Abstract
Reduced monocyte infiltration into the vessel wall and increased macrophage cholesterol efflux are critical components in atherosclerotic plaque regression. During inflammation, monocyte chemotactic protein 1 (MCP-1) signaling activation and cholesterol deposition in macrophages induce endoplasmic reticulum (ER) stress, which promotes an increased inflammatory response. Increased macrophage ER stress shifts macrophages into an M2 macrophage phenotype with increased cholesterol uptake and deposition. In type 2 diabetes, a population with elevated baseline risk of cardiovascular disease (CVD), vitamin D deficiency doubles that risk. We have found that 1,25-dihydroxy vitamin D [1,25(OH)2D] prevents foam cell formation during macrophage differentiation by suppressing ER stress. However, it is unknown whether suppression of ER stress by 1,25(OH)2D decreases monocyte infiltration and reverses atherogenic cholesterol metabolism in previously-differentiated, vitamin D-deplete macrophages. We collected peripheral monocytes from type 2 diabetic patients and differentiated them into macrophages under vitamin D-deplete or 1,25(OH)2D-supplemented conditions. 1, 25(OH)2D supplementation suppressed macrophage migration in response to MCP-1 and mRNA expression of chemokine (C-C motif) receptor 2 (CCR2), the MCP-1 receptor, compared to vitamin D-deplete cells. Furthermore, inhibition of ER stress with phenyl butyric acid resulted in similar effects even in vitamin D-deplete cells, while induction of ER stress with Thapsigargin under 1,25(OH)2D-supplemented conditions increased macrophage migration and CCR2 expression, suggesting that the effects of vitamin D on migration are mediated through ER stress suppression. To determine whether the detrimental pattern of macrophage cholesterol metabolism in vitamin D depletion is reversible, we assessed cholesterol uptake in macrophages differentiated under vitamin D-deplete conditions as described above, then supplemented with 1,25(OH)2D or maintained in vitamin D-deplete conditions. Cholesterol uptake was decreased in 1,25(OH)2D-supplemented compared to vitamin D-deplete cells, suggesting slowed cholesterol deposition with active vitamin D. 1,25(OH)2D supplementation also suppressed cholesteryl ester formation and enhanced cholesterol efflux in M2 macrophages compared to vitamin D-deplete cells, suggesting facilitation of cholesterol egress in the presence of 1,25(OH)2D. We thus provide further evidence that active vitamin D is an ER stress reliever that may have a role in atherosclerotic plaque regression.
Keywords: Vitamin D, macrophage phenotype, migration, cholesterol metabolism, ER stress, diabetes
1. Introduction
In recent years, vitamin D has been shown not only to be important for bone and calcium metabolism but also for homeostasis of critical tissues involved in vascular disease in patients with type 2 diabetes. In diabetics, the prevalence of deficiency of 25-hydroxy vitamin D [25(OH)D], the principal storage form of vitamin D, is almost twice that for nondiabetics, and low vitamin D levels nearly double the relative risk of developing CVD compared to diabetic patients with normal vitamin D levels [1–3]. A growing body of evidence from animal and human studies shows that vitamin D improves peripheral insulin action, suppresses the renin-angiotensin system, decreases systemic inflammatory mediators of vascular disease, and prevents foam cell formation [4–7], revealing the influence of vitamin D on multiple known mechanisms responsible for the increased vascular inflammation seen in diabetic patients.
Atherosclerotic plaque progression depends on the accumulation of monocyte-derived cells within the plaque. This process results from the imbalance of monocyte recruitment, macrophage survival within the plaque, and macrophage egression capabilities. In ApoE−/− mouse models of atherosclerosis, ApoE rescue lowers plasma cholesterol and increases HDL, leading to plaque regression mediated by suppression of monocyte recruitment with stable rates of macrophage apoptosis [8]. In a surgical murine model, transplantation of plaque-bearing ApoE−/− aortae into wild-type mice results in rapid plaque regression mediated by emigration of macrophages expressing CCR7, an M1 macrophage marker [9]. Monocytes recruited to the subendothelial space respond to environmental signals such as cytokines and modified cholesterol to stimulate differentiation into macrophages with diverse functional programs. Interferon (IFN)γ induces the M1 macrophage subtype, characterized by proinflammatory cytokine production to accelerate additional immune cell recruitment [10]. Interleukin (IL)-4, IL-10, and immunocomplex plus lipopolysaccaride (IC) induce the multiple M2 macrophage subtypes, more heterogeneous cells with both pro- and anti-inflammatory functions [11–13], but all with increased cholesterol uptake and cholesteryl ester formation [14]. In mouse models of atherosclerosis, alteration of the cytokine microenvironment triggers the conversion of macrophage subtypes already present in the lesion and changes their location within the plaque [15]. We have demonstrated that ER stress is a functional switch controlling macrophage differentiation in diabetics; suppression of ER stress shifts M2-predominant macrophages to M1-predominant cells and decreases foam cell formation [14]. We have also showed that 1,25(OH)2D suppresses macrophage ER stress. This leads to M1-predominant macrophage differentiation and prevention of foam cell formation through downregulation of scavenger receptors CD36 and SRA-1 [7, 16], suggesting that vitamin D promotes an anti-atherogenic macrophage phenotype. Recently, we found that 25(OH)D deficiency in diabetics is also associated with increased monocyte ER stress, leading to similarly M1-predominant markers and increased monocyte adhesion to the endothelium [16]. However, it is unclear whether suppression of ER stress by vitamin D affects mechanisms implicated in plaque regression, including suppression of monocyte infiltration and shift of differentiated macrophages toward a phenotype with lower cholesterol content.
2. Materials and Methods
Subjects with type 2 diabetes were voluntarily recruited for a single venous blood draw and provided written informed consent, approved by the Human Research Protection Office of Washington University School of Medicine. Peripheral monocytes were isolated by standard Ficoll technique and selected for CD14 marker positivity (Miltenyi Biotec). To induce differentiation into vitamin D-deplete or 1,25(OH)2D-supplemented macrophages, monocytes were cultured for 5 days in vitamin D-deplete media [deplete of both 25(OH)D and 1,25(OH)2D: DMEM plus 10% charcoal/dextran-treated FBS] plus macrophage colony-stimulating factor (M-CSF; 100 ng/ml; Sigma) and with or without 1,25(OH)2D3 supplementation (10−8 M). ER stress inhibition was obtained by adding phenyl butyric acid (PBA; 10 mM; Calbiochem) for 16 hours in macrophages following culture in vitamin D-deplete media. Induction of ER stress was obtained by adding Thapsigargin (0.25 µ M, Sigma) for 24 hours in macrophages following culture in vitamin D-supplemented media. Transwell migration assays were performed (Costar polycarbonate filters, 5 µ m pore size) as previously described [17]. Membranes and 12-well plates were coated with fibronectin (5 µl/mL; Life Technologies) overnight at 4 degrees. Macrophages (0.3 ×105 cells/well) were added to the upper chamber, and MCP-1 (100 ng/well; Sigma) in 0.8% agarose solution was added to the lower chamber to stimulate migration. Cells migrating into the lower chamber after 8 hours of incubation were manually counted. Quantitative RT-PCR (qPCR) analyses for CCR2 expression were performed by Sybrgreen methodologies and normalized to the housekeeping gene L32. Cholesterol uptake and efflux, as well as cholesteryl ester formation were performed as we previously described [7]. Briefly, cholesterol uptake was measured after macrophage incubation with 10 µg/mL oxidized low density lipoprotein (oxLDL) labeled with 1,1'-dioctadecyl-3,3,3',3'-tetramethyl indocarbocyanine percholate (DiI; Invitrogen) for 6 hours. Cholesteryl ester formation was measured after macrophage incubation with oxLDL (200 µg/mL) with 3H oleic acid (0.1 mM) (American Radiolabeled Chemicals Inc.) for 6 hours. Cholesterol efflux was measured after macrophage incubation for 24 hours with labeled oxLDL (300 µg/mL) with 5 mCi of 3H cholesterol and initiation of efflux by media containing high density lipoprotein (HDL; 50 µg/mL) or apolipoprotein A-I (ApoA-I; 25 µg/mL). Cholesterol uptake was assessed in differentiated vitamin D-deplete macrophages subsequently supplemented with 1,25(OH)2D3 (10−8 M) or maintained in vitamin D-deplete conditions for 5 additional days, then incubated with oxLDL. Cholesteryl ester formation and cholesterol efflux were also assessed in differentiated vitamin D-deplete macrophages subsequently supplemented with 1,25(OH)2D3 or maintained in vitamin D-deplete conditions, but prior to oxidized LDL incubation, cells were stimulated with IFNγ to promote M1 macrophage formation or IL-4, IL-10, or IC to promote M2 macrophage subtypes. Experiments were carried out with duplicate or triplicate samples, with results expressed as mean ± SEM for continuous variables. Statistical significance of differences was defined by p ≤0.05 using the paired t-test.
3. Results and Discussion
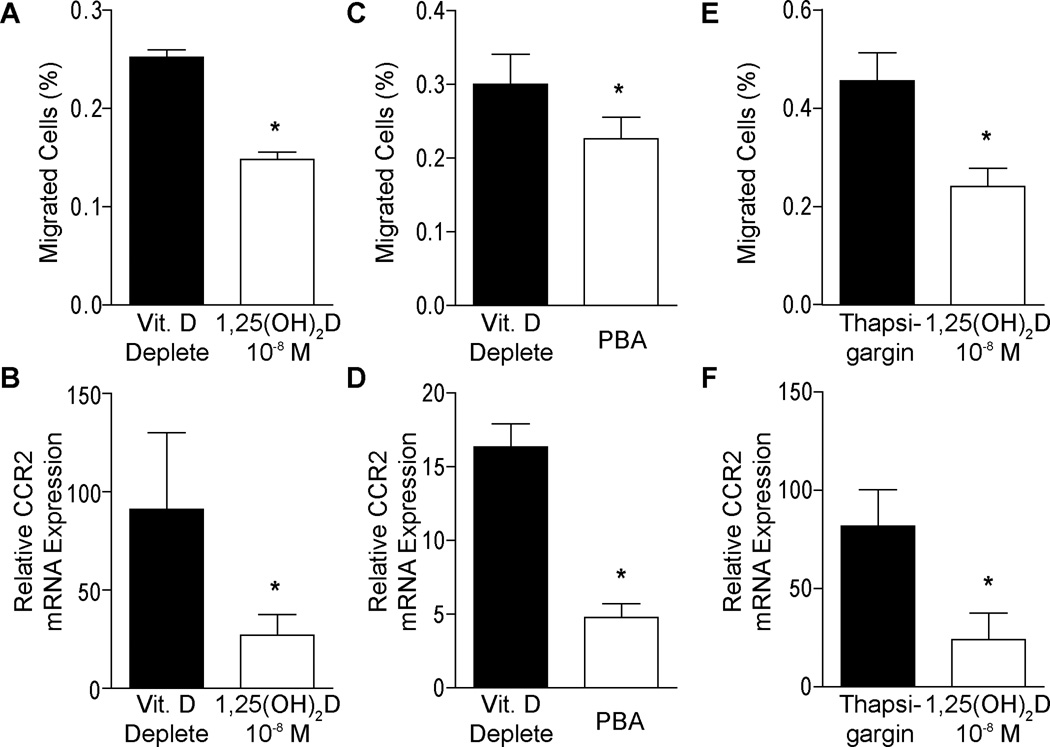
To evaluate mechanisms involved in plaque regression, we performed migration assays in vitamin D-deplete or 1,25(OH)2D-supplemented macrophages. Migration in response to MCP-1 was suppressed by 40% in 1,25(OH)2D-supplemented macrophages compared to vitamin D-deplete conditions (Figure 1A, p<0.01), and expression of the MCP-1 receptor, CCR2, was significantly decreased (Figure 1B, p<0.001), suggesting that vitamin D reduces monocyte recruitment. To test whether these effects are ER stress-dependent, we incubated vitamin D-deplete macrophages with PBA, an inhibitor of ER stress. PBA-treated macrophages showed a 25% decrease in migration in response to MCP-1 (Figure 1C, p<0.05) and suppressed expression of CCR2 (Figure 1D, p<0.001) compared to non-PBA treated macrophages. Conversely, stimulation of ER stress by Thapsigargin, an inducer of ER stress, in 1,25(OH)2D-supplemented macrophages nearly doubled migration in response to MCP-1 compared to non-Thapsigargin-treated cells (Figure 1E, p<0.001), also with a significant rise in CCR2 expression (Figure 1F, p<0.05). Thus, vitamin D suppression of CCR2 expression and macrophage migration through ER stress suppression could be a key mechanism to reduce monocyte infiltration and vascular inflammation in patients with type 2 diabetes.
Figure 1. 1,25(OH)2D suppresses macrophage migration in type 2 diabetics.
For A–B, monocytes from type 2 diabetics were differentiated into macrophages under vitamin D-deplete or 1,25(OH)2D-supplemented culture conditions. A. Transwell migration assay showing percentage of cells migrated in response to MCP-1 (n=9, p<0.01 vs. vitamin Ddeplete). B. qPCR for mRNA of migration receptor CCR2 (n=6, p<0.001 vs. vitamin D-deplete). For C–Dmonocytes from type 2 diabetics were differentiated into macrophages under vitamin D-deplete conditions with or without PBA (ER stress inhibitor). C. Transwell migration assay (n=9, p<0.05 vs. non-PBA-treated). D. qPCR for mRNA of CCR2 (n=6, p<0.001 vs. non-PBA-treated). For E–F, monocytes from type 2 diabetics were differentiated into macrophages under 1,25(OH)2D3-supplemented conditions with or without Thapsigargin (ER stress inducer). E. Transwell migration assay (n=9, p<0.001 vs. Thapsigargin). F. qPCR for mRNA of CCR2 (n=6, p<0.05 vs. Thapsigargin).
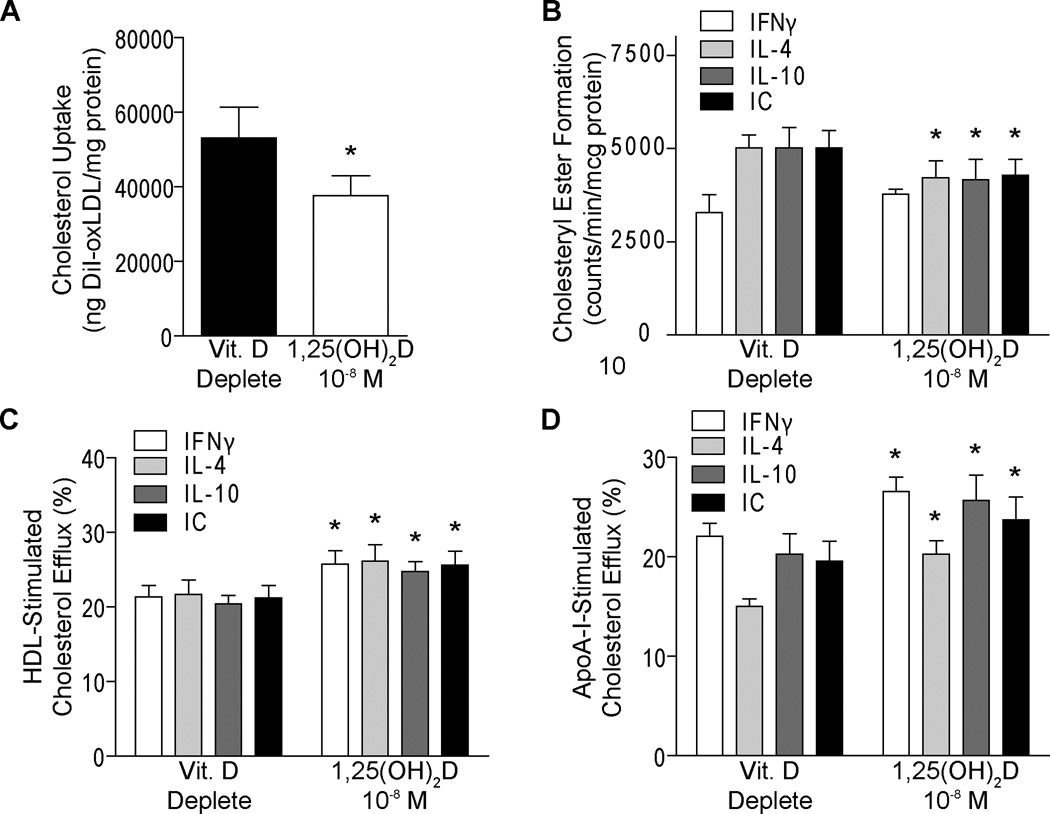
Although it has long been known that cholesterol lowering reduces atherogenesis in animal models [18, 19], it is still unclear which environmental conditions affect macrophage cholesterol metabolism to facilitate plaque regression. In this study, we differentiated monocytes into vitamin D-deplete macrophages over 5 days as described above, and then either replaced 1,25(OH)2D for an additional 5 days or maintained vitamin D-deplete conditions. We found 1,25(OH)2D suppressed oxLDL cholesterol uptake by nearly 30% compared with cells maintained in deplete conditions (Figure 2A, p<0.05), suggesting that active vitamin D could slow cholesterol accumulation. To evaluate whether 1,25(OH)2D facilitates reversal of macrophage cholesterol deposition in cytokine induced-macrophage subtypes, we replaced 1,25(OH)2D or maintained vitamin D-deplete conditions as described above, then stimulated with IFNγ to promote M1 macrophage formation or IL-4, IL-10, or IC to promote M2 macrophage subtypes prior to oxLDL cholesterol exposure. 1,25(OH)2D decreased cholesteryl ester formation by 15–20% compared to cells maintained in vitamin D-deplete conditions in M2 subtypes, while there was no effect on the baseline low levels in the M1 cells (Figure 2B, p<0.05). Additionally, active vitamin D enhanced HDL-induced cholesterol efflux by 20–25% and ApoA-I-induced cholesterol efflux by 20-35% in all macrophage subtypes, M1 and M2, compared to unsupplemented cells (Figure 2C–D, p<0.05). Therefore, active vitamin D reversed cholesterol deposition in differentiated macrophages by slowing cholesterol uptake and enhancing cholesterol egression in macrophages from patients with type 2 diabetes, indicating a possible role of vitamin D in plaque regression.
Figure 2. 1,25(OH)2D suppresses macrophage cholesterol deposition and facilitates cholesterol efflux.
A. Cholesterol uptake in macrophages from type 2 diabetics differentiated into macrophages under vitamin D-deplete conditions, then replaced with 1,25(OH)2D or not (n=4, p<0.05 vs. vitamin D-deplete). For B–Dmacrophages from type 2 diabetics were differentiated into macrophages under vitamin D-deplete conditions, then replaced with 1,25(OH)2D or maintained in vitamin D-deplete conditions, then stimulated with IFNγ to promote M1 macrophage formation or IL-4, IL-10, or IC to promote M2 macrophage subtypes prior to oxLDL exposure. B. Cholesteryl ester formation (n=4, p<0.05 for all vs. same subtype in vitamin D-deplete). Cholesterol efflux induced by C. HDL (n=6, p<0.001 for all vs. same subtype in vitamin D-deplete) or D. ApoA-I (n=6, p<0.05 for all vs. same subtype in vitamin D-deplete)
4. Conclusions
Active vitamin D acts as an ER stress reliever in type 2 diabetes, decreasing macrophage infiltration, reversing macrophage cholesterol deposition, and promoting cholesterol egression. These findings indicate vitamin D supplementation as a potential therapy for atherosclerosis regression.
1,25(OH)2D suppresses macrophage migration induced by MCP-1.
1,25(OH)2D supplementation prevents progression of macrophage cholesterol uptake.
1,25(OH)2D reverses cholesterol deposition in vitamin D-deplete macrophages.
1,25(OH)2D works as an ER stress reliever in diabetic macrophages.
Acknowledgements
This publication was made possible by the American Diabetes Association 1-12-CT-08, the Endocrine Society, the Endocrine Fellows Foundation, and NIH R01HL094818-0, P30DK079333, and UL1TR000448/Sub-Award KL2TR000450 from the NIH-National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations used include
- MCP-1
monocyte chemotactic protein 1
- ER
endoplasmic reticulum
- CVD
cardiovascular disease
- 1,25(OH)2D
1,25-dihydroxy vitamin D
- CCR2
chemokine (C-C motif) receptor 2
- IFN
interferon
- IL
interleukin
- IC
immunocomplex plus lipopolysaccharide
- 25(OH)D
25-hydroxy vitamin D
- M-CSF
macrophage colony-stimulating factor
- PBA
phenyl butyric acid
- qPCR
quantitative RT-PCR
- oxLDL
oxidized low density lipoprotein
- DiI
1,1'-dioctadecyl-3,3,3',3'-tetramethyl indocarbocyanine percholate
- HDL
high density lipoprotein
- ApoA-I
apolipoprotein A-I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amy E. Riek, Email: ariek@dom.wustl.edu.
Jisu Oh, Email: joh@dom.wustl.edu.
Carlos Bernal-Mizrachi, Email: cbernal@dom.wustl.edu.
References
- 1.Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24(8):1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 2.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29(3):722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 5.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77(1):47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120(8):687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/−mice during disease regression. The Journal of clinical investigation. 2011;121(5):2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103(10):3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S. Alternative activation of macrophages. Nature reviews. Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-tomacrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 14.Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, Cella M, Bernal-Mizrachi C. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287(15):11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5(1):e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riek AE, Oh J, Sprague JE, Timpson A, de Las Fuentes L, Bernal-Mizrachi L, Schechtman KB, Bernal-Mizrachi C. Vitamin D Suppression of Endoplasmic Reticulum Stress Promotes an Anti-Atherogenic Monocyte/Macrophage Phenotype in Type 2 Diabetic Patients. J Biol Chem. 2012 doi: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, Shepherd MD, Seibel JA, Kreisberg R, Goldberg R. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2012;18 Suppl(1):1–78. doi: 10.4158/ep.18.s1.1. [DOI] [PubMed] [Google Scholar]
- 18.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353(6341):265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong ML. Evidence of regression of atherosclerosis in primates and man. Postgraduate medical journal. 1976;52(609):456–461. doi: 10.1136/pgmj.52.609.456. [DOI] [PMC free article] [PubMed] [Google Scholar]