers also report the presence of pseudopyloric gland metaplasia in 1 of 2 available terminal ileum biopsies, indicative of a chronic ileitis(7). However, Paneth cell metaplasia in the distal colon is not specific for IBD, as it may be seen in other conditions that result in chronic injury to the colonic mucosa; for example, it is well recognized to occur in collagenous colitis and lymphocytic colitis(30, 31). Features more in keeping with IBD-type chronicity (basal lymphoplasmacytosis and substantial crypt architectural distortion) are only rarely seen in the colon in CCS(7). Similarly, well-formed granulomas are uncommon, and the inflammation is usually lymphohistiocytic to ill-defined granulomatous, as in our case. Overall, we propose that IBD-like features in a patient status post CBT presenting with diarrhea should not dissuade the pathologist from suggesting CCS. The overlap in pathologic features does prompt speculation that CCS results from immunological alterations similar to those seen in Crohn’s disease(32). It would be interesting to see whether patients with CCS are at risk for developing clinicopathologic manifestations of IBD on long-term follow-up. Clearly, further investigation into the pathogenesis and clinical behavior of CCS is needed.
In summary, we present a case of CCS manifesting as granulomatous inflammation in the stomach and colon, in association with mild neutrophilic inflammation, surface erosions, mildly increased apoptosis, and Paneth cell metaplasia in the distal colon. We believe that, in the appropriate clinical setting, pathologists and clinicians should be able to recognize this constellation of findings as characteristic of CCS. Distinguishing CCS from acute GvHD based on endoscopic biopsies is important in order to allow for timely initiation of antibiotic treatment.
Acknowledgments
Nitin K. Gupta is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T32DK007191. Ricard Masia is supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA09216.
Footnotes
Disclosures: The authors have no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eapen M, Wagner JE. Transplant outcomes in acute leukemia. I. Semin Hematol. 2010;47:46–50. doi: 10.1053/j.seminhematol.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 6.Parody R, Martino R, Rovira M, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Herrera AF, Soriano G, Bellizzi AM, et al. Cord colitis syndrome in cord-blood stem-cell transplantation. N Engl J Med. 2011;365:815–824. doi: 10.1056/NEJMoa1104959. [DOI] [PubMed] [Google Scholar]
- 8.Matuchansky C. Cord colitis syndrome in cord-blood stem-cell transplantation. N Engl J Med. 2011;365:2336–2337. doi: 10.1056/NEJMc1111264. author reply 2337-2338. [DOI] [PubMed] [Google Scholar]
- 9.Shidham VB, Chang CC, Shidham G, et al. Colon biopsies for evaluation of acute graft-versus-host disease (A-GVHD) in allogeneic bone marrow transplant patients. BMC Gastroenterol. 2003;3:5. doi: 10.1186/1471-230X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Bekkum DW. Biology of acute and chronic graft-versus-host reactions: predictive value of studies in experimental animals. Bone Marrow Transplant. 1994;14(Suppl 4):S51–55. [PubMed] [Google Scholar]
- 11.Beelen DW, Elmaagacli A, Muller KD, et al. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–3275. [PubMed] [Google Scholar]
- 12.van der Velden WJ, Netea MG, de Haan AF, et al. Role of the Mycobiome in Human Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2012 doi: 10.1016/j.bbmt.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Alousi AM, Bolanos-Meade J, Lee SJ. Graft-versus-Host Disease: The State of the Science. Biol Blood Marrow Transplant. 2012 doi: 10.1016/j.bbmt.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Bekkum D, Vossen J, Zurcher C. Cord colitis syndrome in cord-blood stem-cell transplantation. N Engl J Med. 2011;365:2336. doi: 10.1056/NEJMc1111264. author reply 2337-2338. [DOI] [PubMed] [Google Scholar]
- 15.Akpek G, Chinratanalab W, Lee LA, et al. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46–51. doi: 10.1053/bbmt.2003.49999. [DOI] [PubMed] [Google Scholar]
- 16.Asplund S, Gramlich TL. Chronic mucosal changes of the colon in graft-versus-host disease. Mod Pathol. 1998;11:513–515. [PubMed] [Google Scholar]
- 17.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci. 2012;57:1122–1129. doi: 10.1007/s10620-012-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchet B, Billemont B, Barete S, et al. Toxicity of sorafenib: clinical and molecular aspects. Expert Opin Drug Saf. 2010;9:275–287. doi: 10.1517/14740330903510608. [DOI] [PubMed] [Google Scholar]
- 20.Frieling T, Heise J, Wassilew SW. Multiple colon ulcerations, perforation and death during treatment of malignant melanoma with sorafenib. Dtsch Med Wochenschr. 2009;134:e1–2. 1464–1466. doi: 10.1055/s-0029-1225311. [DOI] [PubMed] [Google Scholar]
- 21.Inoue T, Kinoshita H, Komai Y, et al. Two cases of gastrointestinal perforation after radiotherapy in patients receiving tyrosine kinase inhibitor for advanced renal cell carcinoma. World J Surg Oncol. 2012;10:167. doi: 10.1186/1477-7819-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SG, Chung CH, Park CY. Colon perforation during sorafenib therapy for advanced hepatocelluar carcinoma. A case report. Tumori. 2011;97:794–799. doi: 10.1177/030089161109700618. [DOI] [PubMed] [Google Scholar]
- 23.Loriot Y, Boudou-Rouquette P, Billemont B, et al. Acute exacerbation of hemorrhagic rectocolitis during antiangiogenic therapy with sunitinib and sorafenib. Ann Oncol. 2008;19:1975. doi: 10.1093/annonc/mdn566. [DOI] [PubMed] [Google Scholar]
- 24.Somchit N, Norshahida AR, Hasiah AH, et al. Hepatotoxicity induced by antifungal drugs itraconazole and fluconazole in rats: a comparative in vivo study. Hum Exp Toxicol. 2004;23:519–525. doi: 10.1191/0960327104ht479oa. [DOI] [PubMed] [Google Scholar]
- 25.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 26.Shanahan F. Crohn’s disease. Lancet. 2002;359:62–69. doi: 10.1016/S0140-6736(02)07284-7. [DOI] [PubMed] [Google Scholar]
- 27.Rotterdam H, Korelitz BI, Sommers SC. Microgranulomas in grossly normal rectal mucosa in Crohn’s disease. Am J Clin Pathol. 1977;67:550–554. doi: 10.1093/ajcp/67.6.550. [DOI] [PubMed] [Google Scholar]
- 28.Le Berre N, Heresbach D, Kerbaol M, et al. Histological discrimination of idiopathic inflammatory bowel disease from other types of colitis. J Clin Pathol. 1995;48:749–753. doi: 10.1136/jcp.48.8.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams WJ. Histology of Crohn’s syndrome. Gut. 1964;5:510–516. doi: 10.1136/gut.5.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goff JS, Barnett JL, Pelke T, et al. Collagenous colitis: histopathology and clinical course. Am J Gastroenterol. 1997;92:57–60. [PubMed] [Google Scholar]
- 31.Ayata G, Ithamukkala S, Sapp H, et al. Prevalence and significance of inflammatory bowel disease-like morphologic features in collagenous and lymphocytic colitis. Am J Surg Pathol. 2002;26:1414–1423. doi: 10.1097/00000478-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Masuda S. Cord colitis syndrome in cord-blood stem-cell transplantation. N Engl J Med. 2011;365:2337. doi: 10.1056/NEJMc1111264. author reply 2337-2338. [DOI] [PubMed] [Google Scholar]