Abstract
The human tongue is one of the most important yet least understood structures of the body. One reason for the relative lack of research on the human tongue is its complex anatomy. This is a real barrier to investigators as there are few anatomical resources in the literature that show this complex anatomy clearly. As a result, the diagnosis and treatment of tongue disorders lags behind that for other structures of the head and neck. This report intended to fill this gap by displaying the tongue’s anatomy in multiple ways. The primary material used in this study was serial axial images of the male and female human tongue from the Visible Human (VH) Project of the National Library of Medicine. In addition, thick serial coronal sections of three human tongues were rendered translucent. The VH axial images were computer reconstructed into serial coronal sections and each tongue muscle was outlined. These outlines were used to construct a 3-dimensional computer model of the tongue that allows each muscle to be seen in its in vivo anatomical position. The thick coronal sections supplement the 3-D model by showing details of the complex interweaving of tongue muscles throughout the tongue. The graphics are perhaps the clearest guide to date to aid clinical or basic science investigators in identifying each tongue muscle in any part of the human tongue.
Keywords: tongue, intrinsic and extrinsic tongue muscles, neuromuscular compartments, tongue movement, speech, swallowing, respiration, 3-D reconstruction
INTRODUCTION
In humans, tongue function is critical for normal speech, swallowing and respiration (Hiiemae and Palmer, 2003); and tongue dysfunction can result in aphasia, dysphagia, and obstructive sleep disorders, respectively. At present there is a large discrepancy between the obvious importance of the tongue and our meager understanding of its structure and function. A major reason for this gap in our knowledge is that all tongues fall into the category of muscular hydrostats; muscular organs whose biomechanical properties are more akin to a hydraulic system then the more familiar mechanical levers that constitute the skeletal muscle system (Kier and Smith, 1985). MH is composed of muscle groups oriented in different directions and this makes them particularly hard to study by gross dissection or routine histological methods.
Although the tongues of some birds, amphibians, reptiles and mammals have been studied (Abd-El-Malek, 1938a; Lombard and Wake, 1976; Hellstrand, 1980, 1981; Gans and Gorniak, 1982; Bhattacharyya, 1985; Delheusy et al., 1994; Herrel et al., 1998; Nishikawa et al., 1999; Mu and Sanders, 1999, 2010), we are far from understanding the relationship between muscle structure and function within tongues. Gross tongue motion caused by contraction of any individual muscle is dependent on the activity of surrounding muscles. Because of this complexity there are few studies that demonstrate the activity of any intrinsic tongue muscle, either by electromyographic recording during gross tongue movement, or by electrical stimulation of a single isolated muscle. Therefore, the actions of the individual tongue muscles are largely assumptions based on their anatomical arrangement and extrapolation from animal experiments (Abd-El-Malek, 1938a; Hellstrand, 1981; Gilliam and Goldberg, 1995). At present, gross tongue movements are believed to result from a complex mixture of individual tongue muscles acting as agonists, antagonists or stabilizers (Lowe, 1980). However, the details of how any movement is accomplished are unknown.
Tongue Base, Body and Blade
Anatomists and speech scientists use different terminology to describe the parts of the tongue and this can be a source of confusion. In this study, the tongue is divided into three parts: the blade, body and base. The base, or root, is that part of the tongue posterior to the sulcus terminalis, the line of circumvallate papillae taste receptors. The body extends from the circumvallate papillae to the frenulum, the anterior part of the genioglossus (GG) muscle. The blade is the region of the tongue anterior to the frenulum. The body is the largest segment and it convenient to arbitrarily separate the body into an anterior and posterior part. The anterior body is inferior to the hard palate, the posterior body lies inferior to the soft palate.
Basic Tongue Muscular Anatomy
The tongue muscles of most mammals are divided into two main groups: the extrinsic and the intrinsic (Sonntag, 1925). The extrinsic muscles have one attachment to a bone (mandible, hyoid bone or styloid process) while the other end inserts within the tongue. In contrast, the intrinsic muscles originate and insert within the tongue and have no bony attachments. Generally, the extrinsic muscles tend to move the position of the whole tongue while the intrinsic muscles change its shape.
Some authors describe a greater number of extrinsic muscles than those in this study. We exclueded the palatoglossus (PG), the muscle of the anterior tonsillar pillar; the glossopharyngeus (GP), a slip from the superior pharyngeal constrictor (SPC) that connects to the tongue; and the chondroglossus (CG), a small muscle that is sometimes considered to be part of the hyoglossus (HG) muscle. These muscles are not thought to make large contributions to tongue movements and were excluded as they would make the 3-dimensional model needlessly complex.
In this paper the term tongue is used to refer to the intrinsic tongue, that part of the tongue encapsulated by connective tissue. The intrinsic tongue includes all of the intrinsic muscles plus the internal parts of the extrinsic muscles. The extrinsic tongue refers to those parts of the extrinsic muscles outside the tongue. The whole tongue refers to the intrinsic tongue plus the external parts of the extrinsic muscles. The dorsum refers to the superior surface of the tongue.
The main connective tissue structures in the tongue include: the medium septum, a thick sheet of fascia in the midline of the tongue that serves as origin for the transverse muscle; the paramedian septum, fascia that separate the GG muscle from the inferior longitudinal (IL) muscle; the lateral septum, connective tissue that envelopes the IL muscle.
Basic Concepts of Tongue Biomechanics; The Tongue Is A Muscular Hydrostat
The tongue, like the tentacles of an octopus or trunk of an elephant, is a muscular hydrostat (MH) (Kier and Smith, 1985). MHs are muscular structures without bones whose biomechanics are more like a hydraulic system than the mechanical lever arms used by most skeletal muscles. The connective tissue keeps the volume of the organ constant during muscle contractions. Therefore, if a muscle contracts to shorten the MH, the MH becomes wider. Similarly, contraction of muscles that are oriented in the cross sectional plane of the muscular hydrostat will narrow the MH and simultaneously lengthen it. In addition to shortening and lengthening, MHs can twist in various directions depending on muscle arrangement and selective activation.
The same principles are believed to apply to tongue movement. For example, isolated contraction of the longitudinal muscles would be expected to shorten and thicken the tongue. However, contraction of a muscle oriented in the cross sectional plane of the tongue, such as the vertical and transverse (V/T) muscles, thins and lengthens the tongue. If both groups contract simultaneously, they work against each other and the tongue adopts a rigid position. If the longitudinal muscles on one side are more active the tongue bends to that side.
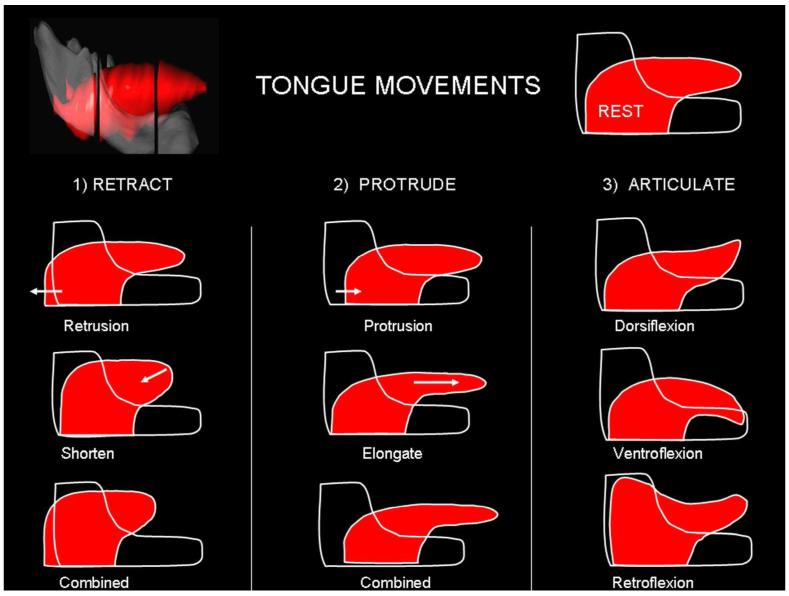
The whole tongue can move as a unit, change its shape to elongate or shorten, or articulate its different parts. Movement of the entire tongue posteriorly is called retrusion while anterior movement is called protrusion. Curving the tongue tip superiorly is called dorsoflexion while inferior curving is called ventroflexion. Movement of the tongue superiorly is called elevation and inferiorly is called depression. Simultaneous depression of the tongue body with elevation of the base is called retroflexion.
Moreover, the anatomy and physiology of the human tongue is more complex than that of other species. Clearly the human tongue performs unique movements during speech and swallowing, however the anatomical specializations that underlie these unique tongue movements are almost completely unknown. All of the named tongue muscles can be identified as discrete entities in some region of the tongue. However, in other parts of the tongue each muscle separates into smaller muscle fascicles that interweave with those other tongue muscles. The result is that in large parts of the tongue, muscle fascicles from two or more muscles are indistinguishable.
Based on what is available in the literature it is difficult for new investigators to understand the 3 dimensional anatomy of human tongue muscles and almost impossible for them to identify individual muscles in histological sections. The most referenced study on the internal anatomy of the human tongue is that of Abd-El Malek (1938b). Abd-El-Marek’s written descriptions of the muscles and fascial planes of the human tongue are highly detailed and include seven photographs of tongue coronal sections at anterior to posterior levels. Although these photographs still are the best depiction of human tongue anatomy their resolution is poor. More recent publications by Miyawaki (1974), Takemoto (2001) and Miller et al., (2002) are all valuable contributions but still do not convey the 3-dimensional complexity of the tongue muscles.
The objective of this study was to provide graphical depictions of the human tongue that present its complex anatomy in a simplified manner. The first aim was to construct a 3-dimensional model of the human tongue that shows the position of each muscle. The second aim was to provide a set of serial reconstructions of the human tongue in all three of the main anatomical axes: axial, coronal and sagittal. The third aim was to provide an easily understandable explanation of tongue anatomy based on these graphical images. The intent was not necessarily to introduce new anatomical information on the human tongue, although some novel observations are reported. Instead the goal was to report a relatively complete but easily understood study of the 3-dimensional anatomy of the human tongue. The lack of this information in the literature appears to be the main obstacle for researchers and clinicians. Hopefully this report will be of value to them and advance the study of normal and abnormal tongue anatomy and physiology.
MATERIALS AND METHODS
Visible Human Project (http://nlm.nih.gov)
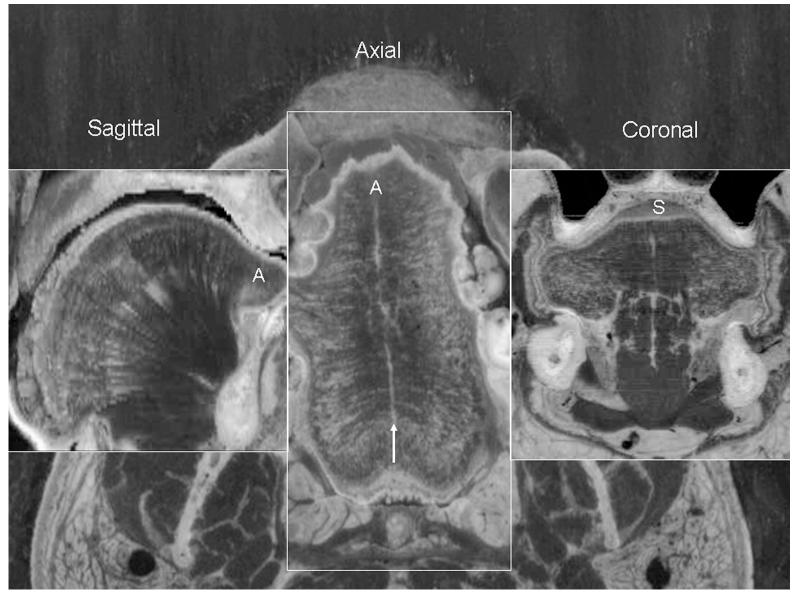
The Visible Human Project was an initiative of the National Library of Medicine that began in 1986 and is still continuing. The aim of the project was to establish a database of normal male and female human anatomy. One male (age 37) and one female (age 59) were embedded in ice and completely serially sectioned in the axial plane. The various anatomical planes are illustrated in Figure 1: the axial plane is perpendicular to the axis of the body and was the original plane of sectioning of the Visible Human; the sagittal plane passes through the midline of the tongue and bisects into two symmetrical hemi-tongues; the coronal plane is perpendicular to the long axis of the tongue.
Fig. 1.
The anatomical planes in the Visible Human: sagittal (left), axial (middle), and coronal (right). The Visible Human Male and Female were frozen in a block of ice and serially sectioned in the axial plane, the cross sectional plane that is perpendicular to the long axis of the body. Each section was digitally photographed at high resolution. The background photograph of this figure is part of section #1184 of the Visible Human Male converted to greyscale. This section passes through the tongue, which is in the middle of the photo. The midline septum of the tongue is marked by an arrow and the transverse muscle passes laterally from this septum throughout the length of the tongue. As the digital photographs were taken at high resolution the area of the tongue can be magnified and cropped from the original image and still show reasonable detail. As the sections from the Visible Humans were precisely cut at constant intervals (1 mm for the male and .33 mm for the female) the serial axial images can be reconstructed into any other plane by computer programs. An example of reconstructed tongue images are shown for the sagittal plane and coronal plane. A, anterior; S, superior.
The male was sectioned at 1 mm intervals resulting in a total of 1,871 sections. Each cryosection was photographed at a resolution of 2,048 × 1,216 pixels with a color depth of 24 bytes, resulting in a data file of 7.5 megabytes for each section (Fig. 1). For the female the section interval was decreased to .33 mm. This interval equals the pixel size in the X and Y-axis so that the X, Y and Z planes were equal, making it easier to develop 3-dimensional reconstructions. There are 5,189 images in the visible female data set, and the total amount of data exceeds 40 gigabytes. In August 2000 a second data set of higher resolution digital photographs was released for the human male. Each is a 70mm photograph digitized at 4,096 × 2,700 pixels in RAW data format with a total file size of 31.8 megabytes each.
The images from the visible human data have the advantages that the original axial photographs can be reconstructed by computer programs into other anatomical planes. Therefore, the same specimen can be examined with serial sections from many different anatomical planes of section (Fig. 1). This has proven to be uniquely valuable, as the extremely complex muscular anatomy of the tongue requires these multiple views. An additional advantage of the visible human data is that it originates from intact cadavers so the tongues are in their natural, in vivo position. This advantage is easy to underestimate, however normal histological processing of excised tongues introduces many artifacts that further complicate the interpretation of the images.
Some computer science departments have developed software specifically to reconstruct the visible human data in various forms. One of the most useful for studying the tongue is the Visible Human Slice and Surface Viewer (VHSSV) located at the Computer Science Department of the Ecole Polytec Federale De Lausanne in Lausanne Switzerland (Director, Professor R.D. Hersch). By accessing their internet site (http://visiblehuman.epfl.ch/) the user can retrieve reconstructed images from the Visible Human data sets in any anatomical plane. Using the VHSSV, total reconstructions of the male and female tongue were retrieved in the coronal, sagittal and axial planes. The clearest tongue data set is that of the female, and the frontal serial section data was used for the 3-D computer reconstruction. Unfortunately, the male has multiple teeth missing from the left side and the tongue is deformed in this area.
3-Dimensional Tongue Model: Surfdriver (http://www.surfdriver.com)
Tongue anatomy is inherently 3-dimensional and it is difficult to understand this anatomy from 3-dimensional images alone. This structure is best communicated by 3-dimensional models, and to our knowledge there are no 3-dimensional models available for the human tongue. Until recently, 3-dimensional reconstructions required the use of expensive software running on specialized computer workstations. A recently released program, Surfdriver (Developed at the University of Hawaii and the University of Alberta by Drs Scott Lazanoff and David Moody, respectively) allows construction of 3-dimensional models from serial sections on a personal computer. The 3-dimentional models could provide valuable anatomical data on the individual tongue muscles which are useful not only for researchers, but also for clinicians and students.
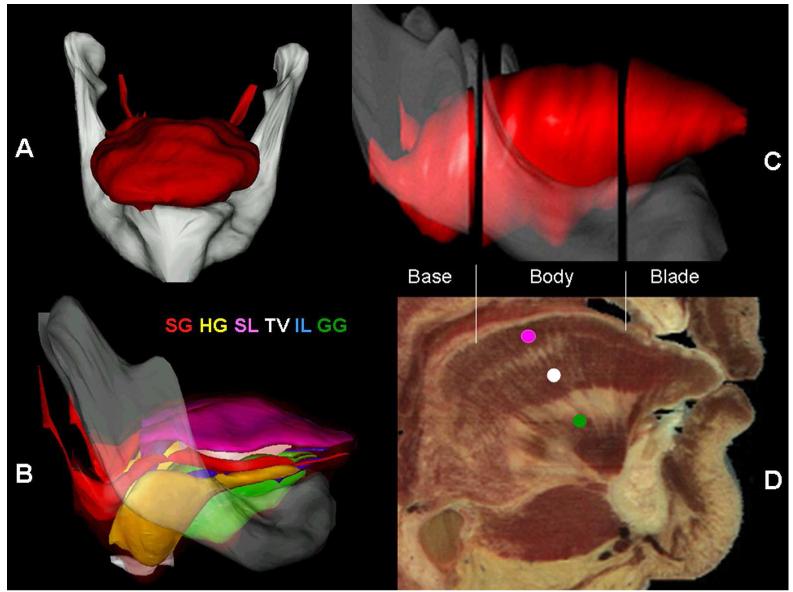
The 3-dimensional model of the tongue was reconstructed from 70 equally spaced digital coronal sections of the visible female data set obtained from the VHSSV (Fig. 2). All images were in jpg format and in direct alignment. Each image was opened in Surfdriver and the regions of interest (ROI) were manually outlined with a closed polygon. As the cross sectional perimeters of each tongue muscle was manually traced, the reproducibility of the method was tested. Each of the tongue muscles or muscle groups was outlined by two investigators, respectively. We revealed a high interobserver agreement (>96%) for demarcation of each muscle or muscle group on the cross sections of the visible humans. After entering all outlines, each ROI was individually reconstructed into a 3-dimensional object. The ROIs were the mandible, the tongue mucosa, the superior longitudinal (SL) muscle, IL, geniohyoid (GH), GG, HG, and styloglossus (SG) muscles, and the midline fascial septum of the tongue. The V and T muscles were outlined as a single unit (V/T) as they were too closely intertwined to be outlined separately. Overlapping volumes are difficult to trace and display so that the model reflects those areas where a muscle exists as a distinct entity. Therefore areas of overlap between muscle groups are ignored, as it would make the model overly complex. For example, the GG muscle has fascicles that pass through practically every other muscle. A different color was given to each muscle to easily differentiate between them (Fig. 2).
Fig. 2.
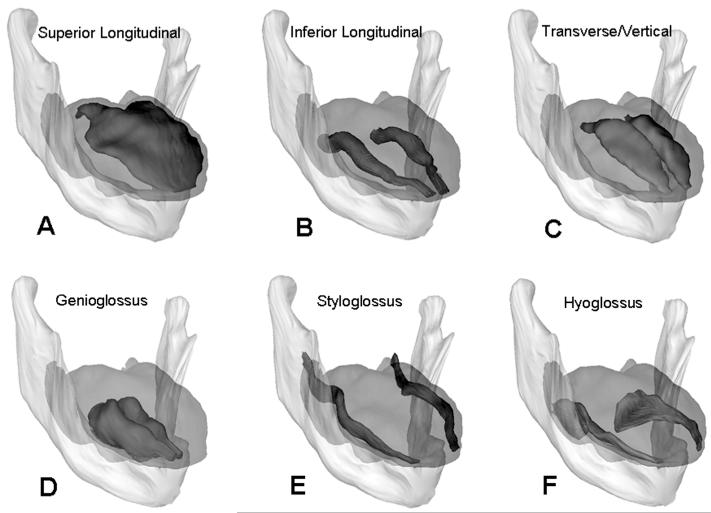
Three dimensional model of the human tongue. (A) Anterior view of the 3-dimensional model of human tongue and mandible. (B) Lateral view of 3-D model with the surface removed. Note that each muscle is rendered in a different color and the legend for each tongue muscle color is shown above the tongue. SG, styloglossus; HG, hyoglossus; SL, Superior longitudinal; TV, transverse/vertical; IL, inferior longitudinal; GG, genioglossus. (C) Lateral view of the 3-D model with the thee boundaries shown between the base, body and blade. (D) A mid-sagittal view of the Visible Human showing the approximate boundaries between base, body and blade, and each muscle marked with its abbreviation in the muscle’s color in the 3-D model.
The Surfdriver software has several features that are useful for manipulating the image to highlight different aspects of tongue structure. One feature is the ability to render any structure invisible, thereby allowing visualization of interior structures. The amount of translucency can be adjusted so that an object such as the surface of the tongue can be retained as a ghost outline that allows the muscles to be seen in their normal position relative to this key landmark. Another feature is that the model can be rotated into any position, magnified or reduced, or has the surface of objects replaced with various textures or colors. One disadvantage of the model is that it is difficult to render muscles with the orientation of the muscle fibers displayed on the surface.
Thick Human Tongue Sections
The images from the visible human are almost too clear and properly aligned to be used as a guide to histological sections, which lack clear landmarks and are almost always skewed and misshapen. A depiction of anatomy intermediate between the visible human and histological sections was obtained from thick tongue sections. Three tongues obtained from autopsies (2 males, ages 55 and 75, and 1 female, age 60) performed at the Mount Sinai Hospital were fixed in formalin and sectioned in the coronal plane at thicknesses from 1-3 millimeters on a rotary microtome. The thick sections were processed as whole mounts by acetylcholinesterase staining as they were initially prepared to study motor endplate locations within the muscles. Although the staining was unsuccessful the process rendered the thick sections translucent. When examined by transilluminated light these sections add a dimension of depth that provides some helpful 3-dimensional information. Moreover, the sections could be examined by dissecting microscopes at higher resolutions than the photographs from the visible human data. In addition the sections have some of the artifact that is present to a much greater extent in histological sections: although they were carefully aligned during sectioning, there is some obliquity introduced during the sectioning process so that one side is slightly more anterior than the other. In some parts of the tongue this introduces surprisingly large anatomical asymmetries. In addition to the asymmetrical artifacts introduced during sectioning, the sections unfold to some degree. In the dorsum of the tongue a large connective tissue boundary keeps the muscles in their original position. However, in the inferior tongue, around where the GG muscle enters the tongue, the muscles are not as well restrained and their position shifts relative to their original in vivo locations.
These technical issues were evident in histologic sections. The sections were between 10 and 50 micrometers in thickness and were more malleable than the thick sections described above. Large technical artifacts were present despite great care in orienting and mounting the specimens correctly on the microtome.
RESULTS AND DISCUSSION
The main goal of this study was to provide a guide to the muscular anatomy of the human tongue. Using images of the tongue from the Visible Human Project (Fig. 1) a 3-dimensional model of the tongue and its muscles was constructed (Fig. 2). Below is a review of terminology regarding the tongue’s anatomy and movements (Figs. 3-5), followed by descriptions of the shape and position of each muscle from the 3-dimensional model (Figs. 2-4) and from serial sections in the coronal plane (Fig. 6), sagittal plane (Fig. 7), and axial plane (Fig. 8).
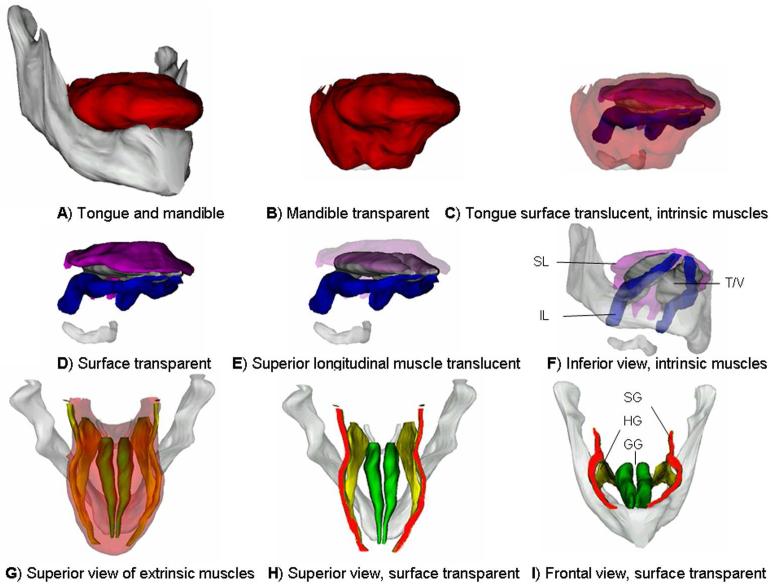
Fig. 3.
This figure shows the positions of the intrinsic and extrinsic muscles in the tongue and also demonstrates the options available for presenting anatomical data with the 3-dimensional model. (A) An oblique frontal view of the complete tongue model including the mandible. (B) The tongue is now seen in the same orientation as A and the mandible is made transparent. (C) The tongue surface is made translucent so that the muscles inside the tongue can be seen. The amount of translucency can be adjusted. The ghost outline of the tongue surface serves as a reference for the internal muscles, although it does obscure them to some extent. (D) The tongue surface is made transparent. Now the tongue muscles can be clearly seen. In this orientation the superior longitudinal (SL) muscle drapes over the transverse/vertical (T/V) muscle group. (E) The SL muscle is made translucent. (F) As the muscles all overlap in E the view is still not optimum so that the model is rotated to an oblique inferior view and the transparent mandible is restored to aid in orientation. (G) The extrinsic muscles are seen from above through a translucent surface. (H) The tongue surface is removed and the muscles are clearly seen. (I) Rotation of the model to a frontal view helps to show the spatial relationship between the muscles. GG, genioglossus muscle; HG, hyoglossus muscle; IL, inferior longitudinal muscle; SG, styloglossus muscle.
Fig. 5.
Movements of the human tongue. The human tongue is capable of changing its shape in multiple ways, however some basic patterns are often described and are a source of confusion. The upper left corner shows the 3D model in relation to the mandible and the upper right corner reproduces this is a simpler schematic form. This is the rest or neutral position and is the reference for all the movements described. When the tongue is moved posterior it is said to retract and the various ways that this can be done are shown in column 1. Retrusion is the posterior movement of the whole tongue with little change in shape. Retrusion is probably performed by extrinsic muscles, specifically the hyoglossus (HG) and styloglossus (SG). However, the appearance of retraction can be obtained by changing tongue shape so that the blade shortens. This shortening is likely due to the action of the muscles that are oriented lengthwise, primarily the superior (SL) and inferior longitudinal (IL) muscles. As the tongue’s volume is constant shortening causes the tongue to become thicker. In actual tongue movements retraction is usually cause by combining retrusion with shortening. When the tongue moves anteriorly in the mouth is said to protrude (column 2). Anterior movement can be done by moving the entire tongue forward (protrusion), which is most likely causes by the posterior fascicles of the genioglossus (GG) muscle. However, a similar appearance can be obtained by elongating the tongue. Elongation is caused by contraction of the muscles oriented in the coronal plane, specifically the vertical and transverse (T/V) muscles. In column 3 basic bending motions of the tongue are shown. Dorsiflexion is the superior bending of the tongue tip. This is likely caused by contraction of the SL muscle. Ventroflexion is the inferior bending of the tongue tip, most likely caused by IL and/or combined longitudinal muscle contractions. Retroflexion is the superior movement of the tongue base. This movement combines the superior and posterior pull of the SG muscle aided by depression of the midtongue by vertically oriented muscle fascicles of the GG.
Fig. 4.
Muscles of the human tongue. (A) The superior longitudinal (SL) muscle is the only unpaired muscle of the tongue which spans the length of the tongue just beneath the mucosa of its superior surface. (B) The inferior longitudinal (IL) muscle spans the length of the tongue just above the mucosa of its inferior surface. (C) The transverse and vertical (T/V) muscles. The T muscle connects the medial septum to the lateral aspect of the tongue. The V muscle connects the inferior surface to the superior surface. (D) The genioglossus (GG) muscle is midline muscle which originates from the posterior surface of the mandible. (E) The styloglossus (SG) muscle originates from the styloid processes and insert along the inferior-lateral margins of the tongue. (F) The hyoglossus (HG) muscle originates from the hyoid bone and also insert into the inferior-lateral margins of the tongue. The muscles are all rendered as separate objects for the sake of clarity, but in reality there is extensive overlap. One example is that the GG muscle becomes the vertical muscle in the medial third of the tongue. The other example is that the T/V muscles are rendered as a single object.
Fig. 6.
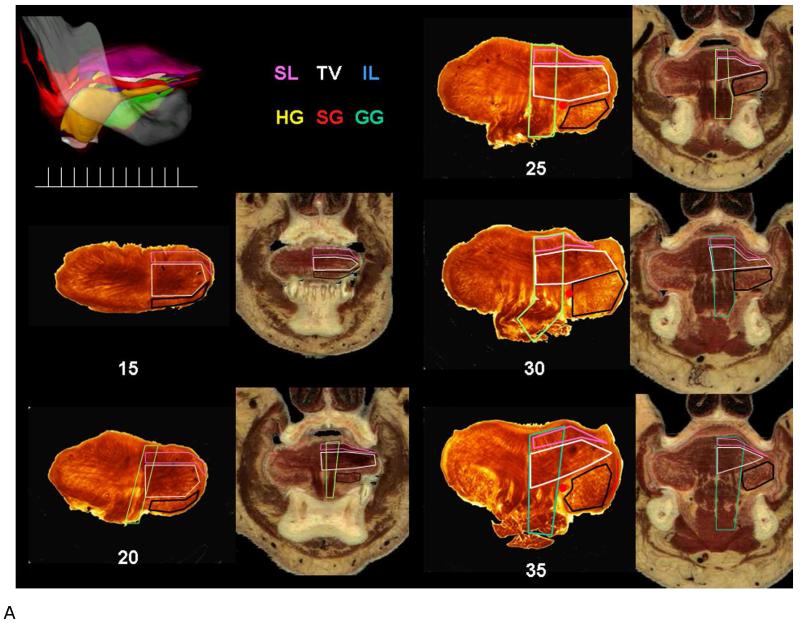
A. Coronal sections of the tongue blade and anterior tongue body. Human tongue muscles are seen best in the coronal plane as most muscles of the tongue are oriented lengthwise and, except for the tongue base, they are seen in cross section. In the left upper corner the lateral view of the 3D model is shown with the color legend for each muscle to its right. Beneath the 3-D model is a line with markers showing the approximate location of the twelve coronal sections seen in this figure. The twelve samples of the human tongue in the coronal plane are arranged from anterior to posterior levels. At each level the thick human tongue (HT) section is shown on the left and the reconstructed images of the visible human (VH) male are shown on the right. Levels are marked by their distance in millimeters from the tongue tip beginning at 10 mm. On the right side of each specimen the muscles are outlined with lines of the same color used for that muscle in the 3-dimensional model. One exception is the combined longitudinal muscle (CL) which is not present in the 3D model and is outlined in black in the sections. Tongue blade (0-15 mm): The tongue blade extends from the tip of the tongue to the frenulum. The anatomy within the human tongue blade most resembles that of other mammals. Specifically, the structure is relatively simple and symmetrical. Longitudinally oriented muscle fascicles line the perimeter of the tongue while the central core contains muscle fascicles oriented in the cross sectional plane. As this basic pattern is present throughout the tongue blade only a single specimen from this level is included (15 mm). Note that the thick sections is skewed such that the left side of the section is from a more posterior level than the right and the size of the left side is therefore larger than the right side. The superior longitudinal (SL) muscle is relatively thin in the tongue blade. Below the SL the transverse and vertical (TV) is quite prominent. Inferior to the TV is the CL, a combined longitudinally oriented muscle largely formed by the inferior longitudinal (IL) and styloglossus (SG). Anterior tongue body (20-35 mm): In this study the tongue body refers to the part of the tongue extending from the frenulum anteriorly to the sulcus terminali posteriorly. The tongue body can be divided into an anterior half, which is shown in this panel (20-35mm), and a posterior half, which is seen in Fig. 6B. The internal anatomy of the tongue body is markedly different from the blade due to the presence of the genioglossus (GG), and the size of the GG as well as the degree to which it is cut obliquely is helpful in identifying the anterior-posterior level of the section. The tongue body has a distinctive mushroom shape: the simple structure of the tongue blade appears to be elevated and displayed by an increasingly enlarging GG muscle. Throughout the tongue body two rows of thick GG fascicles are seen. Anteriorly in the tongue body the GG fascicles are vertically oriented and in coronal sections they are cut lengthwise. The GG fascicles pass through the TV and SL to insert into the dense connective tissue of the dorsum. In most sections the full course of the GG is obscured by the TV but at least in the 25 mm VH and HT sections, some of the GG fascicles can be seen for their entire length. At progressively more posterior levels the GG muscle is seen at more oblique angles and until it appears in cross section at the base of the tongue (compare level 25mm with 50mm). Although a fascial plane called the paramedian septum separates the GG from the CL, it is often difficult to find this plane in histological sections. A good landmark is the lingual artery (red dot) which passes between the GG and CL in the anterior tongue body, and between the GG and the IL in the posterior tongue blade.
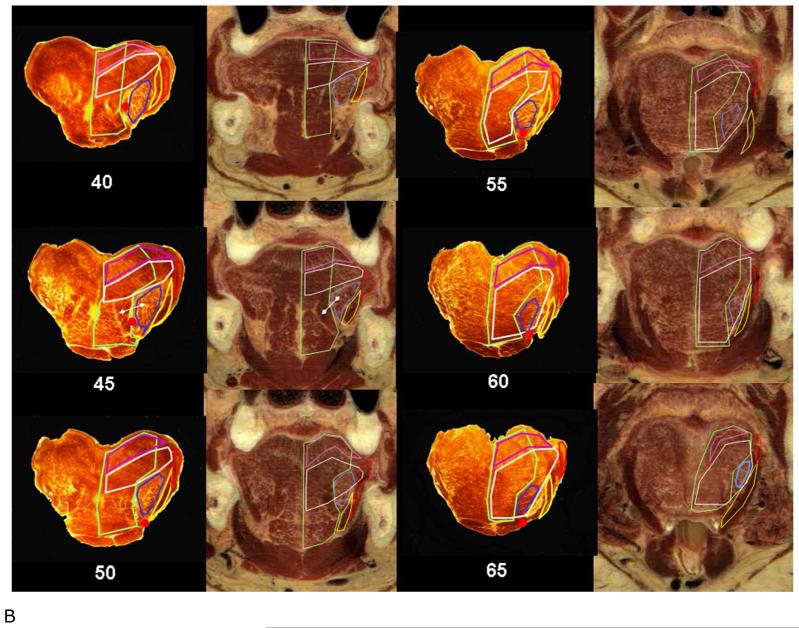
B. Coronal sections of the posterior tongue body and tongue base. Posterior tongue body (40-50 mm): Superiorly, the SL is very sharply delineated and is thickest in the posterior body (see 50 mm). Directly below the SL is the combined TV. The TV is also well demarcated superiorly and inferiorly, although it becomes more obscure laterally. In contrast to the SL, the TV is larger in the anterior tongue body. The CL is a single mass until about 40mm when its individual components begin to separate. The IL is the most difficult muscle to appreciate, and is best seen as a separate muscle at about VH sections 45-55mm. The GG has a large distinct fascicle in its supero-lateral margin that is easy to mistake for the IL (white line). Tongue base (55-65 mm): The tongue base begins at the level of the circumvallate papillae. Overall the structure of the tongue deteriorates as the muscles divide into smaller fascicles and the amount of fat and other soft tissue increases. A midline groove appears in the superior surface of the tongue and progressively enlarges until the tongue separates into two halves posteriorly. This groove is much more pronounced in the HT sections, and may be an artifact of intubation. The transverse muscle is largest in this area and those of the GG and IL separate its fascicles as they pass to insert into the tongue base. The vertical muscle is small in this region. In contrast, the HG and SG muscles are best seen in this area as they are sectioned close to their origins.
Fig. 7.
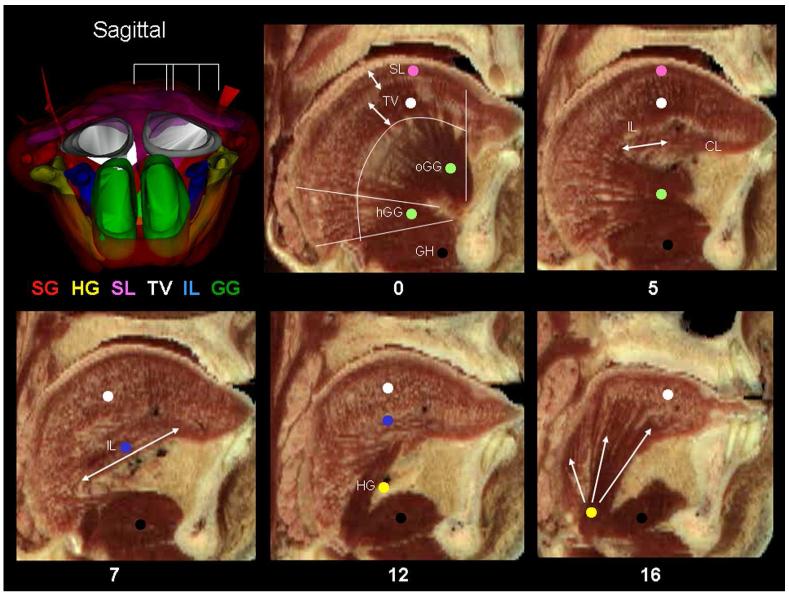
Sagittal sections of the human tongue. This figure shows the appearance of the VH male tongue in the sagittal plane beginning in the mid sagittal plane. Subsequent sections are not evenly spaced but were chosen for to demonstrate specific muscles. The striking aspect of the sagittal view is the curvature of the human tongue. The GG muscle is organized in a fan shape to insert throughout the tongue. At the GG origin a tendon extends from the mandible to allow room for this large muscle. Except for the horizontal GG the fascicles of the GG appear to be of equal length. The overall organization is neat and simple: two muscle groups, the SL and TV follow the curvature of the tongue throughout its length. Both muscles are sharply demarcated throughout most of their lengths. Note that the density of muscle of all types is least in the tongue base. In the 0 mm section, the double headed arrows mark the boundaries of the SL and TV muscles. The SL is thickest in the posterior tongue body while the TV is thinest in the same area. The TV depth is greatest in the tongue base but its density is the least there. The extent of the GG is delineated by the long curved line. The GG has two bellies, the horizontal GG (hGG) inferiorly and the fan shaped oblique GG (oGG) superiorly. The geniohyoid (GH) muscle is beneath the hGG and it is difficult to separate the two muscles. In the 5 mm section, as the sections go laterally the GG disappears anteriorly and is progressively replaced by the CL. The CL is partly composed by the IL which has a small medial part that is horizontally oriented. In the 7 mm section, a lateral, larger part of the IL is oriented more obliquely. Note that the IL does not follow the curvature of the tongue. In the 12 mm section, the HG originates from the hyoid bone and its initial course is lateral to the IL. In the 16 mm section, we discern at least 3 distinct parts of the HG: 1) A small posterior group of fascicles passes superiorly at the tongue base and eventually merge or are continuous with fascicles of the SL muscle; 2) The largest part of the HG inserts into the lateral edge of the tongue; 3) A small part of the HG courses superior and anterior to merge into the CL.
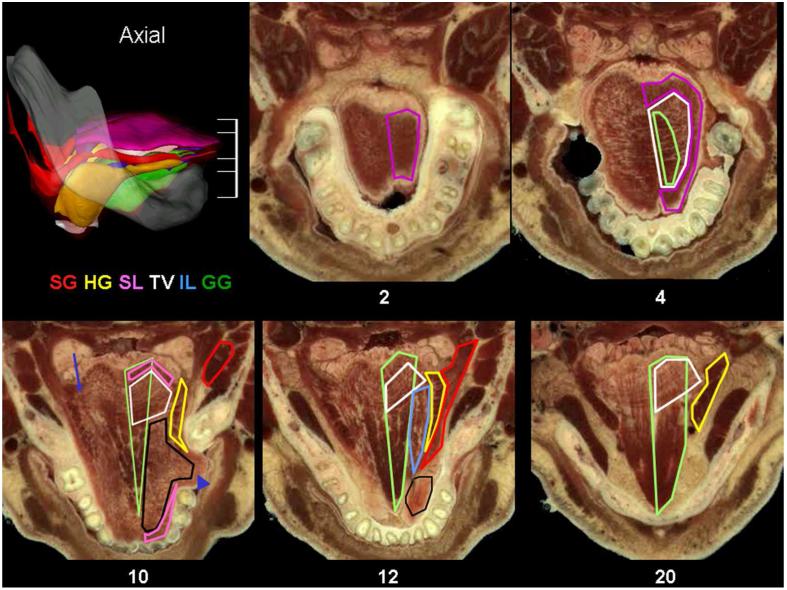
Fig. 8.
Axial sections of the human tongue. The 3D model of the human tongue in the lateral view with the level of five axial sections marked. Sections are arranged from superior to inferior and labeled in mm relative to the most superior part of the tongue. In the 2 mm section the plane passes through the SL. The SL is composed of longitudinally arranged muscle fascicles, yet they interweave so extensively that this longitudinal orientation is not evident in it’s gross appearance. At the 4 mm level most of the tongue is the TV with the SL which is seen around the edges. In the axial plane the transverse muscle is sectioned lengthwise and is more evident then the vertical muscle. Fascicles of the transverse muscle originate from the midline septum and insert into the lateral boundaries of the tongue. A darker area next to the septum reflects the presence of GG muscle fascicles, which compose the vertical muscle in this area and are notably denser than the remainder of the vertical muscle. The 10 mm level passes through middle of the tongue and all tongue muscles are present. Note that in this specimen a regional expansion of the lateral tongue has filled the defect left by the loss of molar teeth (arrowhead). The GG is sharply delineated as a separate entity; it is shaped like a wedge and is composed of thick fascicles arranged in rows. Lateral to the GG the CL is quite prominent. Although this axial plane is below most of the TV, due to the tongue’s curve the TV is seen in the tongue base. A thin rim of SL is present anteriorly and posteriorly. The HG is cut in cross section and appears as a planar muscle with well defined dark muscle fascicles. Finally, the SG is seen close to its origin from the stylohyoid bone on the right side. However in the left side, which is at a lower level, some fascicles of the SG appear to pass through the HG to become transverse muscle fascicles (arrow). At the 12 mm level the most notable changes are that the courses of the IL and SG are seen. The SG has a posterior part that blends into and possible passes through the HG, and an anterior part that joins with the IL to form most of the CL. At the 20 mm level the entire course of the horizontal GG can be seen.
Description of Tongue Muscle Anatomy
The anatomy of each tongue muscle will be described from anterior to posterior in serial coronal sections. The 3-D rendering of each muscle is shown in Figures 3 and 4. Serial coronal sections of the tongue are in Figure 6. Figures 7 and 8 show serial sections from the sagittal and axial planes, respectively.
Some intrinsic tongue muscles overlap so extensively that they are sometimes best considered as single entity rather than separate muscles. The most important example is the T/V muscles where we term the combined entity the T/V. A second important example is in the inferior aspect of the anterior tongue where four of the longitudinally oriented tongue muscles (IL, GG, HG, and SG) interdigitate and become indistinguishable. For convenience we have named this the combined longitudinal muscle (CL).
Extrinsic Tongue Muscles
Genioglossus (GG) muscle (Figs. 2, 3G-I, 4D, 6-8)
Although it is absent from the tongue blade the GG muscle’s large volume, central position, and its passage though most of the intrinsic muscles makes the GG muscle the most important landmark in the coronal plane. As the GG muscle fascicles are oriented at different angles, the degree to which its fascicles appear vertical or horizontal indicates whether a coronal section is from the anterior or posterior aspect of the tongue, respectively.
The GG are paired muscles that originate from the inner midline of the mandible and fan out in a 90° arc. All GG muscle fascicles enter the intrinsic tongue and pass between fascicles of the T/V and SL muscles and terminate in the connective tissue of the dorsum. As such the GG actually constitutes the V muscle in these areas. The lateral fascicles of the oblique GG do not pass directly vertical but course slightly obliquely, and this oblique orientation increases in the tongue base. When it is relevant to distinguish the two parts of the GG, the part of the GG outside the tongue we term the extrinsic GG, and the part within the tongue the intrinsic GG. Although the intrinsic and extrinsic components of the GG appear to be continuous, the components are innervated by different branches of the hypoglossal nerve and may actually be composed of separate muscle fibers arranged in series.
Anteriorly the GG is thin and its fascicles are vertically oriented. The most anterior fascicles compose the frenulum, an external landmark that defines the border between the tongue blade and the tongue body (Figs. 6-8). In coronal sections the anterior GG muscle is narrow, its fascicles are thin and they are sectioned lengthwise. In sections from more posterior levels the entire GG muscle as well as its fascicles become wider and change their orientation from vertical to oblique (Fig. 8).
The most posterior part of the GG has long been considered a separate muscular compartment called the horizontal, and it is separated from the remaining oblique GG by a thin layer of connective tissue (Doran and Baggett, 1972). The horizontal compartment is anatomically simple, it originates from the mandible and courses directly posterior to insert into the hyoid bone or connective tissue above it. The horizontal compartment is believed to function largely in the respiratory actions of the tongue, moving the hyoid and base of tongue anteriorly during inspiration to dilate the airway (Mu and Sanders, 2000).
The oblique compartment of the GG is quite different from the horizontal compartment (Doran and Baggett, 1972; Mu and Sanders, 1999, 2000, 2010). The oblique GG has two parallel rows of large distinct muscle fascicles. These fascicles fan out from their origin on the mandible to insert throughout most of the length of the tongue. The oblique GG is thin anteriorly and becomes progressively wider toward the posterior aspect of the tongue. Around the centerline of the tongue the fascicles of the GG pass directly between the fascicles of the T and SL muscles to insert into the dorsal mucosa of the tongue.
The GG is surrounded by the paramedian septum and is bordered laterally by the IL and HG muscles. The GG is the muscle that is the most different in humans relative to other mammals. In dogs the GG is a thin sheet that is restricted to the midline of the tongue (Mu and Sanders, 1999, 2000). In humans the GG is much larger and has expanded its insertion laterally, especially in the tongue base. The main role ascribed to the GG is tongue protrusion, caused by the horizontal compartment and the posterior fascicles of the oblique compartment (Mu and Sanders, 2010). Additional actions believed to be performed by the GG are: depression of the body of the tongue by the more vertically oriented fascicles in the middle of the oblique GG; ventroflexion and possibly retrusion of the tongue tip by the most anterior muscle fascicles. In addition to these actions, the insertion of the GG around the midline of the tongue dorsum is believed to cause the concave cross sectional shape of the superior tongue surface that is a distinctive feature of speech and swallowing movements.
Hyoglossus (HG) muscle (Figs. 2B, 3G-I, 4F, 6B, 7, 8)
The HG originates from the body and greater cornu of the hyoid bone. The HG is bordered by the GG and IL muscle medially and the SG muscle laterally. It is thin planar muscle near its origin but separates into distinct fascicles that fan out and insert along the length of the tongue. The most posterior fascicles of the HG course superiorly and medially across the posterior surface of tongue base and appear to be continuous with the origin of some of the SL muscle. The middle fascicles of the HG course obliquely superiorly and insert on the lateral edge of the tongue. These fascicles appear continuous with the insertion of some fascicles from the T and GG muscles. The anterior fascicles of the HG course anteriorly along the lateral edge of the tongue to insert into the CL which in turn inserts into the tongue cap. The action of the HG is retrusion and depression of the lateral margin of the tongue.
Styloglossus (SG) muscle (Figs. 2B, 3G-I, 4E, 6B, 8)
The SG originates from the styloid process and stylomandibular ligament and is the most lateral of the tongue muscles. It appears to have two components: a smaller posterior compartment merges into and possibly passes through the lateral surface of the HG muscle at the tongue base; and a larger anterior compartment courses along the lateral aspect of the and merges with the IL muscle and other muscles to form the CL (Figs. 7 and 8). The SG is thin and comparatively small and is often difficult to distinquish from the larger HG muscle. The action of the SG is retrusion and elevation of the lateral margin of the tongue.
Palatoglossus (PG), glossopharyngeus (GP), chondroglossus (CG) muscles
At least three small muscles (PG, GP and CG) insert into lateral margin of the tongue other than the SG and HG. These muscles are generally not thought to significantly contribute to tongue movement (Pernkopf Anatomy, 1989) and were not included in the 3-D model. In Pernkopf Anatomy (1989), these muscles are not considered to be extrinsic tongue muscles or even as distinct muscles at all and will only be briefly mentioned.
The PG muscle, also called the GP muscle, arises from the anterior surface of the soft palate, courses inferiorly to compose the palatopharyngeal arch, and inserts into the lateral margin of the tongue. It originates from the aponeurosis of the soft palate and inserts into the lateral margin of the tongue just medial to the SG muscle. Some of its fibers spreading over the dorsum, and others passing deeply into the substance of the organ to intermingle with the T muscle.
The GP muscle is the most inferior part of the SPC muscle, and it inserts medial to the PG muscle. The CG muscle is sometimes described as a part of the HG muscle, but is separated from it by a lateral fascicle of the GG muscle that passes to the side of the pharynx. It arises from the medial side of the lesser cornu and passes directly upward to blend with the intrinsic muscular fibers of the tongue, between the HG and GG muscles.
Intrinsic Tongue Muscles
Superior longitudinal (SL) muscle (Figs. 2B, D; 3E, F; 4A, 6-8)
The SL muscle spans the length of the tongue just beneath the mucosa of the superior surface of the tongue. It is well demarcated in coronal sections as it is separated from the T muscle inferiorly by a thin layer of connective tissue. Grossly the SL appears as to be a muscular sheet that is thickest in the posterior tongue body and thins to insert into the tongue cap anteriorly, and connective tissue and possibly hyoid bone posteriorly. On closer examination the SL is composed of many individual fascicles oriented longitudinally (Mu and Sanders, 1999, 2010). Between these fascicles the vertical muscle fascicles pass to reach the connective tissue of the tongue dorsum. The SL is distinctive for its high content of connective tissue that appears to couple it biomechanically to the connective tissue of the tongue dorsum. The SL is thick in the posterior body of the tongue but thins anteriorly where it inserts into the cap and also posteriorly in the tongue base. Contraction of the SL shortens the tongue and also dorsiflexes the tip of the tongue. The SL is perhaps the easiest muscle to identify in frontal sections.
Inferior longitudinal (IL) muscle (Figs. 2B, 3D-F, 4B, 6B, 7, 8)
The IL muscle originates near the tongue base and passes anteriorly to join the GG, HG and SG to form the CL. The IL appears to have two parts: a smaller part in the tongue blade lies within the CL and is oriented parallel to the long axis of the tongue; the larger part originates from the hyoid and connective tissue and hyoid medial to the origin of the HG muscle. The larger part courses obliquely straight from the tongue base to the blade without following the curvature of the tongue as the SL does. In most mammals the action of the IL is tongue shortening and ventroflexion. In addition in the human the orientation of the larger part seems ideal for retroflexion of the tongue base. Interestingly, the IL is very prominent and easy to identify in the tongues of non-human mammals, but is probably the most difficult muscle to identify in coronal sections of the human tongue.
Transverse (T) muscle (Figs. 2B, D, 3D-F, 4C, 6-8)
The T muscle originates from the median septum and course laterally. The more superior fascicles of the T muscle pass between the fascicles of the SL to insert into connective tissue of the lateral tongue surface. The more inferiorly situated insert on the connective tissue overlying the IL, HG or CL. The T muscle is relatively enlarged in the human in comparison to other mammalian tongues and it extends further anterior and posterior than the vertical muscle. The action of the transverse is to narrow the tongue thereby simultaneously increasing its sagittal depth and causing elongation of the tongue body and blade.
Vertical (V) muscle (Figs. 2B, D, 3D-F, 4C, 6-8)
The V muscle is a continuation of the GG in the medial third of the tongue. More laterally it originates on the connective tissue overlying the IL, HG and CL. The V muscle fascicles pass between the fascicles of the SL to insert into the dorsum of the tongue. The action of the V muscle is to flatten the tongue thereby simultaneously increasing its width and elongating it.
SUMMARY
Human tongue anatomy, while complex, has certain patterns that make it understandable. One helpful way to visualize the human tongue is that it is composed of two basic parts. A wedge shaped GG in the midline separates the tongue muscles into two longitudinal masses. The easiest muscle to identify in any coronal section of the tongue is the SL muscle. A second important landmark is the GG muscle which composes the bulk of the midtongue posterior to the frenulum. Just lateral to the GG muscle is the paramedian septum and the lingual artery, which separate the GG from the IL muscle. This study introduced the novel concept of the combined longitudinal muscles, the longitudinal muscle mass located inferiorly in the anterior half of the tongue. It was used to describe a complex spatial relationship that has been seldom discussed in previous studies.
ACKNOWLEDGMENTS
This research was supported by a Shannon Award and NIH grant R01 DC004684 from the National Institute on Deafness and Other Communication Disorders (to Dr. Ira Sanders) and by NIH grant 5 R01 DC004728 from the National Institute on Deafness and Other Communication Disorders (to Dr. Liancai Mu).
LITERATURE CITED
- Abd-El-Malek S. A contribution to the study of the movements of the tongue in animals, with special reference to the cat. J Anat. 1938a;73:15–30. [PMC free article] [PubMed] [Google Scholar]
- Abd-El-Marek S. Observation on the morphology of the human tongue. J Anat. 1938b;73:201–210. [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya BN. Functional morphology of the tongue muscles of some Indian insect-eating birds. Gegenbaurs Morphol Jahrb. 1985;131:93–123. [PubMed] [Google Scholar]
- Delheusy V, Toubeau G, Bels VL. Tongue structure and function in Oplurus cuvieri (Reptilia: Iguanidae) Anat Rec. 1994;238:263–276. doi: 10.1002/ar.1092380212. [DOI] [PubMed] [Google Scholar]
- Doran GA, Baggett H. The genioglossus muscle: a reassessment of its anatomy in some mammals, including man. Acta Anat. 1972;83:403–410. doi: 10.1159/000143875. [DOI] [PubMed] [Google Scholar]
- Gans C, Gorniak GC. Functional morphology of lingual protrusion in marine toads (Bufo marinus) Am J Anat. 1982;163:195–222. doi: 10.1002/aja.1001630302. [DOI] [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol. 1995;74:547, 555. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- Hellstrand E. Morphological and histochemical properties of tongue muscles in cat. Acta Physiol Scand. 1980;110:187–198. doi: 10.1111/j.1748-1716.1980.tb06650.x. [DOI] [PubMed] [Google Scholar]
- Hellstrand E. Contraction times of the cat’s tongue muscles measured by light reflection. Innervation of individual tongue muscles. Acta Physiol Scand. 1981;111:417–423. doi: 10.1111/j.1748-1716.1981.tb06757.x. [DOI] [PubMed] [Google Scholar]
- Herrel A, Timmermans JP, De Vree F. Tongue flicking in agamid lizards: morphology, kinematics, and muscle activity patterns. Anat Rec. 1998;252:102–116. doi: 10.1002/(SICI)1097-0185(199809)252:1<102::AID-AR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14:413–429. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- Kier WM, Smith KK. Tongues, tentacles and trunks: The biomechanics of movement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–324. [Google Scholar]
- Lombard RE, Wake DB. Tongue evolution in the lungless salamanders, family plethodontidae. I. Introduction, theory and a general model of dynamics. J Morphol. 1976;148:265–286. doi: 10.1002/jmor.1051480302. [DOI] [PubMed] [Google Scholar]
- Lowe AA. The neural regulation of tongue movements. Prog Neurobiol. 1980;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Miller JL, Watkin KL, Chen MF. Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. J Speech Lang Hear Res. 2002;45:51–62. doi: 10.1044/1092-4388(2002/004). [DOI] [PubMed] [Google Scholar]
- Miyawaki K. A study of the musculature of the human tongue. Ann Bull Res Inst Logoped Phoniatrics. 1974;8:23–50. [Google Scholar]
- Mu L, Sanders I. Neuromuscular organization of the canine tongue. Anat Rec. 1999;256:412–424. doi: 10.1002/(SICI)1097-0185(19991201)256:4<412::AID-AR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Mu L, Sanders I. Neuromuscular specializations of the pharyngeal dilator muscles: II. Compartmentalization of the canine genioglossus muscle. Anat Rec. 2000;260:308–325. doi: 10.1002/1097-0185(20001101)260:3<308::AID-AR70>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mu L, Sanders I. Human tongue neuroanatomy: Nerve supply and motor endplates. Clin Anat. 2010;23:777–791. doi: 10.1002/ca.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa KC, Kier WM, Smith KK. Morphology and mechanics of tongue movement in the African pig-nosed frog Hemisus marmoratum: a muscular hydrostatic model. J Exp Biol. 1999;202:771–780. doi: 10.1242/jeb.202.7.771. [DOI] [PubMed] [Google Scholar]
- Pernkopf Anatomy: 1989. Platzer Werner. Atlas of Topographic and Applied Human Anatomy. vol 1: Head and Neck. Urban & Schwarzenberg; Baltimore, MD: Third edition edited by. [Google Scholar]
- Sonntag CF. The comparative anatomy of the tongues of the mammalia. XII. Summary, classification, and phylogeny. Proc Zool Soc Lond. 1925:701–762. [Google Scholar]
- Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modeling. J Speech Lang Hear Res. 2001;44:95–107. doi: 10.1044/1092-4388(2001/009). [DOI] [PubMed] [Google Scholar]